-
PDF
- Split View
-
Views
-
Cite
Cite
Elżbieta Rytel, Agnieszka Nemś, Anna Pęksa, Agnieszka Kita, Joanna Miedzianka, Agnieszka Tajner-Czopek, Alicja Zofia Kucharska, Anna Sokół-Łętowska, Karel Hamouz, Discolouration of raw and cooked coloured fleshed potatoes differing in anthocyanins and polyphenols content, International Journal of Food Science and Technology, Volume 54, Issue 1, January 2019, Pages 92–101, https://doi.org/10.1111/ijfs.13909
Close - Share Icon Share
Abstract
Despite variations in the content of polyphenols, the tested potato varieties with red and purple flesh contained similar amounts of anthocyanins. Potatoes of the red-fleshed HBr variety were distinguished by a significant content of pelargonidin-3-feruloylrutinoside-5-glucoside, and those of the purple-fleshed Vitelotte variety were characterised by a significant cyanidin-3-rutinoside content. Immediately after cutting, raw potatoes with red flesh showed a small share of yellow (b* parameter) and double the share of red (a*) as compared to purple-fleshed tubers. A reduction in the share of red dye was observed in purple- and red-fleshed potatoes except Vitelotte and Rosalinde tubers, at both 1 and 4 h after being cut. The flesh colour of cooked potatoes was characterised by low susceptibility to darkening, and purple-fleshed tubers were more saturated by chroma than tubers of red-fleshed varieties.
Introduction
Colour is one of the most important quality factors of both fresh and processed food. It often determines the acceptance and preferences for raw material or ready-to-eat products and influences our choice. The colour of raw potatoes as well as those subjected to heat treatment is related to the processes of enzymatic and chemical darkening of the pulp occurring in tubers after cutting. The enzymatic reactions take place after cutting of the raw potatoes, and are catalysed by means of atmospheric oxygen, by the enzyme complex most often referred to as polyphenol oxidase. Cresolase (monophenol monooxygenase), laccase and catecholase most often form part of this complex. Peroxidase and catalase are other potato enzymes oxidising phenols. The newly created quinones undergo non-enzymatic polymerisation reactions and linking to proteins, which leads to the formation of coloured melanin compounds (Stevens & Dewellar, 1996; Stevens et al., 1998). However, the coloured complexes formed as a result of chemical transformations of phenolic acids are responsible for darkening (greying) of cooked potatoes. The molecules of chlorogenic or caffeic acids link to colourless complexes with a divalent iron occurring in potato tissue. In the presence of atmospheric oxygen, oxidation of the divalent iron occurs, leading to the formation of coloured compounds (Hughes & Evans, 1967).
Before consumption, most vegetables are subjected to some culinary treatments, including heat, which affects their quality. During thermal processes many changes take place, including structure, taste, smell and colour, whose intensity depends largely on the initial quality of the raw material. The preservation of the attractive colour of the product, after its technological treatment, is one of the most important problems of food producers due to the high requirements of the modern consumer. Nowadays, consumers are looking for food that is not only characterised by high nutritional value, but also has interesting sensory features. Such a product could be potatoes of different flesh colour, especially purple/blue or red, which are an alternative to potatoes of traditional, yellow flesh varieties. In the last few years, red- or purple-fleshed potatoes as well as products obtained from them (such as chips) have appeared in retail trade, among others in some European countries, e.g. the UK, and on the American market. The potatoes of colourful flesh deserve special attention due to the higher content of polyphenolic compounds as compared to potatoes with yellow coloured flesh (Lewis et al., 1998; Hamouz et al., 2006; Rytel et al., 2014). Polyphenols are found in all parts of plants: flowers, fruits, seeds, leaves and roots. These compounds play an important role in food, especially in determining the sensory characteristics. They not only give a specific sour and bitter aftertaste, but also are responsible for the colour, and moreover can cause sedimentation and turbidity, especially in juices, wines or other drinks (Pietta, 2000; Alasalvar et al., 2001; Jeszka et al., 2010). Furthermore, they increase the nutritional value of food, through their strong antioxidant properties. The most common phenolic acids in plant tissues are hydroxycinnamic acids (Gawlik-Dziki, 2004). This group includes such acids as caffeic, chlorogenic (caffeic and quinic acid ester), o-, m- and p-coumaric, ferulic and sinapic acids. Chlorogenic acid and its isomers constitute about 80–90% of all phenolic compounds found in potato tubers with a traditional creamy flesh. In addition, potatoes contain cinnamic acid, p-coumaric acid, caffeic and ferulic acid (Gawlik-Dziki, 2004). Moreover, potato tubers of red and purple flesh also contain anthocyanins, which give them their characteristic colour. These dyes belong to the flavonoids – the largest and most diverse group of phenolic compounds found in plants (Jeszka et al., 2010). According to their chemical structure, flavonoids can be divided into flavones, flavanols (catechins and proanthocyanidins), isoflavones, anthocyanins and chalcones (King & Young, 1999; Czeczot, 2000; Wilska-Jeszka, 2007). The flavonoids are involved in determining the taste and colour of vegetables, fruits and food products obtained as a result of their processing. These compounds occur not only as free molecules (aglycones), but also, and more often, in a form associated with sugars – glycosides. The plant dyes anthocyanins are present in vegetables as acetylated cyanidin derivatives (Jeszka et al., 2010). Depending on the pH of the environment, the anthocyanins impart a colour from orange to red and from purple to blue (Yao et al., 2004; Mitek & Gasik, 2007; Jeszka et al., 2010). In recent years, studies have been conducted on potato varieties with intensive flesh colour, aimed at using the natural dyes contained therein as natural food additives.
Therefore, the purpose of the present investigation was to study the changes in the colour transformation occurring on the surface of halved red- and purple-fleshed potatoes of different anthocyanin profile and polyphenol content, studied in the fresh and cooked form at 1 and 4 h after cutting.
Materials and methods
Raw material
The material used for the study included four varieties of red-fleshed potatoes – Highland Burgundy Red, Herbie 26, Rosemarie and Rote Emma – and five varieties of purple-fleshed tubers: Blaue Elise, Blaue St. Galler, Blue Congo, Valfi and Vitelotte. The research was conducted during the growing seasons in 2015 and 2016 in the testing station of the Central Institute for Supervising and Testing in Agriculture at Přerov nad Labem (The Czech Republic). The samples of potato tubers were harvested after reaching full maturity. Each sample weighed 10 kg, and all analyses were repeated three times. Characteristics of varieties used for the experiment are shown in Table 1 (Lachman et al., 2013; Pęksa et al., 2013).
| Potato variety . | Maturity . | Flesh colour . | Cooking type* . |
|---|---|---|---|
| Blaue Elise | Medium-early | Purple | A |
| Blaue St. Galler | Medium-early | Purple | B, BC |
| Blue Congo | Medium-early to medium late | Purple | C, CD |
| Valfi | Medium-early to medium late | Purple | BC, C |
| Vitelotte | Late | Purple | AB, B, BC |
| Highland Burgundy Red | Medium-early | Red | BC |
| Herbie 26 | Medium-early | Red | BC |
| Rosalinde | Medium-early | Red | B, BC |
| Rote Emma | Early to medium-early | Red | B |
| Potato variety . | Maturity . | Flesh colour . | Cooking type* . |
|---|---|---|---|
| Blaue Elise | Medium-early | Purple | A |
| Blaue St. Galler | Medium-early | Purple | B, BC |
| Blue Congo | Medium-early to medium late | Purple | C, CD |
| Valfi | Medium-early to medium late | Purple | BC, C |
| Vitelotte | Late | Purple | AB, B, BC |
| Highland Burgundy Red | Medium-early | Red | BC |
| Herbie 26 | Medium-early | Red | BC |
| Rosalinde | Medium-early | Red | B, BC |
| Rote Emma | Early to medium-early | Red | B |
*Cooking type: A – salad, B – general-purpose, C – mealy, D – very mealy (AB, BC, CD –intermediate types).
| Potato variety . | Maturity . | Flesh colour . | Cooking type* . |
|---|---|---|---|
| Blaue Elise | Medium-early | Purple | A |
| Blaue St. Galler | Medium-early | Purple | B, BC |
| Blue Congo | Medium-early to medium late | Purple | C, CD |
| Valfi | Medium-early to medium late | Purple | BC, C |
| Vitelotte | Late | Purple | AB, B, BC |
| Highland Burgundy Red | Medium-early | Red | BC |
| Herbie 26 | Medium-early | Red | BC |
| Rosalinde | Medium-early | Red | B, BC |
| Rote Emma | Early to medium-early | Red | B |
| Potato variety . | Maturity . | Flesh colour . | Cooking type* . |
|---|---|---|---|
| Blaue Elise | Medium-early | Purple | A |
| Blaue St. Galler | Medium-early | Purple | B, BC |
| Blue Congo | Medium-early to medium late | Purple | C, CD |
| Valfi | Medium-early to medium late | Purple | BC, C |
| Vitelotte | Late | Purple | AB, B, BC |
| Highland Burgundy Red | Medium-early | Red | BC |
| Herbie 26 | Medium-early | Red | BC |
| Rosalinde | Medium-early | Red | B, BC |
| Rote Emma | Early to medium-early | Red | B |
*Cooking type: A – salad, B – general-purpose, C – mealy, D – very mealy (AB, BC, CD –intermediate types).
A portion of potatoes of the same size, weighing 0.5 kg, was washed and boiled in 0.7 L of boiling water for about 20 min (until softness). The degree of the tuber's cooking was checked organoleptically.
Analyses
Proximate analysis
In raw potatoes, dry matter according to AOAC methods, anthocyanins and total polyphenol contents were determined. Moreover, the flesh colour of raw and cooked potatoes at the time directly after cutting, and after 1 and 4 h, was examined by tristimulus colorimetry (Hunter Lab).
Sample preparation
Raw potatoes were freeze-dried (using an Edwards Modulyo 4KII freeze dryer, West Sussex, UK). The obtained dry material was ground in an electric grinder and used to determine the concentrations of anthocyanins and total polyphenols.
Extraction of polyphenols and anthocyanins
The samples were prepared according to the method described by Nemś et al. (2015). The freeze-dried raw potatoes were extracted with 70% aqueous acetone (0.1% acetic acid) in a graduated tube. The mixture was homogenised using a vortex and allowed to stand for 2 h at room temperature. The acetone–water solution was partitioned with chloroform to remove the lipophilic compounds. Next, the acetone–water fraction was collected and placed on a Büchi rotary evaporator (Merck, Darmstadt, Germany) until all residual acetone was evaporated. The remaining extract was brought to a known volume with 50% methanol and stored at 20 °C until analysed. The samples were filtered with 0.45-μm and 0.22-μm filters before HPLC-PDA analysis.
Quantification of anthocyanins by HPLC-PDA
Content of anthocyanins was determined according to Kucharska et al. (2017) using a Dionex (Waltham, MA, USA) HPLC system equipped with an Ultimate 3000 model of a diode array detector, an LPG-3400A quaternary pump, an EWPS-3000SI auto sampler and a TCC-3000SD thermos-stated column compartment, controlled by Chromeleon v.6.8 software. The Cadenza Imtakt column C5-C18 (75 × 4.6 mm, 5 μm; Portland, USA) was used. The following solvents constituted the mobile phase: 4.5% formic acid (solvent A) and 100% acetonitrile (solvent B). The following elution conditions were applied: 0–1 min 5% B in A; 1–20 min 25% B in A; 20–27 min 100% B in A; 27–30 min 5% B in A. The flow rate was 1 mL/min, and the injection volume was 40 μL. The column was operated at 30 °C. Anthocyanins were monitored at 520 nm and their content was expressed in cyanidin 3-O-glucoside equivalents (CygE)/100 g dry mass (dm).
Determination of total polyphenols
The total polyphenolic content of the extracts was determined using the Folin–Ciocalteu colorimetric method, as described by Gao et al. (2000). Potato extract (0.1 mL) and Folin–Ciocalteu reagent (0.2 mL) were pipetted into cuvettes. After 3 min, 1 mL of a 20% aqueous solution of sodium carbonate (Na2CO3) and 2 mL of distilled water were added. The absorbance at 765 nm was measured after 1 h, and the results were expressed as mg of gallic acid equivalents per 100 g of dry matter (dm). Data are reported as the mean value for six measurements.
Colour of raw and cooked potatoes
The measurement of darkening the pulp of raw and boiled potatoes was made immediately after cutting, at 1 and 4 h after cutting them (Roztropowicz, 1999; Kołodziejczyk et al., 2005).
A Minolta CM-5 spectrophotometer (Konica Minolta, Tokyo, Japan) was used to measure the colour of potatoes using the Hunter system. The spectrophotometer was calibrated against a white and black reference standard. Mean values of the coordinates L* (lightness-darkness), a* (redness-greenness), and b* (yellowness-blueness) were used to determine the colour of the product through the reflectance mode. Hue angle (h°) and chroma (C) colour space parameters were calculated from a* and b* values:
Hue angle = Arctan (b*/a*)
Chroma = ((a*2)+(b*2))0.5 (Wrolstad et al., 2005)
ΔE was determined using a “CIE 2000 calculator” (http://colormine.org/delta-e-calculator/cie2000).
Statistical analysis
The results obtained in the experiment were subjected to statistical calculations using Statistica 10.0 software (StatSoft Sp. z o.o., Kraków, Poland). A one-way analysis of variance was applied to demonstrate the significance of differences between potato varieties in terms of chemical composition and the impact of time after cutting on tubers’ colour transformations. Duncan's test (P ≤ 0.05) was applied to determine the significance of differences between means. All experiments were performed in three replications from 2 years of investigation and the present results show the mean of all data combined.
Results and discussion
Characteristics of raw material
Based on the present research, the influence of the potato variety on the dry matter content and the amount and composition of polyphenolic compounds, including anthocyanins, was observed. In potatoes of purple flesh varieties, the dry matter content was on average 22%, and was higher than the dry matter content in potatoes of red-fleshed varieties, which was 19.5% on average (Table 2). Most of the analysed potatoes, except the Vitelotte variety, met the requirements for edible tubers. The percentage content of dry matter of potatoes intended for consumption should be between 16 and 22% (Leszczyński, 2000). Also, the total polyphenol content in potatoes varied. The purple-fleshed varieties had more of these compounds – an average of 292.6 mg/100 g dm−1 – than potatoes of red-fleshed tubers – 279.7 mg/100 g dm−1. The anthocyanin content in potatoes of purple- and red-fleshed cultivars was similar, on average 50 mg/100 g dm−1 (Table 2). The amount of total polyphenol as well as anthocyanin content in potatoes depended mainly on their variety. Potatoes of colourful flesh varieties can contain about two to three times more total polyphenols than potatoes with traditional, yellow flesh (Lewis et al., 1998; Hamouz et al., 2006; Rytel et al., 2014). According to Reyes et al. (2005), the content of anthocyanins and total polyphenols in purple- and red-fleshed potatoes varied in a wide range, from 110 to 174 mg of cyanidin-3-glucoside per kg fresh weight and from 760 to 1810 mg of chlorogenic acid per kg fresh weight.
Dry matter (%), total polyphenol and total anthocyanin contents (mg/100 g dm−1) in coloured fleshed potatoes
| Variety . | Colour of flesh . | Dry matter . | Total Polyphenols . | Total Anthocyanins . |
|---|---|---|---|---|
| % . | mg/100 g dm−1 . | mg/100 g dm−1 . | ||
| Blaue Elise | Purple | 20.0C ± 0.14 | 284.5D ± 28.6 | 29.7E ± 0.98 |
| Blaue St Galler | 20.1C ± 0.17 | 246.8E ± 32.8 | 49.0C ± 0.99 | |
| Blue Congo | 21.3B ± 0.11 | 307.8C ± 26.5 | 49.6C ± 0.78 | |
| Valfi | 20.4C ± 0.20 | 219.5F ± 36.7 | 39.4D ± 0.96 | |
| Vitelotte | 27.5A ± 0.25 | 404.6A ± 41.2 | 86.7A ± 1.32 | |
| Highland Burgundy Red | Red | 20.4C ± 0.18 | 348.1B ± 36.7 | 88.8A ± 1.12 |
| Herbie 26 | 19.2D ± 0.14 | 236.9E ± 28.4 | 64.1B ± 0.89 | |
| Rosalinde | 21.0B ± 0.20 | 231.2F ± 32.2 | 16.6F ± 0.45 | |
| Rote Emma | 17.2E ± 0.19 | 302.4C ± 31.9 | 32.2DE ± 0.79 |
| Variety . | Colour of flesh . | Dry matter . | Total Polyphenols . | Total Anthocyanins . |
|---|---|---|---|---|
| % . | mg/100 g dm−1 . | mg/100 g dm−1 . | ||
| Blaue Elise | Purple | 20.0C ± 0.14 | 284.5D ± 28.6 | 29.7E ± 0.98 |
| Blaue St Galler | 20.1C ± 0.17 | 246.8E ± 32.8 | 49.0C ± 0.99 | |
| Blue Congo | 21.3B ± 0.11 | 307.8C ± 26.5 | 49.6C ± 0.78 | |
| Valfi | 20.4C ± 0.20 | 219.5F ± 36.7 | 39.4D ± 0.96 | |
| Vitelotte | 27.5A ± 0.25 | 404.6A ± 41.2 | 86.7A ± 1.32 | |
| Highland Burgundy Red | Red | 20.4C ± 0.18 | 348.1B ± 36.7 | 88.8A ± 1.12 |
| Herbie 26 | 19.2D ± 0.14 | 236.9E ± 28.4 | 64.1B ± 0.89 | |
| Rosalinde | 21.0B ± 0.20 | 231.2F ± 32.2 | 16.6F ± 0.45 | |
| Rote Emma | 17.2E ± 0.19 | 302.4C ± 31.9 | 32.2DE ± 0.79 |
Average values (n = 6) labelled with the same letter in the same column are not statistically different (P ≤ 0.05).
Dry matter (%), total polyphenol and total anthocyanin contents (mg/100 g dm−1) in coloured fleshed potatoes
| Variety . | Colour of flesh . | Dry matter . | Total Polyphenols . | Total Anthocyanins . |
|---|---|---|---|---|
| % . | mg/100 g dm−1 . | mg/100 g dm−1 . | ||
| Blaue Elise | Purple | 20.0C ± 0.14 | 284.5D ± 28.6 | 29.7E ± 0.98 |
| Blaue St Galler | 20.1C ± 0.17 | 246.8E ± 32.8 | 49.0C ± 0.99 | |
| Blue Congo | 21.3B ± 0.11 | 307.8C ± 26.5 | 49.6C ± 0.78 | |
| Valfi | 20.4C ± 0.20 | 219.5F ± 36.7 | 39.4D ± 0.96 | |
| Vitelotte | 27.5A ± 0.25 | 404.6A ± 41.2 | 86.7A ± 1.32 | |
| Highland Burgundy Red | Red | 20.4C ± 0.18 | 348.1B ± 36.7 | 88.8A ± 1.12 |
| Herbie 26 | 19.2D ± 0.14 | 236.9E ± 28.4 | 64.1B ± 0.89 | |
| Rosalinde | 21.0B ± 0.20 | 231.2F ± 32.2 | 16.6F ± 0.45 | |
| Rote Emma | 17.2E ± 0.19 | 302.4C ± 31.9 | 32.2DE ± 0.79 |
| Variety . | Colour of flesh . | Dry matter . | Total Polyphenols . | Total Anthocyanins . |
|---|---|---|---|---|
| % . | mg/100 g dm−1 . | mg/100 g dm−1 . | ||
| Blaue Elise | Purple | 20.0C ± 0.14 | 284.5D ± 28.6 | 29.7E ± 0.98 |
| Blaue St Galler | 20.1C ± 0.17 | 246.8E ± 32.8 | 49.0C ± 0.99 | |
| Blue Congo | 21.3B ± 0.11 | 307.8C ± 26.5 | 49.6C ± 0.78 | |
| Valfi | 20.4C ± 0.20 | 219.5F ± 36.7 | 39.4D ± 0.96 | |
| Vitelotte | 27.5A ± 0.25 | 404.6A ± 41.2 | 86.7A ± 1.32 | |
| Highland Burgundy Red | Red | 20.4C ± 0.18 | 348.1B ± 36.7 | 88.8A ± 1.12 |
| Herbie 26 | 19.2D ± 0.14 | 236.9E ± 28.4 | 64.1B ± 0.89 | |
| Rosalinde | 21.0B ± 0.20 | 231.2F ± 32.2 | 16.6F ± 0.45 | |
| Rote Emma | 17.2E ± 0.19 | 302.4C ± 31.9 | 32.2DE ± 0.79 |
Average values (n = 6) labelled with the same letter in the same column are not statistically different (P ≤ 0.05).
In the conducted studies, differences in the composition of assessed anthocyanins between potato varieties were also found (Fig. 1a, b). Pelargonidin was a dominant anthocyanin in red-fleshed potatoes, whereas petunidin was found in purple-fleshed varieties. In tubers of Rote Emma, Rosalinde and Herbie 26 (red-fleshed potatoes), the largest share in assessed anthocyanins (around 80%) was observed for pelargonidin-3-p-coumaroylrutinoside-5-glucoside, whereas pelargonidin-3-feruloylrutinoside 5-glucoside was predominant in Highland Burgundy Red tubers. The share of the remaining marked anthocyanins, i.e. pelargonidin-3-rutinoside-5-glucoside, pelargonidin-3-rutoside, pelargonidin-3-caffeoylrutinoside-5-glucoside, and pelargonidin in red-fleshed potatoes was at the level of a few percent. However, in purple-fleshed potatoes – Vitelotte, Valfi, Blue Congo, Blaue St. Galler, Blaue Elise – besides petunidin, whose share in the determined anthocyanins was the highest, there were also malvidin, peonidin and cyanidin. In potatoes of purple-fleshed varieties, greater differences in the composition of anthocyanins were observed. An example is the Vitelotte variety, characterised by the highest content of petunidin-3-feruloylrutinoside-5-glucoside – about 70% of total anthocyanins. In the remaining potatoes, the proportion of this anthocyanin was at the level from a few to a dozen or so percent, and the dominant anthocyanin was petunidin-3-coumaroylrutinoside-5-glucoside. In smaller quantities, the purple-fleshed potatoes contained petunidin-3-rutinoside-5-glucoside, malvidin-3-feruloylrutinoside-5-glucoside, cyanidin-3-rutinoside, peonidin-3-rutinoside-5-glucoside and petunidin-3-caffeoylrutinoside-5-glucoside (Fig. 1a).
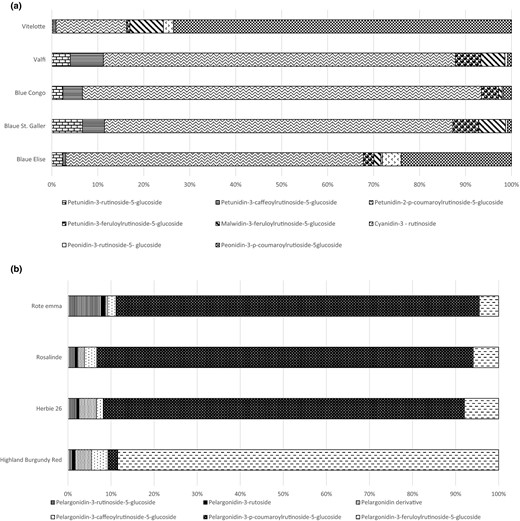
(a) Anthocyanin profile of purple-fleshed potatoes used in experiment. (b) Anthocyanin profile of red-fleshed potatoes used in experiment.
The composition and amount of anthocyanins in potatoes determined the differences in the colour of tuber flesh (Zhao et al., 2009). According to Eichhorn & Winterhalter (2005), petunidin was an anthocyanin found in most purple-fleshed potatoes, whereas pelargonidin was found in Highland Burgundy Red, a red-fleshed variety. Also, according to Nemś et al. (2015), purple-fleshed potatoes contained petunidin and malvidin, whereas red-fleshed varieties contained pelargonidin. In the plant world, the most common anthocyanins are cyanidin derivatives, then pelargonidins, peonidins and delphinidins. Petunidin and malvidin were also identified in plants, which among other things occurred in potatoes with purple-fleshed tubers (Lachman et al., 2009, 2012; Ieri et al., 2011; Michalska et al., 2016). Aside from the six main anthocyanins, more than 540 anthocyanin dyes have been identified in nature (Wrolstad et al., 2005). Anthocyanins differ from each other in structure, but most often they occur in the form of glycosides, i.e. combinations with simple sugars at positions 3 and 5 (a bridge of the structural variation from glycosides and the possible residues of sugar residues with organic acids). The colour of potato flesh varies depending on the type of anthocyanins contained in plants, their combinations with metals and the presence of other dyes (i.e. carotenoids).
The results of the colour measurement of flesh (purple or red) immediately after cutting the tubers and after the first and 4 h after cutting are presented in Table 3. The flesh of the purple-coloured tubers, immediately after cutting, was darker than the flesh of red-coloured potatoes. The lightness (L *) of purple-fleshed tubers ranged from 32 (Vitelotte) to 38.3 (Blue Congo), and that of red potatoes ranged from 50.7 (Rote Emma) to 57.3 (Rosalinde). This was due to the fact that purple-fleshed tubers usually contained more polyphenols, in particular anthocyanins, than the raw material of red varieties, whose composition was different (Kita et al., 2013, 2015; Nemś et al., 2015). Immediately after cutting the colour of the tuber flesh of the purple varieties was characterised by a lower proportion of red (a*), which ranged from 6.5 (Blaue Elise) to 15.4 (Blue Congo), as compared to red-fleshed potatoes, in which the a* value ranged from 19 (Rote Emma) to 23.2 (Rosalinde). In addition, the flesh of red-coloured varieties immediately after being cut was characterised by a small share of yellow (the b* parameter was 3.2 on average), whereas among purple-fleshed varieties the shade was blue (b* on average was −7.2; Table 3).
| Variety . | Colour of flesh . | L* . | a* . | b* . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | ||
| Hour . | Hour . | Hour . | ||||||||
| Blaue Elise | Purple | 32.8B ± 1.5 | 33.7AB ± 1.9 | 35.4A ± 1.9 | 6.5A ± 1.6 | 5.0AB ± 1.9 | 4.4B ± 1.6 | −4.6B ± 0.3 | −2.6A ± 0.6 | −2.3A ± 0.9 |
| Blaue St Galler | 35.4A ± 3.1 | 37.6A ± 2.8 | 38.1A ± 2.9 | 13.4A ± 1.3 | 11.6B ± 1.6 | 10.6B ± 1.4 | −8.2C ± 0.6 | −6.3B ± 0.5 | −5.2A ± 0.8 | |
| Blue Congo | 38.3A ± 2.1 | 39.8A ± 5.5 | 40.9A ± 4.0 | 15.4A ± 2.0 | 13.2AB ± 1.7 | 11.4B ± 2.3 | −8.1B ± 0.6 | −5.8A ± 1.1 | −5.5A ± 0.6 | |
| Valfi | 37.3A ± 4.8 | 38.8A ± 4.5 | 41.7A ± 5.3 | 14.6A ± 2.0 | 13.4AB ± 1.8 | 11.9B ± 1.6 | −8.5B ± 0.9 | −6.2A ± 1.0 | −5.2A ± 1.0 | |
| Vitelotte | 32.0B ± 1.3 | 36.2A ± 2.9 | 37.8A ± 1.9 | 10.4A ± 1.6 | 10.3A ± 2.2 | 8.2A ± 2.3 | −6.6B ± 0.6 | −4.9A ± 1.8 | −4.1A ± 0.8 | |
| Average | 35.2 | 37.2 | 38.8 | 12.1 | 10.7 | 9.3 | −7.2 | −5.2 | −4.5 | |
| Highland Burgundy Red | Red | 55.6A ± 3.9 | 56.5A ± 1.9 | 57.3A ± 3.3 | 19.4A ± 3.2 | 18.3A ± 3.3 | 16.6A ± 2.7 | 3.5A ± 1.2 | 5.1A ± 1.8 | 5.1A ± 1.3 |
| Herbie 26 | 50.9A ± 5.2 | 51.3A ± 2.8 | 52.6A ± 3.2 | 19.4A ± 5.6 | 19.8A ± 4.7 | 18.3A ± 4.2 | 3.1A ± 1.8 | 3.5A ± 2.3 | 4.0A ± 1.6 | |
| Rosalinde | 57.3A ± 2.0 | 56.3A ± 3.3 | 55.9A ± 2.8 | 23.2A ± 0.9 | 21.3B ± 0.8 | 19.3C ± 0.8 | 4.2C ± 0.6 | 7.3A ± 0.3 | 6.0B ± 0.8 | |
| Rote Emma | 50.7A ± 2.9 | 49.8A ± 2.9 | 50.4A ± 2.3 | 19.0A ± 3.7 | 17.4A ± 3.2 | 16.1A ± 3.3 | 1.9C ± 0.5 | 3.9A ± 0.5 | 2.9B ± 0.8 | |
| Average | 53.6 | 53.5 | 54.0 | 20.2 | 19.2 | 17.6 | 3.2 | 4.9 | 4.5 | |
| Variety . | Colour of flesh . | L* . | a* . | b* . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | ||
| Hour . | Hour . | Hour . | ||||||||
| Blaue Elise | Purple | 32.8B ± 1.5 | 33.7AB ± 1.9 | 35.4A ± 1.9 | 6.5A ± 1.6 | 5.0AB ± 1.9 | 4.4B ± 1.6 | −4.6B ± 0.3 | −2.6A ± 0.6 | −2.3A ± 0.9 |
| Blaue St Galler | 35.4A ± 3.1 | 37.6A ± 2.8 | 38.1A ± 2.9 | 13.4A ± 1.3 | 11.6B ± 1.6 | 10.6B ± 1.4 | −8.2C ± 0.6 | −6.3B ± 0.5 | −5.2A ± 0.8 | |
| Blue Congo | 38.3A ± 2.1 | 39.8A ± 5.5 | 40.9A ± 4.0 | 15.4A ± 2.0 | 13.2AB ± 1.7 | 11.4B ± 2.3 | −8.1B ± 0.6 | −5.8A ± 1.1 | −5.5A ± 0.6 | |
| Valfi | 37.3A ± 4.8 | 38.8A ± 4.5 | 41.7A ± 5.3 | 14.6A ± 2.0 | 13.4AB ± 1.8 | 11.9B ± 1.6 | −8.5B ± 0.9 | −6.2A ± 1.0 | −5.2A ± 1.0 | |
| Vitelotte | 32.0B ± 1.3 | 36.2A ± 2.9 | 37.8A ± 1.9 | 10.4A ± 1.6 | 10.3A ± 2.2 | 8.2A ± 2.3 | −6.6B ± 0.6 | −4.9A ± 1.8 | −4.1A ± 0.8 | |
| Average | 35.2 | 37.2 | 38.8 | 12.1 | 10.7 | 9.3 | −7.2 | −5.2 | −4.5 | |
| Highland Burgundy Red | Red | 55.6A ± 3.9 | 56.5A ± 1.9 | 57.3A ± 3.3 | 19.4A ± 3.2 | 18.3A ± 3.3 | 16.6A ± 2.7 | 3.5A ± 1.2 | 5.1A ± 1.8 | 5.1A ± 1.3 |
| Herbie 26 | 50.9A ± 5.2 | 51.3A ± 2.8 | 52.6A ± 3.2 | 19.4A ± 5.6 | 19.8A ± 4.7 | 18.3A ± 4.2 | 3.1A ± 1.8 | 3.5A ± 2.3 | 4.0A ± 1.6 | |
| Rosalinde | 57.3A ± 2.0 | 56.3A ± 3.3 | 55.9A ± 2.8 | 23.2A ± 0.9 | 21.3B ± 0.8 | 19.3C ± 0.8 | 4.2C ± 0.6 | 7.3A ± 0.3 | 6.0B ± 0.8 | |
| Rote Emma | 50.7A ± 2.9 | 49.8A ± 2.9 | 50.4A ± 2.3 | 19.0A ± 3.7 | 17.4A ± 3.2 | 16.1A ± 3.3 | 1.9C ± 0.5 | 3.9A ± 0.5 | 2.9B ± 0.8 | |
| Average | 53.6 | 53.5 | 54.0 | 20.2 | 19.2 | 17.6 | 3.2 | 4.9 | 4.5 | |
Average values (n = 6) labelled with the same letter in the same row are not statistically different (P ≤ 0.05).
| Variety . | Colour of flesh . | L* . | a* . | b* . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | ||
| Hour . | Hour . | Hour . | ||||||||
| Blaue Elise | Purple | 32.8B ± 1.5 | 33.7AB ± 1.9 | 35.4A ± 1.9 | 6.5A ± 1.6 | 5.0AB ± 1.9 | 4.4B ± 1.6 | −4.6B ± 0.3 | −2.6A ± 0.6 | −2.3A ± 0.9 |
| Blaue St Galler | 35.4A ± 3.1 | 37.6A ± 2.8 | 38.1A ± 2.9 | 13.4A ± 1.3 | 11.6B ± 1.6 | 10.6B ± 1.4 | −8.2C ± 0.6 | −6.3B ± 0.5 | −5.2A ± 0.8 | |
| Blue Congo | 38.3A ± 2.1 | 39.8A ± 5.5 | 40.9A ± 4.0 | 15.4A ± 2.0 | 13.2AB ± 1.7 | 11.4B ± 2.3 | −8.1B ± 0.6 | −5.8A ± 1.1 | −5.5A ± 0.6 | |
| Valfi | 37.3A ± 4.8 | 38.8A ± 4.5 | 41.7A ± 5.3 | 14.6A ± 2.0 | 13.4AB ± 1.8 | 11.9B ± 1.6 | −8.5B ± 0.9 | −6.2A ± 1.0 | −5.2A ± 1.0 | |
| Vitelotte | 32.0B ± 1.3 | 36.2A ± 2.9 | 37.8A ± 1.9 | 10.4A ± 1.6 | 10.3A ± 2.2 | 8.2A ± 2.3 | −6.6B ± 0.6 | −4.9A ± 1.8 | −4.1A ± 0.8 | |
| Average | 35.2 | 37.2 | 38.8 | 12.1 | 10.7 | 9.3 | −7.2 | −5.2 | −4.5 | |
| Highland Burgundy Red | Red | 55.6A ± 3.9 | 56.5A ± 1.9 | 57.3A ± 3.3 | 19.4A ± 3.2 | 18.3A ± 3.3 | 16.6A ± 2.7 | 3.5A ± 1.2 | 5.1A ± 1.8 | 5.1A ± 1.3 |
| Herbie 26 | 50.9A ± 5.2 | 51.3A ± 2.8 | 52.6A ± 3.2 | 19.4A ± 5.6 | 19.8A ± 4.7 | 18.3A ± 4.2 | 3.1A ± 1.8 | 3.5A ± 2.3 | 4.0A ± 1.6 | |
| Rosalinde | 57.3A ± 2.0 | 56.3A ± 3.3 | 55.9A ± 2.8 | 23.2A ± 0.9 | 21.3B ± 0.8 | 19.3C ± 0.8 | 4.2C ± 0.6 | 7.3A ± 0.3 | 6.0B ± 0.8 | |
| Rote Emma | 50.7A ± 2.9 | 49.8A ± 2.9 | 50.4A ± 2.3 | 19.0A ± 3.7 | 17.4A ± 3.2 | 16.1A ± 3.3 | 1.9C ± 0.5 | 3.9A ± 0.5 | 2.9B ± 0.8 | |
| Average | 53.6 | 53.5 | 54.0 | 20.2 | 19.2 | 17.6 | 3.2 | 4.9 | 4.5 | |
| Variety . | Colour of flesh . | L* . | a* . | b* . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | ||
| Hour . | Hour . | Hour . | ||||||||
| Blaue Elise | Purple | 32.8B ± 1.5 | 33.7AB ± 1.9 | 35.4A ± 1.9 | 6.5A ± 1.6 | 5.0AB ± 1.9 | 4.4B ± 1.6 | −4.6B ± 0.3 | −2.6A ± 0.6 | −2.3A ± 0.9 |
| Blaue St Galler | 35.4A ± 3.1 | 37.6A ± 2.8 | 38.1A ± 2.9 | 13.4A ± 1.3 | 11.6B ± 1.6 | 10.6B ± 1.4 | −8.2C ± 0.6 | −6.3B ± 0.5 | −5.2A ± 0.8 | |
| Blue Congo | 38.3A ± 2.1 | 39.8A ± 5.5 | 40.9A ± 4.0 | 15.4A ± 2.0 | 13.2AB ± 1.7 | 11.4B ± 2.3 | −8.1B ± 0.6 | −5.8A ± 1.1 | −5.5A ± 0.6 | |
| Valfi | 37.3A ± 4.8 | 38.8A ± 4.5 | 41.7A ± 5.3 | 14.6A ± 2.0 | 13.4AB ± 1.8 | 11.9B ± 1.6 | −8.5B ± 0.9 | −6.2A ± 1.0 | −5.2A ± 1.0 | |
| Vitelotte | 32.0B ± 1.3 | 36.2A ± 2.9 | 37.8A ± 1.9 | 10.4A ± 1.6 | 10.3A ± 2.2 | 8.2A ± 2.3 | −6.6B ± 0.6 | −4.9A ± 1.8 | −4.1A ± 0.8 | |
| Average | 35.2 | 37.2 | 38.8 | 12.1 | 10.7 | 9.3 | −7.2 | −5.2 | −4.5 | |
| Highland Burgundy Red | Red | 55.6A ± 3.9 | 56.5A ± 1.9 | 57.3A ± 3.3 | 19.4A ± 3.2 | 18.3A ± 3.3 | 16.6A ± 2.7 | 3.5A ± 1.2 | 5.1A ± 1.8 | 5.1A ± 1.3 |
| Herbie 26 | 50.9A ± 5.2 | 51.3A ± 2.8 | 52.6A ± 3.2 | 19.4A ± 5.6 | 19.8A ± 4.7 | 18.3A ± 4.2 | 3.1A ± 1.8 | 3.5A ± 2.3 | 4.0A ± 1.6 | |
| Rosalinde | 57.3A ± 2.0 | 56.3A ± 3.3 | 55.9A ± 2.8 | 23.2A ± 0.9 | 21.3B ± 0.8 | 19.3C ± 0.8 | 4.2C ± 0.6 | 7.3A ± 0.3 | 6.0B ± 0.8 | |
| Rote Emma | 50.7A ± 2.9 | 49.8A ± 2.9 | 50.4A ± 2.3 | 19.0A ± 3.7 | 17.4A ± 3.2 | 16.1A ± 3.3 | 1.9C ± 0.5 | 3.9A ± 0.5 | 2.9B ± 0.8 | |
| Average | 53.6 | 53.5 | 54.0 | 20.2 | 19.2 | 17.6 | 3.2 | 4.9 | 4.5 | |
Average values (n = 6) labelled with the same letter in the same row are not statistically different (P ≤ 0.05).
Changes in the colour of raw tuber flesh
At 1 and 4 h after tuber cutting, regardless of the colour of the flesh, the lightness of all tested samples of varieties did not change except potatoes of the Vitelotte and Blaue Elise varieties. In these tubers, the colour after cutting was lighter (L* −37.7 and 35.4, respectively; Table 3). It might result from the partial degradation of anthocyanins and their different susceptibility to polyphenol oxidase and peroxidase. Peonidin-3-p-coumarylrutinoside-5-glucoside was a predominant anthocyanin in the Vitelotte potato variety (Fig. 1a), which was also a significant part of anthocyanin occurring in Blaue Elise tubers. In the other studied purple-fleshed varieties, petunidin-3-p-coumaroylrutinoside-5-glucoside was the main anthocyanin, while in red-fleshed potatoes derivatives of pelargonidin were noted (Fig. 1a, b).
Four hours after cutting of purple-fleshed potatoes, there was observed a reduction in the share of red dye in the colour of flesh, and moreover the degree of these changes depended primarily on the variety. On the other hand, in potatoes of the Vitelotte variety no significant changes in the share of the red dye were observed. Meanwhile, among the potatoes of red-fleshed varieties, the share of the red dye did not change significantly within 4 h after being cut, with the exception of Rosalinde tubers. In these potatoes, the share of red dye in the colour was found to decrease both after 1 and 4 h after being cut (Table 3).
The changes in red colour (factor a*) in potatoes of colourful fleshed varieties differed from the changes taking place in the raw material with the traditional yellow flesh. In the Snoeck et al. (2011) studies, there was observed an increase in the share of red colour during 7-day storage of potatoes with a light flesh. Changes in the colour of the traditional flesh, especially towards brownish yellow, were the result of enzymatic processes of oxidation of tyrosine, caffeic and chlorogenic acid, and other polyphenols by polyphenol oxidase and peroxidase. Initial reactions catalysed by polyphenol oxidase form-brown o-quinones, which are very reactive. The obtained intermediate products then underwent a series of non-enzymatic reactions, resulting in the formation of insoluble, high molecular weight melanin compounds (Thygesen et al., 1995).
In the analysed potatoes of purple-fleshed varieties after being cut, it was also found that the share of blue colour was reduced (Table 3) and yellow slightly increased. These changes occurred in all potatoes of purple cultivars, and the share of factor b* decreased on average from −7.2 to −4.5 (Table 3). In contrast, in red-coloured tubers there was observed a significant increase in saturation of the yellow dye of the potato flesh, especially in the Rosalinde and Rote Emma varieties (Table 3). In other red-fleshed potatoes, no significant changes were observed in the share of yellow colour 1 and 4 h after cutting.
During the analysis, there was also noted a change in the tone of the flesh colour in both purple and red flesh varieties after 1 h of the experiment. The average hue value of purple-fleshed potatoes increased from 328.5 directly after tuber cutting to about 334 1 h after cutting and in tubers of red flesh from 9.9 to about 16 (Fig. 2a), and it was maintained at a similar level 4 h after cutting of potatoes. It was also found that the saturation (chroma) of colour of purple- and red-fleshed potatoes decreased, whereas in the red-fleshed raw material significant changes were visible already after the first hour of the experiment, whereas the saturation of purple-coloured flesh significantly decreased only 4 h after cutting of potatoes.
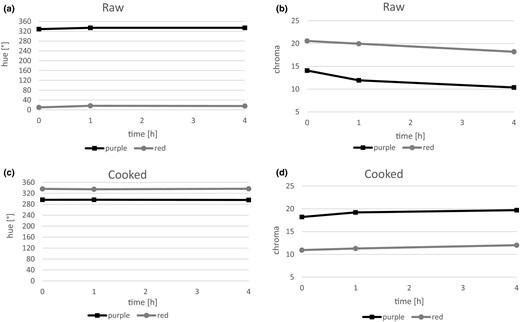
Changes in hunter hue of raw (a) and boiled potatoes (c), and also saturation of colour of raw (b) and cooked potatoes (d) directly, 1 and 4 h after cooking.
Changes in the colour of cooked potatoes
Potatoes must be subjected to thermal treatment before consumption. Cooking, steaming, frying and baking are the most common treatments used to prepare potatoes for consumption. During thermal processing of potatoes, a number of processes take place, such as starch gelatinisation, enzyme inactivation and loss of some nutrients, minerals as well as biologically active compounds (Tian et al., 2016). The thermal treatment process might also cause anthocyanin losses (Patras et al., 2010) and thus changes in the colour of tuber flesh.
On the basis of the conducted studies, it was found that the cooking affected the colour of the tuber flesh (ΔE) in the range from 6.0 (Herbie 26) to 17.9 (Rosalinde). The largest changes in the colour of the flesh were found in potatoes of the varieties Rosalinde (ΔE = 17.91), Blue St Galler (ΔE = 10.45) and Blue Congo (ΔE = 10.27; Table 4). According to other authors (Lachman et al., 2012), the differences in the degree of colour change of the tuber flesh after cooking depended, among other things, on the anthocyanin profile, their quantity in tubers and the presence of other substances that accelerate or inhibit the degradation of anthocyanins. Some of the anthocyanins, as well as water-soluble compounds (Bridle & Timberlake, 1997), during cooking were washed out from the tubers and transferred to the water in which the thermal treatment was carried out.
| Variety . | Colour of flesh . | ΔE . | L* . | a* . | b* . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | |||
| Hour . | Hour . | Hour . | |||||||||
| Blaue Elise | Purple | 7.1 | 34.9A ± 2.6 | 34.0A ± 1.8 | 32.9A ± 1.2 | 4.1A ± 1.4 | 3.9A ± 0.8 | 4.3A ± 1.3 | −12.3A ± 1.6 | −12.8A ± 1.4 | −13.9A ± 1.7 |
| Blaue St Galler | 10.4 | 31.9A ± 2.9 | 31.3A ± 3.3 | 29.6A ± 2.1 | 7.0A0 ± 2.5 | 6.9A ± 1.5 | 7.1A ± 2.1 | −16.3A ± 1.5 | −16.7A ± 1.6 | −16.9A ± 1.5 | |
| Blue Congo | 10.3 | 37.7A ± 3.6 | 36.7A ± 3.6 | 35.9A ± 3.3 | 8.1A ± 1.3 | 8.4A ± 1.4 | 8.4A ± 1.9 | −16.7A ± 1.2 | −17.0AB ± 1.0 | −18.4B ± 1.2 | |
| Valfi | 9.4 | 39.3A ± 4.3 | 39.5A ± 4.4 | 38.0A ± 5.5 | 8.7A ± 1.5 | 9.3A ± 2.0 | 9.0A ± 1.4 | −17.5A ± 1.4 | −18.3A ± 1.4 | −19.3A ± 1.2 | |
| Vitelotte | 8.6 | 41.7A ± 9.1 | 36.4A ± 2.0 | 35.2A ± 1.9 | 14.1A ± 4.1 | 16.1A ± 2.4 | 15.4A ± 2.7 | −16.9A ± 3.4 | −19.0A ± 1.4 | 18.6A ± 1.6 | |
| Average | 9.2 | 37.1 | 35.6 | 34.3 | 8.4 | 8.9 | 8.8 | −15.9 | −16.8 | −17.4 | |
| Highland Burgundy Red | Red | 8.7 | 53.4A ± 2.6 | 54.1A ± 2.6 | 53.4A ± 2.8 | 11.9A ± 2.3 | 12.0A ± 2.9 | 13.4A ± 3.2 | −3.7A ± 0.4 | −3.6A ± 1.0 | −4.4A ± 0.6 |
| Herbie 26 | 6.0 | 53.0A ± 1.4 | 53.0A ± 2.0 | 52.8A ± 1.8 | 9.9A ± 1.7 | 10.6A ± 1.8 | 11.2A ± 2.3 | −5.6A ± 0.3 | −5.9A ± 0.4 | −5.9A ± 0.4 | |
| Rosalinde | 17.9 | 62.0A ± 3.5 | 61.8A ± 4.1 | 59.8A ± 3.1 | 6.7A ± 1.6 | 7.0A ± 1.7 | 8.0A ± 1.0 | −3.3A ± 1.7 | −3.8A ± 1.9 | −3.3A ± 1.2 | |
| Rote Emma | 8.0 | 51.0A ± 1.3 | 52.3A ± 1.8 | 52.0A ± 0.8 | 10.8A ± 1.1 | 11.0A ± 1.3 | 11.4A ± 0.9 | −4.9A ± 0.8 | −5.2A ± 0.9 | −5.3A ± 0.6 | |
| Average | 10.1 | 54.8 | 55.3 ± | 54.5 | 9.8a | 10.1 | 11.0 | −4.4 ± | 4.6 | −4.7 | |
| Variety . | Colour of flesh . | ΔE . | L* . | a* . | b* . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | |||
| Hour . | Hour . | Hour . | |||||||||
| Blaue Elise | Purple | 7.1 | 34.9A ± 2.6 | 34.0A ± 1.8 | 32.9A ± 1.2 | 4.1A ± 1.4 | 3.9A ± 0.8 | 4.3A ± 1.3 | −12.3A ± 1.6 | −12.8A ± 1.4 | −13.9A ± 1.7 |
| Blaue St Galler | 10.4 | 31.9A ± 2.9 | 31.3A ± 3.3 | 29.6A ± 2.1 | 7.0A0 ± 2.5 | 6.9A ± 1.5 | 7.1A ± 2.1 | −16.3A ± 1.5 | −16.7A ± 1.6 | −16.9A ± 1.5 | |
| Blue Congo | 10.3 | 37.7A ± 3.6 | 36.7A ± 3.6 | 35.9A ± 3.3 | 8.1A ± 1.3 | 8.4A ± 1.4 | 8.4A ± 1.9 | −16.7A ± 1.2 | −17.0AB ± 1.0 | −18.4B ± 1.2 | |
| Valfi | 9.4 | 39.3A ± 4.3 | 39.5A ± 4.4 | 38.0A ± 5.5 | 8.7A ± 1.5 | 9.3A ± 2.0 | 9.0A ± 1.4 | −17.5A ± 1.4 | −18.3A ± 1.4 | −19.3A ± 1.2 | |
| Vitelotte | 8.6 | 41.7A ± 9.1 | 36.4A ± 2.0 | 35.2A ± 1.9 | 14.1A ± 4.1 | 16.1A ± 2.4 | 15.4A ± 2.7 | −16.9A ± 3.4 | −19.0A ± 1.4 | 18.6A ± 1.6 | |
| Average | 9.2 | 37.1 | 35.6 | 34.3 | 8.4 | 8.9 | 8.8 | −15.9 | −16.8 | −17.4 | |
| Highland Burgundy Red | Red | 8.7 | 53.4A ± 2.6 | 54.1A ± 2.6 | 53.4A ± 2.8 | 11.9A ± 2.3 | 12.0A ± 2.9 | 13.4A ± 3.2 | −3.7A ± 0.4 | −3.6A ± 1.0 | −4.4A ± 0.6 |
| Herbie 26 | 6.0 | 53.0A ± 1.4 | 53.0A ± 2.0 | 52.8A ± 1.8 | 9.9A ± 1.7 | 10.6A ± 1.8 | 11.2A ± 2.3 | −5.6A ± 0.3 | −5.9A ± 0.4 | −5.9A ± 0.4 | |
| Rosalinde | 17.9 | 62.0A ± 3.5 | 61.8A ± 4.1 | 59.8A ± 3.1 | 6.7A ± 1.6 | 7.0A ± 1.7 | 8.0A ± 1.0 | −3.3A ± 1.7 | −3.8A ± 1.9 | −3.3A ± 1.2 | |
| Rote Emma | 8.0 | 51.0A ± 1.3 | 52.3A ± 1.8 | 52.0A ± 0.8 | 10.8A ± 1.1 | 11.0A ± 1.3 | 11.4A ± 0.9 | −4.9A ± 0.8 | −5.2A ± 0.9 | −5.3A ± 0.6 | |
| Average | 10.1 | 54.8 | 55.3 ± | 54.5 | 9.8a | 10.1 | 11.0 | −4.4 ± | 4.6 | −4.7 | |
Average values (n = 6) labelled with the same letter in the same row are not statistically different (P ≤ 0.05).
| Variety . | Colour of flesh . | ΔE . | L* . | a* . | b* . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | |||
| Hour . | Hour . | Hour . | |||||||||
| Blaue Elise | Purple | 7.1 | 34.9A ± 2.6 | 34.0A ± 1.8 | 32.9A ± 1.2 | 4.1A ± 1.4 | 3.9A ± 0.8 | 4.3A ± 1.3 | −12.3A ± 1.6 | −12.8A ± 1.4 | −13.9A ± 1.7 |
| Blaue St Galler | 10.4 | 31.9A ± 2.9 | 31.3A ± 3.3 | 29.6A ± 2.1 | 7.0A0 ± 2.5 | 6.9A ± 1.5 | 7.1A ± 2.1 | −16.3A ± 1.5 | −16.7A ± 1.6 | −16.9A ± 1.5 | |
| Blue Congo | 10.3 | 37.7A ± 3.6 | 36.7A ± 3.6 | 35.9A ± 3.3 | 8.1A ± 1.3 | 8.4A ± 1.4 | 8.4A ± 1.9 | −16.7A ± 1.2 | −17.0AB ± 1.0 | −18.4B ± 1.2 | |
| Valfi | 9.4 | 39.3A ± 4.3 | 39.5A ± 4.4 | 38.0A ± 5.5 | 8.7A ± 1.5 | 9.3A ± 2.0 | 9.0A ± 1.4 | −17.5A ± 1.4 | −18.3A ± 1.4 | −19.3A ± 1.2 | |
| Vitelotte | 8.6 | 41.7A ± 9.1 | 36.4A ± 2.0 | 35.2A ± 1.9 | 14.1A ± 4.1 | 16.1A ± 2.4 | 15.4A ± 2.7 | −16.9A ± 3.4 | −19.0A ± 1.4 | 18.6A ± 1.6 | |
| Average | 9.2 | 37.1 | 35.6 | 34.3 | 8.4 | 8.9 | 8.8 | −15.9 | −16.8 | −17.4 | |
| Highland Burgundy Red | Red | 8.7 | 53.4A ± 2.6 | 54.1A ± 2.6 | 53.4A ± 2.8 | 11.9A ± 2.3 | 12.0A ± 2.9 | 13.4A ± 3.2 | −3.7A ± 0.4 | −3.6A ± 1.0 | −4.4A ± 0.6 |
| Herbie 26 | 6.0 | 53.0A ± 1.4 | 53.0A ± 2.0 | 52.8A ± 1.8 | 9.9A ± 1.7 | 10.6A ± 1.8 | 11.2A ± 2.3 | −5.6A ± 0.3 | −5.9A ± 0.4 | −5.9A ± 0.4 | |
| Rosalinde | 17.9 | 62.0A ± 3.5 | 61.8A ± 4.1 | 59.8A ± 3.1 | 6.7A ± 1.6 | 7.0A ± 1.7 | 8.0A ± 1.0 | −3.3A ± 1.7 | −3.8A ± 1.9 | −3.3A ± 1.2 | |
| Rote Emma | 8.0 | 51.0A ± 1.3 | 52.3A ± 1.8 | 52.0A ± 0.8 | 10.8A ± 1.1 | 11.0A ± 1.3 | 11.4A ± 0.9 | −4.9A ± 0.8 | −5.2A ± 0.9 | −5.3A ± 0.6 | |
| Average | 10.1 | 54.8 | 55.3 ± | 54.5 | 9.8a | 10.1 | 11.0 | −4.4 ± | 4.6 | −4.7 | |
| Variety . | Colour of flesh . | ΔE . | L* . | a* . | b* . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | 0 . | 1 . | 4 . | |||
| Hour . | Hour . | Hour . | |||||||||
| Blaue Elise | Purple | 7.1 | 34.9A ± 2.6 | 34.0A ± 1.8 | 32.9A ± 1.2 | 4.1A ± 1.4 | 3.9A ± 0.8 | 4.3A ± 1.3 | −12.3A ± 1.6 | −12.8A ± 1.4 | −13.9A ± 1.7 |
| Blaue St Galler | 10.4 | 31.9A ± 2.9 | 31.3A ± 3.3 | 29.6A ± 2.1 | 7.0A0 ± 2.5 | 6.9A ± 1.5 | 7.1A ± 2.1 | −16.3A ± 1.5 | −16.7A ± 1.6 | −16.9A ± 1.5 | |
| Blue Congo | 10.3 | 37.7A ± 3.6 | 36.7A ± 3.6 | 35.9A ± 3.3 | 8.1A ± 1.3 | 8.4A ± 1.4 | 8.4A ± 1.9 | −16.7A ± 1.2 | −17.0AB ± 1.0 | −18.4B ± 1.2 | |
| Valfi | 9.4 | 39.3A ± 4.3 | 39.5A ± 4.4 | 38.0A ± 5.5 | 8.7A ± 1.5 | 9.3A ± 2.0 | 9.0A ± 1.4 | −17.5A ± 1.4 | −18.3A ± 1.4 | −19.3A ± 1.2 | |
| Vitelotte | 8.6 | 41.7A ± 9.1 | 36.4A ± 2.0 | 35.2A ± 1.9 | 14.1A ± 4.1 | 16.1A ± 2.4 | 15.4A ± 2.7 | −16.9A ± 3.4 | −19.0A ± 1.4 | 18.6A ± 1.6 | |
| Average | 9.2 | 37.1 | 35.6 | 34.3 | 8.4 | 8.9 | 8.8 | −15.9 | −16.8 | −17.4 | |
| Highland Burgundy Red | Red | 8.7 | 53.4A ± 2.6 | 54.1A ± 2.6 | 53.4A ± 2.8 | 11.9A ± 2.3 | 12.0A ± 2.9 | 13.4A ± 3.2 | −3.7A ± 0.4 | −3.6A ± 1.0 | −4.4A ± 0.6 |
| Herbie 26 | 6.0 | 53.0A ± 1.4 | 53.0A ± 2.0 | 52.8A ± 1.8 | 9.9A ± 1.7 | 10.6A ± 1.8 | 11.2A ± 2.3 | −5.6A ± 0.3 | −5.9A ± 0.4 | −5.9A ± 0.4 | |
| Rosalinde | 17.9 | 62.0A ± 3.5 | 61.8A ± 4.1 | 59.8A ± 3.1 | 6.7A ± 1.6 | 7.0A ± 1.7 | 8.0A ± 1.0 | −3.3A ± 1.7 | −3.8A ± 1.9 | −3.3A ± 1.2 | |
| Rote Emma | 8.0 | 51.0A ± 1.3 | 52.3A ± 1.8 | 52.0A ± 0.8 | 10.8A ± 1.1 | 11.0A ± 1.3 | 11.4A ± 0.9 | −4.9A ± 0.8 | −5.2A ± 0.9 | −5.3A ± 0.6 | |
| Average | 10.1 | 54.8 | 55.3 ± | 54.5 | 9.8a | 10.1 | 11.0 | −4.4 ± | 4.6 | −4.7 | |
Average values (n = 6) labelled with the same letter in the same row are not statistically different (P ≤ 0.05).
Table 4 presents the results of the colour of flesh of cooked potatoes immediately after cutting, 1 and 4 h after being cut. Immediately after cooking, the colour of the red-fleshed potatoes was lighter than purple-fleshed tubers. The lightness (L*) of cooked potatoes of red-fleshed varieties ranged from 51 (Rote Emma) to 62 (Rosalinde), and of purple from 31.9 (Blue St Galler) to 41.7 (Vitelotte). In both types of cooked potatoes (purple and red fleshed), the share of red dye in the colour increased, with the largest changes occurring in the red varieties (Table 4). The share of blue dye in potatoes of red-fleshed varieties as a result of cooking did not change significantly, while in potatoes with purple colour it increased by 9.4%.
Potatoes of purple- and red-fleshed varieties after cooking were characterised by low susceptibility to darkening within 4 h after cutting of tubers. Also no significant difference in tone of the colour (hue) and saturation (chroma) of cooked purple- and red-fleshed tubers was found (Fig. 2c, d). The lack of changes in the colour of cooked potatoes was associated with the protein denaturation and inactivation of enzymes, such as polyphenol oxidase and peroxidase during thermal processing, whose activity resulted in colour change. Polyphenol oxidase and peroxidase enzymes were relatively heat labile: temperature of higher than 50 °C and proper time of the treatment allow a decrease in activity, whereas they are completely destroyed at 80 °C (Severini et al., 2003). Binding the iron ions with chlorogenic acid might be the main reason for the change in colour of tuber flesh after cooking towards grey-blue colour. In this process, under the influence of air, the free Fe2+ ion was oxidised to the trivalent ion, which reacts with the chlorogenic acid released during cooking and gives a dark blue-grey colour of the flesh (Friedman, 1997; Grudzińska, 2009). The severity of the darkening of boiling potatoes also depended on the ratio of chlorogenic acid to citric acid concentrations in studied samples of potato tubers. A higher ratio usually resulted in darker tubers (Wang-Pruski & Nowak, 2004). The darkening of potatoes after cooking also depended on the size of tubers (Siliciano et al., 1969). The largest tubers were characterised by low citric acid content, a high potassium-citric acid ratio and low citric acid-polyphenol ratios as compared to smaller ones. Citric acid and other chelating agents inhibited the potato blackening by competing with chlorogenic acid for ferrous ion chelating sites (Silva et al., 1991).
Conclusions
The purple-fleshed varieties contained more total polyphenols than red-fleshed varieties, but the anthocyanin content in studied potatoes, regardless of flesh colour, was similar, on average 50 mg/100 g dm−1. Pelargonidin was the dominant anthocyanin in red-fleshed varieties, whereas petunidin was dominant in purple-fleshed varieties. Red-fleshed potatoes of HBr variety were distinguished by a significantly higher content of pelargonidin-3-feruloylrutinoside-5-glucoside, and the Vitelotte variety with purple flesh had a higher cyanidin-3-rutinoside content compared to the other varieties.
Just after cutting, red-fleshed potatoes showed double the share of red (a* parameter) as compared to purple-fleshed varieties. Regardless of the colour of potato flesh, during the first and 4 h after cutting of raw tubers, the proportion of red dye decreased, except tubers of purple-fleshed Vitelotte and red-fleshed Rosalinde varieties. The lightness of the surface of colour fleshed potatoes did not change, with the exception of purple-fleshed Vitelotte and red-fleshed BE varieties. During the first and 4 h after cutting of raw tubers the share of blue colour on the surface of cut potato tubers of purple-fleshed varieties decreased, and on the surface of red-fleshed potatoes an increase of the share of yellow dye was observed. Colour changes of cooked potatoes depended on variety. The largest colour transformations were observed in potatoes of the Rosalinde variety, with a value of 17.91 (ΔE). The share of blue dye in potatoes of red-fleshed varieties as a result of cooking did not change significantly, while in purple potatoes it increased by 9.4%. Cooked potatoes of purple-fleshed tubers were more saturated by chroma than tubers of red-fleshed varieties.
Acknowledgments
This publication was supported by the Wroclaw Centre of Biotechnology programme at the Leading National Research Centre (KNOW) for the years 2014–2018.



