-
PDF
- Split View
-
Views
-
Cite
Cite
Salud Serrano, Roberto Espejo, Marta Villarejo, Manuela Luisa Jodral, Diastase and invertase activities in Andalusian honeys, International Journal of Food Science and Technology, Volume 42, Issue 1, January 2007, Pages 76–79, https://doi.org/10.1111/j.1365-2621.2006.01213.x
Close - Share Icon Share
Abstract
Forty-nine samples of unheated, commercially purchased Andalusian (southern Spain) honeys were examined for diastase (α- and β-amylase) and invertase (α-glucosidase) activities. The honeys were from Citrus sp. (5), Sunflower (2), Rosmarinus (3), Eucalyptus sp. (4) or multifloral in origin (35). Mean value for diastase was 20.48 ± 10.14 (range 3.99–49.42) expressed as diastase number in Gothe's scale, for invertase it was 12.34 ± 8.09 (range 1.2–36.8) expressed as invertase number. Correlation was found between invertase and diastase activities (r = 0.853). These results were compared with literature data and differences are discussed.
Introduction
Honey contains small amounts of different enzymes, the most important ones being diastase (α- and β-amylase), invertase (α-glucosidase), glucose-oxidase, catalase and acid phosphatase, which come from nectar sources, from salivary fluids and from the pharyngeal gland secretions of the honeybee. This enzyme content is one of the characteristics which makes honey a different product from other sweeteners. However, this content can be considerably diminished by processing, heating and prolonged storage (Huidobro et al., 1995).
The study and interpretation of this enzyme activity, together with its content of hydroxymethylfurfural (HMF), can give us information about the intensity of the heat treatment carried out and the aging degree of this natural product. It can thus be affirmed that the enzyme compound of honey and its HMF content is the quality indicator of an authentic, nonadulterated product.
According to Arvanitoyannis et al. (2005), the authenticity of honey has two different aspects: authenticity with respect to honey production, and authenticity with respect to description, for example, its geographical and botanical origin, ‘natural’, ‘organic’, ‘raw’ and ‘unheated’ honey. Honey authenticity determination requires the consideration of both aspects, although the second aspect would be the one in which to include the investigation of chemometrics, which in the past few decades has taken its place in the development of novel analytical methods used in conjunction with multivariate analysis to assess honey authenticity.
The annual honey production in Andalusia is estimated at 4500 tonnes, which represents 14.5% of the annual production in Spain. The large variety of melliferous sources also enables Andalusia to produce characteristic unifloral nectar honeys. The main Andalusian unifloral honeys are sunflower (Helianthus annuus L), orange (Citrus spp.), eucalyptus (Eucalyptus spp.) and rosemary (Rosmarinus officinalis).
This research was carried out to find out the variability in the content of diastase and invertase in Andalusian honeys and to establish the relationship between both enzymes.
Materials and methods
Forty-nine samples of unheated, commercially purchased Andalusian (southern Spain, harvests 1999–2000) honeys were examined for diastase (α- and β-amylase) and invertase (α-glucosidase) activities. The honeys were from Citrus sp. (5), Sunflower (2), Rosmarinus (3), Eucalyptus sp. (4) or multifloral in origin (35). Samples were maintained at 4°C till analysis.
Determination of HMF was made according to the Winkler (1955) method where, to aliquot parts of a honey solution, solutions of p-toluidine and barbituric acid were added and the resultant colour measured against a blank in 1 cm cuvettes at 550 nm.
To measure the invertase activity the Siegenthaler (1977) method, harmonized by the European Honey Commission (Bogdanov et al., 1997), was followed. The enzyme activity was evaluated photometrically by measuring the degradation of the substrate p-nitrophenyl-α-d-glucopyranoside in the product p-nitrophenol (maximum absorbance at 400 nm). The results are expressed as an invertase number (IN). The IN indicates the amount of sucrose per g an hour hydrolysed by the enzymes contained in 100 g of honey.
The diastase activity was determined photometrically by the Phadebas method (Schade et al., 1958). The results are expressed as a diastase number (DN) in Gothe or Schade units. One unit corresponds to the enzyme activity of 1 g of honey, which can hydrolyse 0.01 g of starch in 1 h at 40°C and pH 5.2.
The calculation modified by Bogdanov et al. (1997), DN = 28.2 × ΔA620 + 2.64, was used.
Results and discussion
From the HMF content (mean value of 8.24 mg kg−1) it can be observed that except for sample 32, the rest complied with the limitation of 40 mg kg−1 established by the Council Directive 2001/110/EC (2001) which, in an attempt to fight fraud, has fixed limits regarding the composition criteria for honey.
The range of HMF values found (0.19–41.16 mg kg−1) coincided with those presented by Sousa et al. (2002) in Galician honeys. Some high values of the HMF content (sample 32) should be interpreted by taking into consideration the climatic conditions of Andalusia, which increase the formation of HMF. White (1992) affirmed that honeys from subtropical countries can, in a natural way, have a high HMF content without the honey having been overheated or adulterated. Terrab et al. (2002) found HMF values in the range of 3.8–48.4 mg kg−1 for multifloral honeys in Morocco, where the climate might increase its content.
The diastase activity of the honeys lay in the range of 3.99–49.42 on Gothe's scale (Table 1). Most of the samples are within the limits of European Honey Standards, taking into account that diastase levels of under 8° Goethe correspond to citrus honeys (samples 38, 41, 44 and 47) with a low enzyme content and HMF levels of below 15 mg kg−1.
| Identification samples . | Diastase activitya . | Invertase activityb . | HMF (mg kg−1) . |
|---|---|---|---|
| 1 Eucalyptus | 49.42 | 36.8 | 0.96 |
| 2 Multifloral | 23.68 | 15.2 | 3.84 |
| 3 Rosmarynus | 32.14 | 30.8 | 0.58 |
| 4 Eucalyptus | 17.73 | 13.2 | 11.44 |
| 5 Multifloral | 19.47 | 20.8 | 1.16 |
| 6 Multifloral | 23.70 | 17.3 | 8.53 |
| 7 Multifloral | 21.90 | 11.5 | 4.37 |
| 8 Multifloral | 20.35 | 12.9 | 7.98 |
| 9 Multifloral | 6.05 | 1.2 | 20.72 |
| 10 Multifloral | 16.57 | 9.8 | 11.84 |
| 11 Multifloral | 11.44 | 5.8 | 17.69 |
| 12 Multifloral | 33.77 | 21.1 | 1.92 |
| 13 Multifloral | 20.15 | 6.3 | 19.97 |
| 14 Eucalyptus | 22.86 | 9.5 | 11.48 |
| 15 Multifloral | 11.73 | 12.9 | 10.37 |
| 16 Multifloral | 10.06 | 2.9 | 6.29 |
| 17 Multifloral | 24.86 | 17.4 | 1.53 |
| 18 Multifloral | 15.54 | 12.2 | 8.64 |
| 19 Rosmarynus | 26.05 | 9.1 | 9.97 |
| 20 Multifloral | 11.92 | 7.6 | 6.34 |
| 21 Eucalyptus | 22.83 | 12.0 | 5.32 |
| 22 Rosmarynus | 20.35 | 10.6 | 8.74 |
| 23 Multifloral | 26.92 | 12.6 | 4.22 |
| 24 Multifloral | 40.89 | 27.1 | 1.52 |
| 25 Multifloral | 8.70 | 5.0 | 17.42 |
| 26 Sunflower | 13.92 | 7.4 | 15.02 |
| 27 Multifloral | 37.66 | 18.8 | 0.76 |
| 28 Multifloral | 26.44 | 5.6 | 17.69 |
| 29 Multifloral | 24.61 | 18.0 | 0.19 |
| 30 Multifloral | 40.51 | 27.7 | 0.37 |
| 31 Multifloral | 10.08 | 1.9 | 3.45 |
| 32 Multifloral | 7.91 | 2.9 | 41.16 |
| 33 Multifloral | 19.79 | 11.8 | 7.22 |
| 34 Multifloral | 6.93 | 2.2 | 20.35 |
| 35 Multifloral | 31.12 | 15.7 | 6.14 |
| 36 Multifloral | 26.16 | 14.8 | 13.82 |
| 37 Multifloral | 18.74 | 5.0 | 5.15 |
| 38 Citrus | 3.99 | 2.3 | 6.67 |
| 39 Multifloral | 18.46 | 11.7 | 5.90 |
| 40 Multifloral | 17.59 | 13.2 | 10.6 |
| 41 Citrus | 7.86 | 5.9 | 1.1 |
| 42 Multifloral | 26.61 | 21.9 | 7.5 |
| 43 Sunflower | 29.99 | 8.8 | 8.8 |
| 44 Citrus | 7.46 | 1.4 | 5.5 |
| 45 Multifloral | 18.74 | 14.2 | 2.9 |
| 46 Multifloral | 20.60 | 15.7 | 8.6 |
| 47 Citrus | 7.41 | 2.3 | 9.6 |
| 48 Citrus | 10.79 | 9.1 | 1.9 |
| 49 Multifloral | 30.90 | 24.3 | 0.7 |
| Identification samples . | Diastase activitya . | Invertase activityb . | HMF (mg kg−1) . |
|---|---|---|---|
| 1 Eucalyptus | 49.42 | 36.8 | 0.96 |
| 2 Multifloral | 23.68 | 15.2 | 3.84 |
| 3 Rosmarynus | 32.14 | 30.8 | 0.58 |
| 4 Eucalyptus | 17.73 | 13.2 | 11.44 |
| 5 Multifloral | 19.47 | 20.8 | 1.16 |
| 6 Multifloral | 23.70 | 17.3 | 8.53 |
| 7 Multifloral | 21.90 | 11.5 | 4.37 |
| 8 Multifloral | 20.35 | 12.9 | 7.98 |
| 9 Multifloral | 6.05 | 1.2 | 20.72 |
| 10 Multifloral | 16.57 | 9.8 | 11.84 |
| 11 Multifloral | 11.44 | 5.8 | 17.69 |
| 12 Multifloral | 33.77 | 21.1 | 1.92 |
| 13 Multifloral | 20.15 | 6.3 | 19.97 |
| 14 Eucalyptus | 22.86 | 9.5 | 11.48 |
| 15 Multifloral | 11.73 | 12.9 | 10.37 |
| 16 Multifloral | 10.06 | 2.9 | 6.29 |
| 17 Multifloral | 24.86 | 17.4 | 1.53 |
| 18 Multifloral | 15.54 | 12.2 | 8.64 |
| 19 Rosmarynus | 26.05 | 9.1 | 9.97 |
| 20 Multifloral | 11.92 | 7.6 | 6.34 |
| 21 Eucalyptus | 22.83 | 12.0 | 5.32 |
| 22 Rosmarynus | 20.35 | 10.6 | 8.74 |
| 23 Multifloral | 26.92 | 12.6 | 4.22 |
| 24 Multifloral | 40.89 | 27.1 | 1.52 |
| 25 Multifloral | 8.70 | 5.0 | 17.42 |
| 26 Sunflower | 13.92 | 7.4 | 15.02 |
| 27 Multifloral | 37.66 | 18.8 | 0.76 |
| 28 Multifloral | 26.44 | 5.6 | 17.69 |
| 29 Multifloral | 24.61 | 18.0 | 0.19 |
| 30 Multifloral | 40.51 | 27.7 | 0.37 |
| 31 Multifloral | 10.08 | 1.9 | 3.45 |
| 32 Multifloral | 7.91 | 2.9 | 41.16 |
| 33 Multifloral | 19.79 | 11.8 | 7.22 |
| 34 Multifloral | 6.93 | 2.2 | 20.35 |
| 35 Multifloral | 31.12 | 15.7 | 6.14 |
| 36 Multifloral | 26.16 | 14.8 | 13.82 |
| 37 Multifloral | 18.74 | 5.0 | 5.15 |
| 38 Citrus | 3.99 | 2.3 | 6.67 |
| 39 Multifloral | 18.46 | 11.7 | 5.90 |
| 40 Multifloral | 17.59 | 13.2 | 10.6 |
| 41 Citrus | 7.86 | 5.9 | 1.1 |
| 42 Multifloral | 26.61 | 21.9 | 7.5 |
| 43 Sunflower | 29.99 | 8.8 | 8.8 |
| 44 Citrus | 7.46 | 1.4 | 5.5 |
| 45 Multifloral | 18.74 | 14.2 | 2.9 |
| 46 Multifloral | 20.60 | 15.7 | 8.6 |
| 47 Citrus | 7.41 | 2.3 | 9.6 |
| 48 Citrus | 10.79 | 9.1 | 1.9 |
| 49 Multifloral | 30.90 | 24.3 | 0.7 |
HMF, hydroxymethylfurfural.
aDiastase number.
bInvertase number.
| Identification samples . | Diastase activitya . | Invertase activityb . | HMF (mg kg−1) . |
|---|---|---|---|
| 1 Eucalyptus | 49.42 | 36.8 | 0.96 |
| 2 Multifloral | 23.68 | 15.2 | 3.84 |
| 3 Rosmarynus | 32.14 | 30.8 | 0.58 |
| 4 Eucalyptus | 17.73 | 13.2 | 11.44 |
| 5 Multifloral | 19.47 | 20.8 | 1.16 |
| 6 Multifloral | 23.70 | 17.3 | 8.53 |
| 7 Multifloral | 21.90 | 11.5 | 4.37 |
| 8 Multifloral | 20.35 | 12.9 | 7.98 |
| 9 Multifloral | 6.05 | 1.2 | 20.72 |
| 10 Multifloral | 16.57 | 9.8 | 11.84 |
| 11 Multifloral | 11.44 | 5.8 | 17.69 |
| 12 Multifloral | 33.77 | 21.1 | 1.92 |
| 13 Multifloral | 20.15 | 6.3 | 19.97 |
| 14 Eucalyptus | 22.86 | 9.5 | 11.48 |
| 15 Multifloral | 11.73 | 12.9 | 10.37 |
| 16 Multifloral | 10.06 | 2.9 | 6.29 |
| 17 Multifloral | 24.86 | 17.4 | 1.53 |
| 18 Multifloral | 15.54 | 12.2 | 8.64 |
| 19 Rosmarynus | 26.05 | 9.1 | 9.97 |
| 20 Multifloral | 11.92 | 7.6 | 6.34 |
| 21 Eucalyptus | 22.83 | 12.0 | 5.32 |
| 22 Rosmarynus | 20.35 | 10.6 | 8.74 |
| 23 Multifloral | 26.92 | 12.6 | 4.22 |
| 24 Multifloral | 40.89 | 27.1 | 1.52 |
| 25 Multifloral | 8.70 | 5.0 | 17.42 |
| 26 Sunflower | 13.92 | 7.4 | 15.02 |
| 27 Multifloral | 37.66 | 18.8 | 0.76 |
| 28 Multifloral | 26.44 | 5.6 | 17.69 |
| 29 Multifloral | 24.61 | 18.0 | 0.19 |
| 30 Multifloral | 40.51 | 27.7 | 0.37 |
| 31 Multifloral | 10.08 | 1.9 | 3.45 |
| 32 Multifloral | 7.91 | 2.9 | 41.16 |
| 33 Multifloral | 19.79 | 11.8 | 7.22 |
| 34 Multifloral | 6.93 | 2.2 | 20.35 |
| 35 Multifloral | 31.12 | 15.7 | 6.14 |
| 36 Multifloral | 26.16 | 14.8 | 13.82 |
| 37 Multifloral | 18.74 | 5.0 | 5.15 |
| 38 Citrus | 3.99 | 2.3 | 6.67 |
| 39 Multifloral | 18.46 | 11.7 | 5.90 |
| 40 Multifloral | 17.59 | 13.2 | 10.6 |
| 41 Citrus | 7.86 | 5.9 | 1.1 |
| 42 Multifloral | 26.61 | 21.9 | 7.5 |
| 43 Sunflower | 29.99 | 8.8 | 8.8 |
| 44 Citrus | 7.46 | 1.4 | 5.5 |
| 45 Multifloral | 18.74 | 14.2 | 2.9 |
| 46 Multifloral | 20.60 | 15.7 | 8.6 |
| 47 Citrus | 7.41 | 2.3 | 9.6 |
| 48 Citrus | 10.79 | 9.1 | 1.9 |
| 49 Multifloral | 30.90 | 24.3 | 0.7 |
| Identification samples . | Diastase activitya . | Invertase activityb . | HMF (mg kg−1) . |
|---|---|---|---|
| 1 Eucalyptus | 49.42 | 36.8 | 0.96 |
| 2 Multifloral | 23.68 | 15.2 | 3.84 |
| 3 Rosmarynus | 32.14 | 30.8 | 0.58 |
| 4 Eucalyptus | 17.73 | 13.2 | 11.44 |
| 5 Multifloral | 19.47 | 20.8 | 1.16 |
| 6 Multifloral | 23.70 | 17.3 | 8.53 |
| 7 Multifloral | 21.90 | 11.5 | 4.37 |
| 8 Multifloral | 20.35 | 12.9 | 7.98 |
| 9 Multifloral | 6.05 | 1.2 | 20.72 |
| 10 Multifloral | 16.57 | 9.8 | 11.84 |
| 11 Multifloral | 11.44 | 5.8 | 17.69 |
| 12 Multifloral | 33.77 | 21.1 | 1.92 |
| 13 Multifloral | 20.15 | 6.3 | 19.97 |
| 14 Eucalyptus | 22.86 | 9.5 | 11.48 |
| 15 Multifloral | 11.73 | 12.9 | 10.37 |
| 16 Multifloral | 10.06 | 2.9 | 6.29 |
| 17 Multifloral | 24.86 | 17.4 | 1.53 |
| 18 Multifloral | 15.54 | 12.2 | 8.64 |
| 19 Rosmarynus | 26.05 | 9.1 | 9.97 |
| 20 Multifloral | 11.92 | 7.6 | 6.34 |
| 21 Eucalyptus | 22.83 | 12.0 | 5.32 |
| 22 Rosmarynus | 20.35 | 10.6 | 8.74 |
| 23 Multifloral | 26.92 | 12.6 | 4.22 |
| 24 Multifloral | 40.89 | 27.1 | 1.52 |
| 25 Multifloral | 8.70 | 5.0 | 17.42 |
| 26 Sunflower | 13.92 | 7.4 | 15.02 |
| 27 Multifloral | 37.66 | 18.8 | 0.76 |
| 28 Multifloral | 26.44 | 5.6 | 17.69 |
| 29 Multifloral | 24.61 | 18.0 | 0.19 |
| 30 Multifloral | 40.51 | 27.7 | 0.37 |
| 31 Multifloral | 10.08 | 1.9 | 3.45 |
| 32 Multifloral | 7.91 | 2.9 | 41.16 |
| 33 Multifloral | 19.79 | 11.8 | 7.22 |
| 34 Multifloral | 6.93 | 2.2 | 20.35 |
| 35 Multifloral | 31.12 | 15.7 | 6.14 |
| 36 Multifloral | 26.16 | 14.8 | 13.82 |
| 37 Multifloral | 18.74 | 5.0 | 5.15 |
| 38 Citrus | 3.99 | 2.3 | 6.67 |
| 39 Multifloral | 18.46 | 11.7 | 5.90 |
| 40 Multifloral | 17.59 | 13.2 | 10.6 |
| 41 Citrus | 7.86 | 5.9 | 1.1 |
| 42 Multifloral | 26.61 | 21.9 | 7.5 |
| 43 Sunflower | 29.99 | 8.8 | 8.8 |
| 44 Citrus | 7.46 | 1.4 | 5.5 |
| 45 Multifloral | 18.74 | 14.2 | 2.9 |
| 46 Multifloral | 20.60 | 15.7 | 8.6 |
| 47 Citrus | 7.41 | 2.3 | 9.6 |
| 48 Citrus | 10.79 | 9.1 | 1.9 |
| 49 Multifloral | 30.90 | 24.3 | 0.7 |
HMF, hydroxymethylfurfural.
aDiastase number.
bInvertase number.
The invertase activity expressed in IN of the honeys were in the range of 1.2–36.8 (Table 1). The measurements obtained for both enzymes (mean value of 12.34 ± 8.09 for invertase; mean value of 20.48 ± 10.14 for diastase) coincide with those found by Persano et al. (1999) and Horn & Boehm (2004).
From the study of the ratio IN/DN of the forty-nine samples the correlation observed between the two enzymes was defined by the following parameters: r = 0.853, P = 0.000, Y = 0.681X − 1.612 (Fig. 1 and Table 2).
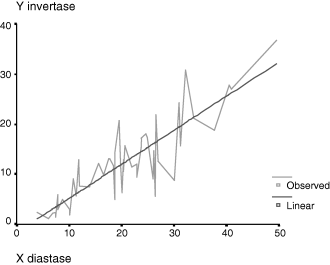
Parameters of the adjusted model, independent term (−1.612) and line slope (0.681)
| Model 1 . | Unstandardized coefficients (B) . | SE . | Standardized coefficients (β) . | t . | Sig. . |
|---|---|---|---|---|---|
| Constant | −1.612 | 1385 | −1165 | 0.250 | |
| X | 0.681 | 0.061 | 0.853 | 11.224 | 0.000 |
| Model 1 . | Unstandardized coefficients (B) . | SE . | Standardized coefficients (β) . | t . | Sig. . |
|---|---|---|---|---|---|
| Constant | −1.612 | 1385 | −1165 | 0.250 | |
| X | 0.681 | 0.061 | 0.853 | 11.224 | 0.000 |
Parameters of the adjusted model, independent term (−1.612) and line slope (0.681)
| Model 1 . | Unstandardized coefficients (B) . | SE . | Standardized coefficients (β) . | t . | Sig. . |
|---|---|---|---|---|---|
| Constant | −1.612 | 1385 | −1165 | 0.250 | |
| X | 0.681 | 0.061 | 0.853 | 11.224 | 0.000 |
| Model 1 . | Unstandardized coefficients (B) . | SE . | Standardized coefficients (β) . | t . | Sig. . |
|---|---|---|---|---|---|
| Constant | −1.612 | 1385 | −1165 | 0.250 | |
| X | 0.681 | 0.061 | 0.853 | 11.224 | 0.000 |
The coefficient of correlation was higher than those obtained by Persano et al. (1999) (r = 0.835), Horn & Boehm (2004) (r = 0.700) and Krauze & Krauze (1991) (r = 0.738), but lower than those proposed by Huidobro et al. (1995) (r = 0.878), and Aldcorn et al. (1985) (r = 0.924). Vit & Pulcini (1996) found a very low correlation between both enzymes in their study on Venezuela honeys, although in this latter case this is due to the low content in diastase typical of stingless bee honey, that requires a different approach for diastase activity. Table 3 shows the results of this study compared to others from different authors.
| Enzyme correlation (r) . | Reference . |
|---|---|
| 0.853 | Present study |
| 0.835 | Persano et al. (1999) |
| 0.700 | Horn & Boehm (2004) |
| 0.738 | Krauze & Krauze (1991) |
| 0.878 | Huidobro et al. (1995) |
| 0.924 | Aldcorn et al. (1985) |
| Nonsignificant | Vit & Pulcini (1996) |
| Enzyme correlation (r) . | Reference . |
|---|---|
| 0.853 | Present study |
| 0.835 | Persano et al. (1999) |
| 0.700 | Horn & Boehm (2004) |
| 0.738 | Krauze & Krauze (1991) |
| 0.878 | Huidobro et al. (1995) |
| 0.924 | Aldcorn et al. (1985) |
| Nonsignificant | Vit & Pulcini (1996) |
| Enzyme correlation (r) . | Reference . |
|---|---|
| 0.853 | Present study |
| 0.835 | Persano et al. (1999) |
| 0.700 | Horn & Boehm (2004) |
| 0.738 | Krauze & Krauze (1991) |
| 0.878 | Huidobro et al. (1995) |
| 0.924 | Aldcorn et al. (1985) |
| Nonsignificant | Vit & Pulcini (1996) |
| Enzyme correlation (r) . | Reference . |
|---|---|
| 0.853 | Present study |
| 0.835 | Persano et al. (1999) |
| 0.700 | Horn & Boehm (2004) |
| 0.738 | Krauze & Krauze (1991) |
| 0.878 | Huidobro et al. (1995) |
| 0.924 | Aldcorn et al. (1985) |
| Nonsignificant | Vit & Pulcini (1996) |
Conclusions
These results confirm the variability of enzyme activity in different honeys, probably due to factors such as the time of collecting the nectar (the physiological state of the colony), the abundance of nectar and sugar content, the age of bees (Al-Khalifa & Al-Arify, 1999), consumption of pollen (Simpson et al., 1968; Crane, 1975; Brouwers, 1982, 1983; Fluri et al., 1982; Huang & Otis, 1989), at the same time demonstrating the correlation between the two enzymes, showing that honeys with a low content in invertase also have a low content in diastase, and vice versa. This correlation should be useful, to some extent, for excluding honeys subjected to some of the common practices of adulteration, as an enzyme content within the ranges obtained, together with a low HMF content, is one of the most relevant factors when gauging the authenticity of honey.
Acknowledgments
This work was supported by the Programa Apícola Nacional from Ministerio de Agricultura, Pesca y Alimentación (project ref. API 99-010-C2-02).



