-
PDF
- Split View
-
Views
-
Cite
Cite
Fatemeh Bamdad, Mahdi Kadivar, Javad Keramat, Evaluation of phenolic content and antioxidant activity of Iranian caraway in comparison with clove and BHT using model systems and vegetable oil, International Journal of Food Science and Technology, Volume 41, Issue Supplement_1, August 2006, Pages 20–27, https://doi.org/10.1111/j.1365-2621.2006.01238.x
Close - Share Icon Share
Abstract
Antioxidant activity (AA) of phenolic compounds in methanolic extract of Iranian caraway was compared with clove. Total phenolic content of caraway and clove extracts determined by the Folin-Ciocalteu method, were 13.76 and 243.91 mg g−1 dry matter respectively, expressed as tannic acid equivalents, and total flavonoid content of extracts were 2.6 and 5.0 mg g−1 dry matter, respectively, expressed as epicatechin equivalents. The extracts were screened for their potency as antioxidant using various in vitro models, such as β-carotene–linoleate, ferric thiocyanate, 1,1-diphenyl-2-picryl hydroxyl (DPPH) radical, hydroxyl radical (•OH) and reducing power model systems. AA of BHT was also determined in model systems. AA of methanolic extracts at different concentrations of total phenolic content according to β-carotene bleaching and ferric thiocyanate methods expressed as AA (per cent inhibition relative to control) ranged from 82% to 96%.The scavenging effect of both extracts on DPPH and •OH radicals were 23–40% and 3.5–12.7%, respectively, which were comparable with BHT as a synthetic antioxidant. Extracts with high content of total phenolic compounds exhibited also good reducing power.
Introduction
Autoxidation of fats and oils not only lowers the nutritional value of foods, but is also associated with aging, membrane damages, heart diseases, diabetes and cancer in living organisms (Scott, 1997; Hollman, 2001). Addition of antioxidants to food is an effective way for retarding the oxidation of fats.
There are two basic categories of antioxidants, namely, synthetic and natural. The most common synthetic antioxidants used in foods, are compounds with phenolic structures of various degrees of substitution, whereas natural antioxidants are primarily plant phenolic and polyphenolic compounds that may occur in all parts of plants (Velioglu et al., 1998; Shahidi & Naczk, 2004).
Most of the antioxidants in use commercially (e.g., Butylated Hydroxy Toluene (BHT), Butylated Hydroxy Anisol (BHA) and Propyl Gallate (PG)) are synthetic (Allen & Hamilton, 1989). Although largely effective, synthetic antioxidants continue to be scrutinised for their safety as food additives; consequently, there is increasing public interest in the use of natural antioxidants. Extracts from spices, rosemary, thyme and sage are reported to possess antioxidant properties comparable with or greater than BHA and BHT (Kramer, 1985; Pokorny, 1991; Cuvelier et al., 1994).
A major group of phytochemicals in foods are the phenolic compounds, which includes simple phenols, phenyl propanoids, benzoic acid and derivatives, flavonoids, stilbenes, tannins, lignans and lignins. Plant phenolics are multifunctional and can act as reducing agents (free radical terminators), metal chelators and singlet oxygen quenchers (Shahidi & Naczk, 2004). Flavonoids are a class of compounds that have been demonstrated to be potent antioxidants based on their phenolic hydroxyl groups. They are known as primary antioxidants and can delay or inhibit the oxidation of lipids or other molecules by inhibiting the initiation or propagation of oxidative chain reactions (Andlauer & Furst, 1998). Structure–activity relationship studies of flavonoids have shown that the o-dihydroxy structure in the B ring and the 2,3 double bond in conjugation with the 4-oxo function in the C ring (as in flavones) are essential for effective free-radical scavenging activity. The presence of a 3-hydroxyl group in the heterocyclic ring also increases the radical scavenging activity of flavonols (Pannala et al., 2001). Some spices like clove buds (Eugenia caryophyllata Tunb.) and particularly caraway seeds (Carum carvi L.), known as black zira, are usually used in Iranian diet. Black zira is one of the most important plants that belong to the Umbeliferae family. It is the native plant of Asia and Europe. In Iran, it grows in such places as Kerman and Balouchestan and western-north of Alborz and some other places. It is known as the most desirable kind of zira and its ripe fruits are consumed as spice. However, whether this spice possesses functional effects such as antioxidative activity has not been reported. In this study, extracts of clove and caraway were prepared using 80% methanol. Total phenolic and flavonoid content of the extracts were determined and then the antioxidant properties of these extracts containing different concentrations of phenolic compounds were assessed by five model systems.
Materials and methods
Chemicals and reagents
Dried clove buds (E. caryophyllata Tunb.) and caraway seed (C. carvi L.) were purchased from Isfahan Pakan Bazr Company, Isfahan, Iran. Linoleic acid, β-carotene and 1,1-diphenyl-2-picryl hydrazyl (DPPH) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Folin-Ciocalteu reagent, BHT and ascorbic acid were purchased from E. Merck (Darmstadt, Germany). Sunflower oil void of any antioxidant was obtained from Naz Veg. Oil Company (Isfahan, Iran). All other reagents and solvents were of the highest grade available and purchased from either Sigma or Merck representatives.
Extraction
Phenolics were extracted according to Kähkönen et al. (1999). Ground spices (500 mg) were weighed into a test tube. A total of 10 ml of 80% aqueous methanol was added and the suspension was stirred slightly. Tubes were vortexed for 1 min and centrifuged (1500 g) for 10 min, and supernatants were collected. Precipitates were re-extracted. Combined supernatants were used as extracts. To calculate the yield of extraction, extracts were concentrated using a rotary evaporator until partial dryness and then completely dried in a vacuum oven (room temperature and 10 mm Hg pressure). The yield of total phenolic content in dried extracts was then calculated.
Determination of total phenolics
The content of total phenolics in extracts was determined according to the Folin-Ciocalteu procedure (Kähkönen et al., 1999). Extracts (1 ml) were poured into the test tubes; 5 ml of Folin-Ciocalteu reagent (1:10 diluted) and 4 ml of sodium carbonate (7.5%) were added. The tubes were mixed and allowed to stand for 30 min. Absorption was measured at 760 nm (M350 Double Beam UV-Visible Comspec Spectrophotometer). Total phenolic content was expressed as tannic acid equivalents in milligrams per gram dry matter. Standard curve was prepared with a range of tannic acid concentrations (10–100 ppm) in 80% aqueous methanol.
Determination of total flavonoid content
Total flavonoid content was determined using a colorimetric method described by Dewanto et al. (2002). Briefly, 0.25 ml of the spice extracts or epicatechin standard solution was mixed with 1.25 ml of distilled water in a test tube followed by addition of 75 μl of a 5% NaNO2 solution. After 6 min, 150 μl of a 10% AlCl3.6H2O solution was added and allowed to stand for another 5 min, before 0.5 ml of 1 m NaOH was added. The mixture was brought to 2.5 ml with distilled water and mixed well. Absorbance was measured immediately against the blank at 510 nm. Standard curve was prepared similarly with known epicatechin concentrations (200–800 ppm). The results are expressed as mean (milligrams of epicatechin equivalent per gram of dry matter).
Antioxidant assay using β-carotene–linoleate model system
Antioxidant activity (AA) of caraway and clove extracts containing 100, 300 and 500 ppm of total phenolic content was evaluated according to Singh et al. (2002). β-carotene (0.2 mg) in 0.2 ml chloroform, linoleic acid (20 mg) and Tween-40 (Polyoxyethylene sorbitan monopalmitate) (200 mg) were mixed. Chloroform was removed at 40 °C under vacuum, and the resulting mixture was diluted with 10 ml of water and mixed well. To this emulsion, 40 ml of oxygenated water (prepared by injection of oxygen in cold water) was added. Four-millilitre aliquots of the emulsion were then pipetted into different test tubes containing 0.2 ml of caraway and clove extracts and BHT (50 and 100 ppm in methanol), respectively. A control containing 0.2 ml of methanol and 4 ml of this emulsion was prepared. The tubes were placed at 50 °C in a water bath, and the absorbance at 470 nm were taken at zero time (t = 0). The absorbance was read at intervals of 15 min until the colour of β-carotene disappeared in the control tube (t = 180 min). A mixture prepared this way without β-carotene served as blank. AA of the extracts was evaluated in terms of bleaching of the β-carotene using the following formula:
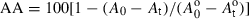
where A0 and A are the absorbance values measured at zero time of the incubation for test sample and control, respectively and At and A
are the absorbance values measured at zero time of the incubation for test sample and control, respectively and At and A are the absorbance values measured in test sample and control, respectively, after incubation for 180 min.
are the absorbance values measured in test sample and control, respectively, after incubation for 180 min.
Antioxidant activity using linoleic acid peroxidation method
AA of caraway and clove extracts was determined using the thiocyanate method (Jayaprakasha et al., 2001). Linoleic acid emulsion was prepared by homogenising 0.28 g of linoleic acid, 0.28 g of Tween-40 as emulsifier and 50 ml of phosphate buffer (0.2 m, pH 7.0). Test samples (0.5 ml) containing 100, 300 and 500 ppm of total phenolic content were mixed with 2.5 ml of linoleic acid emulsion and 2.5 ml of phosphate buffer, incubated at 37 °C for 120 h. The mixture prepared this way without test sample served as control. Aliquots (0.1 ml) were drawn from the incubation mixture at an interval of 20 h and mixed with 5 ml of 75% ethanol, 0.1 ml of 30% ammonium thiocyanate and 0.1 ml of 20 mm ferrous chloride in 3.5% HCl and allowed to stand at room temperature for 3 min. The colour developed was measured at 500 nm. The degree of linoleic acid peroxidation was calculated at 120 h using the following formula:
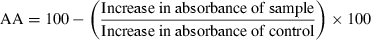
BHT was included as standard antioxidant for comparison.
Radical scavenging activity using 1,1-diphenyl-2-picryl hydroxyl
Five millilitres of a 0.1 mm methanolic solution of DPPH was added to 0.1 ml of caraway and clove extracts (25, 50 and 100 ppm of phenolic compounds) and BHT (50 and 100 ppm), and then shaken vigorously. The tubes were allowed to stand at 27 °C for 20 min. Control was prepared this way without any extracts. Changes in the absorbance of the samples were read at 517 nm. Radical scavenging activity was expressed as inhibition percentage and was calculated using the following formula (Singh et al., 2002):
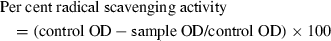
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity was determined according to the method of Singh et al. (2002). Various concentrations (50, 100 and 150 ppm) of total phenolic content in extracts and BHT (50 and 100 ppm) were poured in test tubes. One milliliter of Iron-ehtylenediaminetetraacetic acid (EDTA) solution (0.13% ferrous ammonium sulphate and 0.26% EDTA), 0.5 ml of EDTA (0.018%) and 1 ml of dimethyl sulphoxide (DMSO) (0.85% v/v in 0.1 m phosphate buffer, pH 7.4) were added to the tubes and the reaction was initiated by adding 0.5 ml of 0.22% ascorbic acid. Test tubes were capped tightly and heated on a water bath at 80–90 °C for 15 min. The reaction was terminated by addition of 1 ml of ice-cold trichloroacetic acid (TCA) (17.5% w/v). Three millilitre of Nash reagent (75.0 g of ammonium acetate, 3 ml of glacial acetic acid and 2 ml of acetyl acetone were mixed and raised to 1 L with distilled water) was added to all of the tubes and left at room temperature for 15 min for color development. The intensity of yellow color formed was measured spectrophotometrically at 412 nm against reagent blank. The percentage of the hydroxyl radical's scavenging activity was calculated by the following formula:

Determination of reducing power
The reducing power of test samples was determined by the method of Jayaprakasha et al. (2001). Different concentrations (25, 50, 100 and 150 ppm) of total phenolic content in 1 ml of 80% methanol were mixed with 2.5 ml of 1% potassium ferricyanide in 10-ml test tubes. The mixtures were incubated for 20 min at 50°C. At the end of the incubation, 2.5 ml of 10% TCA was added to the mixtures, followed by centrifugation at 5000 rpm for 10 min. The upper layer (2.5 ml) was mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% ferric chloride, and the absorbance was measured at 700 nm. The reducing power tests were run in triplicate. Increase in absorbance of the reaction mixture indicated the reducing power of the samples.
Evaluation of antioxidant activity in oil
Different concentrations (100, 300 and 500 ppm) of total phenolic content in caraway and clove extracts and BHT (50 and 100 ppm) were added to 45 g of sunflower oil in 100-ml dark bottles, which were then subjected to Schaal oven test at 63 ± 0.5 °C (Zandi & Gordon, 2001). A control sample was prepared under the same conditions, without adding any antioxidants. Samples were taken at different intervals (0, 3rd, 9th and 15th days) and peroxide value was measured (AOAC International, 1990; Method Cd 8-53). AA of extracts was determined according to the following formula:
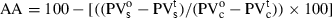
where: PV and PV
and PV are peroxide values of the sample at the zero and 15th days, respectively.
are peroxide values of the sample at the zero and 15th days, respectively.
PV and PV
and PV are peroxide values of control at the zero and 15th days, respectively.
are peroxide values of control at the zero and 15th days, respectively.
Statistical analysis
Statistical analysis involved use of the Statistical Analysis System (SAS, 2000) software package. Analysis of variance was performed by Anova procedures. Significant differences between means were determined by Duncan's multiple-range test. All determinations were carried out in triplicate.
Results and discussion
Yield
The yields of extracts obtained from caraway and clove and their total phenolic and flavonoid contents are shown in Table 1.
| Spices . | Yield (mg g−1 dry spice) . | Phenolic content (mg g−1 dry spice) . | Total flavonoids (mg g−1 dry spice) . |
|---|---|---|---|
| Clove | 306.0 ± 6.1 | 243.9 ± 0.9 | 5.0 ± 0.1 |
| Caraway | 130.3 ± 4.5 | 13.8 ± 0.3 | 2.6 ± 0.2 |
| Spices . | Yield (mg g−1 dry spice) . | Phenolic content (mg g−1 dry spice) . | Total flavonoids (mg g−1 dry spice) . |
|---|---|---|---|
| Clove | 306.0 ± 6.1 | 243.9 ± 0.9 | 5.0 ± 0.1 |
| Caraway | 130.3 ± 4.5 | 13.8 ± 0.3 | 2.6 ± 0.2 |
aValues expressed are the mean ± SD of three replications.
| Spices . | Yield (mg g−1 dry spice) . | Phenolic content (mg g−1 dry spice) . | Total flavonoids (mg g−1 dry spice) . |
|---|---|---|---|
| Clove | 306.0 ± 6.1 | 243.9 ± 0.9 | 5.0 ± 0.1 |
| Caraway | 130.3 ± 4.5 | 13.8 ± 0.3 | 2.6 ± 0.2 |
| Spices . | Yield (mg g−1 dry spice) . | Phenolic content (mg g−1 dry spice) . | Total flavonoids (mg g−1 dry spice) . |
|---|---|---|---|
| Clove | 306.0 ± 6.1 | 243.9 ± 0.9 | 5.0 ± 0.1 |
| Caraway | 130.3 ± 4.5 | 13.8 ± 0.3 | 2.6 ± 0.2 |
aValues expressed are the mean ± SD of three replications.
Major types of phenolic constituents identified in the spice extracts are phenolic acids, phenolic diterpenes, flavonoids and volatile oils (e.g., aromatic compounds) (Shan et al., 2005). Methanolic extracts of caraway and clove contain 10.56% and 79.71% phenolic compounds, respectively, indicating much lower phenolic compounds and flavonoids in caraway extract than that of clove.
Antioxidant assay using β-carotene–linoleate model system
The AA of phenolic compounds in caraway, clove extracts and BHT as measured by bleaching of β-carotene, is presented in Fig. 1.
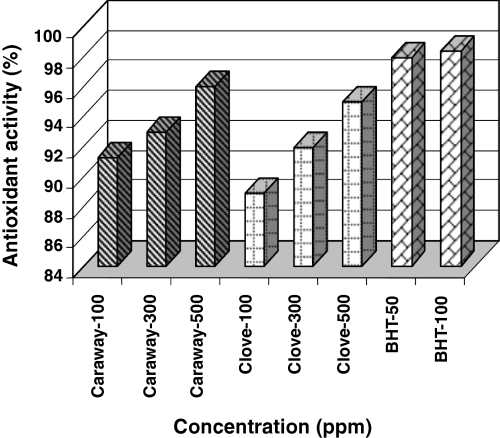
Antioxidant activity of caraway and clove extracts and BHT by β-carotene–linoleate model system at different concentrations.
Both caraway and clove extracts showed increase in AA with increasing concentration of total phenolic content. Caraway and clove extracts at 500 ppm phenolic concentrations indicated that AA was close to BHT at 50 ppm concentration. However, the AA of caraway at all concentrations was greater than those of clove, which might be due to its different phenolic profile. According to Shan et al. (2005), the major phenolic compounds of clove bud are phenolic acids (gallic acid), flavonol glucosides, tannins and phenolic volatile oils (eugenol, acetyl eugenol), and that of caraway fruits are volatile oils, phenolic acids, flavonoids (kaempferol) and coumarins. Caraway phenolic compounds at 100, 300 and 500 ppm concentrations showed 91.3%, 93.0% and 96.0% AA, respectively, in comparison with 88.9%, 92.0% and 95.0% for clove.
The mechanism of bleaching of β-carotene is a free radical-mediated phenomenon, resulting from the hydroperoxides formed from linoleic acid. β-carotene in this model system undergoes rapid discoloration in the absence of antioxidant.
The linoleic acid free radical formed upon the abstraction of a hydrogen atom from one of its diallylic methylene groups attacks the highly unsaturated β-carotene molecules. As β-carotene molecules lose their double bonds by oxidation, the compound loses its chromophore and characteristic orange color, which can be monitored spectrophotometrically. The presence of different extracts can hinder the extent of β-carotene bleaching by neutralizing the linoleate free radical and other free radicals formed in the system (Singh et al., 2002). In this regard, caraway phenolic compounds were able to quench more free radicals as evidenced by high AA. AA of caraway and clove were highly correlated (rclove = 0.99, rcaraway = 0.98) with total phenolic content, indicating they are able to prevent bleaching of β-carotene (Fig. 2).
Inhibition of β-carotene bleaching by natural and synthetic antioxidants at different concentrations in β-carotene–linoleate model system.
Antioxidant activity using linoleic acid peroxidation method
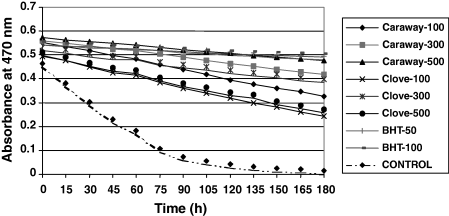
The data of linoleic peroxidation, determined by the thiocyanate method at 37 °C is shown in Fig. 3.
Antioxidant activities of caraway and clove extracts and BHT in linoleate peroxidation model system at different concentrations.
AA of caraway and clove extracts increased with increasing concentrations of total phenolic content. There was a positive and highly significant (P < 0.01) relationship between total phenolics and AA (rclove = 0.99, rcaraway = 0.99). Shan et al. (2005) also reported a highly positive linear relationship between total equivalent antioxidant capacity (TEAC) values and total phenolic content. It shows that phenolic compounds in the spices contribute significantly to their AC. Per cent inhibition of linoleic acid oxidation at 500 ppm phenolic content in caraway and clove extracts were 91% and 94%, respectively, which were higher than that of BHT at 100 ppm concentration.
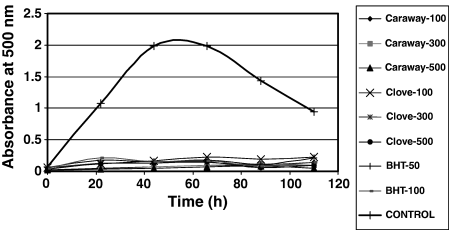
Figure 4 shows a decrease in absorbance of control after an initial increase. In control, absorbance has increased up to 1.99 at 44 h of incubation, and then decreased. This is due to oxidation of linoleic acid that generate hydroperoxides, which are then decomposed to many secondary oxidation products.
Antioxidant activities of caraway and clove extracts and BHT at different concentrations of phenolic compounds by thiocyanate method.
The oxidized products react with ferrous sulphate to form ferric sulphate and then ferric thiocyanate of blood-red colour. After 44 h of incubation of control, the formation of peroxides will be stopped, mainly due to nonavailability of the linoleic acid and this is where the absorbance declines. However, the intermediate products are then converted to stable end products, which do not show any effect on ferrous sulphate. In the presence of antioxidants, oxidation of linoleic acid will be prohibited (Jayaprakasha et al., 2001). Hence, the development of colour because of formation of thiocyanate will be slow. Of the extracts, the highest AA was observed in clove – 500 ppm followed by caraway 500 ppm and BHT – 100 ppm.
Free radical scavenging activity
It is well known that free radicals play an important role in autoxidation of unsaturated lipids in food stuff (Kaur & Perkins, 1991). For example, oxidation of muscle cholesterol may be initiated by free radicals that are generated during the oxidation of polyunsaturated fatty acids (Hoelscher et al., 1998). Reactive oxygen species (ROS), capable of causing damage to DNA, have been associated with carcinogenesis, coronary heart disease and many other health problems related to advancing age. Minimizing oxidative damage may well be one of the most important approaches to the primary prevention of these aging-associated diseases and health problems (Yu et al., 2005). Antioxidants are believed to intercept the free radical chain of oxidation, and to contribute hydrogen from their own phenolic hydroxyl groups, thereby forming stable free radicals, which do not initiate or propagate further oxidation of lipids (Sherwin, 1978). DPPH was used as free radical to evaluate AA present in natural sources (Schwarz et al., 2001). Hydroxyl radical is an extremely reactive free radical formed in biological systems and has been implicated as a highly damaging species in free radical pathology, capable of damaging almost every molecule found in living cells (Cervato et al., 2000). This radical has the capacity to join nucleotides in DNA and cause strand breakage, which contributes to carcinogenesis, mutagenesis and cytotoxicity. In addition, this species is considered to be one of the quick initiators of the lipid peroxidation process, abstracting hydrogen atoms from unsaturated fatty acids.
1,1-diphenyl-2-picryl hydrazyl radical scavenging activity
The scavenging effects of caraway and clove extracts at different concentrations of total phenolic content on DPPH radical are shown in Fig. 5.
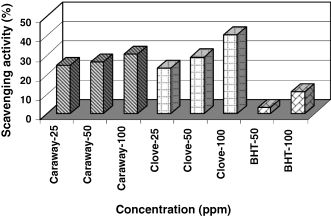
Radical scavenging activity of caraway and clove extracts and BHT by 1,1-diphenyl-2-picryl hydrazyl (DPPH) method at different concentrations (ppm).
The scavenging activity of spice extracts on DPPH radical was significantly related (P < 0.01) to total phenolic content of the extracts (rclove = 0.99, rcaraway = 0.99). Caraway and clove extracts at 100 ppm concentration, show 40.5% and 30.5% inhibition of the DPPH radical, respectively. Figure 4 shows that spice extracts are more effective in DPPH scavenging activity than BHT. BHT showed 11.11% and 3.2% radical scavenging activity at 100 and 50 ppm concentrations, respectively. Similar results have been reported by Chen & Ho (1997), who showed 8.9% inhibition by BHT at 20 μm concentration. The lower scavenging activity of BHT might be related to its structure as it has only one hydroxyl group.
Significantly differences (P < 0.01) were found among the extracts, indicating that they exhibited a potent scavenging effect on free radicals. Activity of the extracts can be attributed to their hydrogen donating ability. The data obtained reveal that the extracts are free radical inhibitors and are classified as primary antioxidants that mostly react with free radicals. Tomaino et al. (2005) Stated that clove essential oil, when maintained at room temperature, was more effective than cinnamon, nutmeg, basil and oregano essential oils in DPPH radical scavenging activity.
Hydroxyl radical scavenging activity
Hydroxyl radicals were generated using ascorbic acid–iron EDTA and then were reacted with DMSO to yield formaldehyde, which provides a convenient method to detect hydroxyl radicals by treatment with Nash reagent (Klein et al., 1981). Hydroxyl radical scavenging activity of the caraway and clove methanolic extracts is shown in Fig. 6.
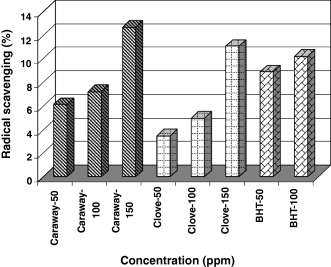
Antioxidant activities of caraway and clove extracts and BHT in hydroxyl radical scavenging model system at different concentrations.
Extracts of caraway and clove at 150 ppm phenolic compounds exhibited 11% and 12.7% hydroxyl radical scavenging activity. Total phenolic content was correlated with AA (rclove = 0.71, rcaraway = 0.88). The ability of these extracts to quench hydroxyl radicals seems to be directly related to the prevention of propagation of lipid peroxidation process, and therefore can be a good scavenger of active oxygen species. However, the AA of caraway at all concentrations was greater than that of clove, which might be due to their different phenolic profiles. Also, caraway and clove indicated higher scavenging effect than BHT although at higher concentrations.
Determination of reducing power
The AA has been reported to be concomitant with the reducing power (Amarowicz et al., 2000). Figure 7 shows the reducing powers of different concentrations of phenolic compounds in caraway and clove extracts using the potassium ferricyanide reduction method. At all concentrations, reducing power of ascorbic acid is greater than those of caraway and clove extracts. However, at lower concentrations, the differences are not significant. Caraway was more effective as a reducing agent than clove at higher concentrations.
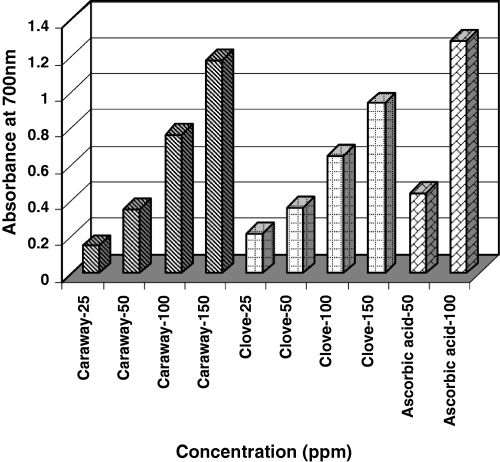
Reducing power of caraway and clove extracts at different concentrations of phenolic compounds.
Reducing properties are generally associated with the presence of reductones (Pin-Der et al., 1999). Gordon (1990) reported that the antioxidative action of reductones is based on the breaking of the free radical chain by the donation of a hydrogen atom. Reductones also react with certain precursors of peroxide, thus preventing peroxide formation.
The observed reducing power significantly correlated (rclove = 0.99, rcaraway = 0.99) with the total phenolic content present in them (P < 0.01).
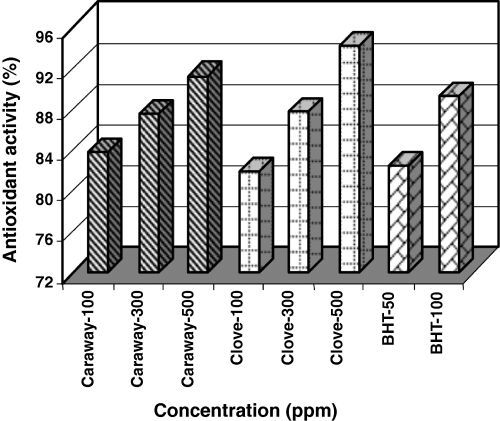
Antioxidant activity in sunflower oil
Extracts of spices at three concentrations (100, 300 and 500 ppm) along with BHT (50 and 100 ppm) were added to antioxidant-free frying oil and their peroxide value were determined on 0, 3rd, 9th and 15th days. AA on the basis of peroxide value on the 15th day is as shown in Fig. 8.
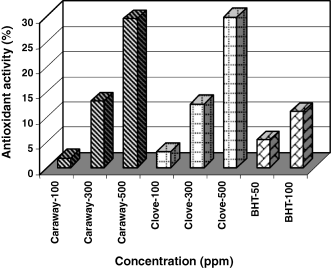
Antioxidant activities of caraway and clove extracts and BHT at different phenolic concentrations in sunflower oil.
BHT is very effective in stabilizing oil. Caraway and clove extracts are also effective in higher concentrations.
Conclusion
Five different model systems clearly showed that AA of phenolic compounds present in caraway is comparable with those of clove as well as BHT. Results obtained from reducing power of these compounds indicate that extracted phenolics were able to change redox condition of metals and therefore lessen their catalytic activity. Chain breakdown of oxidation reaction through reduction of free radicals by phenolics was also evidenced. DPPH and hydroxyl radical model systems were effectively used to show the ability of extracts to chelate free radicals. Regarding their activity in food, phenolic extracts of caraway and clove, although at slightly higher concentration, were comparable with BHT and therefore they can be useful in food applications. Further research is required in order to isolate and identify their active components.
Acknowledgments
We are indebted to L. Tabil, University of Saskatchewan, Canada and M. Shahtalebi, University of Isfahan, Iran for their scientific support on our work. Thanks are due to A. Akbari also for his technical assistance.



