-
PDF
- Split View
-
Views
-
Cite
Cite
Ioannis S Arvanitoyannis, Persefoni Tserkezou, Theodoros Varzakas, An update of US food safety, food technology, GM food and water protection and management legislation, International Journal of Food Science and Technology, Volume 41, Issue Supplement_1, August 2006, Pages 130–159, https://doi.org/10.1111/j.1365-2621.2006.01266.x
Close - Share Icon Share
Abstract
US government was the first to introduce Hazard Analysis Critical Control Point, the system that had a tremendous impact on everybody's life starting from the food and packaging companies up to consumer themselves. The rest of the nations simply followed US approach with a considerable delay both in terms of legislation and implementation. In the case of genetically modified (GM) or genetically engineered foods, the situation was entirely different. United States benefited from the ‘dubious’, and definitely not proved, ‘substantial equivalence’ principle invoked as the most practical approach to assess the safety of GM foods and food ingredients. US legislation appeared to be considerably more lenient than the European Union. The latter required many more analyses and labelling of GM food or food components. In this article, an update is attempted of the entire US legislation falling in fields like food safety, food technology, GM foods and finally legislation referring to specific foods (animal origin – meat, poultry, fish, dairy; and agricultural produces – vegetables, fruits) and water quality by means of fourteen comprehensive tables.
Introduction
The Food Safety and Inspection Service (FSIS) is the public health agency in the US Department of Agriculture responsible for ensuring safety and regulating all raw beef, pork, lamb, chicken, and turkey, as well as processed meat and poultry products, including hams, sausage, soups, stews, pizzas and frozen dinners (any product that contains 2% or more cooked poultry or 3% or more raw meat). Under the Federal Meat Inspection Act, the Poultry Products Inspection Act and the Egg Products Inspection Act, FSIS inspects all meat, poultry and eggs prepared for distribution in commerce, including imported products. FSIS develops and improves analytical procedures for detecting microbiological and chemical adulterants and infectious and toxic agents in meat and poultry products. The agency also develops new methods of inspection to better protect the public health, and it responds to microbiological, residue and other contamination incidents and, when appropriate, seeks voluntary recall of products by firms (http://www.usda.gov/news/pubs/97arp/arp4.htm). In August 1996, Food Quality Protection Act (FQPA) was signed – a comprehensive overhaul of laws that regulated pesticides in food. This law established a single, strong health-based standard by using the best science available, and, for the first time, provided Americans with the ‘right-to-know’ about health risks from pesticides. In August 1996, it was signed into law the Safe Water Drinking Act of 1996, thus strengthening protections to ensure that American families have clean, safe tap water to drink. On 2 December 1997, Food and Drug Administration (FDA) approved irradiation of meat products for controlling disease-causing microorganisms. The approval applied to fresh and frozen meat such as beef, lamb and pork; FDA concluded that irradiation is safe in reducing disease-causing microbes in or on meat, and that it does not compromise the nutritional quality of treated products. Disease-causing microorganisms that can be controlled by irradiation include the Escherichia coli O157:H7 and Salmonella species (http://www.hhs.gov/news/press/2000pres/20000316a.html). 27.4 million pounds of ready-to-eat poultry products were recalled because of fears of Listeria. Since January 2000, all federally inspected meat and poultry slaughtering and processing plants operate under the Hazard Analysis Critical Control Point (HACCP) system to ensure meat safety. Schumer's legislation will give the United States Department of Agriculture (USDA) the authority needed to establish and enforce microbiological standards. These standards will include specific pathogens like Listeria, Salmonella, E. coli and others identified as threats (http://schumer.senate.gov/schumerwebsite/pressroom/press_releases/pr01257.html). Closed US Federal Cases, mainly for meat and poultry, were summarised in Tables 1–3 from which one can have an idea of the changes detected in microorganisms occurrence in specific foods vs. time. Figures 1–3 show the variation of specific parameters: microbiological (Listeria, E. coli and Salmonella), presence of undeclared allergens and undeclared ingredients and presence of foreign materials in foods and detected underprocessed foods. Apart from Salmonella, the rest of the parameters displayed an increase over the period 2001–2003 and, thereafter, a considerable decrease was shown most probably because of the corrective actions undertaken (according to HACCP) and to stricter diligence displayed.
| Failure mode . | Food . | Quantity . |
|---|---|---|
| 1996 | ||
| Listeria | Sliced ham, chunked and formed ham, extra hot beef jerky, cooked beef, sliced roast beef, cooked beef roast | 6 |
| Escherichia coli O157:H7 | Frozen ground beef patties, ground beef | 2 |
| Undeclared allergen | Lasagna, ground beef patties, cooked salami | 3 |
| Undeclared ingredient | Ham, turkey and vegetable pie, various sausage | 3 |
| Salmonella | Smoked sausage | 1 |
| Foreign materials | Smoked poultry sausage, boned chicken thighs | 2 |
| Underprocessed | Boneless smoked ham, roast beef, ham, beef franks, fully cooked hams | 5 |
| 1997 | ||
| Listeria | Filzette salame, dry sausage, cooked chicken | 3 |
| Escherichia coli O157:H7 | Ground beef | 6 |
| Undeclared allergen | Roast beef gravy, cured sliced pork feet, beef steak dinner, chicken nuggets | 4 |
| Undeclared ingredient | Bologna, franks | 2 |
| Salmonella | Cervetal semi-dry sausage | 1 |
| Foreign materials | Boneless chicken, chicken wing, ground beef, breaded chicken patty nuggets, beef and bean chilli roll, grilled chicken breast, turkey breast | 6 |
| Underprocessed | Grilled chicken, chicken salad, ham, smoked sausage | 3 |
| 1998 | ||
| Listeria | Hotdogs, packaged meat, smoked beef strips, dry sausage, ham steaks, frankfurters | 6 |
| Escherichia coli O157:H7 | Ground beef, ground beef patties, coarse and fine ground beef, dry sausage, beef–vegetable protein patties | 13 |
| Undeclared allergen | Burritos, fresh pork, serrano ham, boned chicken dinners, green chilli burritos | 6 |
| Undeclared ingredient | Wieners, summer sausage and beef sticks, chicken breast | 3 |
| Salmonella | Cooked poultry products, chopped and formed beef steak, real cajun dressing mix | 3 |
| Foreign materials | Baby food chicken dinner, ground beef patties, chicken nuggets breast fillet, ham, spam, baked chicken, ravioli | 7 |
| Underprocessed | Jumbo franks, turkey breast roast, cooked chicken breast fillets, dry fermented sausage | 4 |
| 1999 | ||
| Listeria | Pates, luncheon meat, beef franks, Polish sausage, hotdogs, smoked sausage, beef frankfurters, chicken salad, turkey franks, sausage, roasting sausage, sliced or whole ham, bacon chips, frankfurters, skinless franks, weisswurst, pasta with sausage, head cheese, mortedella, chicken burrito, chorizos | 31 |
| Escherichia coli O157:H7 | Ground beef, salami | 10 |
| Undeclared allergen | Meatballs, chicken breast fajitas, cotto salami, chicken breast nuggets | 4 |
| Undeclared ingredient | Chicken nuggets, smoked sausage, cooked chicken breast patties, egg roll | 5 |
| Salmonella | Chicken piroshki, chicken breast nuggets, chorizos, dry sausage, sliced ham, Polish sausage | 6 |
| Foreign materials | Smoked sausage, sausage, ground beef | 3 |
| Underprocessed | Flying chilli sauce and beef, beef tips and gravy, ham, smoked turkey | 4 |
| 2000 | ||
| Listeria | Ham, turkey and chicken products, wiener products, chicken salad, Polish sausage, sliced ready-to-eat products, ready-to-eat products, beef jerky, beef Bologna, sliced ham, chicken enchiladas, franks, luncheon meat, smoked sausage, cooked sausage, pork spread, chicken nuggets, Bologna and salami, sliced meat products, roast beef and ham, bratwurst, pastrami, hotdog, cooked corn beef | 33 |
| Escherichia coli O157:H7 | Ground beef, ground beef patties, ground chuck | 21 |
| Undeclared allergen | Chicken, beef products, chicken salad, chicken fritters, steak product | 5 |
| Undeclared ingredient | Chinese style sausage, bratwurst sausage, pork rinds, franks | 4 |
| Salmonella | Frankfurters, chicken salad, chicken nuggets, mortadella | 3 |
| Foreign materials | Cheese franks, Italian sausage, chicken products, vegetable beef condensed soup | 4 |
| Failure mode . | Food . | Quantity . |
|---|---|---|
| 1996 | ||
| Listeria | Sliced ham, chunked and formed ham, extra hot beef jerky, cooked beef, sliced roast beef, cooked beef roast | 6 |
| Escherichia coli O157:H7 | Frozen ground beef patties, ground beef | 2 |
| Undeclared allergen | Lasagna, ground beef patties, cooked salami | 3 |
| Undeclared ingredient | Ham, turkey and vegetable pie, various sausage | 3 |
| Salmonella | Smoked sausage | 1 |
| Foreign materials | Smoked poultry sausage, boned chicken thighs | 2 |
| Underprocessed | Boneless smoked ham, roast beef, ham, beef franks, fully cooked hams | 5 |
| 1997 | ||
| Listeria | Filzette salame, dry sausage, cooked chicken | 3 |
| Escherichia coli O157:H7 | Ground beef | 6 |
| Undeclared allergen | Roast beef gravy, cured sliced pork feet, beef steak dinner, chicken nuggets | 4 |
| Undeclared ingredient | Bologna, franks | 2 |
| Salmonella | Cervetal semi-dry sausage | 1 |
| Foreign materials | Boneless chicken, chicken wing, ground beef, breaded chicken patty nuggets, beef and bean chilli roll, grilled chicken breast, turkey breast | 6 |
| Underprocessed | Grilled chicken, chicken salad, ham, smoked sausage | 3 |
| 1998 | ||
| Listeria | Hotdogs, packaged meat, smoked beef strips, dry sausage, ham steaks, frankfurters | 6 |
| Escherichia coli O157:H7 | Ground beef, ground beef patties, coarse and fine ground beef, dry sausage, beef–vegetable protein patties | 13 |
| Undeclared allergen | Burritos, fresh pork, serrano ham, boned chicken dinners, green chilli burritos | 6 |
| Undeclared ingredient | Wieners, summer sausage and beef sticks, chicken breast | 3 |
| Salmonella | Cooked poultry products, chopped and formed beef steak, real cajun dressing mix | 3 |
| Foreign materials | Baby food chicken dinner, ground beef patties, chicken nuggets breast fillet, ham, spam, baked chicken, ravioli | 7 |
| Underprocessed | Jumbo franks, turkey breast roast, cooked chicken breast fillets, dry fermented sausage | 4 |
| 1999 | ||
| Listeria | Pates, luncheon meat, beef franks, Polish sausage, hotdogs, smoked sausage, beef frankfurters, chicken salad, turkey franks, sausage, roasting sausage, sliced or whole ham, bacon chips, frankfurters, skinless franks, weisswurst, pasta with sausage, head cheese, mortedella, chicken burrito, chorizos | 31 |
| Escherichia coli O157:H7 | Ground beef, salami | 10 |
| Undeclared allergen | Meatballs, chicken breast fajitas, cotto salami, chicken breast nuggets | 4 |
| Undeclared ingredient | Chicken nuggets, smoked sausage, cooked chicken breast patties, egg roll | 5 |
| Salmonella | Chicken piroshki, chicken breast nuggets, chorizos, dry sausage, sliced ham, Polish sausage | 6 |
| Foreign materials | Smoked sausage, sausage, ground beef | 3 |
| Underprocessed | Flying chilli sauce and beef, beef tips and gravy, ham, smoked turkey | 4 |
| 2000 | ||
| Listeria | Ham, turkey and chicken products, wiener products, chicken salad, Polish sausage, sliced ready-to-eat products, ready-to-eat products, beef jerky, beef Bologna, sliced ham, chicken enchiladas, franks, luncheon meat, smoked sausage, cooked sausage, pork spread, chicken nuggets, Bologna and salami, sliced meat products, roast beef and ham, bratwurst, pastrami, hotdog, cooked corn beef | 33 |
| Escherichia coli O157:H7 | Ground beef, ground beef patties, ground chuck | 21 |
| Undeclared allergen | Chicken, beef products, chicken salad, chicken fritters, steak product | 5 |
| Undeclared ingredient | Chinese style sausage, bratwurst sausage, pork rinds, franks | 4 |
| Salmonella | Frankfurters, chicken salad, chicken nuggets, mortadella | 3 |
| Foreign materials | Cheese franks, Italian sausage, chicken products, vegetable beef condensed soup | 4 |
| Failure mode . | Food . | Quantity . |
|---|---|---|
| 1996 | ||
| Listeria | Sliced ham, chunked and formed ham, extra hot beef jerky, cooked beef, sliced roast beef, cooked beef roast | 6 |
| Escherichia coli O157:H7 | Frozen ground beef patties, ground beef | 2 |
| Undeclared allergen | Lasagna, ground beef patties, cooked salami | 3 |
| Undeclared ingredient | Ham, turkey and vegetable pie, various sausage | 3 |
| Salmonella | Smoked sausage | 1 |
| Foreign materials | Smoked poultry sausage, boned chicken thighs | 2 |
| Underprocessed | Boneless smoked ham, roast beef, ham, beef franks, fully cooked hams | 5 |
| 1997 | ||
| Listeria | Filzette salame, dry sausage, cooked chicken | 3 |
| Escherichia coli O157:H7 | Ground beef | 6 |
| Undeclared allergen | Roast beef gravy, cured sliced pork feet, beef steak dinner, chicken nuggets | 4 |
| Undeclared ingredient | Bologna, franks | 2 |
| Salmonella | Cervetal semi-dry sausage | 1 |
| Foreign materials | Boneless chicken, chicken wing, ground beef, breaded chicken patty nuggets, beef and bean chilli roll, grilled chicken breast, turkey breast | 6 |
| Underprocessed | Grilled chicken, chicken salad, ham, smoked sausage | 3 |
| 1998 | ||
| Listeria | Hotdogs, packaged meat, smoked beef strips, dry sausage, ham steaks, frankfurters | 6 |
| Escherichia coli O157:H7 | Ground beef, ground beef patties, coarse and fine ground beef, dry sausage, beef–vegetable protein patties | 13 |
| Undeclared allergen | Burritos, fresh pork, serrano ham, boned chicken dinners, green chilli burritos | 6 |
| Undeclared ingredient | Wieners, summer sausage and beef sticks, chicken breast | 3 |
| Salmonella | Cooked poultry products, chopped and formed beef steak, real cajun dressing mix | 3 |
| Foreign materials | Baby food chicken dinner, ground beef patties, chicken nuggets breast fillet, ham, spam, baked chicken, ravioli | 7 |
| Underprocessed | Jumbo franks, turkey breast roast, cooked chicken breast fillets, dry fermented sausage | 4 |
| 1999 | ||
| Listeria | Pates, luncheon meat, beef franks, Polish sausage, hotdogs, smoked sausage, beef frankfurters, chicken salad, turkey franks, sausage, roasting sausage, sliced or whole ham, bacon chips, frankfurters, skinless franks, weisswurst, pasta with sausage, head cheese, mortedella, chicken burrito, chorizos | 31 |
| Escherichia coli O157:H7 | Ground beef, salami | 10 |
| Undeclared allergen | Meatballs, chicken breast fajitas, cotto salami, chicken breast nuggets | 4 |
| Undeclared ingredient | Chicken nuggets, smoked sausage, cooked chicken breast patties, egg roll | 5 |
| Salmonella | Chicken piroshki, chicken breast nuggets, chorizos, dry sausage, sliced ham, Polish sausage | 6 |
| Foreign materials | Smoked sausage, sausage, ground beef | 3 |
| Underprocessed | Flying chilli sauce and beef, beef tips and gravy, ham, smoked turkey | 4 |
| 2000 | ||
| Listeria | Ham, turkey and chicken products, wiener products, chicken salad, Polish sausage, sliced ready-to-eat products, ready-to-eat products, beef jerky, beef Bologna, sliced ham, chicken enchiladas, franks, luncheon meat, smoked sausage, cooked sausage, pork spread, chicken nuggets, Bologna and salami, sliced meat products, roast beef and ham, bratwurst, pastrami, hotdog, cooked corn beef | 33 |
| Escherichia coli O157:H7 | Ground beef, ground beef patties, ground chuck | 21 |
| Undeclared allergen | Chicken, beef products, chicken salad, chicken fritters, steak product | 5 |
| Undeclared ingredient | Chinese style sausage, bratwurst sausage, pork rinds, franks | 4 |
| Salmonella | Frankfurters, chicken salad, chicken nuggets, mortadella | 3 |
| Foreign materials | Cheese franks, Italian sausage, chicken products, vegetable beef condensed soup | 4 |
| Failure mode . | Food . | Quantity . |
|---|---|---|
| 1996 | ||
| Listeria | Sliced ham, chunked and formed ham, extra hot beef jerky, cooked beef, sliced roast beef, cooked beef roast | 6 |
| Escherichia coli O157:H7 | Frozen ground beef patties, ground beef | 2 |
| Undeclared allergen | Lasagna, ground beef patties, cooked salami | 3 |
| Undeclared ingredient | Ham, turkey and vegetable pie, various sausage | 3 |
| Salmonella | Smoked sausage | 1 |
| Foreign materials | Smoked poultry sausage, boned chicken thighs | 2 |
| Underprocessed | Boneless smoked ham, roast beef, ham, beef franks, fully cooked hams | 5 |
| 1997 | ||
| Listeria | Filzette salame, dry sausage, cooked chicken | 3 |
| Escherichia coli O157:H7 | Ground beef | 6 |
| Undeclared allergen | Roast beef gravy, cured sliced pork feet, beef steak dinner, chicken nuggets | 4 |
| Undeclared ingredient | Bologna, franks | 2 |
| Salmonella | Cervetal semi-dry sausage | 1 |
| Foreign materials | Boneless chicken, chicken wing, ground beef, breaded chicken patty nuggets, beef and bean chilli roll, grilled chicken breast, turkey breast | 6 |
| Underprocessed | Grilled chicken, chicken salad, ham, smoked sausage | 3 |
| 1998 | ||
| Listeria | Hotdogs, packaged meat, smoked beef strips, dry sausage, ham steaks, frankfurters | 6 |
| Escherichia coli O157:H7 | Ground beef, ground beef patties, coarse and fine ground beef, dry sausage, beef–vegetable protein patties | 13 |
| Undeclared allergen | Burritos, fresh pork, serrano ham, boned chicken dinners, green chilli burritos | 6 |
| Undeclared ingredient | Wieners, summer sausage and beef sticks, chicken breast | 3 |
| Salmonella | Cooked poultry products, chopped and formed beef steak, real cajun dressing mix | 3 |
| Foreign materials | Baby food chicken dinner, ground beef patties, chicken nuggets breast fillet, ham, spam, baked chicken, ravioli | 7 |
| Underprocessed | Jumbo franks, turkey breast roast, cooked chicken breast fillets, dry fermented sausage | 4 |
| 1999 | ||
| Listeria | Pates, luncheon meat, beef franks, Polish sausage, hotdogs, smoked sausage, beef frankfurters, chicken salad, turkey franks, sausage, roasting sausage, sliced or whole ham, bacon chips, frankfurters, skinless franks, weisswurst, pasta with sausage, head cheese, mortedella, chicken burrito, chorizos | 31 |
| Escherichia coli O157:H7 | Ground beef, salami | 10 |
| Undeclared allergen | Meatballs, chicken breast fajitas, cotto salami, chicken breast nuggets | 4 |
| Undeclared ingredient | Chicken nuggets, smoked sausage, cooked chicken breast patties, egg roll | 5 |
| Salmonella | Chicken piroshki, chicken breast nuggets, chorizos, dry sausage, sliced ham, Polish sausage | 6 |
| Foreign materials | Smoked sausage, sausage, ground beef | 3 |
| Underprocessed | Flying chilli sauce and beef, beef tips and gravy, ham, smoked turkey | 4 |
| 2000 | ||
| Listeria | Ham, turkey and chicken products, wiener products, chicken salad, Polish sausage, sliced ready-to-eat products, ready-to-eat products, beef jerky, beef Bologna, sliced ham, chicken enchiladas, franks, luncheon meat, smoked sausage, cooked sausage, pork spread, chicken nuggets, Bologna and salami, sliced meat products, roast beef and ham, bratwurst, pastrami, hotdog, cooked corn beef | 33 |
| Escherichia coli O157:H7 | Ground beef, ground beef patties, ground chuck | 21 |
| Undeclared allergen | Chicken, beef products, chicken salad, chicken fritters, steak product | 5 |
| Undeclared ingredient | Chinese style sausage, bratwurst sausage, pork rinds, franks | 4 |
| Salmonella | Frankfurters, chicken salad, chicken nuggets, mortadella | 3 |
| Foreign materials | Cheese franks, Italian sausage, chicken products, vegetable beef condensed soup | 4 |
| Failure mode . | Food . | Quantity . |
|---|---|---|
| 2001 | ||
| Listeria | Sausage patties, bratwurst, beef products, luncheon meat, beef products, chicken products, pork and corned beef barbecue, sausage links, fajitas, chicken chow mein, ham, turkey barbecue, beef sausage, duck products, salami, duckling products, meat and poultry products, chicken wings | 25 |
| Misbranded | Country hams, frankfurters | 2 |
| Escherichia coli O157:H7 | Ground beef, ground pork, ground beef patties, hamburger patties | 26 |
| Undeclared allergen | Frozen dinners, luncheon meat, soup, meat and poultry products, ground beef products, franks, pork loin chops, chicken products, chicken wings | 11 |
| Undeclared ingredient | Canned corned beef, Italian sausage products, pork skin products | 13 |
| Salmonella | Sliced beef and ham products, sausage product | 2 |
| Foreign materials | Ham products, sausage links, soup, chicken products, chicken nuggets | 6 |
| Underprocessed | Chicken, ham, basterma | 6 |
| Residue contamination | Sausage products, burritos, chicken | 4 |
| 2002 | ||
| Listeria | Pork sausage products, souse products, pork products, luncheon meat, pork shoulder products, poultry products, turkey and chicken products, pork dumplings, ham, beef product, prosciutto hams, sausage products, chicken egg rolls, cooked hams, corned beef products, chicken salad, frankfurters, braunschweiger, imported cured ham, sliced ham, franks and Bologna, franks and hotdogs, pizza, turkey products, beef and pork products, fajita chicken strips, ham product, seasoned beef | 42 |
| Misbranded | Turkey breast, Salisbury steak products, fresh pork tenderloin, chicken base paste, turkey lunch kits, chicken product | 6 |
| Escherichia coli O157:H7 | Ground beef, ground beef products, beef trim | 35 |
| Undeclared allergen | Chicken product, chicken tacos, soup, egg roll products, sausage, meat loaf product, bratwurst, turkey products, pork, chicken salad, sausage products, ravioli, canned soup, meatballs, chicken ravioli, ready-to-cook chicken breasts | 18 |
| Undeclared ingredient | Frozen beef products, beef products, beef franks, pork and chicken sausages, chicken and pork products, chicken base products, pork products, pork loin products, canned corned beef, beef and pork products | 12 |
| Salmonella | Pork products, beef products, sausage | 4 |
| Foreign materials | Chicken, pork products, chilli tortilla product, bratwurst, beef products | 6 |
| Underprocessed | Chicken products | 3 |
| 2003 | ||
| Listeria | Pork shoulder picnic, beef products, ready-to-eat luncheon meat, Thai-style noodle salad, various ready-to-eat sausages, cooked beef products, pork and veal Bologna, cajun chicken salad, Cangialosi brand sweet Italian sausage, beef sausage, sliced fully cooked, chicken frankfurters, smoked pork chops | 12 |
| Misbranded | Roast half duckling with Szechwan style sauce, Cocina Mexicana refried beans with chorizo, ready-to-eat beef products, pork sausage, classic fried chicken white and dark chicken pieces with mashed potatoes, corn and apple–cranberry crumb dessert, food lion ready-to-serve chunky grilled sirloin steak with vegetables, imported Canadian sliced pork pepperoni, old-fashioned bacon end slices and old-fashioned bacon seasoning pieces, el Rio southwestern style, chilli corn carne, maple leaf brand Vienna sausages in beef broth, beef franks, pork egg rolls with shrimp added, head cheese, fresh and frozen raw young chickens, ‘sausage shop’ beef hot links smoked sausage, ready-to-eat pork and beef barbeque with sauce, turkey breakfast patties | 20 |
| Ineligible for import | La Sierra Authentica Cocina Mexicana refried beans with chorzio, chicken biriyani/chicken fried rice, imported Korean frozen dumplings with pork products | 3 |
| Foreign material | Country pride fresh young chickens and fresh young chicken whole wings | 1 |
| Escherichia coli O157:H7 | Ground beef products and pork sausage, frozen ground beef products, various cuts of boneless beef destined for further processing, fresh and frozen ground been products, frozen ground beef patties, beef trim | 8 |
| Insanitary conditions | ‘Mr Meat’ brand ‘meatballs, hamburger, 14–1’ and bulk ‘hamburger’, ground beef and various cuts of beef products | 2 |
| Underprocessed | Roast pork and pastrami products, Kolbassi sausage | 2 |
| Chemical contamination | Savage foods fresh raw beef and pork products | 1 |
| 2004 | ||
| Listeria | Beef Jerkey, ready-to-eat chicken products, fully cooked ham, frozen, fully cooked chicken products, wieners, beef and pork products, scrapple, ring Bologna with variety meat, Deli meat and cheese trays, cooked ham, frankfurters, meat products, the butcher block sliced cooked roast with juices, frozen beef brisket dinners, chicken salad with almonds and cranberries, fully cooked, diced chicken breast | 18 |
| Misbranded | Cooked beef products, frozen dinners, low-fat lunchables cracker stackers, classic shaved roast beef, garlic and herb pork products, cimmaron, premium, beef chilli with beans, roast beef, tubes of pure ground beef, gourmet banger London sausage, meat products, sonic, America's drive-in, chilli, ‘Quickmeal’ brand ‘Premium corn dog’ and ‘Quickmeal’ brand ‘Premium jumbo corn dog’, canned ‘no bean, chilli man chilli’ | 12 |
| Ineligible for import | Frozen ground beef patties | 1 |
| Foreign material | Imported frozen chicken pot pie, pork products, meat dumplings, meatballs, turkey, cooked turkey breasts, fully cooked frozen grilled chicken patties, chilli with meat | 8 |
| Escherichia coli O157:H7 | Frozen beef products, ground beef, frozen ground beef and beef patties, tubes of pure ground beef, fresh ground beef | 7 |
| Salmonella | Hand-made Russian brand blintzes, pork rinds, chef salad with turkey and ham, old Santa Fe beef jerky in original peppered, red chilli and green chilli flavours, jerky in original peppered, red chilli and green chilli flavours | 4 |
| Presumptive BSE positive | Raw beef | 1 |
| Undercooked | Beef wieners | 1 |
| 2005 | ||
| Listeria | Cooked country hams, chorizo, blood sausage, blood pudding, natural proportion cooked chicken meat, ready-to-eat chicken salads, ready-to-eat ham, primera chorizos Spanish brand sausage, turkey and cooked ham capicolla club wrap, sir pizza ham, smoked turkey and pork products, various sausage products, various ready-to-eat meat products, chicken breast wraps, pork blood sausage, chicken products, various sausage products, chicken wrap sandwiches, fully cooked chicken dumplings, cooked pork products, chicken salad, chicken products | 20 |
| Misbranded | Spaghettio's Plus Calcium, cheeseburger-stuffed sandwiches, Deli | 12 |
| Franks, wedding bell soup with meat balls and chicken, day-lee pride beef gyoza potstickers vegetables and beef dumplings, pork gyoza potstickers, pork and vegetable dumplings, frozen crispy chicken strips, chicken biriyani traditional Indian style, turkey, ham and cheddar cheese ready-to-eat complete meals, beef pattie | ||
| Ineligible for import | Imported Ukrainian canned beef, pork and poultry products, various beef, pork, and poultry canned and dried products | 2 |
| Foreign material | Frozen egg rolls, frozen, fully cooked chicken breast strips | 2 |
| Escherichia coli O157:H7 | Frozen ground beef patties and meatballs | 1 |
| Insanitary conditions | Sausage and bacon products | 1 |
| SRM | Bone-in beef products | 1 |
| Undercooked | Fully cooked chicken breast strips, frankfurters | 2 |
| Underprocessed | Canned turkey luncheon meat | 1 |
| Unapproved substance | Ground beef | 1 |
| Failure mode . | Food . | Quantity . |
|---|---|---|
| 2001 | ||
| Listeria | Sausage patties, bratwurst, beef products, luncheon meat, beef products, chicken products, pork and corned beef barbecue, sausage links, fajitas, chicken chow mein, ham, turkey barbecue, beef sausage, duck products, salami, duckling products, meat and poultry products, chicken wings | 25 |
| Misbranded | Country hams, frankfurters | 2 |
| Escherichia coli O157:H7 | Ground beef, ground pork, ground beef patties, hamburger patties | 26 |
| Undeclared allergen | Frozen dinners, luncheon meat, soup, meat and poultry products, ground beef products, franks, pork loin chops, chicken products, chicken wings | 11 |
| Undeclared ingredient | Canned corned beef, Italian sausage products, pork skin products | 13 |
| Salmonella | Sliced beef and ham products, sausage product | 2 |
| Foreign materials | Ham products, sausage links, soup, chicken products, chicken nuggets | 6 |
| Underprocessed | Chicken, ham, basterma | 6 |
| Residue contamination | Sausage products, burritos, chicken | 4 |
| 2002 | ||
| Listeria | Pork sausage products, souse products, pork products, luncheon meat, pork shoulder products, poultry products, turkey and chicken products, pork dumplings, ham, beef product, prosciutto hams, sausage products, chicken egg rolls, cooked hams, corned beef products, chicken salad, frankfurters, braunschweiger, imported cured ham, sliced ham, franks and Bologna, franks and hotdogs, pizza, turkey products, beef and pork products, fajita chicken strips, ham product, seasoned beef | 42 |
| Misbranded | Turkey breast, Salisbury steak products, fresh pork tenderloin, chicken base paste, turkey lunch kits, chicken product | 6 |
| Escherichia coli O157:H7 | Ground beef, ground beef products, beef trim | 35 |
| Undeclared allergen | Chicken product, chicken tacos, soup, egg roll products, sausage, meat loaf product, bratwurst, turkey products, pork, chicken salad, sausage products, ravioli, canned soup, meatballs, chicken ravioli, ready-to-cook chicken breasts | 18 |
| Undeclared ingredient | Frozen beef products, beef products, beef franks, pork and chicken sausages, chicken and pork products, chicken base products, pork products, pork loin products, canned corned beef, beef and pork products | 12 |
| Salmonella | Pork products, beef products, sausage | 4 |
| Foreign materials | Chicken, pork products, chilli tortilla product, bratwurst, beef products | 6 |
| Underprocessed | Chicken products | 3 |
| 2003 | ||
| Listeria | Pork shoulder picnic, beef products, ready-to-eat luncheon meat, Thai-style noodle salad, various ready-to-eat sausages, cooked beef products, pork and veal Bologna, cajun chicken salad, Cangialosi brand sweet Italian sausage, beef sausage, sliced fully cooked, chicken frankfurters, smoked pork chops | 12 |
| Misbranded | Roast half duckling with Szechwan style sauce, Cocina Mexicana refried beans with chorizo, ready-to-eat beef products, pork sausage, classic fried chicken white and dark chicken pieces with mashed potatoes, corn and apple–cranberry crumb dessert, food lion ready-to-serve chunky grilled sirloin steak with vegetables, imported Canadian sliced pork pepperoni, old-fashioned bacon end slices and old-fashioned bacon seasoning pieces, el Rio southwestern style, chilli corn carne, maple leaf brand Vienna sausages in beef broth, beef franks, pork egg rolls with shrimp added, head cheese, fresh and frozen raw young chickens, ‘sausage shop’ beef hot links smoked sausage, ready-to-eat pork and beef barbeque with sauce, turkey breakfast patties | 20 |
| Ineligible for import | La Sierra Authentica Cocina Mexicana refried beans with chorzio, chicken biriyani/chicken fried rice, imported Korean frozen dumplings with pork products | 3 |
| Foreign material | Country pride fresh young chickens and fresh young chicken whole wings | 1 |
| Escherichia coli O157:H7 | Ground beef products and pork sausage, frozen ground beef products, various cuts of boneless beef destined for further processing, fresh and frozen ground been products, frozen ground beef patties, beef trim | 8 |
| Insanitary conditions | ‘Mr Meat’ brand ‘meatballs, hamburger, 14–1’ and bulk ‘hamburger’, ground beef and various cuts of beef products | 2 |
| Underprocessed | Roast pork and pastrami products, Kolbassi sausage | 2 |
| Chemical contamination | Savage foods fresh raw beef and pork products | 1 |
| 2004 | ||
| Listeria | Beef Jerkey, ready-to-eat chicken products, fully cooked ham, frozen, fully cooked chicken products, wieners, beef and pork products, scrapple, ring Bologna with variety meat, Deli meat and cheese trays, cooked ham, frankfurters, meat products, the butcher block sliced cooked roast with juices, frozen beef brisket dinners, chicken salad with almonds and cranberries, fully cooked, diced chicken breast | 18 |
| Misbranded | Cooked beef products, frozen dinners, low-fat lunchables cracker stackers, classic shaved roast beef, garlic and herb pork products, cimmaron, premium, beef chilli with beans, roast beef, tubes of pure ground beef, gourmet banger London sausage, meat products, sonic, America's drive-in, chilli, ‘Quickmeal’ brand ‘Premium corn dog’ and ‘Quickmeal’ brand ‘Premium jumbo corn dog’, canned ‘no bean, chilli man chilli’ | 12 |
| Ineligible for import | Frozen ground beef patties | 1 |
| Foreign material | Imported frozen chicken pot pie, pork products, meat dumplings, meatballs, turkey, cooked turkey breasts, fully cooked frozen grilled chicken patties, chilli with meat | 8 |
| Escherichia coli O157:H7 | Frozen beef products, ground beef, frozen ground beef and beef patties, tubes of pure ground beef, fresh ground beef | 7 |
| Salmonella | Hand-made Russian brand blintzes, pork rinds, chef salad with turkey and ham, old Santa Fe beef jerky in original peppered, red chilli and green chilli flavours, jerky in original peppered, red chilli and green chilli flavours | 4 |
| Presumptive BSE positive | Raw beef | 1 |
| Undercooked | Beef wieners | 1 |
| 2005 | ||
| Listeria | Cooked country hams, chorizo, blood sausage, blood pudding, natural proportion cooked chicken meat, ready-to-eat chicken salads, ready-to-eat ham, primera chorizos Spanish brand sausage, turkey and cooked ham capicolla club wrap, sir pizza ham, smoked turkey and pork products, various sausage products, various ready-to-eat meat products, chicken breast wraps, pork blood sausage, chicken products, various sausage products, chicken wrap sandwiches, fully cooked chicken dumplings, cooked pork products, chicken salad, chicken products | 20 |
| Misbranded | Spaghettio's Plus Calcium, cheeseburger-stuffed sandwiches, Deli | 12 |
| Franks, wedding bell soup with meat balls and chicken, day-lee pride beef gyoza potstickers vegetables and beef dumplings, pork gyoza potstickers, pork and vegetable dumplings, frozen crispy chicken strips, chicken biriyani traditional Indian style, turkey, ham and cheddar cheese ready-to-eat complete meals, beef pattie | ||
| Ineligible for import | Imported Ukrainian canned beef, pork and poultry products, various beef, pork, and poultry canned and dried products | 2 |
| Foreign material | Frozen egg rolls, frozen, fully cooked chicken breast strips | 2 |
| Escherichia coli O157:H7 | Frozen ground beef patties and meatballs | 1 |
| Insanitary conditions | Sausage and bacon products | 1 |
| SRM | Bone-in beef products | 1 |
| Undercooked | Fully cooked chicken breast strips, frankfurters | 2 |
| Underprocessed | Canned turkey luncheon meat | 1 |
| Unapproved substance | Ground beef | 1 |
| Failure mode . | Food . | Quantity . |
|---|---|---|
| 2001 | ||
| Listeria | Sausage patties, bratwurst, beef products, luncheon meat, beef products, chicken products, pork and corned beef barbecue, sausage links, fajitas, chicken chow mein, ham, turkey barbecue, beef sausage, duck products, salami, duckling products, meat and poultry products, chicken wings | 25 |
| Misbranded | Country hams, frankfurters | 2 |
| Escherichia coli O157:H7 | Ground beef, ground pork, ground beef patties, hamburger patties | 26 |
| Undeclared allergen | Frozen dinners, luncheon meat, soup, meat and poultry products, ground beef products, franks, pork loin chops, chicken products, chicken wings | 11 |
| Undeclared ingredient | Canned corned beef, Italian sausage products, pork skin products | 13 |
| Salmonella | Sliced beef and ham products, sausage product | 2 |
| Foreign materials | Ham products, sausage links, soup, chicken products, chicken nuggets | 6 |
| Underprocessed | Chicken, ham, basterma | 6 |
| Residue contamination | Sausage products, burritos, chicken | 4 |
| 2002 | ||
| Listeria | Pork sausage products, souse products, pork products, luncheon meat, pork shoulder products, poultry products, turkey and chicken products, pork dumplings, ham, beef product, prosciutto hams, sausage products, chicken egg rolls, cooked hams, corned beef products, chicken salad, frankfurters, braunschweiger, imported cured ham, sliced ham, franks and Bologna, franks and hotdogs, pizza, turkey products, beef and pork products, fajita chicken strips, ham product, seasoned beef | 42 |
| Misbranded | Turkey breast, Salisbury steak products, fresh pork tenderloin, chicken base paste, turkey lunch kits, chicken product | 6 |
| Escherichia coli O157:H7 | Ground beef, ground beef products, beef trim | 35 |
| Undeclared allergen | Chicken product, chicken tacos, soup, egg roll products, sausage, meat loaf product, bratwurst, turkey products, pork, chicken salad, sausage products, ravioli, canned soup, meatballs, chicken ravioli, ready-to-cook chicken breasts | 18 |
| Undeclared ingredient | Frozen beef products, beef products, beef franks, pork and chicken sausages, chicken and pork products, chicken base products, pork products, pork loin products, canned corned beef, beef and pork products | 12 |
| Salmonella | Pork products, beef products, sausage | 4 |
| Foreign materials | Chicken, pork products, chilli tortilla product, bratwurst, beef products | 6 |
| Underprocessed | Chicken products | 3 |
| 2003 | ||
| Listeria | Pork shoulder picnic, beef products, ready-to-eat luncheon meat, Thai-style noodle salad, various ready-to-eat sausages, cooked beef products, pork and veal Bologna, cajun chicken salad, Cangialosi brand sweet Italian sausage, beef sausage, sliced fully cooked, chicken frankfurters, smoked pork chops | 12 |
| Misbranded | Roast half duckling with Szechwan style sauce, Cocina Mexicana refried beans with chorizo, ready-to-eat beef products, pork sausage, classic fried chicken white and dark chicken pieces with mashed potatoes, corn and apple–cranberry crumb dessert, food lion ready-to-serve chunky grilled sirloin steak with vegetables, imported Canadian sliced pork pepperoni, old-fashioned bacon end slices and old-fashioned bacon seasoning pieces, el Rio southwestern style, chilli corn carne, maple leaf brand Vienna sausages in beef broth, beef franks, pork egg rolls with shrimp added, head cheese, fresh and frozen raw young chickens, ‘sausage shop’ beef hot links smoked sausage, ready-to-eat pork and beef barbeque with sauce, turkey breakfast patties | 20 |
| Ineligible for import | La Sierra Authentica Cocina Mexicana refried beans with chorzio, chicken biriyani/chicken fried rice, imported Korean frozen dumplings with pork products | 3 |
| Foreign material | Country pride fresh young chickens and fresh young chicken whole wings | 1 |
| Escherichia coli O157:H7 | Ground beef products and pork sausage, frozen ground beef products, various cuts of boneless beef destined for further processing, fresh and frozen ground been products, frozen ground beef patties, beef trim | 8 |
| Insanitary conditions | ‘Mr Meat’ brand ‘meatballs, hamburger, 14–1’ and bulk ‘hamburger’, ground beef and various cuts of beef products | 2 |
| Underprocessed | Roast pork and pastrami products, Kolbassi sausage | 2 |
| Chemical contamination | Savage foods fresh raw beef and pork products | 1 |
| 2004 | ||
| Listeria | Beef Jerkey, ready-to-eat chicken products, fully cooked ham, frozen, fully cooked chicken products, wieners, beef and pork products, scrapple, ring Bologna with variety meat, Deli meat and cheese trays, cooked ham, frankfurters, meat products, the butcher block sliced cooked roast with juices, frozen beef brisket dinners, chicken salad with almonds and cranberries, fully cooked, diced chicken breast | 18 |
| Misbranded | Cooked beef products, frozen dinners, low-fat lunchables cracker stackers, classic shaved roast beef, garlic and herb pork products, cimmaron, premium, beef chilli with beans, roast beef, tubes of pure ground beef, gourmet banger London sausage, meat products, sonic, America's drive-in, chilli, ‘Quickmeal’ brand ‘Premium corn dog’ and ‘Quickmeal’ brand ‘Premium jumbo corn dog’, canned ‘no bean, chilli man chilli’ | 12 |
| Ineligible for import | Frozen ground beef patties | 1 |
| Foreign material | Imported frozen chicken pot pie, pork products, meat dumplings, meatballs, turkey, cooked turkey breasts, fully cooked frozen grilled chicken patties, chilli with meat | 8 |
| Escherichia coli O157:H7 | Frozen beef products, ground beef, frozen ground beef and beef patties, tubes of pure ground beef, fresh ground beef | 7 |
| Salmonella | Hand-made Russian brand blintzes, pork rinds, chef salad with turkey and ham, old Santa Fe beef jerky in original peppered, red chilli and green chilli flavours, jerky in original peppered, red chilli and green chilli flavours | 4 |
| Presumptive BSE positive | Raw beef | 1 |
| Undercooked | Beef wieners | 1 |
| 2005 | ||
| Listeria | Cooked country hams, chorizo, blood sausage, blood pudding, natural proportion cooked chicken meat, ready-to-eat chicken salads, ready-to-eat ham, primera chorizos Spanish brand sausage, turkey and cooked ham capicolla club wrap, sir pizza ham, smoked turkey and pork products, various sausage products, various ready-to-eat meat products, chicken breast wraps, pork blood sausage, chicken products, various sausage products, chicken wrap sandwiches, fully cooked chicken dumplings, cooked pork products, chicken salad, chicken products | 20 |
| Misbranded | Spaghettio's Plus Calcium, cheeseburger-stuffed sandwiches, Deli | 12 |
| Franks, wedding bell soup with meat balls and chicken, day-lee pride beef gyoza potstickers vegetables and beef dumplings, pork gyoza potstickers, pork and vegetable dumplings, frozen crispy chicken strips, chicken biriyani traditional Indian style, turkey, ham and cheddar cheese ready-to-eat complete meals, beef pattie | ||
| Ineligible for import | Imported Ukrainian canned beef, pork and poultry products, various beef, pork, and poultry canned and dried products | 2 |
| Foreign material | Frozen egg rolls, frozen, fully cooked chicken breast strips | 2 |
| Escherichia coli O157:H7 | Frozen ground beef patties and meatballs | 1 |
| Insanitary conditions | Sausage and bacon products | 1 |
| SRM | Bone-in beef products | 1 |
| Undercooked | Fully cooked chicken breast strips, frankfurters | 2 |
| Underprocessed | Canned turkey luncheon meat | 1 |
| Unapproved substance | Ground beef | 1 |
| Failure mode . | Food . | Quantity . |
|---|---|---|
| 2001 | ||
| Listeria | Sausage patties, bratwurst, beef products, luncheon meat, beef products, chicken products, pork and corned beef barbecue, sausage links, fajitas, chicken chow mein, ham, turkey barbecue, beef sausage, duck products, salami, duckling products, meat and poultry products, chicken wings | 25 |
| Misbranded | Country hams, frankfurters | 2 |
| Escherichia coli O157:H7 | Ground beef, ground pork, ground beef patties, hamburger patties | 26 |
| Undeclared allergen | Frozen dinners, luncheon meat, soup, meat and poultry products, ground beef products, franks, pork loin chops, chicken products, chicken wings | 11 |
| Undeclared ingredient | Canned corned beef, Italian sausage products, pork skin products | 13 |
| Salmonella | Sliced beef and ham products, sausage product | 2 |
| Foreign materials | Ham products, sausage links, soup, chicken products, chicken nuggets | 6 |
| Underprocessed | Chicken, ham, basterma | 6 |
| Residue contamination | Sausage products, burritos, chicken | 4 |
| 2002 | ||
| Listeria | Pork sausage products, souse products, pork products, luncheon meat, pork shoulder products, poultry products, turkey and chicken products, pork dumplings, ham, beef product, prosciutto hams, sausage products, chicken egg rolls, cooked hams, corned beef products, chicken salad, frankfurters, braunschweiger, imported cured ham, sliced ham, franks and Bologna, franks and hotdogs, pizza, turkey products, beef and pork products, fajita chicken strips, ham product, seasoned beef | 42 |
| Misbranded | Turkey breast, Salisbury steak products, fresh pork tenderloin, chicken base paste, turkey lunch kits, chicken product | 6 |
| Escherichia coli O157:H7 | Ground beef, ground beef products, beef trim | 35 |
| Undeclared allergen | Chicken product, chicken tacos, soup, egg roll products, sausage, meat loaf product, bratwurst, turkey products, pork, chicken salad, sausage products, ravioli, canned soup, meatballs, chicken ravioli, ready-to-cook chicken breasts | 18 |
| Undeclared ingredient | Frozen beef products, beef products, beef franks, pork and chicken sausages, chicken and pork products, chicken base products, pork products, pork loin products, canned corned beef, beef and pork products | 12 |
| Salmonella | Pork products, beef products, sausage | 4 |
| Foreign materials | Chicken, pork products, chilli tortilla product, bratwurst, beef products | 6 |
| Underprocessed | Chicken products | 3 |
| 2003 | ||
| Listeria | Pork shoulder picnic, beef products, ready-to-eat luncheon meat, Thai-style noodle salad, various ready-to-eat sausages, cooked beef products, pork and veal Bologna, cajun chicken salad, Cangialosi brand sweet Italian sausage, beef sausage, sliced fully cooked, chicken frankfurters, smoked pork chops | 12 |
| Misbranded | Roast half duckling with Szechwan style sauce, Cocina Mexicana refried beans with chorizo, ready-to-eat beef products, pork sausage, classic fried chicken white and dark chicken pieces with mashed potatoes, corn and apple–cranberry crumb dessert, food lion ready-to-serve chunky grilled sirloin steak with vegetables, imported Canadian sliced pork pepperoni, old-fashioned bacon end slices and old-fashioned bacon seasoning pieces, el Rio southwestern style, chilli corn carne, maple leaf brand Vienna sausages in beef broth, beef franks, pork egg rolls with shrimp added, head cheese, fresh and frozen raw young chickens, ‘sausage shop’ beef hot links smoked sausage, ready-to-eat pork and beef barbeque with sauce, turkey breakfast patties | 20 |
| Ineligible for import | La Sierra Authentica Cocina Mexicana refried beans with chorzio, chicken biriyani/chicken fried rice, imported Korean frozen dumplings with pork products | 3 |
| Foreign material | Country pride fresh young chickens and fresh young chicken whole wings | 1 |
| Escherichia coli O157:H7 | Ground beef products and pork sausage, frozen ground beef products, various cuts of boneless beef destined for further processing, fresh and frozen ground been products, frozen ground beef patties, beef trim | 8 |
| Insanitary conditions | ‘Mr Meat’ brand ‘meatballs, hamburger, 14–1’ and bulk ‘hamburger’, ground beef and various cuts of beef products | 2 |
| Underprocessed | Roast pork and pastrami products, Kolbassi sausage | 2 |
| Chemical contamination | Savage foods fresh raw beef and pork products | 1 |
| 2004 | ||
| Listeria | Beef Jerkey, ready-to-eat chicken products, fully cooked ham, frozen, fully cooked chicken products, wieners, beef and pork products, scrapple, ring Bologna with variety meat, Deli meat and cheese trays, cooked ham, frankfurters, meat products, the butcher block sliced cooked roast with juices, frozen beef brisket dinners, chicken salad with almonds and cranberries, fully cooked, diced chicken breast | 18 |
| Misbranded | Cooked beef products, frozen dinners, low-fat lunchables cracker stackers, classic shaved roast beef, garlic and herb pork products, cimmaron, premium, beef chilli with beans, roast beef, tubes of pure ground beef, gourmet banger London sausage, meat products, sonic, America's drive-in, chilli, ‘Quickmeal’ brand ‘Premium corn dog’ and ‘Quickmeal’ brand ‘Premium jumbo corn dog’, canned ‘no bean, chilli man chilli’ | 12 |
| Ineligible for import | Frozen ground beef patties | 1 |
| Foreign material | Imported frozen chicken pot pie, pork products, meat dumplings, meatballs, turkey, cooked turkey breasts, fully cooked frozen grilled chicken patties, chilli with meat | 8 |
| Escherichia coli O157:H7 | Frozen beef products, ground beef, frozen ground beef and beef patties, tubes of pure ground beef, fresh ground beef | 7 |
| Salmonella | Hand-made Russian brand blintzes, pork rinds, chef salad with turkey and ham, old Santa Fe beef jerky in original peppered, red chilli and green chilli flavours, jerky in original peppered, red chilli and green chilli flavours | 4 |
| Presumptive BSE positive | Raw beef | 1 |
| Undercooked | Beef wieners | 1 |
| 2005 | ||
| Listeria | Cooked country hams, chorizo, blood sausage, blood pudding, natural proportion cooked chicken meat, ready-to-eat chicken salads, ready-to-eat ham, primera chorizos Spanish brand sausage, turkey and cooked ham capicolla club wrap, sir pizza ham, smoked turkey and pork products, various sausage products, various ready-to-eat meat products, chicken breast wraps, pork blood sausage, chicken products, various sausage products, chicken wrap sandwiches, fully cooked chicken dumplings, cooked pork products, chicken salad, chicken products | 20 |
| Misbranded | Spaghettio's Plus Calcium, cheeseburger-stuffed sandwiches, Deli | 12 |
| Franks, wedding bell soup with meat balls and chicken, day-lee pride beef gyoza potstickers vegetables and beef dumplings, pork gyoza potstickers, pork and vegetable dumplings, frozen crispy chicken strips, chicken biriyani traditional Indian style, turkey, ham and cheddar cheese ready-to-eat complete meals, beef pattie | ||
| Ineligible for import | Imported Ukrainian canned beef, pork and poultry products, various beef, pork, and poultry canned and dried products | 2 |
| Foreign material | Frozen egg rolls, frozen, fully cooked chicken breast strips | 2 |
| Escherichia coli O157:H7 | Frozen ground beef patties and meatballs | 1 |
| Insanitary conditions | Sausage and bacon products | 1 |
| SRM | Bone-in beef products | 1 |
| Undercooked | Fully cooked chicken breast strips, frankfurters | 2 |
| Underprocessed | Canned turkey luncheon meat | 1 |
| Unapproved substance | Ground beef | 1 |
| Failure mode . | 1996 . | 1997 . | 1998 . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Listeria | 6 | 3 | 6 | 31 | 33 | 25 | 42 | 12 | 18 | 20 |
| Escherichia coli O157:H7 | 2 | 6 | 13 | 10 | 21 | 26 | 35 | 8 | 7 | 1 |
| Salmonella | 1 | 1 | 3 | 6 | 3 | 2 | 4 | 0 | 4 | 0 |
| Undeclared allergen | 3 | 4 | 6 | 4 | 5 | 11 | 18 | 0 | 0 | 0 |
| Undeclared ingredient | 3 | 2 | 3 | 5 | 4 | 13 | 12 | 0 | 0 | 0 |
| Foreign materials | 2 | 6 | 7 | 3 | 4 | 6 | 6 | 1 | 8 | 2 |
| Underprocessed | 5 | 3 | 4 | 4 | 0 | 6 | 3 | 2 | 0 | 1 |
| Failure mode . | 1996 . | 1997 . | 1998 . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Listeria | 6 | 3 | 6 | 31 | 33 | 25 | 42 | 12 | 18 | 20 |
| Escherichia coli O157:H7 | 2 | 6 | 13 | 10 | 21 | 26 | 35 | 8 | 7 | 1 |
| Salmonella | 1 | 1 | 3 | 6 | 3 | 2 | 4 | 0 | 4 | 0 |
| Undeclared allergen | 3 | 4 | 6 | 4 | 5 | 11 | 18 | 0 | 0 | 0 |
| Undeclared ingredient | 3 | 2 | 3 | 5 | 4 | 13 | 12 | 0 | 0 | 0 |
| Foreign materials | 2 | 6 | 7 | 3 | 4 | 6 | 6 | 1 | 8 | 2 |
| Underprocessed | 5 | 3 | 4 | 4 | 0 | 6 | 3 | 2 | 0 | 1 |
| Failure mode . | 1996 . | 1997 . | 1998 . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Listeria | 6 | 3 | 6 | 31 | 33 | 25 | 42 | 12 | 18 | 20 |
| Escherichia coli O157:H7 | 2 | 6 | 13 | 10 | 21 | 26 | 35 | 8 | 7 | 1 |
| Salmonella | 1 | 1 | 3 | 6 | 3 | 2 | 4 | 0 | 4 | 0 |
| Undeclared allergen | 3 | 4 | 6 | 4 | 5 | 11 | 18 | 0 | 0 | 0 |
| Undeclared ingredient | 3 | 2 | 3 | 5 | 4 | 13 | 12 | 0 | 0 | 0 |
| Foreign materials | 2 | 6 | 7 | 3 | 4 | 6 | 6 | 1 | 8 | 2 |
| Underprocessed | 5 | 3 | 4 | 4 | 0 | 6 | 3 | 2 | 0 | 1 |
| Failure mode . | 1996 . | 1997 . | 1998 . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Listeria | 6 | 3 | 6 | 31 | 33 | 25 | 42 | 12 | 18 | 20 |
| Escherichia coli O157:H7 | 2 | 6 | 13 | 10 | 21 | 26 | 35 | 8 | 7 | 1 |
| Salmonella | 1 | 1 | 3 | 6 | 3 | 2 | 4 | 0 | 4 | 0 |
| Undeclared allergen | 3 | 4 | 6 | 4 | 5 | 11 | 18 | 0 | 0 | 0 |
| Undeclared ingredient | 3 | 2 | 3 | 5 | 4 | 13 | 12 | 0 | 0 | 0 |
| Foreign materials | 2 | 6 | 7 | 3 | 4 | 6 | 6 | 1 | 8 | 2 |
| Underprocessed | 5 | 3 | 4 | 4 | 0 | 6 | 3 | 2 | 0 | 1 |
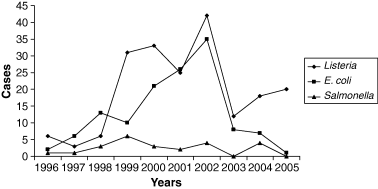
Listeria (◆), Escherichia coli (■) and Salmonella (▲) determined values in US vs. time (1996–2005).
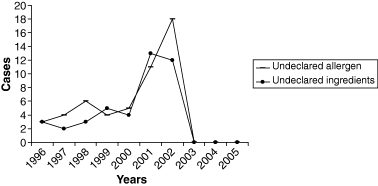
Undeclared allergen (–––) and undeclared ingredients (•) determined values in US vs. time (1996–2005).
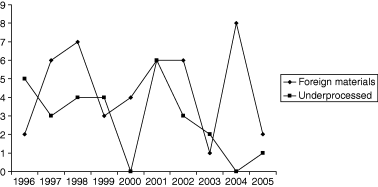
Foreign materials (◆) and underprocessed (■) determined cases in US vs. time (1996–2005).
Topics/categories covered under US legislation
Introduction in food safety
The purpose of food safety legislation is to ensure the health and safety of the consumer by providing controls over the production, storage, preparation and sale of food throughout the country. A food business is defined as being any undertaking regarding food, whether carried out for profit or not, carrying out one or more of the following operations: preparation, processing, manufacturing, packaging, storage, transportation/distribution, handling, offering for sale or supplying a consumer (http://www.eastherts.gov.uk/buisiness%20guide%20to%20law%20and%20practise/legislation.htm). Regulating food safety in the United States is complex. This complexity is due largely to the historical division of food safety responsibility among different Federal agencies and to evolving public attitudes towards the safety of food and concern about the changing nature of foodborne illnesses. Although this overview focuses on Federal regulation of food safety, it is important to note that state regulatory agencies also play an important role in food safety regulation, primarily with food sanitation and safe food handling by food retailers, foodservice providers and food-vending operations (http://www.nationalaglawcenter.org/readindrooms/foodsafety/). The Food Safety Act 1990 and regulations specified certain safety standards for the processing and sale of food. It is an offense for anyone to process or sell food that is harmful to health. The regulations also placed an obligation on businesses to ensure that their activities were carried out in a hygienic way. Running a food business means that one has a particular responsibility to protect the health of your customers (http://www.torridge.gov.uk/index.cfm?articleid=3967). The FQPA is one of the most significant pieces of legislation enacted in the past two decades. The FQPA includes sweeping new food safety protections and requires major changes in how pesticides are regulated, with the goal of improving environmental and public health protection, especially for children (http://www.epa.gov/history/topics/fqpa/02.htm).
Food safety legislation
In Consumer Product Safety Act (1972), the term ‘consumer product’ means any article or component part thereof, produced or distributed (i) for sale to a consumer for use in or around a permanent or temporary household or residence, a school, in recreation, or otherwise, or (ii) for the personal use, consumption or enjoyment of a consumer in or around a permanent or temporary household or residence, a school, in recreation or otherwise; but such term does not include (i) any article that is not customarily produced or distributed for sale to, or use or consumption by, or enjoyment of, a consumer, (ii) tobacco and tobacco products, (iii) motor vehicles or motor vehicle equipment, and (iv) pesticides. A consumer product safety standard shall consist of one or more of any of the following types of requirements:
- 1
Requirements expressed in terms of performance requirements.
- 2
Requirements that a consumer product be marked with or accompanied by clear and adequate warnings or instructions or requirements respecting the form of warnings or instructions. Any requirement of such a standard shall be reasonably necessary to prevent or reduce an unreasonable risk of injury associated with such product.
Whenever the Commission finds that (i) a consumer product is being, or will be, distributed in commerce and such consumer product presents an unreasonable risk of injury and (ii) no feasible consumer product safety standard under this Act would adequately protect the public from the unreasonable risk of injury associated with such product, the Commission may, in accordance with this section, promulgate a rule declaring such product a banned hazardous product. Every manufacturer of a product, which is subject to a consumer product safety standard under this Act and which is distributed in commerce, shall issue a certificate which shall certify that such product conforms to all applicable consumer product safety standards and shall specify any standard which is applicable. Such certificate shall accompany the product or shall otherwise be furnished to any distributor or retailer to whom the product is delivered. Not less than 30 days before any person exports to a foreign country any product: (i) which is not in conformity with an applicable consumer product safety standard in effect under this Act or (ii) which is declared to be a banned hazardous substance by a rule promulgated under this section, such person shall file a statement with the Commission notifying the Commission of such exportation, and the Commission, upon receipt of such statement, shall promptly notify the government of such country of such exportation and the basis for such safety standard or rule. Any statement filed with the Commission under the preceding sentence shall specify the anticipated date of shipment of such product, the country and port of destination of such product, and the quantity of such product that will be exported, and shall contain such other information as the Commission may by regulation require.
Following FQPA (1996), key issues revolved around: the roles of state, local and tribal governments in pesticide regulation and Federal law enforcement; the cost of scientific tests required by the US Environmental Protection Agency (EPA) to support pesticide registration and reregistration; delays in processing applications for new or amended registrations, especially for minor uses; and long delays in reregistration of older pesticides and the need for fees to support the effort. A lesser issue involved state authority to require training of persons who regularly apply non-restricted pesticides in urban and suburban areas. Cancelling a pesticide registration, when it is found to cause ‘unreasonable adverse effects,’ can be a prolonged process, lasting 4–8 years or more. To prevent an ‘imminent hazard’ during this period, EPA can suspend registration, meaning that use of the pesticide is immediately prohibited, but not before EPA has published, or provided the registrant with a notice of its intention to cancel the registration. While not a particularly large proportion of total pesticide use by volume, household and business uses of pesticides in urban and suburban settings may lead to exposure of larger and more diverse populations than in agricultural applications. Concerns about such exposures include immediate toxic reactions in sensitive individuals, as well as less visible but longer term health effects such as cancer. Pesticides that end up in rivers, lakes and groundwater are considered a significant water pollution problem. This can result from excessive and improper application or improper disposal of pesticide containers, for example. While these concerns apply to agricultural applications as well, urban/suburban uses are sometimes at higher concentrations and may be applied by persons unfamiliar with risks or proper application practices. Moreover, when pesticides are applied to lawns and gardens, golf courses, roadside, public buildings, apartment buildings and single-family homes, the people exposed may be very young or elderly and in robust or fragile health. People may be exposed to pesticides in such settings unknowingly or at least without prior warning, as for example, when entering a recently treated roadside area or public building.
According to Food Safety Issues in the 106th Congress (2000), the US food supply is among the safest in the world; every year foodborne pathogens in the food supply make many people ill. Consumers have expressed increasing concerns about microbial contaminants in their foods and are asking whether the Federal regulatory system adequately deals with those problems. The nation's food safety system consists of activities carried out by several Federal, state and local government agencies that inspect, test, research and monitor the food supply. For the most part, these agencies monitor whether food manufacturers are adhering to their legal responsibility of assuring the production of safe food. However, reported occurrences of outbreaks of foodborne illnesses have been increasing, and current safety efforts are not providing the confidence in the food supply that US consumers demand. The US food industry makes available to US consumers a wide variety of food that is produced domestically or imported. The Federal government attempts to ensure that the food supply is safe from the farm and ports to consumer tables under statutory mandates and regulatory policies. Federal regulatory jurisdiction depends on the type of food, the way the food is processed, or the type of adulterant to be found in a particular food. Critics charge that the agencies’ overlapping jurisdictions and duplication of effort are wasting taxpayers’ money. Overlapping jurisdictional responsibilities inhibit efforts to focus where the risk of adulteration and contamination is the greatest, they claim. Administration officials, however, argue that by recently working cooperatively and through formal understandings among the agencies, Federal agencies now avoid duplicating efforts, for the most part. Congressional oversight for food safety is shared among several committees. In the Senate, food safety issues are considered by the Committees on Agriculture, Nutrition and Forestry; Commerce, Science and Transportation; Environment and Public Works; Government Affairs and Health, Education, Labor and Pensions. In the House, food safety is considered by the Committees on Agriculture; Commerce; Government Reform and Oversight and Science. The Appropriations Committees also serve an oversight role in how the major agencies set and carry out policies affecting the safety of foods.
Another Public Health Security and Bioterrorism Preparedness and Response Act (2002) claimed that the Secretary of Agriculture shall by regulation establish and maintain a list of each biological agent and each toxin that the Secretary determines has the potential to pose a severe threat to animal or plant health or to animal or plant products. In determining whether to include an agent or toxin, the Secretary shall (1) consider (i) the effect of exposure to the agent or toxin on animal or plant health, and on the production and marketability of animal or plant products; (ii) the pathogenicity of the agent or the toxicity of the toxin and the methods by which the agent or toxin is transferred to animals or plants; (iii) the availability and effectiveness of pharmacotherapies and prophylaxis to treat and prevent any illness caused by the agent or toxin and (iv) any other criteria that the Secretary considers appropriate to protect animal or plant health or animal or plant products; and (2) consult with appropriate Federal departments and agencies and with scientific experts representing appropriate professional groups. For use in inspections of food under this section, the Secretary shall provide for research on the development of tests and sampling methodologies: (i) whose purpose is to test food in order to rapidly detect the adulteration of the food, with the greatest priority given to detect the intentional adulteration of food and (ii) whose results offer significant improvements over the available technology in terms of accuracy, timing or costs. An article of food held under this paragraph may be delivered to a person who is not a debarred person under this section if such person affirmatively establishes, at the expense of the person, that the article complies with the requirements of this Act, as determined by the Secretary. The Secretary shall by regulation require that any facility engaged in manufacturing, processing, packing or holding food for consumption in the United States be registered with the Secretary. To be registered (i) for a domestic facility, the owner, operator or agent in charge of the facility shall submit a registration to the Secretary and (ii) for a foreign facility, the owner, operator or agent in charge of the facility shall submit a registration to the Secretary and shall include with the registration the name of the United States agent for the facility.
In Food Safety Act (2002), ‘food’ means food or drink for human consumption and includes (i) any substance or thing that is manufactured, sold or represented for use as food or drink for human consumption, (ii) any substance or thing that is manufactured, sold or represented for use as an additive, ingredient or processing aid in a substance or thing referred to in paragraph (i) and (iii) any agricultural or aquatic product that is grown, raised, cultivated, harvested or kept for the purpose of producing food or drink for human consumption. An operator is responsible for ensuring that the food in his or her food establishment is safe for human consumption. To determine whether there is compliance with this Act and the regulations, an inspector may do any of the following: (i) enter at reasonable times the premises of a food establishment or any place that the inspector believes on reasonable grounds is being used as a food establishment, (ii) inspect the premises and the equipment, facilities and food in or on those premises, (iii) make a record, including a record on film, audiotape, videotape or otherwise, of the premises and the equipment, facilities and food in or on those premises, (iv) suspend the operation of any enterprise or activity in or on those premises, (v) stop and inspect a vehicle in which the inspector believes on reasonable grounds, i.e. whether there is food or an inspection item to which this Act or the regulations apply, (vi) open any container that the inspector believes on reasonable grounds to contain an inspection item, (vii) require to be produced for inspection, or for the purpose of obtaining copies of it or extracts from it, any record that the inspector believes on reasonable grounds contains information relevant to the administration of this Act and the regulations, (viii) require to be produced for inspection any food or other inspection item, (ix) take and remove samples of any inspection item, (x) examine or test samples referred to in this paragraph, or have either of these things done and (xi) take any other steps the inspector considers necessary. An inspector who believes on reasonable grounds (i) that food does not meet a prescribed standard for that food or (ii) that food is contaminated or otherwise unfit for human consumption, (iii) to seize the food or have it seized and (iv) to detain it, or have it detained, for examination or inspection that the inspector considers necessary to determine whether that standard has been met or whether the food is contaminated or unfit for human consumption.
Major points of US legislation focused on biological safety are summarised in Table 4.
| Title . | Main points . | Comments . |
|---|---|---|
| Consumer Product Safety Act, 1972 | • Definitions (consumer product, manufacturer, etc.) • Consumer product safety standards • Commission responsibility – petition for consumer product safety rule • Product certification and labelling • Financial penalties | Amendments |
| • 1976 (Consumer Product Safety Commission Improvement Act) • 1978 (Consumer Product Safety Authorization Act) • 1981 (Consumer Product Safety Amendments) • 1983 (Lead Contamination Control Act) • 1988 (Anti-drug Abuse Act) • 1990 (Consumer Product Safety Improvement Act) • 1994 (Child Safety Protection Act) | ||
| Food Quality Protection Act, 1996 | • It related to pesticide uses • The new law will facilitate registrations and reregistrations of pesticides for special (the so-called ‘minor’) uses and authorise collection of maintenance fees of support pesticide reregistration • Food safety provisions will establish a single standard of safety for pesticide residue on raw and processed foods | |
| Food Safety Issues in the 106th Congress, 2000 | • The 106th Congress debated a number of food safety proposals • The Federal agencies are implementing several presidential food safety initiatives • The food safety activities of these agencies consist of inspecting, testing, researching and monitoring the food supply • Consumers have become aware of the serious consequences of illnesses linked to a growing variety of foods, produced domestically or imported | |
| Food Safety Act, 2002 | • Definitions (food, food establishment, etc.) • Licences for designated food establishments • Seizure and destruction of food • Designation of food establishment | |
| Public Health Security and Bioterrorism Preparedness and Response Act, 2002 | • Regulation of certain biological agents and toxins • Food safety and security strategy • Notices to States regarding imported food • Maintenance and inspection of records for foods |
| Title . | Main points . | Comments . |
|---|---|---|
| Consumer Product Safety Act, 1972 | • Definitions (consumer product, manufacturer, etc.) • Consumer product safety standards • Commission responsibility – petition for consumer product safety rule • Product certification and labelling • Financial penalties | Amendments |
| • 1976 (Consumer Product Safety Commission Improvement Act) • 1978 (Consumer Product Safety Authorization Act) • 1981 (Consumer Product Safety Amendments) • 1983 (Lead Contamination Control Act) • 1988 (Anti-drug Abuse Act) • 1990 (Consumer Product Safety Improvement Act) • 1994 (Child Safety Protection Act) | ||
| Food Quality Protection Act, 1996 | • It related to pesticide uses • The new law will facilitate registrations and reregistrations of pesticides for special (the so-called ‘minor’) uses and authorise collection of maintenance fees of support pesticide reregistration • Food safety provisions will establish a single standard of safety for pesticide residue on raw and processed foods | |
| Food Safety Issues in the 106th Congress, 2000 | • The 106th Congress debated a number of food safety proposals • The Federal agencies are implementing several presidential food safety initiatives • The food safety activities of these agencies consist of inspecting, testing, researching and monitoring the food supply • Consumers have become aware of the serious consequences of illnesses linked to a growing variety of foods, produced domestically or imported | |
| Food Safety Act, 2002 | • Definitions (food, food establishment, etc.) • Licences for designated food establishments • Seizure and destruction of food • Designation of food establishment | |
| Public Health Security and Bioterrorism Preparedness and Response Act, 2002 | • Regulation of certain biological agents and toxins • Food safety and security strategy • Notices to States regarding imported food • Maintenance and inspection of records for foods |
| Title . | Main points . | Comments . |
|---|---|---|
| Consumer Product Safety Act, 1972 | • Definitions (consumer product, manufacturer, etc.) • Consumer product safety standards • Commission responsibility – petition for consumer product safety rule • Product certification and labelling • Financial penalties | Amendments |
| • 1976 (Consumer Product Safety Commission Improvement Act) • 1978 (Consumer Product Safety Authorization Act) • 1981 (Consumer Product Safety Amendments) • 1983 (Lead Contamination Control Act) • 1988 (Anti-drug Abuse Act) • 1990 (Consumer Product Safety Improvement Act) • 1994 (Child Safety Protection Act) | ||
| Food Quality Protection Act, 1996 | • It related to pesticide uses • The new law will facilitate registrations and reregistrations of pesticides for special (the so-called ‘minor’) uses and authorise collection of maintenance fees of support pesticide reregistration • Food safety provisions will establish a single standard of safety for pesticide residue on raw and processed foods | |
| Food Safety Issues in the 106th Congress, 2000 | • The 106th Congress debated a number of food safety proposals • The Federal agencies are implementing several presidential food safety initiatives • The food safety activities of these agencies consist of inspecting, testing, researching and monitoring the food supply • Consumers have become aware of the serious consequences of illnesses linked to a growing variety of foods, produced domestically or imported | |
| Food Safety Act, 2002 | • Definitions (food, food establishment, etc.) • Licences for designated food establishments • Seizure and destruction of food • Designation of food establishment | |
| Public Health Security and Bioterrorism Preparedness and Response Act, 2002 | • Regulation of certain biological agents and toxins • Food safety and security strategy • Notices to States regarding imported food • Maintenance and inspection of records for foods |
| Title . | Main points . | Comments . |
|---|---|---|
| Consumer Product Safety Act, 1972 | • Definitions (consumer product, manufacturer, etc.) • Consumer product safety standards • Commission responsibility – petition for consumer product safety rule • Product certification and labelling • Financial penalties | Amendments |
| • 1976 (Consumer Product Safety Commission Improvement Act) • 1978 (Consumer Product Safety Authorization Act) • 1981 (Consumer Product Safety Amendments) • 1983 (Lead Contamination Control Act) • 1988 (Anti-drug Abuse Act) • 1990 (Consumer Product Safety Improvement Act) • 1994 (Child Safety Protection Act) | ||
| Food Quality Protection Act, 1996 | • It related to pesticide uses • The new law will facilitate registrations and reregistrations of pesticides for special (the so-called ‘minor’) uses and authorise collection of maintenance fees of support pesticide reregistration • Food safety provisions will establish a single standard of safety for pesticide residue on raw and processed foods | |
| Food Safety Issues in the 106th Congress, 2000 | • The 106th Congress debated a number of food safety proposals • The Federal agencies are implementing several presidential food safety initiatives • The food safety activities of these agencies consist of inspecting, testing, researching and monitoring the food supply • Consumers have become aware of the serious consequences of illnesses linked to a growing variety of foods, produced domestically or imported | |
| Food Safety Act, 2002 | • Definitions (food, food establishment, etc.) • Licences for designated food establishments • Seizure and destruction of food • Designation of food establishment | |
| Public Health Security and Bioterrorism Preparedness and Response Act, 2002 | • Regulation of certain biological agents and toxins • Food safety and security strategy • Notices to States regarding imported food • Maintenance and inspection of records for foods |
Technological aspects
Factory-made foods have made chemical additives a significant part of our diet. Most people may not be able to pronounce the names of many of these chemicals, but they still want to know what the chemicals do and which ones are safe and which are poorly tested or possibly dangerous. This listing provides that information for most common additives. The food and chemical industries have said for decades that all food additives are well tested and safe. And most additives are safe. However, the history of food additives is riddled with additives that, after many years of use, were found to pose health risks (http://www.cspinet.org/reports/chemcuisine.htm). Over the years, however, improvements have been made in increasing the efficiency and ensuring the safety of all additives. Nowadays, food and colour additives are more strictly regulated that at any other time in history. The basis of modern food law is the Federal Food, Drug and Cosmetic Act of 1938, which gives the FDA authority over food and food ingredients and defines requirements for truthful labelling of ingredients (http://www.cfsan.fda.gov/~lrd/foodaddi.html). Another technological aspect is food irradiation. Food irradiation is one means of food preservation that may not be familiar to many, but it has been in development since the early decades of the twentieth century. If properly applied, irradiation can be an effective way to treat a variety of problems in our food supply, such as insect infestation of grains, sprouting of potatoes, rapid ripening of fruits and bacterial growth. However, it has not yet obtained a significant place in the US food industry (http://www.fcs.uga.edu/pubs/current/fdns-e-3.html).
Technological aspects legislation
Following Toxic Substances Control Act (TSCA, 1976), the term ‘chemical substance’ means any organic or inorganic substance of a particular molecular identity, including (i) any combination of such substances occurring in whole or in part as a result of a chemical reaction or occurring in nature and (ii) any element or uncombined radical. Such term does not include (a) any mixture, (b) any pesticide when manufactured, processed or distributed in commerce for use as a pesticide, (c) tobacco or any tobacco product, (d) any source material, special nuclear material or by-product material, (e) any food, food additive, drug, cosmetic or device when manufactured, processed or distributed in commerce for use as a food, food additive, drug, cosmetic or device. Within 6 months after 1 January 1977, the Administrator shall promulgate rules to (i) prescribe methods for the disposal of polychlorinated biphenyls and (ii) require polychlorinated biphenyls to be marked with clear and adequate warnings, and instructions with respect to their processing, distribution in commerce, use or disposal or with respect to any combination of such activities. One year after 1 January 1977, no person may manufacture, process or distribute in commerce or use any polychlorinated biphenyl in any manner other than in a totally enclosed manner. The Administrator may by rule authorise the manufacture, processing, distribution in commerce or use (or any combination of such activities) of any polychlorinated biphenyl in a manner other than in a totally enclosed manner if the Administrator finds that such manufacture, processing, distribution in commerce or use (or combination of such activities) will not present an unreasonable risk of injury to health or the environment. For the purposes of this paragraph, the term ‘totally enclosed manner’ means any manner which will ensure that any exposure of humans or the environment to a polychlorinated biphenyl will be insignificant as determined by the Administrator by rule. No person may manufacture any polychlorinated biphenyl after 2 years after 1 January 1977, and no person may process or distribute in commerce any polychlorinated biphenyl after 2 and a half years after such date. The Administrator may grant by rule such an exemption if the Administrator finds that (i) an unreasonable risk of injury to health or environment would not result and (ii) good faith efforts have been made to develop a chemical substance that does not present an unreasonable risk of injury to health or the environment and that may be substituted for such polychlorinated biphenyl. An exemption granted under this paragraph shall be subject to such terms and conditions as the Administrator may prescribe and shall be in effect for such period (but not more than 1 year from the date it is granted) as the Administrator may prescribe. The term ‘imminently hazardous chemical substance or mixture’ means a chemical substance or mixture that presents an imminent and unreasonable risk of serious or widespread injury to health or the environment. Such a risk to health or the environment shall be considered imminent if it is shown that the manufacture, processing, distribution in commerce, use or disposal of the chemical substance or mixture, or that any combination of such activities, is likely to result in such injury to health or the environment before a final rule can protect against such risk.
In US Regulatory Requirements for Irradiating Foods (1986), Congress explicitly defined a source of radiation as a food additive. In a report accompanying the legislation, Congress explicitly stated ‘Sources of radiation (including radioactive isotopes, particle accelerators, and X-ray machines) intended for use in processing food are included in the term ‘‘food additive’’ as defined in this legislation’. In early work on food irradiation, sources of sufficiently high energies to induce radioactivity in foods were sometimes used. As research continued, sources whose energies are too low to induce detectable radioactivity were adopted by the international community. Therefore, this issue is of no concern when currently approved sources of radiation are used, but must be addressed if other sources are being considered. Toxicological safety of typical food additives has traditionally been assessed by feeding large amounts of purified substances to laboratory animals and applying a safety factor to the highest dose of a tested substance that causes no toxic effects in any species. Moreover, standards for the conduct of such studies have evolved over time. For substances like irradiated whole foods, which may become a large proportion of diet, application of a 100-fold safety factor is impossible; attempts to exaggerate the amount of irradiated food in the diet have produced adverse nutritional effects that have confounded the results of many feeding studies. It is recommended that foods irradiated at doses of less than 1 kGy, or foods representing a very small fraction of the diet, should be exempt from requirements for toxicological testing because the types and amounts of radiolytic products would not show any toxic effects in well-conducted tests and their presence in the diet did not justify such testing. For foods irradiated at higher doses that were consumed in significant amounts, the Committee recommended a testing regime. Under the general labelling requirements, FDA has found it necessary to inform the consumer that an irradiated food has been processed, because irradiation, like other forms of processing, can affect the characteristics of food. For situations where the processing is not obvious, such as whole foods that have been irradiated, FDA requires that the label bear the radura symbol and the phrase ‘treated with radiation’ or ‘treated by irradiation’. If irradiated ingredients are added to foods that have not been irradiated, no special labelling is required on retail packages because it is obvious that such foods have been processed. Special labelling is required for foods not yet in the retail market that may undergo further processing, however, to ensure that foods are not irradiated multiple times. In promulgating this regulation, FDA advised that other truthful statements, such as the reason for irradiating the food, could be added to the statement and encouraged food manufacturers to do so. Irradiation can cause chemical change in packaging, as well as in food, and this can affect migration of the package components (or degradation products of those components) to food. Irradiation can cause cross-linking, which would likely reduce migration, but it also can cause decomposition to lower molecular weight entities with increased migration characteristics. Sometimes, irradiation has been used in the manufacture (or sterilisation) of packaging before food is added. FDA considers this use the same as any other manufacturing process, namely the final irradiated packaging must comply with the appropriate regulations and must not otherwise adulterate food.
Food irradiation (1999) is a technology for controlling spoilage and eliminating foodborne pathogens, such as Salmonella. The result is similar to conventional pasteurisation and is often called ‘cold pasteurisation’ or ‘irradiation pasteurisation’. Like pasteurisation, irradiation kills bacteria and other pathogens, which could otherwise result in spoilage or food poisoning. The fundamental difference between the two methods is the source of the energy they rely on to destroy the microbes. While conventional pasteurisation relies on heat, irradiation relies on the energy of ionising radiation. The FDA emphasises that no preservation method is a substitute for safe food handling procedures. The food irradiation process uses three types of ionising radiation sources: (i) cobalt-60 gamma sources, (ii) electron beam generators, (iii) X-ray generators. Cobalt-60 emits ionising radiation in the form of intense gamma rays. ‘Gamma facilities’ store it in stainless steel capsules, in underwater tanks. Cobalt-60 has several advantages: (i) up to 95% of its emitted energy is available for use, (ii) penetrates deeply, (iii) yields substantial uniformity of the dose in the food product, (iv) decays to non-radioactive nickel, (v) considered to pose low risk to the environment. However, its 5.3-year half-life offers disadvantages: cobalt-60 ‘pencils’ require frequent replenishment and treatment of the food is relatively slow. Cesium-137 is a gamma source that is also used for irradiation. Cesium-137 has a less penetrating gamma beam and a longer half-life, making it more suitable under certain circumstances. Electron beam facilities generate e-beams with an electron beam linear accelerator. (It works on the same principle as a television tube.) The electrons are concentrated and accelerated to 99% of the speed of light and energies of up to 10 MeV. Because e-beams are generated electrically, they offer certain advantages: (i) they can be turned on only as needed, (ii) they do not require replenishment of the source as does cobalt-60, (iii) there is no radioactive waste.
Food Additives Guide (2005) makes clear that food additives are an important component of our food supply. They mean that we can enjoy a wide variety of foods throughout the year. They also have an important role in ensuring that our food lasts longer and is easier to use. There are good reasons for the use of food additives. They can be used to improve the keeping quality or stability of a food. For example, sorbitol – humectant (420) – may be added to mixed dried fruit to maintain the moisture level and softness of the fruit, preserve food when this is the most practical way of extending its storage life. For example, sulphur dioxide – preservative (220) – is added to some meat products such as sausage meat to prevent the bugs that cause food poisoning from growing and improve the taste or appearance of a processed food. For example, lecithin – emulsifier (322) – may be added to margarine to help maintain texture. Additives are used in processed foods in relatively small quantities. Many substances used as additives also occur naturally, such as vitamin C or ascorbic acid (300) in fruit and lecithin (322) in eggs or soy beans. Some food additives have more than one use. Food additives are listed according to their functional or class names: (i) colourings add or restore colour to foods, (ii) colour retention agents retain or intensify the colour of a food, (iii) preservatives help protect against deterioration caused by microorganisms, (iv) artificial sweetening substances are substances that impart a sweet taste for fewer kilojoules/calories than sugar, (v) flavour enhancers improve the flavour and/or aroma of food, (vi) flavourings restore taste losses as a result of processing, maintain uniformity and make food more palatable, (vii) anticaking agents keep powdered products, such as salt, flow freely when poured, (viii) emulsifiers help prevent oil and water mixtures separating into layers, (ix) food acids help maintain a constant level of sourness in food, (x) humectants prevent foods such as dried fruits from drying out, (xi) mineral salts improve the texture of foods, such as processed meat, (xii) thickeners and vegetable gums improve texture and maintain uniform consistency, (xiii) stabilisers maintain the uniform dispersion of substances in a food, (xiv) flour treatment agents are substances added to flour to improve baking quality or appearance, (xv) glazing agents impart a shiny appearance or provide a protective coating to a food and (xvi) propellants are gases which help propel food from a container.
Federal Food, Drug and Cosmetic Act (2005) claims that Director of the Center shall (i) conduct postmarket risk assessment of drugs approved under this Act and of biological products licensed under the Public Health Service Act, (ii) conduct and improve postmarket surveillance of approved drugs and licensed biological products using postmarket surveillance programmes and activities risk–benefit analyses, adverse event reports, the scientific literature, any clinical or observational studies and any other resources that the Director of the Center determines appropriate, (iii) determine whether a study is required under this section and consult with the sponsors of drugs and biological products to ensure that such studies are completed by the date, and according to the terms, specified by the Director of the Center, (iv) contract, or require the sponsor of an application or the holder of an approved application or license to contract, with the holders of domestic and international surveillance databases to conduct epidemiological and other observational studies, (v) determine, based on postmarket surveillance programmes and activities, risk–benefit analyses, adverse event reports, the scientific literature, and any clinical or observational studies and any other resources that the Director of the Center determines appropriate, whether a drug or biological product may present an unreasonable risk to the health of patients or the general public, and take corrective action if such an unreasonable risk may exist, (vi) make information about the safety and effectiveness of approved drugs and licensed biological products available to the public and healthcare providers in a timely manner and (vii) conduct other activities as the Director of the Center determines appropriate to ensure the safety and effectiveness of all drugs approved under this section and all biological products licensed under the Public Health Service Act. Not later than 90 days after the date of enactment of the Food and Drug Administration Safety Act of 2005, the Director of the Center shall (i) review and publish a list in the Federal Register of any postmarketing studies outstanding on the date of enactment of the Food and Drug Administration Safety Act of 2005 and (ii) as the Director determines appropriate, require the sponsor of a study described in (i) to conduct such study under this section. The Director of the Center shall require that the sponsor of a study under this section submit to the Secretary: (i) not less frequently than every 90 days, an up-to-date report describing the progress of such study and (ii) upon the completion date of such study, the results of such study. The civil penalty ordered under this paragraph shall be $250 000 for the first 30-day period after the date specified by the Secretary that the study is not completed and shall double in amount for every 30-day period thereafter that the study is not completed.
Some representative points and comments of the US legislation about technological aspects are given in Table 5.
| Title . | Main points . | Comments . |
|---|---|---|
| Toxic Substances Control Act (TSCA), 1976 | • It establishes the EPA's toxic substances programme • Under this law manufacturers, may be required to conduct tests to evaluate the characteristics of the substance • The Act establishes a system for the prioritised listing of chemical substances to be tested • Under TSCA, manufacturers must notify EPA of their intentions to mass-produce a new chemical substance • TSCA also regulates polychlorinated biphenyls (PCBs) | Amendments |
| • In 1986, TSCA was amended to incorporate the Asbestos Hazard Emergency Response Act to address matters relating to asbestos products in public schools and other buildings | ||
| US Regulatory Requirements for Irradiating Foods, 1986 | • Legal requirements • Safety issues (radiological, toxicological, microbiological, nutritional adequacy) • Labelling of irradiating foods • Packaging of irradiating foods | • 1997 (Federal Register, 62) • 1999 (Federal Register, 64) |
| Food Irradiation, 1999 | • Food irradiation is the process of exposing food to ionising radiation • Food irradiation is a technology for controlling spoilage and eliminating foodborne pathogens, such as salmonella • FDA approved irradiation for the control of pathogenic microorganisms in red meat | |
| Food Additives Guide, 2005 | • Use of food additives • Food additives are listed according to their functional or class names • Intolerance and food additives • Food additive safety | |
| Federal Food, Drug and Cosmetic Act, 2005 | • Duties of the centre for postmarket drug evaluation and research • Publication of progress reports and completed studies • Amount of penalties |
| Title . | Main points . | Comments . |
|---|---|---|
| Toxic Substances Control Act (TSCA), 1976 | • It establishes the EPA's toxic substances programme • Under this law manufacturers, may be required to conduct tests to evaluate the characteristics of the substance • The Act establishes a system for the prioritised listing of chemical substances to be tested • Under TSCA, manufacturers must notify EPA of their intentions to mass-produce a new chemical substance • TSCA also regulates polychlorinated biphenyls (PCBs) | Amendments |
| • In 1986, TSCA was amended to incorporate the Asbestos Hazard Emergency Response Act to address matters relating to asbestos products in public schools and other buildings | ||
| US Regulatory Requirements for Irradiating Foods, 1986 | • Legal requirements • Safety issues (radiological, toxicological, microbiological, nutritional adequacy) • Labelling of irradiating foods • Packaging of irradiating foods | • 1997 (Federal Register, 62) • 1999 (Federal Register, 64) |
| Food Irradiation, 1999 | • Food irradiation is the process of exposing food to ionising radiation • Food irradiation is a technology for controlling spoilage and eliminating foodborne pathogens, such as salmonella • FDA approved irradiation for the control of pathogenic microorganisms in red meat | |
| Food Additives Guide, 2005 | • Use of food additives • Food additives are listed according to their functional or class names • Intolerance and food additives • Food additive safety | |
| Federal Food, Drug and Cosmetic Act, 2005 | • Duties of the centre for postmarket drug evaluation and research • Publication of progress reports and completed studies • Amount of penalties |
| Title . | Main points . | Comments . |
|---|---|---|
| Toxic Substances Control Act (TSCA), 1976 | • It establishes the EPA's toxic substances programme • Under this law manufacturers, may be required to conduct tests to evaluate the characteristics of the substance • The Act establishes a system for the prioritised listing of chemical substances to be tested • Under TSCA, manufacturers must notify EPA of their intentions to mass-produce a new chemical substance • TSCA also regulates polychlorinated biphenyls (PCBs) | Amendments |
| • In 1986, TSCA was amended to incorporate the Asbestos Hazard Emergency Response Act to address matters relating to asbestos products in public schools and other buildings | ||
| US Regulatory Requirements for Irradiating Foods, 1986 | • Legal requirements • Safety issues (radiological, toxicological, microbiological, nutritional adequacy) • Labelling of irradiating foods • Packaging of irradiating foods | • 1997 (Federal Register, 62) • 1999 (Federal Register, 64) |
| Food Irradiation, 1999 | • Food irradiation is the process of exposing food to ionising radiation • Food irradiation is a technology for controlling spoilage and eliminating foodborne pathogens, such as salmonella • FDA approved irradiation for the control of pathogenic microorganisms in red meat | |
| Food Additives Guide, 2005 | • Use of food additives • Food additives are listed according to their functional or class names • Intolerance and food additives • Food additive safety | |
| Federal Food, Drug and Cosmetic Act, 2005 | • Duties of the centre for postmarket drug evaluation and research • Publication of progress reports and completed studies • Amount of penalties |
| Title . | Main points . | Comments . |
|---|---|---|
| Toxic Substances Control Act (TSCA), 1976 | • It establishes the EPA's toxic substances programme • Under this law manufacturers, may be required to conduct tests to evaluate the characteristics of the substance • The Act establishes a system for the prioritised listing of chemical substances to be tested • Under TSCA, manufacturers must notify EPA of their intentions to mass-produce a new chemical substance • TSCA also regulates polychlorinated biphenyls (PCBs) | Amendments |
| • In 1986, TSCA was amended to incorporate the Asbestos Hazard Emergency Response Act to address matters relating to asbestos products in public schools and other buildings | ||
| US Regulatory Requirements for Irradiating Foods, 1986 | • Legal requirements • Safety issues (radiological, toxicological, microbiological, nutritional adequacy) • Labelling of irradiating foods • Packaging of irradiating foods | • 1997 (Federal Register, 62) • 1999 (Federal Register, 64) |
| Food Irradiation, 1999 | • Food irradiation is the process of exposing food to ionising radiation • Food irradiation is a technology for controlling spoilage and eliminating foodborne pathogens, such as salmonella • FDA approved irradiation for the control of pathogenic microorganisms in red meat | |
| Food Additives Guide, 2005 | • Use of food additives • Food additives are listed according to their functional or class names • Intolerance and food additives • Food additive safety | |
| Federal Food, Drug and Cosmetic Act, 2005 | • Duties of the centre for postmarket drug evaluation and research • Publication of progress reports and completed studies • Amount of penalties |
Genetically engineered food in US trade
Advocates of genetically modified (GM) foods often assert that the processes of laboratory genetic engineering are really no different from those of plant and animal husbandry. This argument is not as convincing as they expect. Those who express concern about the safety of GM food claim that genetic engineering allows humans to do what nature will not – they worry that scientists cut and paste genes and can now transfer genes between species. This gene transfer raises new safety questions, making the production and marketing of GM foods a matter for consideration by public health authorities (http://www.mja.com.au/public/issues/172_04_210200/huppleed/leeder.html). Whenever official approval for the introduction of GM foods has been given in Europe or the United States, regulatory committees have invoked the concept of ‘substantial equivalence’ (http://www.greenpeace.org.br/). The European Union (EU), the World Health Organisation (WHO) and the Food and Agriculture Organisation (FAO) of the United Nations agreed on a methodology referred to as ‘substantial equivalence’ as the most practical approach to assess the safety of GM foods and food ingredients. (http://www.eufic.org/gb/food/pag/food37/food374.htm). The substantial equivalence concept was developed following an initial FAO/WHO consultation exercise (FAO/WHO, 1991) and further refined by OECD (1993) and WHO/FAO (1996). The concept has recently been re-evaluated at an OECD Workshop (OECD, 1998). Substantial equivalence is based on the premise that if a novel or modified food or food ingredient can be shown to be essentially equivalent in composition to an existing food or food ingredient, then it can be assumed that the new food is as safe as its conventional equivalent (http://www.mrc.ac.uk/pdf-strategy-gm_foods.pdf). ‘Substantial equivalence’ focuses on the product rather than the production process. It is a rigorous procedure including a detailed list of parameters and characteristics that need to be considered including molecular characterisation of the genetic modification, agronomic characterisation, nutritional and toxicological assessments. The ‘substantial equivalence’ approach acknowledges that the goal of the assessment cannot be to establish absolute safety (http://www.eufic.org/gb/food/pag/food37/food374.htm). Safety is established by the demonstration that there is no significant difference in a range of characteristics, including both phenotype and composition, between the new/modified food and its conventional equivalent. Any effect of genetic modification is considered in the context of the normal variation in phenotype and composition that exists in the conventional counterpart (http://www.mrc.ac.uk/pdf-strategy-gm_foods.pdf). However, if the GM product has new traits or characteristics that make it no longer substantially equivalent (such as a higher level of a vitamin), then an additional assessment is required (http://www.eufic.org/gb/food/pag/food37/food374.htm). Safety is established by the demonstration that there is no significant difference in a range of characteristics, including both phenotype and composition, between the new/modified food and its conventional equivalent. Any effect of genetic modification is considered in the context of the normal variation in phenotype and composition that exists in the conventional counterpart (http://www.mrc.ac.uk/pdf-strategy-gm_foods.pdf). GM foods are placed in three classes, based on the results of substantial equivalence testing: (1) the food is considered to be substantially equivalent in all respects and no more information is requested, (2) the food is considered to be different only in the GM characteristics – for example, the gene products that make the crop resistant to insects or tolerant to a herbicide, (3) the food is not considered to be substantially equivalent, so more toxicological and nutritional data are required. Scientists critical of this approach have previously argued that gross chemical comparisons between GM foods and conventional counterparts are not sufficient to detect unexpected changes (http://www.the-scientist.com/news/20020206/04). The use of substantial equivalence is a ‘pseudo-scientific concept because it is a commercial and political judgement masquerading as if it were scientific. It is, moreover, inherently antiscientific because it was created primarily to provide an excuse for not requiring biochemical or toxicological tests. It therefore serves to discourage and inhibit informative scientific inquiry’ (Millstone et al., 1999). One of the more difficult aspects of the substantial equivalence approach relates to unintended effects. This issue is addressed by the evaluation of comparative data on composition and phenotype. Current regulatory bodies take a decision on the extent of scientific data required to satisfy concern that a significant secondary effect might create a safety hazard. For any established crop plant, there may be known toxicological or nutritional concerns; for example, it may produce natural toxicants. In conventional plant breeding, this is an established safety issue and analysis of the levels of such substances in a GM plant provides reassurance that gene introduction has not created an unexpected change that might cause harm (http://www.mrc.ac.uk/pdf-strategy-gm_foods.pdf). Some commentators (Millstone et al., 1999; Von Schomberg, 1999) have argued that substantial equivalence will never serve the purpose of reassuring consumers and that toxicological and biochemical tests should always be required. Substantial equivalence has been accused of being a pseudo-scientific concept embodying commercial and political judgement and that the definition of the concept is too vague to serve as a benchmark for public health policy. Counter to this, it has been explained that substantial equivalence is not intended to be a substitute for safety assessment but is simply a guiding principle, which is a useful tool for regulatory scientists engaged in food safety assessments (Burke, 1999; Kearns & Mayers, 1999). The cornerstones in GM acceptance are given in Table 6. Table 7 compares various approaches to risk assessment and safety testing. The first column lists requirements in ascending order of stringency, the middle column indicates the types of testing required in each set and the third column indicates the relative costs of the tests. Under the terms of the Cartagena Protocol on Biosafety – the framework for international regulation of GM organisms or GMOs – governments may require these costs to be met by the organisation applying for approval. In relation to GM foods and crops, the types of tests required have changed over time. The first two rows of the table represent first recent, and secondly current, practice in the United States and the EU. Current testing requirements for GM foods and crops, especially in the EU, are more elaborate than those introduced in the late 1990s. The third row in the table refers to a set of testing requirements for GM foods that the European Food Safety Authority's expert scientific advisory committee – the Scientific Panel on GMO – is proposing for the not-too-distant future (EFSA, 2004). The fourth row represents recent and current requirements for new food additives and new active ingredients for pesticide products. Those chemicals are tested far more stringently than are GM foods. The final row shows current requirements for pharmaceutical products – the most stringent of all those listed (http://www.twnside.org.sg/title2/service169.htm). One of the first novel foods to be formally assessed in the UK was the mycoprotein ‘Quorn’. At roughly, the same time the safety of irradiated foods was assessed through a vast array of animal feeding studies. In both cases, practical difficulties were encountered. In contrast to many non-nutritive substances, foods are intended to be consumed by human at levels which approach the maximum dose that could be used in animal studies. Toxicological studies are designed to characterise the toxicological profile of individual chemical substances not complex substances such as foods. Long-term feeding of high levels of individual ‘foods’ to animals can result in nutritional imbalances that make interpretation of such studies extremely difficult. Table 8 compares the differences between the safety assessment of chemicals and foods (http://www.acnfp.gov.uk/acnfppapers/inforelatass/toxrev?view=printerfriendly).
| Year . | Institution . | Verdict . | Reference . |
|---|---|---|---|
| 1990 | UN Food and Agriculture Organization (FAO) and World Health Organization (WHO) | The challenge of how to deal with the issue from consuming GM foods was first confronted in 1990 at an international meeting | http://www.greenpeace.org.br |
| Mid-1990s | World Trade Organization (WTO) | Genetics and molecular biology were dominated by a set of assumptions generally presumed to be unproblematic. One was that there was a one-to-one correspondence between genes and proteins, in other words that each gene directs the expression of a single protein | http://www.twnside.org.sg/title2/service169.htm |
| Late 1990s | WTO | Compelling evidence emerged indicating that, across all species, the number of proteins considerably exceeded the number of genes. That anomaly suggests that the expression of proteins may be controlled by combinations of genes, rather than just by individual genes | http://www.twnside.org.sg/title2/service169.htm |
| 1996 | UN FAO and WHO | The concept of substantial equivalence was first introduced and was subsequently endorsed | http://www.greenpeace.org.br |
| 1997 | European Union (EU) | Novel Food Regulation included a simplified procedure stipulating that when a novel food was considered to be substantially equivalent to an existing food, a company was required only to provide a justification for the claim of substantial equivalence, rather than a formal risk assessment. That procedure was used to authorise several GM foods for sale in the EU | Levidow & Murphy (2002) |
| May 1999 | UK Government's Chief Medical Officer (CMO) and Chief Scientific Adviser (CSA) | • There is no current evidence to suggest that genetic modification technologies, used to produce food, are inherently harmful • The precautionary nature and rigour of the current procedures used to assess the safety of individual GM foods are reassuring | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| May 2000 | FAO, WHO Expert Consultation | • The application of the concept of substantial equivalence contributes to a robust safety assessment framework. The approach used to assess the safety of the GM foods that have been approved for commercial use is satisfactory | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| June 2000 | Medical Research Council Expert Group | • Many of the potential effects of GM foods on human health also apply to food or food ingredients produced by conventional plant and animal breeding • Current regulatory procedures, using the principle of substantial equivalence, addressed the theoretically possible health risks of known toxins and allergens in GM foods | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| 2001 | European Commission (EC) | A revised Novel Food Regulation explained that this proposal does not include a notification (simplified) procedure or GM foods that are substantially equivalent to existing foods. The use of this regulatory short cut for the so-called ‘substantially equivalent’ GM foods has been very controversial in the community in recent years and there is consensus at the international level that while substantial equivalence is a key step in the safety assessment process of GM foods, it is not a safety assessment in itself | CEC (2001) |
| 2002 | Royal Society | • There is no evidence at present that GM foods cause allergic reactions • It seems reasonable to assume that this poses no significant risk to human health and eating GM DNA will have no ill effects | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| 2003 | EU | The EC indicated that chemical analyses would no longer be considered a sufficient basis for making a decision about the safety of GM foods, but that such analyses should provide a starting point for a more sophisticated approach | EU, 2003, Recital 6 |
| Year . | Institution . | Verdict . | Reference . |
|---|---|---|---|
| 1990 | UN Food and Agriculture Organization (FAO) and World Health Organization (WHO) | The challenge of how to deal with the issue from consuming GM foods was first confronted in 1990 at an international meeting | http://www.greenpeace.org.br |
| Mid-1990s | World Trade Organization (WTO) | Genetics and molecular biology were dominated by a set of assumptions generally presumed to be unproblematic. One was that there was a one-to-one correspondence between genes and proteins, in other words that each gene directs the expression of a single protein | http://www.twnside.org.sg/title2/service169.htm |
| Late 1990s | WTO | Compelling evidence emerged indicating that, across all species, the number of proteins considerably exceeded the number of genes. That anomaly suggests that the expression of proteins may be controlled by combinations of genes, rather than just by individual genes | http://www.twnside.org.sg/title2/service169.htm |
| 1996 | UN FAO and WHO | The concept of substantial equivalence was first introduced and was subsequently endorsed | http://www.greenpeace.org.br |
| 1997 | European Union (EU) | Novel Food Regulation included a simplified procedure stipulating that when a novel food was considered to be substantially equivalent to an existing food, a company was required only to provide a justification for the claim of substantial equivalence, rather than a formal risk assessment. That procedure was used to authorise several GM foods for sale in the EU | Levidow & Murphy (2002) |
| May 1999 | UK Government's Chief Medical Officer (CMO) and Chief Scientific Adviser (CSA) | • There is no current evidence to suggest that genetic modification technologies, used to produce food, are inherently harmful • The precautionary nature and rigour of the current procedures used to assess the safety of individual GM foods are reassuring | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| May 2000 | FAO, WHO Expert Consultation | • The application of the concept of substantial equivalence contributes to a robust safety assessment framework. The approach used to assess the safety of the GM foods that have been approved for commercial use is satisfactory | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| June 2000 | Medical Research Council Expert Group | • Many of the potential effects of GM foods on human health also apply to food or food ingredients produced by conventional plant and animal breeding • Current regulatory procedures, using the principle of substantial equivalence, addressed the theoretically possible health risks of known toxins and allergens in GM foods | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| 2001 | European Commission (EC) | A revised Novel Food Regulation explained that this proposal does not include a notification (simplified) procedure or GM foods that are substantially equivalent to existing foods. The use of this regulatory short cut for the so-called ‘substantially equivalent’ GM foods has been very controversial in the community in recent years and there is consensus at the international level that while substantial equivalence is a key step in the safety assessment process of GM foods, it is not a safety assessment in itself | CEC (2001) |
| 2002 | Royal Society | • There is no evidence at present that GM foods cause allergic reactions • It seems reasonable to assume that this poses no significant risk to human health and eating GM DNA will have no ill effects | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| 2003 | EU | The EC indicated that chemical analyses would no longer be considered a sufficient basis for making a decision about the safety of GM foods, but that such analyses should provide a starting point for a more sophisticated approach | EU, 2003, Recital 6 |
| Year . | Institution . | Verdict . | Reference . |
|---|---|---|---|
| 1990 | UN Food and Agriculture Organization (FAO) and World Health Organization (WHO) | The challenge of how to deal with the issue from consuming GM foods was first confronted in 1990 at an international meeting | http://www.greenpeace.org.br |
| Mid-1990s | World Trade Organization (WTO) | Genetics and molecular biology were dominated by a set of assumptions generally presumed to be unproblematic. One was that there was a one-to-one correspondence between genes and proteins, in other words that each gene directs the expression of a single protein | http://www.twnside.org.sg/title2/service169.htm |
| Late 1990s | WTO | Compelling evidence emerged indicating that, across all species, the number of proteins considerably exceeded the number of genes. That anomaly suggests that the expression of proteins may be controlled by combinations of genes, rather than just by individual genes | http://www.twnside.org.sg/title2/service169.htm |
| 1996 | UN FAO and WHO | The concept of substantial equivalence was first introduced and was subsequently endorsed | http://www.greenpeace.org.br |
| 1997 | European Union (EU) | Novel Food Regulation included a simplified procedure stipulating that when a novel food was considered to be substantially equivalent to an existing food, a company was required only to provide a justification for the claim of substantial equivalence, rather than a formal risk assessment. That procedure was used to authorise several GM foods for sale in the EU | Levidow & Murphy (2002) |
| May 1999 | UK Government's Chief Medical Officer (CMO) and Chief Scientific Adviser (CSA) | • There is no current evidence to suggest that genetic modification technologies, used to produce food, are inherently harmful • The precautionary nature and rigour of the current procedures used to assess the safety of individual GM foods are reassuring | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| May 2000 | FAO, WHO Expert Consultation | • The application of the concept of substantial equivalence contributes to a robust safety assessment framework. The approach used to assess the safety of the GM foods that have been approved for commercial use is satisfactory | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| June 2000 | Medical Research Council Expert Group | • Many of the potential effects of GM foods on human health also apply to food or food ingredients produced by conventional plant and animal breeding • Current regulatory procedures, using the principle of substantial equivalence, addressed the theoretically possible health risks of known toxins and allergens in GM foods | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| 2001 | European Commission (EC) | A revised Novel Food Regulation explained that this proposal does not include a notification (simplified) procedure or GM foods that are substantially equivalent to existing foods. The use of this regulatory short cut for the so-called ‘substantially equivalent’ GM foods has been very controversial in the community in recent years and there is consensus at the international level that while substantial equivalence is a key step in the safety assessment process of GM foods, it is not a safety assessment in itself | CEC (2001) |
| 2002 | Royal Society | • There is no evidence at present that GM foods cause allergic reactions • It seems reasonable to assume that this poses no significant risk to human health and eating GM DNA will have no ill effects | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| 2003 | EU | The EC indicated that chemical analyses would no longer be considered a sufficient basis for making a decision about the safety of GM foods, but that such analyses should provide a starting point for a more sophisticated approach | EU, 2003, Recital 6 |
| Year . | Institution . | Verdict . | Reference . |
|---|---|---|---|
| 1990 | UN Food and Agriculture Organization (FAO) and World Health Organization (WHO) | The challenge of how to deal with the issue from consuming GM foods was first confronted in 1990 at an international meeting | http://www.greenpeace.org.br |
| Mid-1990s | World Trade Organization (WTO) | Genetics and molecular biology were dominated by a set of assumptions generally presumed to be unproblematic. One was that there was a one-to-one correspondence between genes and proteins, in other words that each gene directs the expression of a single protein | http://www.twnside.org.sg/title2/service169.htm |
| Late 1990s | WTO | Compelling evidence emerged indicating that, across all species, the number of proteins considerably exceeded the number of genes. That anomaly suggests that the expression of proteins may be controlled by combinations of genes, rather than just by individual genes | http://www.twnside.org.sg/title2/service169.htm |
| 1996 | UN FAO and WHO | The concept of substantial equivalence was first introduced and was subsequently endorsed | http://www.greenpeace.org.br |
| 1997 | European Union (EU) | Novel Food Regulation included a simplified procedure stipulating that when a novel food was considered to be substantially equivalent to an existing food, a company was required only to provide a justification for the claim of substantial equivalence, rather than a formal risk assessment. That procedure was used to authorise several GM foods for sale in the EU | Levidow & Murphy (2002) |
| May 1999 | UK Government's Chief Medical Officer (CMO) and Chief Scientific Adviser (CSA) | • There is no current evidence to suggest that genetic modification technologies, used to produce food, are inherently harmful • The precautionary nature and rigour of the current procedures used to assess the safety of individual GM foods are reassuring | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| May 2000 | FAO, WHO Expert Consultation | • The application of the concept of substantial equivalence contributes to a robust safety assessment framework. The approach used to assess the safety of the GM foods that have been approved for commercial use is satisfactory | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| June 2000 | Medical Research Council Expert Group | • Many of the potential effects of GM foods on human health also apply to food or food ingredients produced by conventional plant and animal breeding • Current regulatory procedures, using the principle of substantial equivalence, addressed the theoretically possible health risks of known toxins and allergens in GM foods | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| 2001 | European Commission (EC) | A revised Novel Food Regulation explained that this proposal does not include a notification (simplified) procedure or GM foods that are substantially equivalent to existing foods. The use of this regulatory short cut for the so-called ‘substantially equivalent’ GM foods has been very controversial in the community in recent years and there is consensus at the international level that while substantial equivalence is a key step in the safety assessment process of GM foods, it is not a safety assessment in itself | CEC (2001) |
| 2002 | Royal Society | • There is no evidence at present that GM foods cause allergic reactions • It seems reasonable to assume that this poses no significant risk to human health and eating GM DNA will have no ill effects | http://www.food.gov.uk/gmdebate/aboutgm/gm_safety?view=gm+microsite |
| 2003 | EU | The EC indicated that chemical analyses would no longer be considered a sufficient basis for making a decision about the safety of GM foods, but that such analyses should provide a starting point for a more sophisticated approach | EU, 2003, Recital 6 |
| Approaches . | Test options . | Lost . | Reference . |
|---|---|---|---|
| Recent practice for GM foods and crops in the United States and European Union (EU) | Coarse chemical analyses | Low | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for GM foods and crops in the EU | Slightly finer chemical analysis and some short-term farm-animal feeding studies | Low | http://www.twnside.org.sg/title2/service169.htm |
| Officially envisaged future (in the EU) for GM foods and crops | Far finer chemical analyses: including proteomics and metabolomics as well as laboratory animal feeding studies, farm-scale cultivation trials for crops | Medium | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for additives and pesticides | Chemical analyses plus toxicological tests with bacteria and studies on (400) live animals, and some immunological testing, but no human trials | High | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for pharmaceutical products | Chemical analyses plus toxicological tests with bacteria and live animal studies, some immunological testing and some clinical trials | Very high | http://www.twnside.org.sg/title2/service169.htm |
| Approaches . | Test options . | Lost . | Reference . |
|---|---|---|---|
| Recent practice for GM foods and crops in the United States and European Union (EU) | Coarse chemical analyses | Low | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for GM foods and crops in the EU | Slightly finer chemical analysis and some short-term farm-animal feeding studies | Low | http://www.twnside.org.sg/title2/service169.htm |
| Officially envisaged future (in the EU) for GM foods and crops | Far finer chemical analyses: including proteomics and metabolomics as well as laboratory animal feeding studies, farm-scale cultivation trials for crops | Medium | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for additives and pesticides | Chemical analyses plus toxicological tests with bacteria and studies on (400) live animals, and some immunological testing, but no human trials | High | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for pharmaceutical products | Chemical analyses plus toxicological tests with bacteria and live animal studies, some immunological testing and some clinical trials | Very high | http://www.twnside.org.sg/title2/service169.htm |
| Approaches . | Test options . | Lost . | Reference . |
|---|---|---|---|
| Recent practice for GM foods and crops in the United States and European Union (EU) | Coarse chemical analyses | Low | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for GM foods and crops in the EU | Slightly finer chemical analysis and some short-term farm-animal feeding studies | Low | http://www.twnside.org.sg/title2/service169.htm |
| Officially envisaged future (in the EU) for GM foods and crops | Far finer chemical analyses: including proteomics and metabolomics as well as laboratory animal feeding studies, farm-scale cultivation trials for crops | Medium | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for additives and pesticides | Chemical analyses plus toxicological tests with bacteria and studies on (400) live animals, and some immunological testing, but no human trials | High | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for pharmaceutical products | Chemical analyses plus toxicological tests with bacteria and live animal studies, some immunological testing and some clinical trials | Very high | http://www.twnside.org.sg/title2/service169.htm |
| Approaches . | Test options . | Lost . | Reference . |
|---|---|---|---|
| Recent practice for GM foods and crops in the United States and European Union (EU) | Coarse chemical analyses | Low | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for GM foods and crops in the EU | Slightly finer chemical analysis and some short-term farm-animal feeding studies | Low | http://www.twnside.org.sg/title2/service169.htm |
| Officially envisaged future (in the EU) for GM foods and crops | Far finer chemical analyses: including proteomics and metabolomics as well as laboratory animal feeding studies, farm-scale cultivation trials for crops | Medium | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for additives and pesticides | Chemical analyses plus toxicological tests with bacteria and studies on (400) live animals, and some immunological testing, but no human trials | High | http://www.twnside.org.sg/title2/service169.htm |
| Current practice for pharmaceutical products | Chemical analyses plus toxicological tests with bacteria and live animal studies, some immunological testing and some clinical trials | Very high | http://www.twnside.org.sg/title2/service169.htm |
Differences between chemical and food toxicity evaluation (adapted from http://www.acnfp.gov.uk/acnfppapers/inforelatass/toxrev?view=printerfriendly)
| Chemical . | Food . |
|---|---|
| Simple and rather pure substance | Blend of numerous compounds |
| Highest dose level should produce an effect | Dubious effect even at maximum dose of under study test animals |
| Low intake (less than 1% of diet) | High intake (higher than 10%) |
| Easy to feed excessive dose | Intakes above those normally present in the diet difficult |
| Independent of nutrition in most cases | Nutrition dependent |
| Specific route of metabolism simple to follow | Complicated metabolism difficult to follow |
| Cause/effect quite clear to correlate | Cause/effect very difficult to trace down |
| Chemical . | Food . |
|---|---|
| Simple and rather pure substance | Blend of numerous compounds |
| Highest dose level should produce an effect | Dubious effect even at maximum dose of under study test animals |
| Low intake (less than 1% of diet) | High intake (higher than 10%) |
| Easy to feed excessive dose | Intakes above those normally present in the diet difficult |
| Independent of nutrition in most cases | Nutrition dependent |
| Specific route of metabolism simple to follow | Complicated metabolism difficult to follow |
| Cause/effect quite clear to correlate | Cause/effect very difficult to trace down |
Differences between chemical and food toxicity evaluation (adapted from http://www.acnfp.gov.uk/acnfppapers/inforelatass/toxrev?view=printerfriendly)
| Chemical . | Food . |
|---|---|
| Simple and rather pure substance | Blend of numerous compounds |
| Highest dose level should produce an effect | Dubious effect even at maximum dose of under study test animals |
| Low intake (less than 1% of diet) | High intake (higher than 10%) |
| Easy to feed excessive dose | Intakes above those normally present in the diet difficult |
| Independent of nutrition in most cases | Nutrition dependent |
| Specific route of metabolism simple to follow | Complicated metabolism difficult to follow |
| Cause/effect quite clear to correlate | Cause/effect very difficult to trace down |
| Chemical . | Food . |
|---|---|
| Simple and rather pure substance | Blend of numerous compounds |
| Highest dose level should produce an effect | Dubious effect even at maximum dose of under study test animals |
| Low intake (less than 1% of diet) | High intake (higher than 10%) |
| Easy to feed excessive dose | Intakes above those normally present in the diet difficult |
| Independent of nutrition in most cases | Nutrition dependent |
| Specific route of metabolism simple to follow | Complicated metabolism difficult to follow |
| Cause/effect quite clear to correlate | Cause/effect very difficult to trace down |
Genetic engineering, the ability to insert a novel gene in an organism, is a developing science that offers possible benefits and hazards. Genetically engineered (GE) foods present new issues of food safety. Given the consensus among the scientific community that genetic engineering can potentially introduce hazards, such as allergens or toxins, GE foods need to be evaluated on a case-by-case basis and cannot be presumed to be generally recognised as safe (http://www.mindfully.org/ge/ge4/ge-legislation-rep-kucinich11jun02.htm). The US FDA does not currently require GE organisms (GEOs) to be identified because it has determined that GE foods are substantially equivalent to conventional crops and as such they are safe. There remain, however, concerns that GE components could contaminate traditional food crops. Although regulation in this area continues to evolve, under current FDA rules, GE foods would require labelling only if modifications altered food composition significantly, changed the nutritional value or introduced an allergen (http://www.uschamber.com/issues/index/agriculture/geneticallyengineeredfood.htm). FDA proposed regulations that would require a mandatory notification before a GE plant intended for food or feed is marketed, in part because FDA believes that the next generation of GE crops is more likely to raise new food safety concerns. Although that proposal improves upon the current process by mandating a transparent agency review, it does not materially change the agency's scientific review and will not result in an official safety determination (http://www.cspinet.org/biotech/gefood_approval.html).
Genetically engineered food legislation
According to Genetically Engineered Food Safety Act (2003), the Congress finds as follows: (1) genetic engineering is an artificial gene transfer process wholly different from traditional breeding, (2) genetic engineering can be used to produce new versions of virtually all plant and animal foods. Thus, within a short time, the food supply could consist almost entirely of GE products, (3) this conversion from a food supply based on traditionally bred organisms to the one based on organisms produced through genetic engineering could be one of the most important changes in our food supply in this century, (4) GE foods present new issues of safety, which have not been adequately studied, (5) the Congress has previously required that food additives be analysed for their safety prior to their placement on the market, (6) adding new genes into a food should be considered as adding a food additive, thus requiring an analysis of safety factors, (7) Federal agencies have failed to uphold congressional intent of the Food Additives Amendment of 1958 by allowing GE foods to be marketed, sold and otherwise used without requiring premarket safety testing addressing their unique characteristics, (8) the food additive process gives the FDA discretion in applying the safety factors that are generally recognised as appropriate to evaluate the safety of food and food ingredients. The term ‘GEO’ means (i) an organism that has been altered at the molecular or cellular level by means that are not possible under natural conditions or processes (including but not limited to recombinant DNA and RNA techniques, cell fusion, microencapsulation, macroencapsulation, gene deletion and doubling, introducing a foreign gene and changing the positions of genes), other than a means consisting exclusively of breeding, conjugation, fermentation, hybridisation, in vitro fertilisation or tissue culture and (ii) an organism made through sexual or asexual reproduction (or both) involving an organism described in clause (i), if possessing any of the altered molecular or cellular characteristics of the organism so described. In the case of a genetic food additive, the factors considered by the Secretary regarding safety for use shall include (but not be limited to) the results of the following analyses: (i) allergenicity effects resulting from the added proteins, including proteins not found in the food supply, (ii) pleiotropic effects – the Secretary shall require tests to determine the potential for such effects, (iii) appearance of new toxins or increased levels of existing toxins, (iv) changes in the functional characteristics of food, (v) changes in the levels of important nutrients. Generally, in the case of a genetic food additive that in the United States was in commercial use in food as of the day before the date on which the final rule under this section is promulgated, the amendments made by this Act apply to the additive upon the expiration of the 2-year period beginning on the date on which the final rule is promulgated.
In Genetically Engineered Crop and Animal Farmer Protection Act (2003), ‘GE animal’ means an animal that contains a GE material or was produced with a GE material. An animal shall be considered to contain a GE material or to have been produced with a GE material if the animal has been injected or otherwise treated with a GE material or is the offspring of an animal that has been so injected or treated and ‘GE plant’ means a plant that contains a GE material or was produced from a GE seed. A plant shall be considered to contain a GE material if the plant has been injected or otherwise treated with a GE material. A biotech company that sells any GE animal, GE plant or GE seed that the biotech company knows, or has reason to believe, will be used by the purchaser in the United States to produce an agricultural commodity shall provide written notice to the purchaser that fully and clearly discloses the possible legal and environmental risks that the use of the GE animal, GE plant or GE seed may pose to the purchaser. The provisions referred to in this section are any of the following: (1) in the case of a sale of GE plants or GE seeds, a provision that prohibits the purchaser from retaining a portion of the harvested crop for future crop planting by the purchaser or that charges a fee to retain a portion of the harvested crop for future crop planting, (2) a provision that limits the ability of the purchaser to recover damages from the biotech company for a GE animal, GE plant or GE seed that does not perform as advertised, (3) a provision that shifts any liability from the biotech company to the purchaser, (4) a provision that requires the purchaser to grant agents of the seller access to the purchaser's property, (5) a provision that mandates arbitration of any disputes between the biotech company and the purchaser, (6) a provision that mandates any court of jurisdiction for settlement of disputes, (7) a provision that mandates that the purchaser pay liquidated damages of more than a technology fee or similar fee itself, plus interest, (8) a provision that imposes any unfair condition upon the purchaser, as determined by the Secretary or a court. A seed company or other person may not sell, or offer for sale, seeds for planting that are labelled as non-GE or otherwise represented as not containing GE material if the Secretary finds that any sample of the seeds contains GE material. Notwithstanding any other provision of law, effective 45 days after the date of the enactment of this Act, a person may not manufacture, distribute, sell, plant or otherwise use any seed that is GE to produce a plant whose seeds are not fertile or are rendered infertile by the application of an external chemical inducer.
Furthermore, another Act (Genetically Engineered Food Right to Know Act, 2003) claims that ‘GE material’ means material derived from any part of a GEO, without regard to whether the altered molecular or cellular characteristics of the organism are detectable in the material. In the case of a recipient who with respect to a food establishes a guaranty or undertaking in accordance with this paragraph, the exclusion under such paragraph from being subject to penalties applies to the recipient without regard to the use of the food by the recipient, including (i) processing the food, (ii) using the food as an ingredient in a food product, (iii) repacking the food or (iv) growing, raising or otherwise producing the food. For purposes of this Act, a meat food is misbranded if it (i) contains a GE material or was produced with a GE material and (ii) does not bear a label (or include labelling, in the case of a meat food that is not packaged in a container) that provides, in a clearly legible and conspicuous manner. A poultry product shall be considered to have been produced with a GE material if (a) the poultry from which the food is derived has been injected or otherwise treated with a GE material, (b) the poultry from which the food is derived has been fed GE material or (c) the food contains an ingredient that is a food to which this paragraph applies.
For the purpose of Genetically Engineered Pharmaceutical and Industrial Crop Safety Act (2003), ‘pharmaceutical crop’ means a GE plant that is designed to produce medical products, including human and veterinary drugs and biologics. The term includes a crop intentionally treated with GE material that, in turn, produces a medical substance. Pharmaceutical crops and industrial crops also pose substantial liability and other economic risks to farmers, grain handlers, food companies and other persons in the food and feed supply chain. These risks include liability for contamination episodes, costly food recalls, losses in export markets, reduced prices for a contaminated food or feed crop and loss of confidence in the safety of the American food supply among foreign importers and consumers of American agricultural commodities. No pharmaceutical crop or industrial crop may be grown, raised or otherwise cultivated until the final regulations and tracking system required by this section are in effect. The USDA shall establish a tracking system to regulate the growing, handling, transportation and disposal of all pharmaceutical and industrial crops and their by-products to prevent contamination. The maximum amount that may be accessed under this section for a violation may not exceed $1 000 000. In determining the amount of the civil penalty, the Secretary shall take into account: (i) the gravity of the violation, (ii) the degree of culpability, (iii) the size and type of the business and (iv) any history of prior offenses under such section or other laws administered by the Secretary.
A summary of the US Acts related to GE food is found in Table 9.
| Title . | Main points . | Comments . |
|---|---|---|
| Genetically Engineered Food Safety Act, 2003 | • Definitions (genetically engineered organism, genetically engineered material, etc.) • Federal determination of safety of genetically engineered food, regulation as food additive • Rulemaking, effective date, previously unregulated marketed additives | |
| Genetically Engineered Crop and Animal Farmer Protection Act, 2003 | • Definitions (genetically engineered plant, genetically engineered animal, genetically engineered material, etc.) • Contract limitations regarding sale of genetically engineered seeds, plants and animals • Prohibition on labelling certain seeds as non-genetically engineered • Prohibition on certain non-fertile plant seeds | |
| Genetically Engineered Food Right to Know Act, 2003 | • Definitions (genetically engineered organism, genetically engineered material, etc.) • Requirements for labelling regarding genetically engineered material • Misbranding of food with respect to genetically engineered material | |
| Genetically Engineered Pharmaceutical and Industrial Crop Safety Act, 2003 | • A pharmaceutical crop or industrial crop is a plant that has been genetically engineered to produce a medical or industrial product, including a human or veterinary drug, biological, industrial or research chemical, or enzyme • Definitions (genetically engineered plant, genetically engineered animal, genetically engineered material, etc.) • Report to Congress on alternative methods to produce pharmaceutical and industrial crops |
| Title . | Main points . | Comments . |
|---|---|---|
| Genetically Engineered Food Safety Act, 2003 | • Definitions (genetically engineered organism, genetically engineered material, etc.) • Federal determination of safety of genetically engineered food, regulation as food additive • Rulemaking, effective date, previously unregulated marketed additives | |
| Genetically Engineered Crop and Animal Farmer Protection Act, 2003 | • Definitions (genetically engineered plant, genetically engineered animal, genetically engineered material, etc.) • Contract limitations regarding sale of genetically engineered seeds, plants and animals • Prohibition on labelling certain seeds as non-genetically engineered • Prohibition on certain non-fertile plant seeds | |
| Genetically Engineered Food Right to Know Act, 2003 | • Definitions (genetically engineered organism, genetically engineered material, etc.) • Requirements for labelling regarding genetically engineered material • Misbranding of food with respect to genetically engineered material | |
| Genetically Engineered Pharmaceutical and Industrial Crop Safety Act, 2003 | • A pharmaceutical crop or industrial crop is a plant that has been genetically engineered to produce a medical or industrial product, including a human or veterinary drug, biological, industrial or research chemical, or enzyme • Definitions (genetically engineered plant, genetically engineered animal, genetically engineered material, etc.) • Report to Congress on alternative methods to produce pharmaceutical and industrial crops |
| Title . | Main points . | Comments . |
|---|---|---|
| Genetically Engineered Food Safety Act, 2003 | • Definitions (genetically engineered organism, genetically engineered material, etc.) • Federal determination of safety of genetically engineered food, regulation as food additive • Rulemaking, effective date, previously unregulated marketed additives | |
| Genetically Engineered Crop and Animal Farmer Protection Act, 2003 | • Definitions (genetically engineered plant, genetically engineered animal, genetically engineered material, etc.) • Contract limitations regarding sale of genetically engineered seeds, plants and animals • Prohibition on labelling certain seeds as non-genetically engineered • Prohibition on certain non-fertile plant seeds | |
| Genetically Engineered Food Right to Know Act, 2003 | • Definitions (genetically engineered organism, genetically engineered material, etc.) • Requirements for labelling regarding genetically engineered material • Misbranding of food with respect to genetically engineered material | |
| Genetically Engineered Pharmaceutical and Industrial Crop Safety Act, 2003 | • A pharmaceutical crop or industrial crop is a plant that has been genetically engineered to produce a medical or industrial product, including a human or veterinary drug, biological, industrial or research chemical, or enzyme • Definitions (genetically engineered plant, genetically engineered animal, genetically engineered material, etc.) • Report to Congress on alternative methods to produce pharmaceutical and industrial crops |
| Title . | Main points . | Comments . |
|---|---|---|
| Genetically Engineered Food Safety Act, 2003 | • Definitions (genetically engineered organism, genetically engineered material, etc.) • Federal determination of safety of genetically engineered food, regulation as food additive • Rulemaking, effective date, previously unregulated marketed additives | |
| Genetically Engineered Crop and Animal Farmer Protection Act, 2003 | • Definitions (genetically engineered plant, genetically engineered animal, genetically engineered material, etc.) • Contract limitations regarding sale of genetically engineered seeds, plants and animals • Prohibition on labelling certain seeds as non-genetically engineered • Prohibition on certain non-fertile plant seeds | |
| Genetically Engineered Food Right to Know Act, 2003 | • Definitions (genetically engineered organism, genetically engineered material, etc.) • Requirements for labelling regarding genetically engineered material • Misbranding of food with respect to genetically engineered material | |
| Genetically Engineered Pharmaceutical and Industrial Crop Safety Act, 2003 | • A pharmaceutical crop or industrial crop is a plant that has been genetically engineered to produce a medical or industrial product, including a human or veterinary drug, biological, industrial or research chemical, or enzyme • Definitions (genetically engineered plant, genetically engineered animal, genetically engineered material, etc.) • Report to Congress on alternative methods to produce pharmaceutical and industrial crops |
Food quality
The goal of the labelling requirement is to increase consumption of domestic commodities and improve the market for US producers. USDA officials appear to have consulted only with opponents of mandatory country-of-origin labelling before developing cost burden estimates associated with the implementation of the labelling requirements contained in the farm bill (http://enzi.senate.gov/usdaco.htm). The new US rules require that the FDA conducts inspections to ensure that food manufacturers are complying with practices to reduce or eliminate cross-contact of a food with any major food allergens that are not intentional ingredients of the food (http://www.foodnavigator-usa.com/news/ng.asp?n=58569-food-labeling-must). Most mandatory labelling legislation around the world has either not yet been fully implemented or has been implemented in combination with informal moratoria on GE foods – the result being that few, if any, GE foods actually carry a label yet. GE food labels are only mandated if the food has any known nutritional difference or food safety risk (including increased toxicity or likelihood of causing allergies) (http://www.geo-pie.cornell.edu/issues/intllabeling.html).
Labelling and Packaging legislation
Fair Packaging and Labeling Act (1967) specifies that packages and their labels should enable consumers to obtain accurate information as to the quantity of the contents and should facilitate value comparisons. Therefore, it is hereby declared to be the policy of the Congress to assist consumers and manufacturers in reaching these goals in the marketing of consumer goods. The separate label statement of net quantity of contents appearing upon or affixed to any package: (A) (i) if on a package labelled in terms of weight, shall be expressed in pounds, with any remainder in terms of ounces or common or decimal fractions of the pound; or in the case of liquid measure, in the largest whole unit (quarts, quarts and pints or pints, as appropriate) with any remainder in terms of fluid ounces or common or decimal fractions of the pint or quart, (ii) if on a random package, may be expressed in terms of pounds and decimal fractions of the pound carried out to not more than three decimal places and is not required to, but may, include a statement in terms of the SI metric system carried out to not more than three decimal places, (iii) if on a package labelled in terms of linear measure, shall be expressed in terms of the largest whole unit (yards, yards and feet or feet, as appropriate) with any remainder in terms of inches or common or decimal fractions of the foot or yard, (iv) if on a package labelled in terms of measure of area, shall be expressed in terms of the largest whole square unit (square yards, square yards and square feet or square feet, as appropriate) with any remainder in terms of square inches or common or decimal fractions of the square foot or square yard; (B) shall appear in conspicuous and easily legible type in distinct contrast (by topography, layout, colour, embossing or moulding) with other matter on the package; (C) shall contain letters or numerals in a type size which shall be (i) established in relationship to the area of the principal display panel of the package and (ii) uniform for all packages of substantially the same size and (D) shall be so placed that the lines of printed matter included in that statement are generally parallel to the base on which the package rests as it is designed to be displayed. The label of any package of a consumer commodity which bears a representation as to the number of servings of such commodity contained in such package shall bear a statement of the net quantity (in terms of weight or mass, measure or numerical count) of each such serving. The term ‘package’ means any container or wrapping in which any consumer commodity is enclosed for use in the delivery or display of that consumer commodity to retail purchasers, but does not include (i) shipping containers or wrappings used solely for the transportation of any consumer commodity in bulk or in quantity to manufacturers, packers or processors, or to wholesale or retail distributors thereof, (ii) shipping containers or outer wrappings used by retailers to ship or deliver any commodity to retail customers if such containers and wrappings bear no printed matter pertaining to any particular commodity or (iii) containers subject to the provisions of this Act. The term ‘label’ means any written, printed or graphic matter affixed to any consumer commodity or affixed to or appearing upon a package containing any consumer commodity.
Biotech Food Labeling illustrates three observations made in the theory section of this report. First, to establish successful mandatory labelling requirements, the government must provide or arrange for standards, testing, certification and enforcement. Secondly, labelling of complex, unclear information will not reduce information and search costs. Thirdly, labelling is not the best policy tool for redressing externalities (even theoretical externalities). Labelling requirements are established by USDA for meat and poultry and by FDA for all other food products. Both agencies require labelling of a biotech food if the food's composition differs significantly from that of its conventional counterpart. Most biotech foods on the market have been found to be essentially equivalent to their conventional counterparts; hence, most biotech foods are unlabelled. Despite assurances from the government (and many other organisations) about the safety of biotech foods on the market, some consumers have expressed a desire to be able to distinguish between foods and food products containing biotech ingredients and those that are biotech free. In this article, we examine the costs and benefits of meeting this demand. The second set of costs that arises in pursuing a non-biotech marketing strategy are the costs of keeping non-biotech commodities and food products free of biotech material. This segregation could be achieved by either specialising in biotech or non-biotech, establishing separate facilities for biotech and non-biotech, or taking precautions to sequence or separate biotech and non-biotech production (including a thorough cleaning of equipment and storage facilities after each biotech variety). As an alternative to segregation, processors could choose to reformulate their products to use ingredients from crops that are exclusively non-biotech, thus minimising the risk of inadvertently using a biotech variety. For example, corn emulsifiers could be replaced with rice emulsifiers. The cost of any of these options varies greatly depending on the flexibility of the production and marketing systems, the tolerance level for biotech content, the volume of biotech and non-biotech commodities and products processed by the system and the likelihood of achieving economies of scale.
Following Biotech Labeling Guidance (2001) in comments submitted to the FDA, the National Food Processors Association (NFPA) has commended FDA for its draft guidance on voluntary labelling for foods that have or have not been derived through biotechnology. Consumers will benefit only if voluntary biotechnology labelling conforms to standards assuring the information is truthful and not misleading. In this regard, the FDA has provided appropriate and much needed guidance to the food industry through its authority under the misbranding provisions of the Federal Food, Drug and Cosmetics Act. Among NFPA's specific comments: (i) NFPA supports FDA's preference for the terms ‘biotechnology’ and ‘GE’ to describe plants derived through recombinant DNA technology. NFPA recommends that they be used consistently in label claims to clarify that it is the plant sources of foods and food ingredients that are developed through biotechnology and not the ingredients themselves. For example, FDA cites a sample biotechnology presence claim: ‘This product contains cornmeal that was produced using biotechnology’. NFPA believes that this example should be worded: ‘This product contains cornmeal from corn that was produced using biotechnology’. (ii) NFPA agrees with FDA that claims such as ‘GMO free’ may be inaccurate, misleading and not well understood by consumers. NFPA supports FDA's conclusion that terms such as ‘GMO free’, ‘not GM’ and ‘free of GMO’, which include the word modified, are not technically accurate as traditional techniques of plant development also result in products that are ‘GM’. (iii) Further, NFPA agrees that use of the term ‘free’ of bioengineered materials may be potentially misleading because consumers will assume that the food contains zero adventitious bioengineered material. NFPA agrees with FDA that currently there is insufficient scientific information to establish a threshold for use of the term ‘free’. (iv) NFPA supports FDA's advice that a statement that a food was not bioengineered, or does not contain bioengineered ingredients, may be misleading if it implies that the labelled food is superior to foods that are not so labelled. Likewise, NFPA believes that such statements would be misleading if they state or imply any type of warning regarding foods developed through biotechnology. (v) NFPA agrees with FDA's advice that a ‘certified organic’ statement in compliance with the recently promulgated National Organic Program standard likely would be sufficient to substantiate a claim that a food was not produced through biotechnology, as biotechnology is a method excluded from ‘organic’ production and handling. NFPA also urged FDA to be vigilant that ‘organic’ foods comply with the guidance on food biotechnology claims.
According to Food Labeling (2004), GE food is becoming the rule, rather than the exception, on American grocery store shelves. By some estimates, two thirds or more of processed foods now contain ingredients derived from GE corn and soy crops. Unlike the old botanical roulette involved in combining plants to see what traits emerge in their hybrids, genetic engineering is precise. Single traits of one species are spliced into another. The new label, reflecting the definition that organic farmers themselves pushed for, will certify that organic food, in addition to being grown without pesticides, contains no GE ingredients. The USDA embarked on this organic certification programme because of the chaos that resulted from the previous jumble of definitions, with consumers getting sold different things in different states. The USDA labels should give consumers enough information to steer clear of GE food if they want to. And that is their right. People do not choose food on a strictly scientific basis. They pick and avoid foods every day for religious, political and personal reasons. They should have the information to do so, even if others might judge their choices to be irrational or whimsical. At a time when the Federal Department of Agriculture is launching a national programme, aiming at consistency, it makes no sense for Oregon to cobble together its own variation on labelling. Far from satisfying the hunger for information, a single-state system would likely succeed, mainly, in feeding consumer confusion.
The titles, main points and comments of the US legislation about labelling and packaging are summarised in Table 10.
US legislation (title, main points, comments) dealing with labelling and packaging
| Title . | Main points . | Comments . |
|---|---|---|
| Fair Packaging and Labeling Act, 1967 | • Unfair and deceptive packaging and labelling: scope of prohibition • Requirements of labelling, placement, form and contents of statement of quantity, supplemental statement of quantity • Annual reports to Congress • Definitions (consumer commodity, package, label) | Amendments |
| 1992 (The quantity disclosure on labels of consumer commodities be expressed in both the metric system and the customary inch/pound system of measurement) | ||
| Biotech Food Labeling, 1999 | • The biotech labelling example illustrates three observations • Labelling requirements are established by USDA for meat and poultry and by FDA for all other food products • Numerous private costs could be incurred in the process of establishing a credible non-biotech product label | |
| Biotech Labeling Guidance, 2001 | • Labelling of biotechnology food products • Definitions (biotechnology, genetically engineered, etc.) • Words using in labelling | |
| Labelling Food, 2004 | • Genetically engineered food is becoming the rule • The new label, reflecting the definition that organic farmers themselves pushed for, will certify that organic food, in addition to being grown without pesticides, contains no genetically engineered ingredients • Labelling of genetically engineered food |
| Title . | Main points . | Comments . |
|---|---|---|
| Fair Packaging and Labeling Act, 1967 | • Unfair and deceptive packaging and labelling: scope of prohibition • Requirements of labelling, placement, form and contents of statement of quantity, supplemental statement of quantity • Annual reports to Congress • Definitions (consumer commodity, package, label) | Amendments |
| 1992 (The quantity disclosure on labels of consumer commodities be expressed in both the metric system and the customary inch/pound system of measurement) | ||
| Biotech Food Labeling, 1999 | • The biotech labelling example illustrates three observations • Labelling requirements are established by USDA for meat and poultry and by FDA for all other food products • Numerous private costs could be incurred in the process of establishing a credible non-biotech product label | |
| Biotech Labeling Guidance, 2001 | • Labelling of biotechnology food products • Definitions (biotechnology, genetically engineered, etc.) • Words using in labelling | |
| Labelling Food, 2004 | • Genetically engineered food is becoming the rule • The new label, reflecting the definition that organic farmers themselves pushed for, will certify that organic food, in addition to being grown without pesticides, contains no genetically engineered ingredients • Labelling of genetically engineered food |
US legislation (title, main points, comments) dealing with labelling and packaging
| Title . | Main points . | Comments . |
|---|---|---|
| Fair Packaging and Labeling Act, 1967 | • Unfair and deceptive packaging and labelling: scope of prohibition • Requirements of labelling, placement, form and contents of statement of quantity, supplemental statement of quantity • Annual reports to Congress • Definitions (consumer commodity, package, label) | Amendments |
| 1992 (The quantity disclosure on labels of consumer commodities be expressed in both the metric system and the customary inch/pound system of measurement) | ||
| Biotech Food Labeling, 1999 | • The biotech labelling example illustrates three observations • Labelling requirements are established by USDA for meat and poultry and by FDA for all other food products • Numerous private costs could be incurred in the process of establishing a credible non-biotech product label | |
| Biotech Labeling Guidance, 2001 | • Labelling of biotechnology food products • Definitions (biotechnology, genetically engineered, etc.) • Words using in labelling | |
| Labelling Food, 2004 | • Genetically engineered food is becoming the rule • The new label, reflecting the definition that organic farmers themselves pushed for, will certify that organic food, in addition to being grown without pesticides, contains no genetically engineered ingredients • Labelling of genetically engineered food |
| Title . | Main points . | Comments . |
|---|---|---|
| Fair Packaging and Labeling Act, 1967 | • Unfair and deceptive packaging and labelling: scope of prohibition • Requirements of labelling, placement, form and contents of statement of quantity, supplemental statement of quantity • Annual reports to Congress • Definitions (consumer commodity, package, label) | Amendments |
| 1992 (The quantity disclosure on labels of consumer commodities be expressed in both the metric system and the customary inch/pound system of measurement) | ||
| Biotech Food Labeling, 1999 | • The biotech labelling example illustrates three observations • Labelling requirements are established by USDA for meat and poultry and by FDA for all other food products • Numerous private costs could be incurred in the process of establishing a credible non-biotech product label | |
| Biotech Labeling Guidance, 2001 | • Labelling of biotechnology food products • Definitions (biotechnology, genetically engineered, etc.) • Words using in labelling | |
| Labelling Food, 2004 | • Genetically engineered food is becoming the rule • The new label, reflecting the definition that organic farmers themselves pushed for, will certify that organic food, in addition to being grown without pesticides, contains no genetically engineered ingredients • Labelling of genetically engineered food |
Food products
The ‘fork-to-farm’ philosophy underlines the fact that the quality and safety of food for those who eat it is a major priority for the industry. Research is focused on making food as safe and clean as possible (Corlett, 1998). However, these high standards may not be easy to meet in other parts of the world (http://www.onderzoekinformatie.nl/en/oi/nod/onderzoek/OND1305164/). Initiative-proposed legislation requiring the FDA to inspect foreign farms and block imports of fruits and vegetables from countries that do not have safety standards equal to those of the United States; proposed increased funds to expand FDA's international food inspection force; directed the Department of Agriculture (USDA) and the Department of Health and Human Services (HHS) to help countries improve their food safety systems to prevent importation of unsafe produce and directed USDA and HHS to develop guidelines for good agricultural and manufacturing practices (http://www.ncsenoline.org/nle/crsreports/agriculture/ag_70.cfm). The history of meat inspection is summarised in Table 11. The Water Act regulates the legal status of water and water estate, the methods and conditions of water management (water use, water protection, regulation of watercourses and other water bodies and protection from adverse effects of water), the method of organising and performing water management tasks and functions, basic conditions for carrying out of water management activities; powers and duties of Government administration and other Government bodies, local authorities and other legal subjects and other issues of importance to water management (http://en.gmo.hr/index.php/zakonska_regulativa/hrvatski_zakoni).
| Year . | Event . |
|---|---|
| 1891 | USDA ensuring exports to European countries |
| 1906 | Meat inspection law provided for continuous USDA inspection of processing operations |
| 1946 | Meat and poultry were being used for canned soups and other processed foods |
| 1958 | Passed food additives amendment of the Federal Food, Drug and Cosmetic Act |
| 1967 | FSIS established the National Residue Program |
| 1971 | Pillsbury presented the Hazard Analysis and Critical Control Points (HACCP) at the US National Conference of Food Protection |
| 1973 | FDA incorporated HACCP principles to address serious botulism problems in the canning industry |
| 1976 | • The volume of processed foods and products quadrupled • A study recommended the establishment of microbiological criteria for finished products |
| 1980s | Numerous organisations characterised the traditional inspection system as overwhelmed by the practical realities of modern meat and poultry slaughter and processing |
| 1990s | Outbreak of Escherichia coli O157:H7 foodborne illness in the northwest of the United States |
| 1992 | NACMCF endorsed HACCP as an effective and national means of assuring food safety from harvest to consumption |
| 1996 | Published as a final rule the HACCP Systems |
| Year . | Event . |
|---|---|
| 1891 | USDA ensuring exports to European countries |
| 1906 | Meat inspection law provided for continuous USDA inspection of processing operations |
| 1946 | Meat and poultry were being used for canned soups and other processed foods |
| 1958 | Passed food additives amendment of the Federal Food, Drug and Cosmetic Act |
| 1967 | FSIS established the National Residue Program |
| 1971 | Pillsbury presented the Hazard Analysis and Critical Control Points (HACCP) at the US National Conference of Food Protection |
| 1973 | FDA incorporated HACCP principles to address serious botulism problems in the canning industry |
| 1976 | • The volume of processed foods and products quadrupled • A study recommended the establishment of microbiological criteria for finished products |
| 1980s | Numerous organisations characterised the traditional inspection system as overwhelmed by the practical realities of modern meat and poultry slaughter and processing |
| 1990s | Outbreak of Escherichia coli O157:H7 foodborne illness in the northwest of the United States |
| 1992 | NACMCF endorsed HACCP as an effective and national means of assuring food safety from harvest to consumption |
| 1996 | Published as a final rule the HACCP Systems |
| Year . | Event . |
|---|---|
| 1891 | USDA ensuring exports to European countries |
| 1906 | Meat inspection law provided for continuous USDA inspection of processing operations |
| 1946 | Meat and poultry were being used for canned soups and other processed foods |
| 1958 | Passed food additives amendment of the Federal Food, Drug and Cosmetic Act |
| 1967 | FSIS established the National Residue Program |
| 1971 | Pillsbury presented the Hazard Analysis and Critical Control Points (HACCP) at the US National Conference of Food Protection |
| 1973 | FDA incorporated HACCP principles to address serious botulism problems in the canning industry |
| 1976 | • The volume of processed foods and products quadrupled • A study recommended the establishment of microbiological criteria for finished products |
| 1980s | Numerous organisations characterised the traditional inspection system as overwhelmed by the practical realities of modern meat and poultry slaughter and processing |
| 1990s | Outbreak of Escherichia coli O157:H7 foodborne illness in the northwest of the United States |
| 1992 | NACMCF endorsed HACCP as an effective and national means of assuring food safety from harvest to consumption |
| 1996 | Published as a final rule the HACCP Systems |
| Year . | Event . |
|---|---|
| 1891 | USDA ensuring exports to European countries |
| 1906 | Meat inspection law provided for continuous USDA inspection of processing operations |
| 1946 | Meat and poultry were being used for canned soups and other processed foods |
| 1958 | Passed food additives amendment of the Federal Food, Drug and Cosmetic Act |
| 1967 | FSIS established the National Residue Program |
| 1971 | Pillsbury presented the Hazard Analysis and Critical Control Points (HACCP) at the US National Conference of Food Protection |
| 1973 | FDA incorporated HACCP principles to address serious botulism problems in the canning industry |
| 1976 | • The volume of processed foods and products quadrupled • A study recommended the establishment of microbiological criteria for finished products |
| 1980s | Numerous organisations characterised the traditional inspection system as overwhelmed by the practical realities of modern meat and poultry slaughter and processing |
| 1990s | Outbreak of Escherichia coli O157:H7 foodborne illness in the northwest of the United States |
| 1992 | NACMCF endorsed HACCP as an effective and national means of assuring food safety from harvest to consumption |
| 1996 | Published as a final rule the HACCP Systems |
Meat, poultry, fish and their products
Following Federal Meat Inspection Act (1908) ‘meat food product’ means any product capable of use as human food which is made wholly or in part from any meat or other portion of the carcass of any cattle, sheep, swine or goats, excepting products which contain meat or other portions of such carcasses only in a relatively small proportion or historically have not been considered by consumers as products of the meat food industry, and which are exempted from definition as a meat food product by the Secretary under such conditions as he/she may prescribe to assure that the meat or other portions of such carcasses contained in such product are not adulterated and that such products are not represented as meat food products. This term as applied to food products of equines shall have a meaning comparable to that provided in this paragraph with respect to cattle, sheep, swine and goats. For the purpose of preventing the use in commerce of meat and meat food products which are adulterated, the Secretary shall cause to be made, by inspectors appointed for that purpose, an examination and inspection of all cattle, sheep, swine, goats, horses, mules and other equines before they shall be allowed to enter into any slaughtering, packing, meat canning, rendering or similar establishment, in which they are to be slaughtered and the meat and meat food products thereof are to be used in commerce; and all cattle, sheep, swine, goats, horses, mules and other equines found on such inspection to show symptoms of disease shall be set apart and slaughtered separately from all other cattle, sheep, swine, goats, horses, mules or other equines, and when so slaughtered the carcasses of said cattle, sheep, swine, goats, horses, mules or other equines shall be subject to a careful examination and inspection, all as provided by the rules and regulations to be prescribed by the Secretary. No person, firm or corporation shall, with respect to any cattle, sheep, swine, goats, horses, mules or other equines, or any carcasses, parts of carcasses, meat or meat food products of any such animals (i) slaughtering animals or preparation of articles capable of use as human food, (ii) humane methods of slaughter, (iii) sales, transportation and other transactions and (iv) adulteration or misbranding. No carcasses, parts of carcasses, meat or meat food products of cattle, sheep, swine, goats, horses, mules or other equines which are capable of use as human food, shall be imported into the United States if such articles are adulterated or misbranded and unless they comply with all the inspection, building, construction standards and all other provisions of this article and regulations issued thereunder applicable to such articles in commerce within the United States. No such carcasses, parts of carcasses, meat or meat food products shall be imported into the United States unless the livestock from which they were produced was slaughtered and handled in connection with slaughter in accordance with the Act.
In Poultry Products Inspection Act (1968), ‘poultry’ means any domesticated bird, whether live or dead and ‘poultry product’ means any poultry carcass or part thereof; or any product that is made wholly or in part from any poultry carcass or part thereof, excepting products that contain poultry ingredients only in a relatively small proportion or historically have not been considered by consumers as products of the poultry food industry, and which are exempted by the Secretary from definition as a poultry product under such conditions as the Secretary may prescribe to assure that the poultry ingredients in such products are not adulterated and that such products are not represented as poultry products. For the purpose of preventing the entry into or flow or movement in commerce of, or the burdening of commerce by, any poultry product which is capable of use as human food and is adulterated, the Secretary shall, where and to the extent considered by him/her necessary, cause to be made by inspectors antemortem inspection of poultry in each official establishment processing poultry or poultry products for commerce or otherwise subject to inspection under this article. Each official establishment slaughtering poultry or processing poultry products for commerce or otherwise subject to inspection under this article shall have such premises, facilities and equipment, and be operated in accordance with such sanitary practices, as are required by regulations promulgated by the Secretary for the purpose of preventing the entry into or flow or movement in commerce or burdensome effect upon commerce, of poultry products that are adulterated. No slaughtered poultry, or parts or products thereof, of any kind shall be imported to the United States unless they are healthful, wholesome, fit for human food, not adulterated and contain no dye, chemical, preservative or ingredient which renders them unhealthful, unwholesome, adulterated or unfit for human food and unless they also comply with the rules and regulations made by the Secretary of Agriculture to assure that imported poultry or poultry products comply with the standards provided for in this article. Inspection shall not be provided under this article at any establishment for the slaughter of poultry or the processing of any carcasses or parts or products of poultry, which are not intended for use as human food, but such articles shall, prior to their offer for sale or transportation in commerce, unless naturally inedible by humans, be denatured or otherwise identified as prescribed by regulations of the Secretary to deter their use for human food. No person shall buy, sell, transport or offer for sale or transportation, or receive for transportation, in commerce, or import, any poultry carcasses or parts or products thereof, which are not intended for use as human food unless they are denatured or otherwise identified as required by the regulations of the Secretary or are naturally inedible by humans.
For the purpose of Egg Products Inspection (1970), ‘egg product’ means any dried, frozen or liquid eggs, with or without added ingredients, excepting products that contain eggs only in a relatively small proportion or historically have not been, in the judgement of the Secretary, considered by consumers as products of the egg food industry, and which may be exempted by the Secretary under such conditions as he/she may prescribe to assure that the egg ingredients are not adulterated and such products are not represented as egg products and ‘egg’ means the shell egg of the domesticated chicken, turkey, duck, goose or guinea. Eggs and egg products found to be adulterated at official plants shall be condemned and, if no appeal be taken from such determination of condemnation, such articles shall be destroyed for human food purposes under the supervision of an inspector: Provided that articles which may by reprocessing be made not adulterated need not be condemned and destroyed if so reprocessed under the supervision of an inspector and thereafter found to be not adulterated. If an appeal be taken from such determination, the eggs or egg products shall be appropriately marked and segregated pending completion of an appeal inspection, which appeal shall be at the cost of the appellant if the Secretary determines that the appeal is frivolous. If the determination of condemnation is sustained, the eggs or egg products shall be destroyed for human food purposes under the supervision of an inspector. Egg products inspected at any official plant under the authority of this article and found to be not adulterated shall be pasteurised before they leave the official plant, except as otherwise permitted by regulations of the Secretary, and shall at the time they leave the official plant, bear in distinctly legible form on their shipping containers or immediate containers, or both, when required by regulations of the Secretary, the official inspection legend and official plant number, of the plant where the products were processed, and such other information as the Secretary may require by regulations to describe the products adequately and to assure that they will not have false or misleading labelling. No labelling or container shall be used for egg products at official plants if it is false or misleading or has not been approved as required by the regulations of the Secretary. If the Secretary has reason to believe that any labelling or the size or form of any container in use or proposed for use with respect to egg products at any official plant is false or misleading in any particular, he/she may direct that such use be withheld unless the labelling or container is modified in such manner as he/she may prescribe so that it will not be false or misleading. If the person using or proposing to use the labelling or container does not accept the determination of the Secretary, such person may request a hearing, but the use of the labelling or container shall, if the Secretary so directs, be withheld pending hearing and final determination by the Secretary.
According to procedures for the safe and sanitary processing and importing of fish and fishery products (1995), ‘fish’ means fresh or saltwater finfish, crustaceans, other forms of aquatic animal life (including, but not limited to, aligator, frog, aquatic turtle, jellyfish, sea cucumbers and sea urchin and the roe of such animals) other than birds or mammals, and all molluscs, where such animal life is intended for human consumption, ‘fishery products’ means any human food product in which fish is a characterising ingredient. The HACCP plan shall at a minimum list the food safety hazards that are reasonably likely to occur and that thus must be controlled for each fish and fishery product. Consideration should be given to whether any food safety hazards are reasonably likely to occur as a result of the following: (i) natural toxins, (ii) microbiological contamination, (iii) chemical contamination, (iv) pesticides, (v) drug residues, (vi) decomposition in scombroid toxin, (vii) parasites, (viii) unapproved use of direct or indirect food or colour additives and (ix) physical hazards (Corlett, 1998). Whenever a deviation from a critical limit occurs, a processor shall take corrective action by following a corrective action plan that is appropriate for the particular deviation. No product enters commerce that is either injurious to health or is otherwise adulterated as a result of the deviation and the cause of the deviation is corrected. Each processor should have and implement a written sanitation standard operating procedure (herein referred to as SSOP) or similar document that is specific to each location where fish and fishery products are produced. The SSOP should specify how the processor will meet those sanitation conditions and practices that are to be monitored in accordance with this section. Prevention of cross-contamination from insanitary objects to food, food packaging material and other food surfaces, including utensils, gloves and outer garments, and from raw product to cooked product. Every importer of fish and fishery products shall either (i) obtain the fish or fishery product from a country that has an active memorandum of understanding or similar agreement with the FDA, that covers the fish or fishery product and documents the equivalency or compliance of the inspection system of the foreign country with the US system, accurately reflects the current situation between the signing parties and is functioning and enforceable in its entirety or (ii) have and implement written verification procedures for ensuring that the fish and fishery products that they offer for import to the United States were processed in accordance with the requirements of this part. Processors of smoked or smoke-flavoured fishery products shall include in their HACCP plans how they are controlling the food safety hazard associated with the formation of toxin by Clostridium botulinum for at least as long as the shelf life of the product under normal and moderate abuse conditions. Processors shall only process molluscan shellfish harvested from growing waters approved for harvesting by a shellfish control authority. In the case of molluscan shellfish harvested from US Federal waters, the requirements of this part will be met so long as the shellfish have not been harvesting by an agency of the Federal government.
For the purpose of Sustainable Fishery Act (1996), ‘commercial fishing’ means fishing in which the fish harvested, either in whole or in part, are intended to enter commerce or enter commerce through sale, barter or trade and ‘fishing community’ means a community which is substantially dependent on or substantially engaged in the harvest or processing of fishery resources to meet social and economic needs, and includes fishing vessel owners, operators and crew and United States fish processors that are based in such community. The North Pacific Council and the Secretary shall establish a western Alaska community development quota programme under which a percentage of the total allowable catch of any Bering Sea fishery is allocated to the programme. To be eligible to participate in the western Alaska community development quota programme under paragraph, a community shall (i) be located within 50 nautical miles from the baseline from which the breadth of the territorial sea is measured along the Bering Sea coast from the Bering Strait to the westernmost of the Aleutian Islands, or on an island within the Bering Sea, (ii) not be located on the Gulf of Alaska coast of the north Pacific Ocean, (iii) meet criteria developed by the Governor of Alaska, approved by the Secretary and published in the Federal Register, (iv) be certified by the Secretary of the Interior pursuant to the Alaska Native Claims Settlement Act to be a Native village, (v) consist of residents who conduct more than one half of their current commercial or subsistence fishing effort in the waters of the Bering Sea or waters surrounding the Aleutian Islands and (vi) not have previously developed harvesting or processing capability sufficient to support substantial participation in the groundfish fisheries in the Bering Sea, unless the community can show that the benefits from an approved Community Development Plan would be the only way for the community to realise a return from previous investments. The Secretary shall, in cooperation with the Secretary of the department in which the Coast Guard is operating, the States, the Councils and Marine Fisheries Commissions, develop recommendations for implementation of a standardised fishing vessel registration and information management system on a regional basis. The recommendations shall be developed after consultation with interested governmental and non-governmental parties and shall (i) be designed to standardise the requirements of vessel registration and information collection systems required by this Act, the Marine Mammal Protection Act and any other marine resource law implemented by the Secretary, and, with the permission of a State, any marine resource law implemented by such State, (ii) integrate information collection programmes under existing fishery management plans into a non-duplicative information collection and management system, (iii) avoid duplication of existing State, tribal or Federal systems and shall utilise, to the maximum extent practicable, information collected from existing systems, (iv) provide for implementation of the system through cooperative agreements with appropriate State, regional or tribal entities and Marine Fisheries Commissions, (v) provide for funding (subject to appropriations) to assist appropriate State, regional or tribal entities and Marine Fisheries Commissions in implementation, (vi) establish standardised units of measurement, nomenclature and formats for the collection and submission of information, (vii) minimise the paperwork required for vessels registered under the system, (viii) include all species of fish within the geographic areas of authority of the Councils and all fishing vessels including charter fishing vessels, but excluding recreational fishing vessels, (ix) require United States fish processors, and fish dealers and other first ex-vessel purchasers of fish that are subject to the proposed system, to submit information (other than economic information) that may be necessary to meet the goals of the proposed system.
Following meat and poultry pathogen reduction and HACCP systems (1996), each official establishment that slaughters cattle and/or hogs shall test for E. coli Biotype I (E. coli) and shall (i) collect samples in accordance with the sampling techniques and methodology, (ii) obtain analytic results. An establishment, which is annually slaughtering no more than 6000 bovines, 20 000 swine or a combination of bovines and swine not exceeding 6000 bovines and 20 000 animals total, shall collect one sample per week starting from the first full week of June and continuing through August of each year. An establishment annually slaughtering no more than 440 000 chickens, 60 000 turkeys or a combination of chickens and turkeys not exceeding 60 000 turkeys and 440 000 birds total, shall collect one sample per week starting the first full week of June and continuing through August of each year. An establishment slaughtering both species shall collect samples from the species it slaughters in larger numbers. Weekly samples shall be collected and tested until the establishment has completed and recorded one series opf 13 tests. An establishment's raw meat products or raw poultry products, when sampled and tested by FSIS for Salmonella may not test positive for Salmonella at a rate exceeding the applicable national pathogen reduction performance standard. Food safety hazards might be expected to arise from the following: (i) natural toxins, (ii) microbiological contamination, (iii) chemical contamination, (iv) pesticides, (v) drug residues, (vi) zoonotic diseases, (vii) decomposition, (viii) parasites, (ix) unapproved use of direct or indirect food or colour additives and (x) physical hazards. Every establishment shall develop and implement whenever a hazard analysis reveals one or more food safety hazards that are reasonably likely to occur including products in the following processing categories: (i) slaughter – all species, (ii) raw product – ground, (iii) raw product – not ground, (iv) thermally processed – commercially sterile, (v) not heat treated – shelf stable, (vi) heat treated – shelf stable, (vii) fully cooked – not shelf stable, (viii) heat treated but not fully cooked – not shelf stable, (ix) product with secondary inhibitors – not shelf stable.
Meat, Poultry and Egg Products Inspection (1999) (i) requires that each plant develop and implement written standard operating procedures for sanitation to reduce the likelihood that harmful bacteria will contaminate the finished product, (ii) requires regular microbial testing by slaughter establishments to verify the adequacy of their process controls for preventing and removing faecal contamination, (iii) established pathogen reduction performance standards for Salmonella that slaughter plants and plants producing raw ground products had to meet and (iv) required that all meat and poultry plants develop and implement HACCP systems to prevent food safety problems, by addressing microbial, chemical and physical hazards reasonably likely to occur. FSIS began conducting a risk assessment for E. coli O157:H7 in ground beef and carcass trimmings. The risk assessment will estimate the risk of foodborne illness from E. coli O157:H7 both with existing programmes and practices and with alternative mitigation strategies. FSIS is exploring whether further changes are needed in its policy regarding E. coli O157:H7 in the light of new information that is emerging about the pathogen and its relation to human health. FSIS will re-evaluate its policy on E. coli O157:H7 through an open, participatory process, soliciting input from all of its various constituents. At the request of FSIS, USDA's Agricultural Research Service designed a study to examine the prevalence of Listeria in a ready-to-eat product (hotdogs) supplied by volunteer plants over a 12-month period. Product will be held at a temperature representing typical retail storage and at an ‘abuse’ temperature, and will be tested for the presence of Listeria at several time points over a 3-month period. FSIS completed the fourth full year of an agreement with the Centers for Disease Control and Prevention to conduct active population-based surveillance for foodborne diseases (Campylobacter, E. coli O157:H7, Listeria, Salmonella, Shigella, Vibrio, Yersinia, Cryptosporidium and Cyclospora) in Minnesota, Oregon and selected counties in California, Connecticut, Georgia, Maryland, New York and Tennessee (total population: 30 million). This multiyear study is providing much needed data regarding the burden of foodborne illness in the United States. The FDA and the EPA are also partners in this effort.
In Meat and Poultry Inspection Issues (2000), no meat or poultry establishment can slaughter or process products for human consumption until FSIS approves in advance its plans and specifications for the premises, equipment and operating procedures. Once this approval is granted and operations begin, the plant must continue to follow a detailed set of rules that cover such things as proper lighting, ventilation and water supply; cleanliness of equipment and structural features and employee sanitation procedures. A key feature of the programme is that FSIS must inspect all meat and poultry animals at slaughter on a continuous basis; that is, no animal may be slaughtered and dressed unless an inspector has examined each carcass. One or more Federal inspectors are ‘on the line’ during all hours the plant is operating; the appropriate number depends upon each plant's production level. As the programme's inception, inspectors have relied mostly on organoleptic detection procedures: sight, touch and smell. They conduct both antemortem (before slaughter) and postmortem (after slaughter) inspections. Inspectors look for signs of disease, contamination and/or other abnormal conditions. FSIS's legal inspection responsibilities do not begin until animals arrive at slaughterhouses, and they generally end once products leave processing plants. The agency has no regulatory jurisdiction at the farm level. In addition, certain custom slaughter and most retail store and restaurant activities are exempt from Federal inspection. In addition, many flesh foods are not subject to mandatory inspection under the meat and poultry acts, including venison, rabbit, quail, buffalo and seafoods (even those fish and shellfish raised through aquaculture), although they are subject to less intensive FDA regulatory oversight under the Food, Drug, and Cosmetic Act. USDA states that generic E. coli was chosen because it is the best microbial indicator of faecal contamination, the primary vehicle for such potentially dangerous bacteria as Salmonella, Campylobactor and E. coli O157:H7. USDA inspectors conduct the Salmonella testing. Plants were required to begin meeting the standards when they implemented their HACCP plans.
US legislation dealing with meat, poultry, fish and their products are given in Table 12.
US legislation (title, main points and comments) related to meat, poultry, fish and their products
| Title . | Main points . | Comments . |
|---|---|---|
| Federal Meat Inspection Act, 1908 | • Definitions (meat food product, etc.) • Inspection of meat and meat food products Sanitary inspection and regulation of slaughtering and packing establishments; rejection of adulterated meat or meat food products • Marking, labelling or other identification of kinds of animals of articles’ derivation; separate establishments for preparation and slaughtering activities | Amendments |
| • 1958 (changes in slaughtering and handling methods) • 1967 (amendments in the slaughter, storage, handling and distribution of carcasses) • 1970 (repeal two sections of the Act) • 1999 (replacements of sections) | ||
| Poultry Products Inspection Act, 1968 | • Poultry and poultry products are an important source of the Nation's total supply of food • Definitions (poultry, poultry products, etc.) • Federal and State cooperation in development and administration of State poultry product inspection programmes • Sanitary practices • Storage and handling of poultry products | |
| Egg Products Inspection, 1970 | • Eggs and egg products are an important source of the Nation's total supply of food, and are used in food in various forms • Definitions (egg product, egg, etc.) • Inspection of egg products • Pasteurisation and labelling of egg products at official plants | |
| Procedures for the safe and sanitary processing and importing of fish and fishery products, 1995 | • Definitions (fish, fishery products, etc.) • Current good manufacturing practice • Sanitation control procedures • Special requirements for imported products • Specific requirements for processing smoked and smoke-flavoured fishery products • Specific requirements for processing fresh and frozen molluscan shellfish | |
| Sustainable Fishery Act, 1996 | • Definitions (commercial fishing, fishing community, etc.) • Fishery monitoring and research • Fishery management plans • Fisheries financing and capacity reduction | |
| Meat and poultry pathogen reduction and Hazard Analysis and Critical Control Points (HACCP) Systems, 1996 | • Contamination with microorganisms, pathogen reduction performance standards for Salmonella • Poultry products inspection regulations • Sanitation control procedures for meat and poultry | |
| Meat, Poultry and Egg Products Inspection, 1999 | • The Food Safety and Inspection Service (FSIS), a public health regulatory agency within the US Department of Agriculture (USDA), is responsible for ensuring that the commercial supply of meat, poultry and egg products in the United States is safe, wholesome and accurately labelled • FSIS and FDA worked together on a Listeria monocytogenes (Listeria) risk ranking | |
| Meat and poultry inspection issues, 2000 | • The USDA's FSIS is responsible for inspecting most meat, poultry and processed egg products for safety, wholesomeness and proper labelling • No meat or poultry establishment can slaughter or process products for human consumption until FSIS approves in advance its plans and specifications for the premises, equipment and operating procedures • FSIS's legal inspection responsibilities do not begin until animals arrive at slaughterhouses, and they generally end once products leave processing plants |
| Title . | Main points . | Comments . |
|---|---|---|
| Federal Meat Inspection Act, 1908 | • Definitions (meat food product, etc.) • Inspection of meat and meat food products Sanitary inspection and regulation of slaughtering and packing establishments; rejection of adulterated meat or meat food products • Marking, labelling or other identification of kinds of animals of articles’ derivation; separate establishments for preparation and slaughtering activities | Amendments |
| • 1958 (changes in slaughtering and handling methods) • 1967 (amendments in the slaughter, storage, handling and distribution of carcasses) • 1970 (repeal two sections of the Act) • 1999 (replacements of sections) | ||
| Poultry Products Inspection Act, 1968 | • Poultry and poultry products are an important source of the Nation's total supply of food • Definitions (poultry, poultry products, etc.) • Federal and State cooperation in development and administration of State poultry product inspection programmes • Sanitary practices • Storage and handling of poultry products | |
| Egg Products Inspection, 1970 | • Eggs and egg products are an important source of the Nation's total supply of food, and are used in food in various forms • Definitions (egg product, egg, etc.) • Inspection of egg products • Pasteurisation and labelling of egg products at official plants | |
| Procedures for the safe and sanitary processing and importing of fish and fishery products, 1995 | • Definitions (fish, fishery products, etc.) • Current good manufacturing practice • Sanitation control procedures • Special requirements for imported products • Specific requirements for processing smoked and smoke-flavoured fishery products • Specific requirements for processing fresh and frozen molluscan shellfish | |
| Sustainable Fishery Act, 1996 | • Definitions (commercial fishing, fishing community, etc.) • Fishery monitoring and research • Fishery management plans • Fisheries financing and capacity reduction | |
| Meat and poultry pathogen reduction and Hazard Analysis and Critical Control Points (HACCP) Systems, 1996 | • Contamination with microorganisms, pathogen reduction performance standards for Salmonella • Poultry products inspection regulations • Sanitation control procedures for meat and poultry | |
| Meat, Poultry and Egg Products Inspection, 1999 | • The Food Safety and Inspection Service (FSIS), a public health regulatory agency within the US Department of Agriculture (USDA), is responsible for ensuring that the commercial supply of meat, poultry and egg products in the United States is safe, wholesome and accurately labelled • FSIS and FDA worked together on a Listeria monocytogenes (Listeria) risk ranking | |
| Meat and poultry inspection issues, 2000 | • The USDA's FSIS is responsible for inspecting most meat, poultry and processed egg products for safety, wholesomeness and proper labelling • No meat or poultry establishment can slaughter or process products for human consumption until FSIS approves in advance its plans and specifications for the premises, equipment and operating procedures • FSIS's legal inspection responsibilities do not begin until animals arrive at slaughterhouses, and they generally end once products leave processing plants |
US legislation (title, main points and comments) related to meat, poultry, fish and their products
| Title . | Main points . | Comments . |
|---|---|---|
| Federal Meat Inspection Act, 1908 | • Definitions (meat food product, etc.) • Inspection of meat and meat food products Sanitary inspection and regulation of slaughtering and packing establishments; rejection of adulterated meat or meat food products • Marking, labelling or other identification of kinds of animals of articles’ derivation; separate establishments for preparation and slaughtering activities | Amendments |
| • 1958 (changes in slaughtering and handling methods) • 1967 (amendments in the slaughter, storage, handling and distribution of carcasses) • 1970 (repeal two sections of the Act) • 1999 (replacements of sections) | ||
| Poultry Products Inspection Act, 1968 | • Poultry and poultry products are an important source of the Nation's total supply of food • Definitions (poultry, poultry products, etc.) • Federal and State cooperation in development and administration of State poultry product inspection programmes • Sanitary practices • Storage and handling of poultry products | |
| Egg Products Inspection, 1970 | • Eggs and egg products are an important source of the Nation's total supply of food, and are used in food in various forms • Definitions (egg product, egg, etc.) • Inspection of egg products • Pasteurisation and labelling of egg products at official plants | |
| Procedures for the safe and sanitary processing and importing of fish and fishery products, 1995 | • Definitions (fish, fishery products, etc.) • Current good manufacturing practice • Sanitation control procedures • Special requirements for imported products • Specific requirements for processing smoked and smoke-flavoured fishery products • Specific requirements for processing fresh and frozen molluscan shellfish | |
| Sustainable Fishery Act, 1996 | • Definitions (commercial fishing, fishing community, etc.) • Fishery monitoring and research • Fishery management plans • Fisheries financing and capacity reduction | |
| Meat and poultry pathogen reduction and Hazard Analysis and Critical Control Points (HACCP) Systems, 1996 | • Contamination with microorganisms, pathogen reduction performance standards for Salmonella • Poultry products inspection regulations • Sanitation control procedures for meat and poultry | |
| Meat, Poultry and Egg Products Inspection, 1999 | • The Food Safety and Inspection Service (FSIS), a public health regulatory agency within the US Department of Agriculture (USDA), is responsible for ensuring that the commercial supply of meat, poultry and egg products in the United States is safe, wholesome and accurately labelled • FSIS and FDA worked together on a Listeria monocytogenes (Listeria) risk ranking | |
| Meat and poultry inspection issues, 2000 | • The USDA's FSIS is responsible for inspecting most meat, poultry and processed egg products for safety, wholesomeness and proper labelling • No meat or poultry establishment can slaughter or process products for human consumption until FSIS approves in advance its plans and specifications for the premises, equipment and operating procedures • FSIS's legal inspection responsibilities do not begin until animals arrive at slaughterhouses, and they generally end once products leave processing plants |
| Title . | Main points . | Comments . |
|---|---|---|
| Federal Meat Inspection Act, 1908 | • Definitions (meat food product, etc.) • Inspection of meat and meat food products Sanitary inspection and regulation of slaughtering and packing establishments; rejection of adulterated meat or meat food products • Marking, labelling or other identification of kinds of animals of articles’ derivation; separate establishments for preparation and slaughtering activities | Amendments |
| • 1958 (changes in slaughtering and handling methods) • 1967 (amendments in the slaughter, storage, handling and distribution of carcasses) • 1970 (repeal two sections of the Act) • 1999 (replacements of sections) | ||
| Poultry Products Inspection Act, 1968 | • Poultry and poultry products are an important source of the Nation's total supply of food • Definitions (poultry, poultry products, etc.) • Federal and State cooperation in development and administration of State poultry product inspection programmes • Sanitary practices • Storage and handling of poultry products | |
| Egg Products Inspection, 1970 | • Eggs and egg products are an important source of the Nation's total supply of food, and are used in food in various forms • Definitions (egg product, egg, etc.) • Inspection of egg products • Pasteurisation and labelling of egg products at official plants | |
| Procedures for the safe and sanitary processing and importing of fish and fishery products, 1995 | • Definitions (fish, fishery products, etc.) • Current good manufacturing practice • Sanitation control procedures • Special requirements for imported products • Specific requirements for processing smoked and smoke-flavoured fishery products • Specific requirements for processing fresh and frozen molluscan shellfish | |
| Sustainable Fishery Act, 1996 | • Definitions (commercial fishing, fishing community, etc.) • Fishery monitoring and research • Fishery management plans • Fisheries financing and capacity reduction | |
| Meat and poultry pathogen reduction and Hazard Analysis and Critical Control Points (HACCP) Systems, 1996 | • Contamination with microorganisms, pathogen reduction performance standards for Salmonella • Poultry products inspection regulations • Sanitation control procedures for meat and poultry | |
| Meat, Poultry and Egg Products Inspection, 1999 | • The Food Safety and Inspection Service (FSIS), a public health regulatory agency within the US Department of Agriculture (USDA), is responsible for ensuring that the commercial supply of meat, poultry and egg products in the United States is safe, wholesome and accurately labelled • FSIS and FDA worked together on a Listeria monocytogenes (Listeria) risk ranking | |
| Meat and poultry inspection issues, 2000 | • The USDA's FSIS is responsible for inspecting most meat, poultry and processed egg products for safety, wholesomeness and proper labelling • No meat or poultry establishment can slaughter or process products for human consumption until FSIS approves in advance its plans and specifications for the premises, equipment and operating procedures • FSIS's legal inspection responsibilities do not begin until animals arrive at slaughterhouses, and they generally end once products leave processing plants |
Agricultural products
The purpose of Organic Food Production Act (1990) is (i) to establish national standards governing the marketing of certain agricultural products as organically produced products, (ii) to assure consumers that organically produced products meet a consistent standard and (iii) to facilitate interstate commerce in fresh and processed food that is organically produced. The Secretary shall establish an organic certification programme for producers and handlers of agricultural products that have been produced using organic methods as provided for in this article. To be sold or labelled as an organically produced agricultural product under this article, an agricultural product shall (i) have been produced and handled without the use of synthetic chemicals, except as otherwise provided in this article, (ii) except as otherwise provided in this article and excluding livestock, not be produced on land to which any prohibited substances, including synthetic chemicals, have been applied during the 3 years immediately preceding the harvest of the agricultural products and (iii) be produced and handled in compliance with an organic plan agreed to by the producer and handler of such product and the certifying agent. A person may sell or label an agricultural product as organically produced only if such product is produced and handled in accordance with this article and no person may affix a label to, or otherwise provide market information concerning, an agricultural product if such label or information implies, directly or indirectly, that such product is produced and handled using organic methods, except in accordance with this article. Organic plan may provide for the application of raw manure only to (i) any green manure crop, (ii) any perennial crop, (iii) any crop not for human consumption and (iv) any crop for human consumption, if such crop is harvested after a reasonable period of time determined by the certifying agent to ensure the safety of such crop, after the most recent application of raw manure, but in no event shall such period be less than 60 days after such application.
Fruits and Vegetables
Ongoing Issues for Congress (2000) addressed agricultural emergency assistance (disasters and market losses) for specific fruits, vegetables and nursery; contingency funding for APHIS to control crop diseases; funding for the methyl bromide transition programme and funding for the National Organic Program. It also would provide for the establishment of a marketing order for Hass avocados and would redirect the duties charged foreign countries for dumping to injured farmers, ranchers and others. It would make mandatory certain practices to ensure the safety of both domestically produced and imported food. This bill and several others would increase border inspections of imported food, including fresh fruits and vegetables, among other provisions. Supporters of country-of-origin labelling for imported produce say that consumers have a right to know the countries where the produce that they purchase is grown. This information will enable consumers to make informed decisions if they have food safety concerns or if they have concerns about labour, pesticide or environmental practices in specific countries.
According to Agriculture, Conservation, and Rural Enhancement Act (2001), the Administration believes in providing a solid safety net for all farmers and ranchers – including a savings account for farmers that grows each year. The Administration recognises that agriculture is a unique industry, buffeted not only by natural forces but also by the forces of economic policies and markets all around the world. The safety net should protect producers from events and circumstances, which are beyond their control, and enable them to better manage individual financial situations. The Administration believes that farm legislation in the best long-term interests of our producers is market-oriented and minimally distorting of planting and investment decisions. Agriculture functions best when it is responsive to the market and to the needs of users and consumers, not to artificial government signals. The Act increases government payments to farmers on a per bushel basis when prices are low. This will further encourage overproduction and depress prices, deepening the problem of recent years. The Administration supports policies that help producers work with markets, rather than policies that create harmful distortion of markets and further dependency on Federal programmes.
This guide to minimise Microbial Food Safety Hazards for fresh fruits and vegetables (2004) is intended to assist the US and foreign produce industry in enhancing the safety of domestic and imported produce by addressing common areas of concern in the growing, harvesting, sorting, packing and distribution of fresh produce. The guide identifies the broad microbial hazards associated with each area of concern, the scientific basis of that concern and good agricultural and management practices for reducing the risk of microbial contamination in fresh produce. This guidance document is based upon certain basic principles and practices associated with minimising microbial food safety hazards from the field through distribution of fresh fruits and vegetables. By identifying basic principles of microbial food safety within the realm of growing, harvesting, packing and transporting fresh produce, users of this guide will be better prepared to recognise and address the principal elements known to give rise to microbial food safety concerns.
1st Principle: prevention of microbial contamination of fresh produce is favoured over reliance on corrective actions once contamination has occurred.
2nd Principle: to minimise microbial food safety hazards in fresh produce, growers, packers or shippers should use good agricultural and management practices in those areas over which they have control.
3rd Principle: fresh produce can become microbiologically contaminated at any point along the farm-to-table food chain. The major source of microbial contamination with fresh produce is associated with human or animal faeces.
4th Principle: whenever water comes in contact with produce, its source and quality dictates the potential for contamination. Minimise the potential of microbial contamination from water used with fresh fruits and vegetables.
5th Principle: practices using animal manure or municipal biosolid wastes should be managed closely to minimise the potential for microbial contamination of fresh produce.
6th Principle: worker hygiene and sanitation practices during production, harvesting, sorting, packing and transport play a critical role in minimising the potential for microbial contamination of fresh produce.
7th Principle: follow all applicable local, state and Federal laws and regulations, or corresponding or similar laws, regulations or standards for operators outside the United States, for agricultural practices.
8th Principle: accountability at all levels of the agricultural environment (farm, packing facility, distribution centre and transport operation) is important to a successful food safety programme.
There must be qualified personnel and effective monitoring to ensure that all elements of the programme function correctly and to help track produce back through the distribution channels to the producer. Water can be a carrier of many microorganisms, including pathogenic strains of E. coli, Salmonella spp., Vibrio cholerae, Shigella spp., Cryptosporidium parvum, Giardia lamblia, Cyclospora cayetanensis, Toxiplasma gondii, and the Norwalk and hepatitis A viruses. Even small amounts of contamination with some of these organisms can result in foodborne illness.
A summary of the US legislation focused on agricultural products is given in Table 13.
| Title . | Main points . | Comments . |
|---|---|---|
| Organic Food Production Act, 1990 | • Definitions (agricultural product, botanical pesticides, etc.) • National standards for organic production • Prohibited crop production practices and materials • Other production and handling practices | |
| Fruits and Vegetables: Ongoing Issues for Congress, 2000 | • The act addressed agricultural emergency assistance (disasters and market losses) for specific fruits and vegetables • The law reformed the crop insurance programme and approved funds to help fruit and vegetable growers suffering from low market prices and from specific crop diseases • The growing concern for food safety has fuelled discussion among US government health and agriculture officials on the use of irradiation for fresh fruits and vegetables | |
| Agriculture, Conservation, and Rural Enhancement Act, 2001 | • The act would expand the government's role in agriculture in a way that threatens the financial health of the farm sector • The Administration believes in providing a solid safety net for all farmers and ranchers • The Administration supports increasing resource conservation in ways that will enhance environment | |
| Guide to minimise Microbial Food Safety Hazards for fresh fruits and vegetables, 2004 | • Use of this Guide • Basic principles of microbial food safety • Definitions (agricultural water, etc.) • Control of potential hazards Packing facility sanitation |
| Title . | Main points . | Comments . |
|---|---|---|
| Organic Food Production Act, 1990 | • Definitions (agricultural product, botanical pesticides, etc.) • National standards for organic production • Prohibited crop production practices and materials • Other production and handling practices | |
| Fruits and Vegetables: Ongoing Issues for Congress, 2000 | • The act addressed agricultural emergency assistance (disasters and market losses) for specific fruits and vegetables • The law reformed the crop insurance programme and approved funds to help fruit and vegetable growers suffering from low market prices and from specific crop diseases • The growing concern for food safety has fuelled discussion among US government health and agriculture officials on the use of irradiation for fresh fruits and vegetables | |
| Agriculture, Conservation, and Rural Enhancement Act, 2001 | • The act would expand the government's role in agriculture in a way that threatens the financial health of the farm sector • The Administration believes in providing a solid safety net for all farmers and ranchers • The Administration supports increasing resource conservation in ways that will enhance environment | |
| Guide to minimise Microbial Food Safety Hazards for fresh fruits and vegetables, 2004 | • Use of this Guide • Basic principles of microbial food safety • Definitions (agricultural water, etc.) • Control of potential hazards Packing facility sanitation |
| Title . | Main points . | Comments . |
|---|---|---|
| Organic Food Production Act, 1990 | • Definitions (agricultural product, botanical pesticides, etc.) • National standards for organic production • Prohibited crop production practices and materials • Other production and handling practices | |
| Fruits and Vegetables: Ongoing Issues for Congress, 2000 | • The act addressed agricultural emergency assistance (disasters and market losses) for specific fruits and vegetables • The law reformed the crop insurance programme and approved funds to help fruit and vegetable growers suffering from low market prices and from specific crop diseases • The growing concern for food safety has fuelled discussion among US government health and agriculture officials on the use of irradiation for fresh fruits and vegetables | |
| Agriculture, Conservation, and Rural Enhancement Act, 2001 | • The act would expand the government's role in agriculture in a way that threatens the financial health of the farm sector • The Administration believes in providing a solid safety net for all farmers and ranchers • The Administration supports increasing resource conservation in ways that will enhance environment | |
| Guide to minimise Microbial Food Safety Hazards for fresh fruits and vegetables, 2004 | • Use of this Guide • Basic principles of microbial food safety • Definitions (agricultural water, etc.) • Control of potential hazards Packing facility sanitation |
| Title . | Main points . | Comments . |
|---|---|---|
| Organic Food Production Act, 1990 | • Definitions (agricultural product, botanical pesticides, etc.) • National standards for organic production • Prohibited crop production practices and materials • Other production and handling practices | |
| Fruits and Vegetables: Ongoing Issues for Congress, 2000 | • The act addressed agricultural emergency assistance (disasters and market losses) for specific fruits and vegetables • The law reformed the crop insurance programme and approved funds to help fruit and vegetable growers suffering from low market prices and from specific crop diseases • The growing concern for food safety has fuelled discussion among US government health and agriculture officials on the use of irradiation for fresh fruits and vegetables | |
| Agriculture, Conservation, and Rural Enhancement Act, 2001 | • The act would expand the government's role in agriculture in a way that threatens the financial health of the farm sector • The Administration believes in providing a solid safety net for all farmers and ranchers • The Administration supports increasing resource conservation in ways that will enhance environment | |
| Guide to minimise Microbial Food Safety Hazards for fresh fruits and vegetables, 2004 | • Use of this Guide • Basic principles of microbial food safety • Definitions (agricultural water, etc.) • Control of potential hazards Packing facility sanitation |
Water protection and management
The Clean Water Act (CWA, 1972), formerly known as the Federal Water Pollution Control Act, intended to restore and maintain the chemical, physical and biological integrity of the Nation's waters. To accomplish that objective, the act aimed to attain a level of water quality that ‘provides for the protection and propagation of fish, shellfish, and wildlife, and provides for recreation in and on the water’ by 1983 and to eliminate the discharge of pollutants into navigable waters by 1985. The CWA has five main elements: (i) a system of minimum national effluent standards for each industry, (ii) water quality standards, (iii) a discharge permit programme that translates these standards into enforceable limits, (iv) provisions for special problems such as toxic chemicals and oil spills and (iv) a revolving construction loan programme (formerly a grant programme) for publicly owned treatment works. The CWA requires the EPA to establish effluent limitations for the amounts of specific pollutants that may be discharged by municipal sewage plants and industrial facilities. The two-step approach to setting the standards includes (i) establishing a nationwide, base-level treatment through an assessment of what is technologically and economically achievable for a particular industry and (ii) requiring more stringent levels of treatment for specific plants if necessary to achieve water quality objectives for the particular body of water into which that plant discharges. For example, EPA sets limits based on water quality to control pollution in waters designated by the states for drinking, swimming or fishing. The primary method by which the act imposes limitations on pollutant discharges is the nationwide permit programme established under Section 402 and referred to as the National Pollutant Discharge Elimination System (NPDES). Under the NPDES programme, any person responsible for the discharge of a pollutant or pollutants into any waters of the United States from any point source must apply for and obtain a permit. For industrial facilities that existed before 1 July 1977, the ‘best conventional technology’ must be applied to the discharge stream for conventional pollutants. For facilities built after 1 July 1977, the so-called ‘new’ facilities, the National Standards of Performance apply. When either an existing or new facility discharges toxic pollutants, more stringent controls are required. The regulations for toxics are based on ‘best available technology economically achievable’. In all cases, NPDES permits can be made even more stringent than the above standards, if the specific water body in question requires lower discharges of pollutants to meet water quality standards. Facilities that discharge to a municipal or publicly owned wastewater system do not have to obtain an NPDES permit, but they must follow the pretreatment regulations. These pretreatment regulations require that industrial dischargers remove or treat all pollutants that could pass through the municipal system untreated or could adversely affect the performance of the municipal system. Toxic pollutants are the primary concern of these regulations. Note that, quite apart from the CWA, states may under certain circumstances exercise a limited role in the regulation of these materials. Until this section was added to the AEA in 1959, states had no role in the licensing and regulation of source, by-product or special nuclear materials. Section 274, however, provided a statutory basis by which states could assume from the NRC a measure of authority over the regulation of by-product and source materials and special nuclear materials in quantities not sufficient to form a critical mass. To effect this transfer of authority, (i) the NRC must find that the state's radiation control programme is compatible with the NRC's and that it is adequate to protect public health and safety, (ii) the state must establish its authority to enter into an agreement with the NRC and (iii) the NRC must enter into an agreement with the governor of the state desiring such authority.
According to Safe Drinking Water Act (SDWA, 1974), the term ‘primary drinking water regulation’ means a regulation that (i) applies to public water systems, (ii) specifies contaminants which, in the judgement of the Administrator, may have any adverse effect on the health of persons, (iii) specifies for each such contaminant either a maximum contaminant level, if, in the judgement of the Administrator, it is economically and technologically feasible to ascertain the level of such contaminant in water in public water systems or if, in the judgement of the Administrator, it is not economically or technologically feasible to so ascertain the level of such contaminant, each treatment technique known to the Administrator, which leads to a reduction in the level of such contaminant sufficient to satisfy the requirements of this section and (iv) contains criteria and procedures to assure a supply of drinking water which dependably complies with such maximum contaminant levels; including accepted methods for quality control and testing procedures to ensure compliance with such levels and to ensure proper operation and maintenance of the system, and requirements as to the minimum quality of water, which may be taken into the system and siting for new facilities for public water systems. At any time after promulgation of a regulation referred to in this paragraph, the Administrator may add equally effective quality control and testing procedures by guidance published in the Federal Register. Such procedures shall be treated as an alternative for public water systems to the quality control and testing procedures listed in the regulation. For purposes of this Act, a State has primary enforcement responsibility for public water systems during any period for which the Administrator determines (pursuant to regulations prescribed under this section) that such State (i) has adopted drinking water regulations that are no less stringent than the national primary drinking water regulations promulgated by the Administrator under this section not later than 2 years after the date on which the regulations are promulgated by the Administrator, except that the Administrator may provide for an extension of not more than 2 years if, after submission and review of appropriate, adequate documentation from the State, the Administrator determines that the extension is necessary and justified; (ii) has adopted and is implementing adequate procedures for the enforcement of such State regulations, including conducting such monitoring and making such inspections as the Administrator may require by regulation, (iii) will keep such records and make such reports with respect to its activities under (i) and (ii) as the Administrator may require by regulation, (iv) if it permits variances or exemptions, or both, from the requirements of its drinking water regulations which meet the requirements of (i), permits such variances and exemptions under conditions and in a manner which is not less stringent than the conditions under, and the manner in which variances and exemptions may be granted under this section of this title, (v) has adopted and can implement an adequate plan for the provision of safe drinking water under emergency circumstances including earthquakes, floods, hurricanes and other natural disasters, as appropriate and (vi) has adopted authority for administrative penalties (unless the constitution of the State prohibits the adoption of the authority) in a maximum amount: (i) in the case of a system serving a population of more than 10 000 that is not less than $1000 per day per violation and (ii) in the case of any other system that is adequate to ensure compliance (as determined by the State); except that a State may establish a maximum limitation on the total amount of administrative penalties that may be imposed on a public water system per violation.
The main points and comments of CWA and SDWA are given in Table 14.
| Title . | Main points . | Comments . |
|---|---|---|
| Clean Water Act (CWA), 1972 | • The CWA was established to restore and maintain the chemical, physical and biological integrity of the nation's waters • The CWA sets goals to eliminate discharges of pollutants into navigable water, protect fish and wildlife and prohibit the discharge of toxic pollutants in quantities that could adversely affect the environment • The CWA also provides for the construction of publicly owned wastewater treatment facilities | Amendments |
| The CWA was reauthorised in 1987 | ||
| Safe Drinking Water Act (SDWA), 1974 | • It seeks to protect sources of the nation's drinking water and to protect public health to the maximum extent possible, using proper water treatment techniques • SDWA establishes national primary drinking water standards based upon maximum contaminant levels, and establishes state management programmes to enforce the standards • SDWA also establishes procedures for the development, implementation and assessment of demonstration programmes designed to project critical aquifer protection areas located within areas designated as sole or principal source aquifers | 1996 (Amendments to the SDWA) |
| Title . | Main points . | Comments . |
|---|---|---|
| Clean Water Act (CWA), 1972 | • The CWA was established to restore and maintain the chemical, physical and biological integrity of the nation's waters • The CWA sets goals to eliminate discharges of pollutants into navigable water, protect fish and wildlife and prohibit the discharge of toxic pollutants in quantities that could adversely affect the environment • The CWA also provides for the construction of publicly owned wastewater treatment facilities | Amendments |
| The CWA was reauthorised in 1987 | ||
| Safe Drinking Water Act (SDWA), 1974 | • It seeks to protect sources of the nation's drinking water and to protect public health to the maximum extent possible, using proper water treatment techniques • SDWA establishes national primary drinking water standards based upon maximum contaminant levels, and establishes state management programmes to enforce the standards • SDWA also establishes procedures for the development, implementation and assessment of demonstration programmes designed to project critical aquifer protection areas located within areas designated as sole or principal source aquifers | 1996 (Amendments to the SDWA) |
| Title . | Main points . | Comments . |
|---|---|---|
| Clean Water Act (CWA), 1972 | • The CWA was established to restore and maintain the chemical, physical and biological integrity of the nation's waters • The CWA sets goals to eliminate discharges of pollutants into navigable water, protect fish and wildlife and prohibit the discharge of toxic pollutants in quantities that could adversely affect the environment • The CWA also provides for the construction of publicly owned wastewater treatment facilities | Amendments |
| The CWA was reauthorised in 1987 | ||
| Safe Drinking Water Act (SDWA), 1974 | • It seeks to protect sources of the nation's drinking water and to protect public health to the maximum extent possible, using proper water treatment techniques • SDWA establishes national primary drinking water standards based upon maximum contaminant levels, and establishes state management programmes to enforce the standards • SDWA also establishes procedures for the development, implementation and assessment of demonstration programmes designed to project critical aquifer protection areas located within areas designated as sole or principal source aquifers | 1996 (Amendments to the SDWA) |
| Title . | Main points . | Comments . |
|---|---|---|
| Clean Water Act (CWA), 1972 | • The CWA was established to restore and maintain the chemical, physical and biological integrity of the nation's waters • The CWA sets goals to eliminate discharges of pollutants into navigable water, protect fish and wildlife and prohibit the discharge of toxic pollutants in quantities that could adversely affect the environment • The CWA also provides for the construction of publicly owned wastewater treatment facilities | Amendments |
| The CWA was reauthorised in 1987 | ||
| Safe Drinking Water Act (SDWA), 1974 | • It seeks to protect sources of the nation's drinking water and to protect public health to the maximum extent possible, using proper water treatment techniques • SDWA establishes national primary drinking water standards based upon maximum contaminant levels, and establishes state management programmes to enforce the standards • SDWA also establishes procedures for the development, implementation and assessment of demonstration programmes designed to project critical aquifer protection areas located within areas designated as sole or principal source aquifers | 1996 (Amendments to the SDWA) |
Conclusions
This update of US food-related legislation revealed several aspects that can be summarised as follows:
- (i)
According to Tables 1–3 and Figs 1–3, the effectiveness of HACCP implementation was tested on several occasions (especially over the period years 2001–2003) and gave satisfactory results taking into account its rapid ‘restabilisation’.
- (ii)
Another issue is the number of US food-related laws, which when compared with EU are very few. This is definitely an advantage for United States in terms of practicality. However, updates in US legislation are equally rare compared with EU and this is definitely not an advantage as legislators should take into account the latest advances and findings in order to improve the current legislation.
- (iii)
Last but not least is the GMO issue where totally different approach was adopted by US and EU, thus leading to commercial disputes.



