-
PDF
- Split View
-
Views
-
Cite
Cite
Wisal H Idris, Samia M AbdelRahaman, Hagir B ElMaki, Elfadil E Babiker, Abdullahi H El Tinay, Effect of malt pretreatment on phytate and tannin level of two sorghum (Sorghum bicolor) cultivars, International Journal of Food Science and Technology, Volume 41, Issue 10, December 2006, Pages 1229–1233, https://doi.org/10.1111/j.1365-2621.2006.01190.x
Close - Share Icon Share
Abstract
The seeds of two cultivars of Sudanese sorghum (Sorghum bicolor), namely Wad Ahmed and Tabat, were germinated for 4 days to obtain 1-, 2- and 4-day-old malts. Sorghum malt (5% and 10%) was added to sorghum flour. The mixtures were incubated at 30 °C with shaking for 30, 60, 90 and 120 min. Malting loss was very slight for both cultivars and for all incubation periods. Phytic acid and tannin contents were assayed for all treatments. The results revealed that phytate and tannin contents were significantly (P ≤ 0.05) reduced when sorghum flour was pretreated with malt. When a mixture containing 10%, 4-day-old malt and sorghum flour was incubated for 120 min, it significantly (P ≤ 0.05) reduced phytate and tannin contents by 92% and 98%, respectively, for Wad Ahmed cultivar, while for Tabat they were reduced by 93% and 96%, respectively. The rate of reduction of phytate and tannin content increased with incubation time and malt age and concentration.
Introduction
Sorghum is a staple food in many African countries and it contains a reasonable amount of protein (7.5–10.8%), ash (1.2–1.8%), oil (3.4–3.5%), fibre (2.3–2.7%) and carbohydrate (71.4–80.7%) with a dry matter ranging from 89.2% to 95.3% (Samia et al., 2005). Sorghum nutritional quality is dictated mainly by its chemical composition and one of the constraints on the utilisation of sorghum grain as food or feed is the occurrence of phytate and tannins. Phytate binds minerals that are necessary as cofactors, thus interfering with several essential metabolic processes, especially the utilisation of protein (Harland & Oberleas, 1986) and also minerals that are not cofactors, e.g. calcium and iron. Malting of cereals is a processing procedure traditionally used in many African countries for the manufacture of alcoholic and soft drinks (Taylor, 1983). Malting is a process involving germination and drying of cereal seeds, the prime objective being to promote the development of hydrolytic enzymes that are not active in raw seeds (Dewar et al., 1997). The methods employed to improve the nutritional quality and organoleptic properties of cereal-based foods include genetic improvement, amino acid fortification, supplementation or complementation with protein rich sources and processing technologies that include milling, malting, fermentation or sprouting (Chavan & Kadam, 1989). Attempts to reduce the phytate content have been tried by different means including milling (Mahgoub & Elhag, 1998) and soaking of sorghum grains (Elmaki et al., 1999), malting of oats (Larrson & Sandberg, 1995) and pea (Beal & Mehta, 1985), fermentation of sorghum, maize, soybean, cowpea and yam (Marfo et al., 1990) and activation of the indigenous enzyme phytase and/or addition of microbial phytase (Barrier et al., 1996). Moreover, vigorous efforts are directed towards coupling the beneficial effects of tannins in sorghum as a field crop with methods for overcoming the antinutritional effects of tannins in seeds by direct removal of seed testa, inactivation or by extraction. Extractable tannin content was markedly reduced when sorghum grains were soaked in water and germinated for different periods of time (Elmaki et al., 1999). In feeding trials with rats (Yasaman et al., 1990) and chicks (Teeter et al., 1986), tannins reduced weight gain and feed conversion depending on the type of animal and feeding system. Elimination or inactivation of such antinutritional compounds is absolutely necessary to improve the nutritional quality of sorghum and effectively utilise its full potential as human food. Therefore, in this study we would like to evaluate the efficiency of malt pretreatment on phytate and tannin level of sorghum cultivars.
Materials and methods
Sample preparation
The seeds of two cultivars of Sudanese sorghum (Sorghum bicolor), locally known as Wad Ahmed and Tabat, were obtained from Pioneer Company, Khartoum, Sudan. The seeds of Wad Ahmed cultivars are small in size, round in shape and slightly brown in colour, while those of Tabat are slightly big in size, round in shape and white in colour. About 5 kg of each cultivar was cleaned from damaged seeds and foreign objects, then subjected separately to the following treatments:
- 1
Three kilograms of whole seed of each cultivar was milled to whole grain flour using a falling number laboratory mill to pass a 0.4-mm sieve to obtain 95% flour yield and kept at 4 °C before use.
- 2
One kilogram of whole seed of each cultivar was immersed in water overnight and then the grains were spread on trays lined with cloth. It was kept wet by frequent spraying of water. After 1, 2 or 4 days, the germinated grains were removed from the trays, sun-dried, milled and termed as 1-, 2- and 4 -day-old-malt.
Addition and incubation of malt to sorghum flour
One-, 2- or 4-day-old malt was added to sorghum flour at different concentrations (5% or 10%) in triplicate and mixed well. Water was added to the mixture in the ratio of 1:2 (w/v). Then the mixtures were shaken for 30 min and incubated at 30 °C in a shaker for 30, 60, 90 and 120 min, thereafter the mixture was dried at 65 °C and ground to pass a 0.4-mm sieve and kept at 4 °C before use.
Determination of soaking and malting losses
Soaking and malting losses were indicated by the ratio of the weight difference before and after soaking of the seeds or addition of malt to that of the original flour weight (on dry weight basis).
Determination of phytic acid and phosphorus
Phytic acid content of the malt, treated and untreated sorghum flour was determined by the method described by Wheeler & Ferrel (1971) using 2.0 g of a dried sample. A standard curve was prepared expressing the results as Fe(NO3)3 equivalent. Phytate phosphorus was calculated from the standard curve assuming 4:6 iron to phosphorus molar ratio. Phosphorus content was determined according to the ashing method of Chapman & Pratt (1961)). About 5 mL of the ash extract were pipetted into a 50-mL volumetric flask and mixed with 10 mL of the ammonium molybdate and ammonium vanadate reagent. Phosphorus was determined by spectrophotometer (Perkin–Elmer 2380, Beaconsfield, UK). A standard curve of phosphorus was prepared and then phosphorus content (mg 100 g−1) was determined from the standard curve according to the equation:
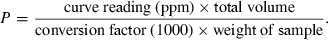
Determination of Tannin
Quantitative estimation of tannins was carried out using the modified vanillin–HCl method (Price et al., 1978). A 200 mg sample was extracted using 10 mL 1% (v/v) concentrated HCl in methanol for 20 min in capped rotating test tubes. Vanillin reagent (0.5%, 5 mL) was added to the extract (1 mL) and the absorbance of the colour developed after 20 min at 30 °C was read at 500 nm. A standard curve was prepared expressing the results as catechin equivalents, i.e. amount of catechin (mg mL−1) which gives a colour intensity equivalent to that given by tannins after correcting for blank. Then tannin content (%) was calculated according to the equation:

where C, concentration obtained from the standard curve (mg mL−1).
Statistical analysis
Each determination was carried out on three separate samples, on dry weight basis and analysed in triplicate, the figures were then averaged. Data were assessed using Anova (Snedecor & Cochran, 1987). Mean comparisons for treatments were made using Duncan's multiple range test (Duncan, 1955). Significance was accepted at P ≤ 0.05.
Results and discussion
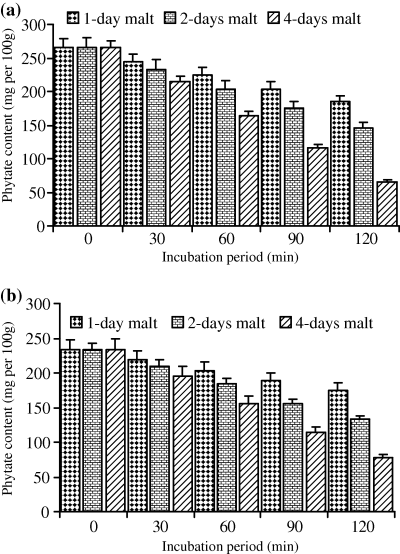
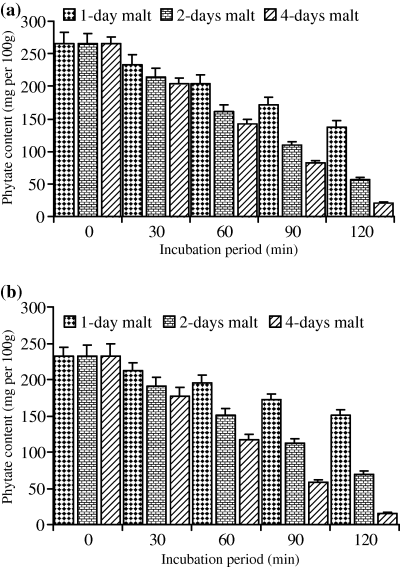
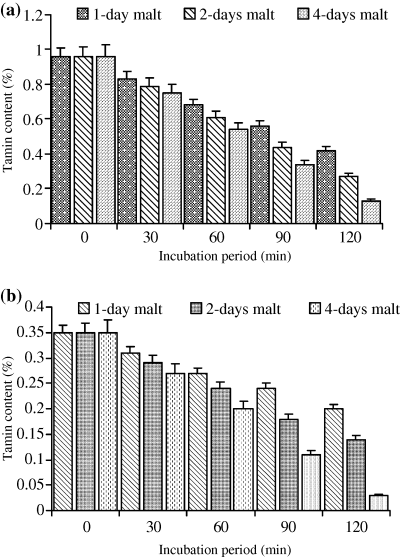
For both cultivars (Wad Ahmed and Tabat), the loss in dry matter occurred when the whole seeds were soaked prior to germination because germination itself had no effect on the sample dry matter. As the period of germination increased, the percentage of loss in dry matter was minor and constant, therefore, the loss was found to be due to soaking prior to germination. Soaking of the cultivars seeds overnight caused a slight reduction in the dry matter and it was found to be of no significant importance (data not shown). Moreover, in this study although malting had no effect on dry matter, we apply only 5% and 10% of the malt produced and in the meantime reduced the incubation period to minimise the loss in dry matter. Results obtained for dry matter loss are similar to those reported by Duhan et al. (2002) on pigeon seeds. Table 1 shows phytate, tannin, phosphate contents and phytate/P percentage of untreated sorghum cultivars. Results showed that phytate, tannin and phosphate contents of Wad Ahmed cultivar were greater than those of Tabat. The percentages of phytic acid/total P for Wad Ahmed and Tabat cultivars were 87% and 82%, respectively. The percentage of phytate/P obtained in this study agree with Chauhan & Mahjan's (1988) finding who stated that phytic acid represents more than 70% of total P in cereals and those of Raboy et al. (1991) who concluded that, in various seeds, phytic acid positively correlates with total P, correlation coefficients being greater than 0.90. Variation in phytate/P percentage between the two cultivars is likely due to variation in soil as well as fertilizers applied which affect total P content and accordingly influences phytic acid concentration (Miller et al., 1980). Malt was found to contain traces of phytate and tannin (data not shown) and had no significant effect on phytate and tannin contents of the treated flour. Low level of phytate in the malt is likely due to the action of the enzyme phytase on phytate while that of tannin is due to leaching during soaking and germination of the seeds as indicated by the significant browning of supporting filter paper during germination. Figure 1 shows the effect of malt pretreatment (5%) followed by incubation for different periods of time on phytate level of Wad Ahmed and Tabat cultivars. Addition of 5% malt of different age to Wad Ahmed cultivar flour with incubation for different periods of time (30, 60, 90 or 120 min) was found to decrease the phytic acid content with time (Fig. 1a). A maximum reduction level (from 265 to 66 mg 100 g−1) was obtained when the 4-day-old malt was added to sorghum flour and incubated for 120 min. The reduction of phytate content is more likely due to incubation time rather than addition of malt. Phytate content of Tabat cultivar (Fig. 1b) was also significantly (P ≤ 0.05) affected by the addition of malt but the rate of reduction was slightly low compared with Wad Ahmed cultivar. Phytate level of Tabat cultivar was decreased from 233 to 77 mg 100 g−1 during incubation of the flour with malt (4 days old) for 120 min (Fig. 1b). Increment of malt concentration to 10%, significantly (P ≤ 0.05) reduced phytic acid content with increasing time of incubation (Fig. 2). Incubation of Wad Ahmed flour with malt (10%) for 120 min reduced phytate content from 265 to 21 mg 100 g−1, i.e. about 92% was lost (Fig. 2a), while that of Tabat reduced from 233 to 16 mg 100 g−1, i.e. about 93% was lost (Fig. 2b). The results indicated that phytic acid reduction was significantly affected by the addition of malt because the germination stage had a substantial effect on the reduction in phytate content as a result of the action of endogenous phytases obtained during germination that degrade the phytate into inorganic phosphorus and inositol and its intermediate forms. The rate of reduction depends upon the age as well as the amount of malt (Elkhalil et al., 2001). Valverde et al. (1994) reported that germination of lentils greatly reduced phytate content compared with soaking or cooking. Similar results were also reported when sorghum flour was treated with malt and incubated for different time intervals (Elkhalil et al., 2001). Figure 3 shows the effect of malt pretreatment on tannin level during incubation of sorghum flour with 5% malt (1, 2 or 4 days old) for different periods of time (30, 60, 90 or 120 min). Wad Ahmed cultivar, which can be classified as a high-tannin cultivar as indicated by the seed coat colour, showed a progressive reduction in tannin content with incubation time (Fig. 3a). Tannin level of the cultivar reduced from 0.96% to 0.13%, when 5% malt was added to the flour and incubated for 120 min. Although Tabat cultivar was low in tannin content but the level of reduction in tannin was similar to that of Wad Ahmed cultivar (Fig. 3b). Tannin level of Tabat cultivar reduced from 0.35% to 0.03%, indicating that most of tannin of the cultivar was soluble and about 90% of it leached out. Increment of malt concentration significantly (P ≤ 0.05) reduced tannin content with increasing time of incubation (Fig. 4). Incubation of Wad Ahmed flour with malt (10%) for 120 min reduced tannin content from 0.96% to 0.02%, i.e. 98% of tannin was leached out (Fig. 4a), while that of Tabat reduced from 0.35% to 0.01%, i.e. 96% was leached out (Fig. 4b). Results indicated that malting of sorghum flour was very efficient in reducing tannin content. It has been reported that soaking of seeds of sorghum grains for 10 h, followed by germination significantly reduced the tannin content and the rate of reduction depends on germination time. When the grains were germinated for 24 h, about 74% of the total tannin was leached out from the grains and the loss significantly increased up to 98% after 72 h germination (Elmaki et al., 1999) because tannins are located in the seed coat (Jambunathan & Mertz, 1973). The loss of tannin during malting can be attributed to solubilisation by enzymes because the prime objective of malting is to promote the development of hydrolytic enzymes that are not active in raw seeds (Dewar et al., 1997).
Antinutritional factors content, phosphorus and phytate/P percentage of raw seeds of two sorghum cultivars
| Parameter . | Cultivars . | |
|---|---|---|
| Wad Ahmed . | Tabat . | |
| Dry matter (%) | 89.20 (±0.20)c | 95.30 (±0.20)a |
| Phytate (mg 100 g−1) | 265.00 (±0.30)a | 233.00 (±0.40)b |
| Phosphorus (mg 100 g−1) | 303.0 (±2.00)a | 283.0 (±2.31)b |
| Phytate/phosphorus (%) | 87a | 82b |
| Tannins (%) | 0.96 (±0.04)a | 0.35 (±0.01)b |
| Parameter . | Cultivars . | |
|---|---|---|
| Wad Ahmed . | Tabat . | |
| Dry matter (%) | 89.20 (±0.20)c | 95.30 (±0.20)a |
| Phytate (mg 100 g−1) | 265.00 (±0.30)a | 233.00 (±0.40)b |
| Phosphorus (mg 100 g−1) | 303.0 (±2.00)a | 283.0 (±2.31)b |
| Phytate/phosphorus (%) | 87a | 82b |
| Tannins (%) | 0.96 (±0.04)a | 0.35 (±0.01)b |
Values are means (±SD). Means not sharing a common superscript letter(s) in a row are significantly different at (P ≤ 0.05) as assessed by Duncan's multiple range test.
Antinutritional factors content, phosphorus and phytate/P percentage of raw seeds of two sorghum cultivars
| Parameter . | Cultivars . | |
|---|---|---|
| Wad Ahmed . | Tabat . | |
| Dry matter (%) | 89.20 (±0.20)c | 95.30 (±0.20)a |
| Phytate (mg 100 g−1) | 265.00 (±0.30)a | 233.00 (±0.40)b |
| Phosphorus (mg 100 g−1) | 303.0 (±2.00)a | 283.0 (±2.31)b |
| Phytate/phosphorus (%) | 87a | 82b |
| Tannins (%) | 0.96 (±0.04)a | 0.35 (±0.01)b |
| Parameter . | Cultivars . | |
|---|---|---|
| Wad Ahmed . | Tabat . | |
| Dry matter (%) | 89.20 (±0.20)c | 95.30 (±0.20)a |
| Phytate (mg 100 g−1) | 265.00 (±0.30)a | 233.00 (±0.40)b |
| Phosphorus (mg 100 g−1) | 303.0 (±2.00)a | 283.0 (±2.31)b |
| Phytate/phosphorus (%) | 87a | 82b |
| Tannins (%) | 0.96 (±0.04)a | 0.35 (±0.01)b |
Values are means (±SD). Means not sharing a common superscript letter(s) in a row are significantly different at (P ≤ 0.05) as assessed by Duncan's multiple range test.
Effect of incubation of sorghum flour with 5% malt on phytate content (mg 100 g−1) of (a) WadAhmed and (b) Tabat cultivars.
Effect of incubation of sorghum flour with 10% malt on phytate content (mg 100 g−1) of (a) WadAhmed and (b) Tabat cultivars.
Effect of incubation of sorghum flour with 5% malt on tannin content (%) of (a) WadAhmed and (b) Tabat cultivars.
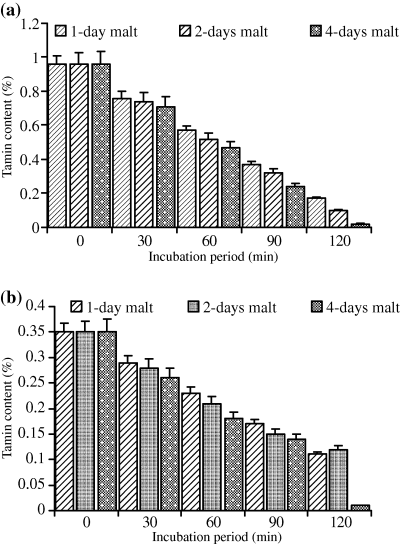
Effect of incubation of sorghum flour with 10% malt on tannin content (%) of (a) WadAhmed and (b) Tabat cultivars.
Conclusion
Utilisation of sorghum malt to lower phytic acid and tannin contents is a promising and simple method. The rate of reduction of phytic acid and tannin depends on the malt age and concentration as well as the incubation period. Short incubation period was useful to avoid the loss of dry matter.



