-
PDF
- Split View
-
Views
-
Cite
Cite
Gabriele Bislenghi, Julie Van Den Bossch, Steffen Fieuws, Albert Wolthuis, Marc Ferrante, Gert de Hertogh, Severine Vermeire, André D’Hoore, Appearance of the Bowel and Mesentery During Surgery Is Not Predictive of Postoperative Recurrence After Ileocecal Resection for Crohn’s Disease: A Prospective Monocentric Study, Inflammatory Bowel Diseases, Volume 30, Issue 10, October 2024, Pages 1686–1695, https://doi.org/10.1093/ibd/izad227
Close - Share Icon Share
Abstract
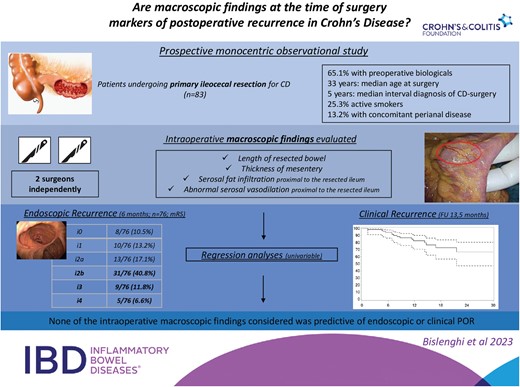
Very few risk factors for postoperative recurrence (POR) of Crohn’s Disease (CD) after ileocecal resection have been identified. The aim of the present study was to verify the association between an a priori defined list of intraoperative macroscopic findings and POR.
This was a prospective observational study including patients undergoing primary ileocecal resection for CD. Four intraoperative factors were independently evaluated by 2 surgeons: length of resected ileum, mesentery thickness, presence of areas of serosal fat infiltration, or abnormal serosal vasodilation on normal bowel proximal to the resected bowel. The primary end point was early endoscopic POR at month 6 and defined as modified Rutgeerts score ≥i2b. Secondary end points were clinical and surgical recurrence.
Between September 2020 and November 2022, 83 consecutive patients were included. Early endoscopic recurrence occurred in 45 of 76 patients (59.2%). Clinical and biochemical recurrence occurred in 17.3% (95% confidence interval, [CI], 10.4%-28.0%) and 14.6% of the patients after 12 months. The risk of developing endoscopic and clinical recurrence was 1.127 (95% CI, 0.448;2.834, P = .799) and 0.896 (95% CI, 0.324-2.478, P = .832) when serosal fat infiltration was observed, and 1.388 (95% CI, 0.554-3.476, P = .484), and 1.153 (95% CI, 0.417;3.187, P = .783) when abnormal serosal vasodilation was observed. Similarly, length of the resected bowel and mesentery thickness showed no association with POR. A subgroup analysis on patients who received no postoperative medical prophylaxis did not identify any risk factor for endoscopic POR.
The macroscopic appearance of the bowel and associated mesentery during surgery does not seem to be predictive of POR after ileocecal resection for CD.
Lay Summary
Prospective studies investigating risk factors for Crohn’s disease recurrence after surgery are scarce. In a prospective cohort of 83 patients undergoing primary ileocecal resection, no association between few intraoperative macroscopic findings and postoperative endoscopic/clinical recurrence was observed.
The relation between the intraoperative macroscopic appearance of the bowel and the mesentery and Crohn’s disease recurrence after ileocecal resection has rarely been investigated.
In the present study, length of resected bowel, mesentery thickness, and initial mesenteric alterations on normal bowel proximal to the resected ileum (defined as serosal fat infiltration and abnormal serosal vasodilation) showed no association with postoperative recurrence.
The macroscopic aspect of both affected and normal bowel during surgery is not predictive of early Crohn’s recurrence.
Introduction
Despite the significant advances in the medical treatment of Crohn’s disease (CD) and the availability of new advanced therapies with novel mechanisms of action,1 a significant proportion of patients still requires surgery.2 Moreover, the role of surgery has been recently extended beyond the treatment of complicated and therapy resistant disease, including the upfront treatment of uncomplicated, limited disease of the terminal ileum.3 Although surgery can induce deep remission in about 30% of patients,4,5 approximately 70% experience endoscopic postoperative recurrence (POR) within 1 year; and 30% require reoperation within 10 years after surgery.2,6 In this view, international guidelines advocate for risk stratification, prophylactic medical therapy in high-risk patients, and tight endoscopic surveillance to inform potential treatment escalation.7–9 Nevertheless, recent population-based data indicate that the use of biological therapies has only had a partial effect in mitigating the risk of re-resections.10 Recently, theories have been advanced proposing the mesentery as main trigger of inflammation in CD. In this view, CD should be viewed as a primary mesenteropathy and recurrence after surgery as a process arising in the mesentery and then extending to the contiguous bowel. Despite the considerable body of literature published on the topic, only a few risk factors for POR have been identified.11 With the exception of surgical margins and length of bowel involvement, the macroscopic appearance of the bowel and of the associated mesentery during surgery have never been investigated as possible predictors of early POR. In particular, this study originates from the observation during surgery of a broad variability in disease extension, mesentery thickness, and occurrence of initial and isolated alterations of the mesentery on normal bowel proximal to the resected segment.
Therefore, the aim of the present study was to verify a possible association between a predefined list of intraoperative macroscopic findings during ileocecal resection (ICR) and postoperative CD recurrence.
Methods
Study Design, Patients, Surgical Procedures, and Follow-up
All consecutive CD patients who underwent primary ICR for terminal ileitis between September 2020 and November 2022 at the University Hospital of Leuven, Belgium, were considered eligible for this prospective monocentric observational study. Informed consent was obtained from all patients. The institutional ethics commission approved the study (S-64382). Approval was obtained from the ethics committee of University Hospitals Leuven (S-64338).
Decision for surgery was taken during the weekly IBD multidisciplinary meeting and was based on endoscopic and/or radiologic proven terminal ileitis. No selection based on previous exposure to preoperative medications (including advanced therapies) and on surgical indication (penetrating, structuring, or inflammatory disease) was made.
Routinely, a partial excision of the mesentery (intramesenteric) dissection was performed. A wide side-to-side ileocolic anastomosis was performed with a 75-mm linear cutter stapler. In case of an isoperistaltic anastomosis, the enterotomy was closed using interrupted coated absorbable 3/0 stitches. Kono-S anastomosis was fashioned in 1 layer using an absorbable 3/0 suture, running for the posterior side and interrupted for the anterior anastomotic wall. No closure of the mesenteric defect was performed. Routinely, no abdominal drain was placed.
Four intraoperative factors were predefined to picture the macroscopic appearance of the bowel and the mesentery during the surgery:
length of the macroscopic affected ileum requiring resection
thickness of the mesentery at the insertion of the bowel wall (scored as <5 mm, 5-10mm, and ≥10 mm)
presence of areas of serosal fat infiltration on normal bowel proximal to the macroscopic affected ileum (Figure 1, Supplementary Figure 1)
presence of areas of abnormal serosal vasodilation on normal bowel proximal to the macroscopic affected ileum (Figure 1, Supplementary Figure 1)
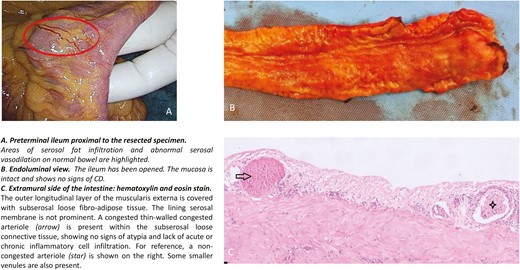
Macroscopic and histological view of a segment of ileum showing areas of serosal fat infiltration and abnormal serosal vasodilation.
Intraoperative videos of the mobilized ileocecal region previous to bowel and mesentery transection are available for the majority of patients included.
The primary objective was to verify the association between each of these variables and endoscopic recurrence 6 months after surgery. Secondary objectives were to verify whether the same variables showed association with clinical, biological, and surgical recurrence.
Intraoperative scoring occurred after complete surgical mobilization (either laparoscopic or open) of the right colon and prior to any vascular ligation or bowel transection and was performed independently by 2 colorectal surgeons with expertise in inflammatory bowel disease (IBD) to assess possible interobserver variability.
Prophylactic medical therapy was initiated (≤4 weeks) or continued postoperatively on a case-by-case basis if (1) active disease outside the site of the resection was present at the time of surgery or (2) active disease was limited to the site of resection in patients at high risk for recurrence based on physician global assessment.11 None of the 4 identified preoperative findings investigated in the present study influenced the decision on the initiation of postoperative prophylactic therapy.
An ileocolonoscopy was scheduled in all patients 6 months after surgery. All endoscopies were performed at University Hospitals Leuven by a dedicated IBD gastroenterologist with extensive experience in the evaluation of endoscopic findings after surgical resection. Patients and caregivers involved in the postoperative phase were blinded for the intraoperative surgeons’ findings.
According to the endoscopic and clinical findings, medical treatment was escalated and further follow-up scheduled.
Outcomes Definition
Postoperative complications were reported according to Dindo-Clavien12 and included radiological or surgically proven anastomotic leakage and perianastomotic abscess within 30 postoperative days.
Endoscopic disease activity was assessed according to the modified Rutgeerts score 6 months after surgery.13 Endoscopic recurrence was defined as mRS ≥i2b.14 Biological recurrence was defined as a C-reactive protein (CRP) level above 5 mg/L or a fecal calprotectin level above 250 µg/g. Clinical recurrence was defined as the occurrence of CD-related symptoms associated to a CRP level above 5 mg/L, a fecal calprotectin level above 250 µg/g, endoscopic recurrence >i2a, and/or radiologic evidence of neoterminal ileitis. Surgical recurrence was defined as symptomatic endoscopically (mRS ≥i2b) or radiologically confirmed stricture/inflammatory disease at the level of the anastomosis/neoterminal ileum requiring redo ileocolic resection or strictureplasty. Modified surgical recurrence was defined as the need for reoperation or balloon-dilation. Clinical, biological, and surgical recurrence were assessed at the longest follow-up available for each patient.
Patients and disease characteristics (penetrating disease, concomitant perianal disease, active smoking, microscopic resection margins, prophylactic medical postoperative treatment) related to disease recurrence were evaluated as independent risk factors for CD recurrence. A positive microscopic margin was defined for the presence of aftoid, broad, or fissural ulcers; signs of chronic inflammation in the mucosa (eg, disrupted architecture, pseudo pyloric metaplasia in the ileum, patchy increased infiltrate, basal plasmacytosis); fibromuscular, neural, or lymphoid hyperplasia; and epithelioid granulomas.15
Statistical Analysis
Descriptive statistics were performed on all data. Values are expressed as median and interquartile range. Cohen’s Kappa statistic (with 95% confidence interval [CI]) was used to test interrater reliability for the assessment of some intraoperative variables (thickness of the mesentery, initial creeping fat, and aberrant vascular pattern). Biological and clinical recurrence were estimated using Kaplan-Meier analysis. Univariable logistic and Cox regressions were performed for categorical and continuous variables to evaluate the relation with endoscopic and clinical recurrence, respectively. Considering the observed interobserver variability for the investigated intraoperative factors, the scores of the first observer (main surgeon) were used to assess risk factors related to endoscopic and clinical POR. Given the relative limited number of recurrences and the high number of variables under investigation and potential confounders, it was deemed inappropriate to report results from a classical multivariable analysis. Moreover, none of the investigated variables showed any form of association with endoscopic or clinical POR at univariable analysis. The SAS system for Windows, version 9.4, was used for statistical analysis.
Results
General Results
Eighty-three patients (40 women [48.2%]) were included. Patients and disease characteristics are reported in Table 1. Median age at surgery was 33 years (interquartile range [IQR], 25-49). Median time between diagnosis of CD and surgery was 5 years (IQR, 25.0-49.0). Twenty-one patients (25.3%) were active smokers at the time of surgery. Indication for surgery was stricturing CD in 42 patients (50.6%) and penetrating CD in 34 patients (41.0%). Concomitant perianal disease was present in 11 patients (13.2%). Fifty-four patients (65.1%) had received advanced therapies preoperatively.
| Variable . | Statistic . | All . |
|---|---|---|
| Sex | ||
| Male | n/N (%) | 43/83 (51.8%) |
| Female | n/N (%) | 40/83 (48.2%) |
| Age at CD diagnosis (years) | Median | 26.8 |
| IQR | (19.0; 37.1) | |
| Age at surgery (years) | Median | 33.0 |
| IQR | (25.0; 49.0) | |
| Years from CD diagnosis to surgery | Median | 5.0 |
| IQR | (0.8; 13.9) | |
| Montreal A (Age) | ||
| A1 | n/N (%) | 9/83 (10.8%) |
| A2 | n/N (%) | 58/83 (69.9%) |
| A3 | n/N (%) | 16/83 (19.3%) |
| Montreal B (Behavior) | ||
| B1 | n/N (%) | 7/83 (8.4%) |
| B2 | n/N (%) | 42/83 (50.6%) |
| B3 | n/N (%) | 34/83 (41.0%) |
| Concomitant perianal disease | ||
| No | n/N (%) | 72/83 (86.7%) |
| Yes | n/N (%) | 11/83 (13.2%) |
| Montreal L (Location) | ||
| L1 | n/N (%) | 70/83 (84.3%) |
| L2 | n/N (%) | 1/83 (1.2%) |
| L3 | n/N (%) | 12/83 (14.5%) |
| ASA Physical Status | ||
| 1 | n/N (%) | 2/83 (2.4%) |
| 2 | n/N (%) | 64/83 (77.1%) |
| 3 | n/N (%) | 17/83 (20.5%) |
| BMI (kg/m2) | Median | 22.9 |
| IQR | (20.3; 26.3) | |
| Smoking | ||
| No | n/N (%) | 48/83 (57.8%) |
| Yes | n/N (%) | 21/83 (25.3%) |
| Ex | n/N (%) | 14/83 (16.9%) |
| Preoperative CD treatment: anti-TNF | ||
| No | n/N (%) | 38/83 (45.8%) |
| Yes | n/N (%) | 45/83 (54.2%) |
| Preoperative CD treatment: other advanced therapies | ||
| No | n/N (%) | 53/83 (63.9%) |
| Yes | n/N (%) | 30/83 (36.1%) |
| Variable . | Statistic . | All . |
|---|---|---|
| Sex | ||
| Male | n/N (%) | 43/83 (51.8%) |
| Female | n/N (%) | 40/83 (48.2%) |
| Age at CD diagnosis (years) | Median | 26.8 |
| IQR | (19.0; 37.1) | |
| Age at surgery (years) | Median | 33.0 |
| IQR | (25.0; 49.0) | |
| Years from CD diagnosis to surgery | Median | 5.0 |
| IQR | (0.8; 13.9) | |
| Montreal A (Age) | ||
| A1 | n/N (%) | 9/83 (10.8%) |
| A2 | n/N (%) | 58/83 (69.9%) |
| A3 | n/N (%) | 16/83 (19.3%) |
| Montreal B (Behavior) | ||
| B1 | n/N (%) | 7/83 (8.4%) |
| B2 | n/N (%) | 42/83 (50.6%) |
| B3 | n/N (%) | 34/83 (41.0%) |
| Concomitant perianal disease | ||
| No | n/N (%) | 72/83 (86.7%) |
| Yes | n/N (%) | 11/83 (13.2%) |
| Montreal L (Location) | ||
| L1 | n/N (%) | 70/83 (84.3%) |
| L2 | n/N (%) | 1/83 (1.2%) |
| L3 | n/N (%) | 12/83 (14.5%) |
| ASA Physical Status | ||
| 1 | n/N (%) | 2/83 (2.4%) |
| 2 | n/N (%) | 64/83 (77.1%) |
| 3 | n/N (%) | 17/83 (20.5%) |
| BMI (kg/m2) | Median | 22.9 |
| IQR | (20.3; 26.3) | |
| Smoking | ||
| No | n/N (%) | 48/83 (57.8%) |
| Yes | n/N (%) | 21/83 (25.3%) |
| Ex | n/N (%) | 14/83 (16.9%) |
| Preoperative CD treatment: anti-TNF | ||
| No | n/N (%) | 38/83 (45.8%) |
| Yes | n/N (%) | 45/83 (54.2%) |
| Preoperative CD treatment: other advanced therapies | ||
| No | n/N (%) | 53/83 (63.9%) |
| Yes | n/N (%) | 30/83 (36.1%) |
Abbreviations: CD, Crohn’s disease; BMI, Body mass index; TNF, Tumor necrosis factor.
| Variable . | Statistic . | All . |
|---|---|---|
| Sex | ||
| Male | n/N (%) | 43/83 (51.8%) |
| Female | n/N (%) | 40/83 (48.2%) |
| Age at CD diagnosis (years) | Median | 26.8 |
| IQR | (19.0; 37.1) | |
| Age at surgery (years) | Median | 33.0 |
| IQR | (25.0; 49.0) | |
| Years from CD diagnosis to surgery | Median | 5.0 |
| IQR | (0.8; 13.9) | |
| Montreal A (Age) | ||
| A1 | n/N (%) | 9/83 (10.8%) |
| A2 | n/N (%) | 58/83 (69.9%) |
| A3 | n/N (%) | 16/83 (19.3%) |
| Montreal B (Behavior) | ||
| B1 | n/N (%) | 7/83 (8.4%) |
| B2 | n/N (%) | 42/83 (50.6%) |
| B3 | n/N (%) | 34/83 (41.0%) |
| Concomitant perianal disease | ||
| No | n/N (%) | 72/83 (86.7%) |
| Yes | n/N (%) | 11/83 (13.2%) |
| Montreal L (Location) | ||
| L1 | n/N (%) | 70/83 (84.3%) |
| L2 | n/N (%) | 1/83 (1.2%) |
| L3 | n/N (%) | 12/83 (14.5%) |
| ASA Physical Status | ||
| 1 | n/N (%) | 2/83 (2.4%) |
| 2 | n/N (%) | 64/83 (77.1%) |
| 3 | n/N (%) | 17/83 (20.5%) |
| BMI (kg/m2) | Median | 22.9 |
| IQR | (20.3; 26.3) | |
| Smoking | ||
| No | n/N (%) | 48/83 (57.8%) |
| Yes | n/N (%) | 21/83 (25.3%) |
| Ex | n/N (%) | 14/83 (16.9%) |
| Preoperative CD treatment: anti-TNF | ||
| No | n/N (%) | 38/83 (45.8%) |
| Yes | n/N (%) | 45/83 (54.2%) |
| Preoperative CD treatment: other advanced therapies | ||
| No | n/N (%) | 53/83 (63.9%) |
| Yes | n/N (%) | 30/83 (36.1%) |
| Variable . | Statistic . | All . |
|---|---|---|
| Sex | ||
| Male | n/N (%) | 43/83 (51.8%) |
| Female | n/N (%) | 40/83 (48.2%) |
| Age at CD diagnosis (years) | Median | 26.8 |
| IQR | (19.0; 37.1) | |
| Age at surgery (years) | Median | 33.0 |
| IQR | (25.0; 49.0) | |
| Years from CD diagnosis to surgery | Median | 5.0 |
| IQR | (0.8; 13.9) | |
| Montreal A (Age) | ||
| A1 | n/N (%) | 9/83 (10.8%) |
| A2 | n/N (%) | 58/83 (69.9%) |
| A3 | n/N (%) | 16/83 (19.3%) |
| Montreal B (Behavior) | ||
| B1 | n/N (%) | 7/83 (8.4%) |
| B2 | n/N (%) | 42/83 (50.6%) |
| B3 | n/N (%) | 34/83 (41.0%) |
| Concomitant perianal disease | ||
| No | n/N (%) | 72/83 (86.7%) |
| Yes | n/N (%) | 11/83 (13.2%) |
| Montreal L (Location) | ||
| L1 | n/N (%) | 70/83 (84.3%) |
| L2 | n/N (%) | 1/83 (1.2%) |
| L3 | n/N (%) | 12/83 (14.5%) |
| ASA Physical Status | ||
| 1 | n/N (%) | 2/83 (2.4%) |
| 2 | n/N (%) | 64/83 (77.1%) |
| 3 | n/N (%) | 17/83 (20.5%) |
| BMI (kg/m2) | Median | 22.9 |
| IQR | (20.3; 26.3) | |
| Smoking | ||
| No | n/N (%) | 48/83 (57.8%) |
| Yes | n/N (%) | 21/83 (25.3%) |
| Ex | n/N (%) | 14/83 (16.9%) |
| Preoperative CD treatment: anti-TNF | ||
| No | n/N (%) | 38/83 (45.8%) |
| Yes | n/N (%) | 45/83 (54.2%) |
| Preoperative CD treatment: other advanced therapies | ||
| No | n/N (%) | 53/83 (63.9%) |
| Yes | n/N (%) | 30/83 (36.1%) |
Abbreviations: CD, Crohn’s disease; BMI, Body mass index; TNF, Tumor necrosis factor.
Surgical details are reported in Table 2. Ileocecal resection was performed laparoscopically in 80 patients (96.4%). In 25 patients (30.1%), this was associated with other procedures, of which 15 (62.5%) were additional bowel resections and 4 (16.7%) were strictureplasties. Kono-S anastomosis was made in 39 patients (47.0%), side-to-side (S-S) isoperistaltic anastomosis in 43 patients (51.8%), and side-to-end (S-E) anastomosis in the remaining patient (1.2%). During follow-up, 7 patients refused to undergo colonoscopy. Assessment of endoscopic POR was therefore possible in 76 patients (91.6%).
| Variable . | Statistic . | All . |
|---|---|---|
| Surgical setting | ||
| Elective | n/N (%) | 73/83 (87.9%) |
| Urgent | n/N (%) | 10/83 (12.0%) |
| Surgical approach | ||
| Open | n/N (%) | 3/83 (3.6%) |
| Minimally invasive | n/N (%) | 80/83 (96.4%) |
| Associated procedures | ||
| No | n/N (%) | 58/83 (69.9%) |
| Yes | n/N (%) | 25/83 (30.1%) |
| Type of associated procedure | ||
| Additional bowel resection | n/N (%) | 15/24 (62.5%) |
| Strictureplasty | n/N (%) | 4/24 (16.7%) |
| Suturing interloop/sigmoid fistula | n/N (%) | 4/24 (16.%) |
| Other | n/N (%) | 1/24 (4.2%) |
| Type of anastomosis | ||
| Kono-S | n/N (%) | 39/83 (47.0%) |
| Side-to-Side | n/N (%) | 43/83 (51.8%) |
| Side-to-End | n/N (%) | 1/83 (1.2%) |
| Variable . | Statistic . | All . |
|---|---|---|
| Surgical setting | ||
| Elective | n/N (%) | 73/83 (87.9%) |
| Urgent | n/N (%) | 10/83 (12.0%) |
| Surgical approach | ||
| Open | n/N (%) | 3/83 (3.6%) |
| Minimally invasive | n/N (%) | 80/83 (96.4%) |
| Associated procedures | ||
| No | n/N (%) | 58/83 (69.9%) |
| Yes | n/N (%) | 25/83 (30.1%) |
| Type of associated procedure | ||
| Additional bowel resection | n/N (%) | 15/24 (62.5%) |
| Strictureplasty | n/N (%) | 4/24 (16.7%) |
| Suturing interloop/sigmoid fistula | n/N (%) | 4/24 (16.%) |
| Other | n/N (%) | 1/24 (4.2%) |
| Type of anastomosis | ||
| Kono-S | n/N (%) | 39/83 (47.0%) |
| Side-to-Side | n/N (%) | 43/83 (51.8%) |
| Side-to-End | n/N (%) | 1/83 (1.2%) |
| Variable . | Statistic . | All . |
|---|---|---|
| Surgical setting | ||
| Elective | n/N (%) | 73/83 (87.9%) |
| Urgent | n/N (%) | 10/83 (12.0%) |
| Surgical approach | ||
| Open | n/N (%) | 3/83 (3.6%) |
| Minimally invasive | n/N (%) | 80/83 (96.4%) |
| Associated procedures | ||
| No | n/N (%) | 58/83 (69.9%) |
| Yes | n/N (%) | 25/83 (30.1%) |
| Type of associated procedure | ||
| Additional bowel resection | n/N (%) | 15/24 (62.5%) |
| Strictureplasty | n/N (%) | 4/24 (16.7%) |
| Suturing interloop/sigmoid fistula | n/N (%) | 4/24 (16.%) |
| Other | n/N (%) | 1/24 (4.2%) |
| Type of anastomosis | ||
| Kono-S | n/N (%) | 39/83 (47.0%) |
| Side-to-Side | n/N (%) | 43/83 (51.8%) |
| Side-to-End | n/N (%) | 1/83 (1.2%) |
| Variable . | Statistic . | All . |
|---|---|---|
| Surgical setting | ||
| Elective | n/N (%) | 73/83 (87.9%) |
| Urgent | n/N (%) | 10/83 (12.0%) |
| Surgical approach | ||
| Open | n/N (%) | 3/83 (3.6%) |
| Minimally invasive | n/N (%) | 80/83 (96.4%) |
| Associated procedures | ||
| No | n/N (%) | 58/83 (69.9%) |
| Yes | n/N (%) | 25/83 (30.1%) |
| Type of associated procedure | ||
| Additional bowel resection | n/N (%) | 15/24 (62.5%) |
| Strictureplasty | n/N (%) | 4/24 (16.7%) |
| Suturing interloop/sigmoid fistula | n/N (%) | 4/24 (16.%) |
| Other | n/N (%) | 1/24 (4.2%) |
| Type of anastomosis | ||
| Kono-S | n/N (%) | 39/83 (47.0%) |
| Side-to-Side | n/N (%) | 43/83 (51.8%) |
| Side-to-End | n/N (%) | 1/83 (1.2%) |
Macroscopic Intraoperative Findings
Median length of resected bowel (terminal ileum) was 21 cm (IQR, 15-32). Thickness of the mesentery was <5mm in 27 patients (32.5%), 5-10mm in 36 patients (43.4%), and >10mm in 20 patients (24.1%). Serosal fat infiltration and abnormal serosal vasodilation proximal to the resected bowel were observed in 44 (53.0%) and in 41 patients (49.4%), respectively. Agreement between observer 1 and observer 2 was 0.71 (95% CI, 0.57-0.84) for the thickness of the mesentery, 0.92 (95% CI, 0.84-1.00) for serosal fat infiltration, and 0.87 (95% CI, 0.77-0.98) for abnormal serosal vasodilation proximal to the resected segment of ileum. Intraoperative scoring was performed independently by 2 colorectal surgeons in 79 of 83 patients. In the remaining 4 patients, double and independent scoring were not performed due to the unavailability of the second surgeon during ICR (Table 3). Endoluminal assessment and full thickness biopsies taken on normal intestine located proximal to the macroscopic-affected ileum where serosal fat infiltration and an abnormal serosal vasodilation were observed revealed a normal mucosa and no histological signs of chronic inflammation related to CD (Figure 1, Supplementary Figure 1).
| . | . | Observer 1 . | Observer 2 . | Kappa Statistics (95% CI,) . |
|---|---|---|---|---|
| Length of resected bowel (cm) | Median | 21.00 | 21.00 | - |
| IQR | (15.00; 32.00) | (15.00; 32.00) | ||
| Thickness of the mesentery | 0.71 (0.57-0.84) | |||
| <5mm | n/N (%) | 27/83 (32.53%) | 21/79 (26.58%) | |
| 5-10mm | n/N (%) | 36/83 (43.37%) | 38/79 (48.10%) | |
| >10mm | n/N (%) | 20/83 (24.10%) | 20/79 (25.32%) | |
| Serosal fat infiltration proximal to resected bowel | 0.92 (0.84-1.00) | |||
| No | n/N (%) | 39/83 (46.99%) | 36/79 (45.57%) | |
| Yes | n/N (%) | 44/83 (53.01%) | 43/79 (54.43%) | |
| Abnormal serosal vasodilation proximal to resected bowel | 0.87 (0.77-0.98) | |||
| No | n/N (%) | 42/83 (50.60%) | 38/79 (48.10%) | |
| Yes | n/N (%) | 41/83 (49.40%) | 41/79 (51.90%) |
| . | . | Observer 1 . | Observer 2 . | Kappa Statistics (95% CI,) . |
|---|---|---|---|---|
| Length of resected bowel (cm) | Median | 21.00 | 21.00 | - |
| IQR | (15.00; 32.00) | (15.00; 32.00) | ||
| Thickness of the mesentery | 0.71 (0.57-0.84) | |||
| <5mm | n/N (%) | 27/83 (32.53%) | 21/79 (26.58%) | |
| 5-10mm | n/N (%) | 36/83 (43.37%) | 38/79 (48.10%) | |
| >10mm | n/N (%) | 20/83 (24.10%) | 20/79 (25.32%) | |
| Serosal fat infiltration proximal to resected bowel | 0.92 (0.84-1.00) | |||
| No | n/N (%) | 39/83 (46.99%) | 36/79 (45.57%) | |
| Yes | n/N (%) | 44/83 (53.01%) | 43/79 (54.43%) | |
| Abnormal serosal vasodilation proximal to resected bowel | 0.87 (0.77-0.98) | |||
| No | n/N (%) | 42/83 (50.60%) | 38/79 (48.10%) | |
| Yes | n/N (%) | 41/83 (49.40%) | 41/79 (51.90%) |
Abbreviation: CI, Confident Interval.
| . | . | Observer 1 . | Observer 2 . | Kappa Statistics (95% CI,) . |
|---|---|---|---|---|
| Length of resected bowel (cm) | Median | 21.00 | 21.00 | - |
| IQR | (15.00; 32.00) | (15.00; 32.00) | ||
| Thickness of the mesentery | 0.71 (0.57-0.84) | |||
| <5mm | n/N (%) | 27/83 (32.53%) | 21/79 (26.58%) | |
| 5-10mm | n/N (%) | 36/83 (43.37%) | 38/79 (48.10%) | |
| >10mm | n/N (%) | 20/83 (24.10%) | 20/79 (25.32%) | |
| Serosal fat infiltration proximal to resected bowel | 0.92 (0.84-1.00) | |||
| No | n/N (%) | 39/83 (46.99%) | 36/79 (45.57%) | |
| Yes | n/N (%) | 44/83 (53.01%) | 43/79 (54.43%) | |
| Abnormal serosal vasodilation proximal to resected bowel | 0.87 (0.77-0.98) | |||
| No | n/N (%) | 42/83 (50.60%) | 38/79 (48.10%) | |
| Yes | n/N (%) | 41/83 (49.40%) | 41/79 (51.90%) |
| . | . | Observer 1 . | Observer 2 . | Kappa Statistics (95% CI,) . |
|---|---|---|---|---|
| Length of resected bowel (cm) | Median | 21.00 | 21.00 | - |
| IQR | (15.00; 32.00) | (15.00; 32.00) | ||
| Thickness of the mesentery | 0.71 (0.57-0.84) | |||
| <5mm | n/N (%) | 27/83 (32.53%) | 21/79 (26.58%) | |
| 5-10mm | n/N (%) | 36/83 (43.37%) | 38/79 (48.10%) | |
| >10mm | n/N (%) | 20/83 (24.10%) | 20/79 (25.32%) | |
| Serosal fat infiltration proximal to resected bowel | 0.92 (0.84-1.00) | |||
| No | n/N (%) | 39/83 (46.99%) | 36/79 (45.57%) | |
| Yes | n/N (%) | 44/83 (53.01%) | 43/79 (54.43%) | |
| Abnormal serosal vasodilation proximal to resected bowel | 0.87 (0.77-0.98) | |||
| No | n/N (%) | 42/83 (50.60%) | 38/79 (48.10%) | |
| Yes | n/N (%) | 41/83 (49.40%) | 41/79 (51.90%) |
Abbreviation: CI, Confident Interval.
No association between any of the investigated intraoperative macroscopic findings with disease phenotype or duration of the disease (interval between date of diagnosis of CD and date of surgery) was observed. Interestingly, a significant association between body mass index (BMI) and the presence of serosal fat infiltration on normal bowel proximal to the resected ileum and thickness of the mesentery was observed. Patients with higher BMI had therefore a significant tendence to develop serosal fat infiltration (P = .042) and to have a thicker mesentery (P = .005). No relation between BMI and length of resected bowel (P = .143) or abnormal serosal vasodilation (P = .238) was found (Supplementary Table 1).
Surgical Outcomes and Postoperative Recurrence
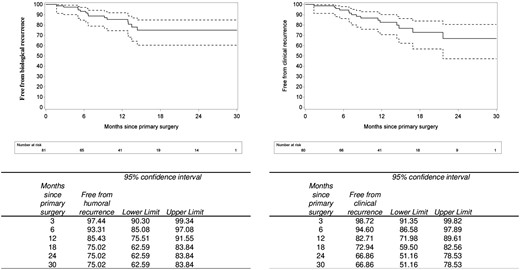
Postoperative outcomes are reported in Table 4. Anastomotic leakage occurred in 5 patients (6.0%). A positive proximal (ileal) microscopic resection margin was observed in 21 patients (25.3%) and a distal (colonic) positive margin in 7 patients (8.4%). Immediate prophylactic medical treatment was initiated or restarted in 37 patients (44.6%). Early endoscopic recurrence (mRS ≥i2b) was observed in 45 out of 76 patients (59.2%). During a median follow-up of 13.4 months, biological recurrence occurred in 14 patients (17.2%) and clinical recurrence in 15 patients (18.7%), respectively (Figure 2). One patient needed new surgery due CD recurrence at the level of anastomosis (surgical recurrence).
| Variable . | Statistic . | All . |
|---|---|---|
| Microscopic resection margin | ||
| Both Negative | n/N (%) | 50/83 (60.2%) |
| Ileal+ | n/N (%) | 18/83 (21.7%) |
| Colonic+ | n/N (%) | 4/83 (4.8%) |
| Both+ | n/N (%) | 3/83 (3.6%) |
| Not reported | n/N (%) | 8/83 (9.6%) |
| Endoscopic recurrence (Modified Rutgeerts score) | ||
| i0 | n/N (%) | 8/76 (10.5%) |
| i1 | n/N (%) | 10/76 (13.2%) |
| i2a | n/N (%) | 13/76 (17.1%) |
| i2b | n/N (%) | 31/76 (40.8%) |
| i3 | n/N (%) | 9/76 (11.8%) |
| i4 | n/N (%) | 5/76 (6.6%) |
| Endoscopic Recurrence (i ≥2b) | ||
| No | n/N (%) | 31/76 (40.8%) |
| Yes | n/N (%) | 45/76 (59.2%) |
| Length of hospital stay (days) | Median | 7.0 |
| IQR | (5.0; 8.0) | |
| Anastomotic leak (<30 days) | ||
| No | n/N (%) | 78/83 (94.0%) |
| Yes | n/N (%) | 5/83 (6.0%) |
| Clavien-Dindo classification | ||
| 0 | n/N (%) | 30/83 (36.1%) |
| 1 | n/N (%) | 5/83 (6.0%) |
| 2 | n/N (%) | 39/83 (47.0%) |
| 3 | n/N (%) | 2/83 (2.4%) |
| 4 | n/N (%) | 7/83 (8.4%) |
| Blood transfusion | ||
| No | n/N (%) | 79/83 (95.2%) |
| Yes | n/N (%) | 4/83 (4.8%) |
| Early post-operative prophylactic treatment | ||
| No | n/N (%) | 46/83 (55.4%) |
| Yes | n/N (%) | 37/83 (44.6%) |
| Type of early post-operative prophylactic treatment | ||
| anti-TNF | n/N (%) | 11/37 (29.7%) |
| anti-TNF + azathioprine | n/N (%) | 3/37 (8.1%) |
| Vedolizumab | n/N (%) | 6/37 (16.2%) |
| Ustekinumab | n/N (%) | 9/37 (24.3%) |
| Other | n/N (%) | 3/37 (8.1%) |
| Budesonide | n/N (%) | 5/37 (13.5%) |
| Variable . | Statistic . | All . |
|---|---|---|
| Microscopic resection margin | ||
| Both Negative | n/N (%) | 50/83 (60.2%) |
| Ileal+ | n/N (%) | 18/83 (21.7%) |
| Colonic+ | n/N (%) | 4/83 (4.8%) |
| Both+ | n/N (%) | 3/83 (3.6%) |
| Not reported | n/N (%) | 8/83 (9.6%) |
| Endoscopic recurrence (Modified Rutgeerts score) | ||
| i0 | n/N (%) | 8/76 (10.5%) |
| i1 | n/N (%) | 10/76 (13.2%) |
| i2a | n/N (%) | 13/76 (17.1%) |
| i2b | n/N (%) | 31/76 (40.8%) |
| i3 | n/N (%) | 9/76 (11.8%) |
| i4 | n/N (%) | 5/76 (6.6%) |
| Endoscopic Recurrence (i ≥2b) | ||
| No | n/N (%) | 31/76 (40.8%) |
| Yes | n/N (%) | 45/76 (59.2%) |
| Length of hospital stay (days) | Median | 7.0 |
| IQR | (5.0; 8.0) | |
| Anastomotic leak (<30 days) | ||
| No | n/N (%) | 78/83 (94.0%) |
| Yes | n/N (%) | 5/83 (6.0%) |
| Clavien-Dindo classification | ||
| 0 | n/N (%) | 30/83 (36.1%) |
| 1 | n/N (%) | 5/83 (6.0%) |
| 2 | n/N (%) | 39/83 (47.0%) |
| 3 | n/N (%) | 2/83 (2.4%) |
| 4 | n/N (%) | 7/83 (8.4%) |
| Blood transfusion | ||
| No | n/N (%) | 79/83 (95.2%) |
| Yes | n/N (%) | 4/83 (4.8%) |
| Early post-operative prophylactic treatment | ||
| No | n/N (%) | 46/83 (55.4%) |
| Yes | n/N (%) | 37/83 (44.6%) |
| Type of early post-operative prophylactic treatment | ||
| anti-TNF | n/N (%) | 11/37 (29.7%) |
| anti-TNF + azathioprine | n/N (%) | 3/37 (8.1%) |
| Vedolizumab | n/N (%) | 6/37 (16.2%) |
| Ustekinumab | n/N (%) | 9/37 (24.3%) |
| Other | n/N (%) | 3/37 (8.1%) |
| Budesonide | n/N (%) | 5/37 (13.5%) |
Abbreviation: TNF, Tumor necrosis factor.
| Variable . | Statistic . | All . |
|---|---|---|
| Microscopic resection margin | ||
| Both Negative | n/N (%) | 50/83 (60.2%) |
| Ileal+ | n/N (%) | 18/83 (21.7%) |
| Colonic+ | n/N (%) | 4/83 (4.8%) |
| Both+ | n/N (%) | 3/83 (3.6%) |
| Not reported | n/N (%) | 8/83 (9.6%) |
| Endoscopic recurrence (Modified Rutgeerts score) | ||
| i0 | n/N (%) | 8/76 (10.5%) |
| i1 | n/N (%) | 10/76 (13.2%) |
| i2a | n/N (%) | 13/76 (17.1%) |
| i2b | n/N (%) | 31/76 (40.8%) |
| i3 | n/N (%) | 9/76 (11.8%) |
| i4 | n/N (%) | 5/76 (6.6%) |
| Endoscopic Recurrence (i ≥2b) | ||
| No | n/N (%) | 31/76 (40.8%) |
| Yes | n/N (%) | 45/76 (59.2%) |
| Length of hospital stay (days) | Median | 7.0 |
| IQR | (5.0; 8.0) | |
| Anastomotic leak (<30 days) | ||
| No | n/N (%) | 78/83 (94.0%) |
| Yes | n/N (%) | 5/83 (6.0%) |
| Clavien-Dindo classification | ||
| 0 | n/N (%) | 30/83 (36.1%) |
| 1 | n/N (%) | 5/83 (6.0%) |
| 2 | n/N (%) | 39/83 (47.0%) |
| 3 | n/N (%) | 2/83 (2.4%) |
| 4 | n/N (%) | 7/83 (8.4%) |
| Blood transfusion | ||
| No | n/N (%) | 79/83 (95.2%) |
| Yes | n/N (%) | 4/83 (4.8%) |
| Early post-operative prophylactic treatment | ||
| No | n/N (%) | 46/83 (55.4%) |
| Yes | n/N (%) | 37/83 (44.6%) |
| Type of early post-operative prophylactic treatment | ||
| anti-TNF | n/N (%) | 11/37 (29.7%) |
| anti-TNF + azathioprine | n/N (%) | 3/37 (8.1%) |
| Vedolizumab | n/N (%) | 6/37 (16.2%) |
| Ustekinumab | n/N (%) | 9/37 (24.3%) |
| Other | n/N (%) | 3/37 (8.1%) |
| Budesonide | n/N (%) | 5/37 (13.5%) |
| Variable . | Statistic . | All . |
|---|---|---|
| Microscopic resection margin | ||
| Both Negative | n/N (%) | 50/83 (60.2%) |
| Ileal+ | n/N (%) | 18/83 (21.7%) |
| Colonic+ | n/N (%) | 4/83 (4.8%) |
| Both+ | n/N (%) | 3/83 (3.6%) |
| Not reported | n/N (%) | 8/83 (9.6%) |
| Endoscopic recurrence (Modified Rutgeerts score) | ||
| i0 | n/N (%) | 8/76 (10.5%) |
| i1 | n/N (%) | 10/76 (13.2%) |
| i2a | n/N (%) | 13/76 (17.1%) |
| i2b | n/N (%) | 31/76 (40.8%) |
| i3 | n/N (%) | 9/76 (11.8%) |
| i4 | n/N (%) | 5/76 (6.6%) |
| Endoscopic Recurrence (i ≥2b) | ||
| No | n/N (%) | 31/76 (40.8%) |
| Yes | n/N (%) | 45/76 (59.2%) |
| Length of hospital stay (days) | Median | 7.0 |
| IQR | (5.0; 8.0) | |
| Anastomotic leak (<30 days) | ||
| No | n/N (%) | 78/83 (94.0%) |
| Yes | n/N (%) | 5/83 (6.0%) |
| Clavien-Dindo classification | ||
| 0 | n/N (%) | 30/83 (36.1%) |
| 1 | n/N (%) | 5/83 (6.0%) |
| 2 | n/N (%) | 39/83 (47.0%) |
| 3 | n/N (%) | 2/83 (2.4%) |
| 4 | n/N (%) | 7/83 (8.4%) |
| Blood transfusion | ||
| No | n/N (%) | 79/83 (95.2%) |
| Yes | n/N (%) | 4/83 (4.8%) |
| Early post-operative prophylactic treatment | ||
| No | n/N (%) | 46/83 (55.4%) |
| Yes | n/N (%) | 37/83 (44.6%) |
| Type of early post-operative prophylactic treatment | ||
| anti-TNF | n/N (%) | 11/37 (29.7%) |
| anti-TNF + azathioprine | n/N (%) | 3/37 (8.1%) |
| Vedolizumab | n/N (%) | 6/37 (16.2%) |
| Ustekinumab | n/N (%) | 9/37 (24.3%) |
| Other | n/N (%) | 3/37 (8.1%) |
| Budesonide | n/N (%) | 5/37 (13.5%) |
Abbreviation: TNF, Tumor necrosis factor.
Relation Between Macroscopic Intraoperative Findings and Postoperative Recurrence
At univariable analysis, none of the investigated macroscopic intraoperative findings or other considered patient- or disease-related factors showed association with endoscopic, clinical (Table 5), or biological recurrence (Supplementary Table 2). In particular, the risk of developing endoscopic POR when serosal fat infiltration and abnormal serosal vasodilatation proximal to the resected bowel were present was 1.12 (95% CI, 0.45-2.83, P = .80) and 1.39 (95% CI, 0.55-3.48, P = .48), respectively. Hazard ratios of clinical recurrence were 0.90 (95% CI, 0.32-2.48, P = .83) and 1.153 (95% CI, 0.42-3.19, P = .78) in presence of serosal fat infiltration and abnormal serosal vasodilation, respectively. Hazard ratios of biochemical recurrence were 1.53 (95% CI, 0.51-4.59, P = .44) and 0.86 (95% CI, 0.30-2.49, P = .78) in the presence of serosal fat infiltration and abnormal serosal vasodilation, respectively. Similarly, a subgroup analysis performed on those patients who did not receive any immediate postoperative medical prophylaxis did not identify any risk factor for early endoscopic POR (Supplementary Table 3).
| . | Endoscopic recurrencea . | Clinical recurrenceb . | |||
|---|---|---|---|---|---|
| Odds ratio (95%CI) . | P . | Hazard ratio . | P . | ||
| Length of resected bowel segment | (cm) | 1.02 (0.99;1.04) | .203 | 1.00 (0.98;1.03) | .905 |
| Thickness of the mesentery | .923 | .071 | |||
| 5-10 mm | 0.98 (0.30;3.18) | .972 | 6.32 (0.81;49.43) | .079 | |
| <5 mm | 0.81 (0.24;2.78) | .738 | 3.11 (0.35;27.93) | .310 | |
| >10 mm | - | - | |||
| Serosal fat infiltration proximal to resected bowel | |||||
| Yes | 1.13 (0.45;2.83) | .799 | 0.90 (0.32;2.45) | .833 | |
| No | - | - | |||
| Abnormal serosal vasodilation proximal to resected bowel | |||||
| Yes | 1.38 (0.55;3.48) | .484 | 1.15 (0.42;3.19) | .784 | |
| No | - | - | |||
| Montreal (Behaviour) | .661 | .026 | |||
| B1 | 1.17 (0.22;6.13) | .861 | 2.13 (0.19;23.58) | .537 | |
| B2 | 1.56 (0.59;4.12) | .367 | 5.542 (1.24;24.84) | .025 | |
| B3 | - | - | |||
| Concomitant peri-anal disease | |||||
| Yes | 2.02 (0.49;8.31) | .331 | 1.86 (0.52;6.61) | .340 | |
| No | - | - | |||
| Smoking | .823 | .286 | |||
| Ex | 1.06 (0.29;3.88) | .925 | 0.97 (0.20;4.67) | .969 | |
| Yes | 1.41 (0.47;4.22) | .538 | 2.37 (0.79;7.09) | .122 | |
| No | - | - | |||
| BMI (Kg/m2) | 1.01 (0.92;1.11) | .892 | 0.99 (0.88;1.12) | .930 | |
| Type of anastomosis | SS | 1.03 (0.41;2.58) | .955 | 1.29 (0.45;3.74) | .634 |
| Kono-S | |||||
| Years from diagnosis to surgery | 0.99 (0.95;1.04) | .681 | 1.05 (1.00;1.10) | .031 | |
| >2 years | 0.86 (0.33;2.27) | .765 | 8.12 (1.07;61.89) | .043 | |
| <2 years | - | - | |||
| Microscopic resection margins | .654 | .380 | |||
| At least one positive | 1.06 (0.37;3.05) | .907 | 2.01 (0.69;5.83) | .197 | |
| Not reported | 0.491 (0.10;2.45) | .385 | 0.72 (0.09;5.81) | .761 | |
| Both Negative | - | - | |||
| Prophylactic medical treatment | |||||
| Yes | 0.33 (0.13;0.85) | .021 | 0.63 (0.21;1.85) | .399 | |
| No | - | - | |||
| . | Endoscopic recurrencea . | Clinical recurrenceb . | |||
|---|---|---|---|---|---|
| Odds ratio (95%CI) . | P . | Hazard ratio . | P . | ||
| Length of resected bowel segment | (cm) | 1.02 (0.99;1.04) | .203 | 1.00 (0.98;1.03) | .905 |
| Thickness of the mesentery | .923 | .071 | |||
| 5-10 mm | 0.98 (0.30;3.18) | .972 | 6.32 (0.81;49.43) | .079 | |
| <5 mm | 0.81 (0.24;2.78) | .738 | 3.11 (0.35;27.93) | .310 | |
| >10 mm | - | - | |||
| Serosal fat infiltration proximal to resected bowel | |||||
| Yes | 1.13 (0.45;2.83) | .799 | 0.90 (0.32;2.45) | .833 | |
| No | - | - | |||
| Abnormal serosal vasodilation proximal to resected bowel | |||||
| Yes | 1.38 (0.55;3.48) | .484 | 1.15 (0.42;3.19) | .784 | |
| No | - | - | |||
| Montreal (Behaviour) | .661 | .026 | |||
| B1 | 1.17 (0.22;6.13) | .861 | 2.13 (0.19;23.58) | .537 | |
| B2 | 1.56 (0.59;4.12) | .367 | 5.542 (1.24;24.84) | .025 | |
| B3 | - | - | |||
| Concomitant peri-anal disease | |||||
| Yes | 2.02 (0.49;8.31) | .331 | 1.86 (0.52;6.61) | .340 | |
| No | - | - | |||
| Smoking | .823 | .286 | |||
| Ex | 1.06 (0.29;3.88) | .925 | 0.97 (0.20;4.67) | .969 | |
| Yes | 1.41 (0.47;4.22) | .538 | 2.37 (0.79;7.09) | .122 | |
| No | - | - | |||
| BMI (Kg/m2) | 1.01 (0.92;1.11) | .892 | 0.99 (0.88;1.12) | .930 | |
| Type of anastomosis | SS | 1.03 (0.41;2.58) | .955 | 1.29 (0.45;3.74) | .634 |
| Kono-S | |||||
| Years from diagnosis to surgery | 0.99 (0.95;1.04) | .681 | 1.05 (1.00;1.10) | .031 | |
| >2 years | 0.86 (0.33;2.27) | .765 | 8.12 (1.07;61.89) | .043 | |
| <2 years | - | - | |||
| Microscopic resection margins | .654 | .380 | |||
| At least one positive | 1.06 (0.37;3.05) | .907 | 2.01 (0.69;5.83) | .197 | |
| Not reported | 0.491 (0.10;2.45) | .385 | 0.72 (0.09;5.81) | .761 | |
| Both Negative | - | - | |||
| Prophylactic medical treatment | |||||
| Yes | 0.33 (0.13;0.85) | .021 | 0.63 (0.21;1.85) | .399 | |
| No | - | - | |||
Abbreviations: BMI, Body Mass Index; SS: side-to-side. - sign means “blanco”. The analysis is reported a row higher.
aUnivariable logistic regression.
bUnivariable Cox regression.
| . | Endoscopic recurrencea . | Clinical recurrenceb . | |||
|---|---|---|---|---|---|
| Odds ratio (95%CI) . | P . | Hazard ratio . | P . | ||
| Length of resected bowel segment | (cm) | 1.02 (0.99;1.04) | .203 | 1.00 (0.98;1.03) | .905 |
| Thickness of the mesentery | .923 | .071 | |||
| 5-10 mm | 0.98 (0.30;3.18) | .972 | 6.32 (0.81;49.43) | .079 | |
| <5 mm | 0.81 (0.24;2.78) | .738 | 3.11 (0.35;27.93) | .310 | |
| >10 mm | - | - | |||
| Serosal fat infiltration proximal to resected bowel | |||||
| Yes | 1.13 (0.45;2.83) | .799 | 0.90 (0.32;2.45) | .833 | |
| No | - | - | |||
| Abnormal serosal vasodilation proximal to resected bowel | |||||
| Yes | 1.38 (0.55;3.48) | .484 | 1.15 (0.42;3.19) | .784 | |
| No | - | - | |||
| Montreal (Behaviour) | .661 | .026 | |||
| B1 | 1.17 (0.22;6.13) | .861 | 2.13 (0.19;23.58) | .537 | |
| B2 | 1.56 (0.59;4.12) | .367 | 5.542 (1.24;24.84) | .025 | |
| B3 | - | - | |||
| Concomitant peri-anal disease | |||||
| Yes | 2.02 (0.49;8.31) | .331 | 1.86 (0.52;6.61) | .340 | |
| No | - | - | |||
| Smoking | .823 | .286 | |||
| Ex | 1.06 (0.29;3.88) | .925 | 0.97 (0.20;4.67) | .969 | |
| Yes | 1.41 (0.47;4.22) | .538 | 2.37 (0.79;7.09) | .122 | |
| No | - | - | |||
| BMI (Kg/m2) | 1.01 (0.92;1.11) | .892 | 0.99 (0.88;1.12) | .930 | |
| Type of anastomosis | SS | 1.03 (0.41;2.58) | .955 | 1.29 (0.45;3.74) | .634 |
| Kono-S | |||||
| Years from diagnosis to surgery | 0.99 (0.95;1.04) | .681 | 1.05 (1.00;1.10) | .031 | |
| >2 years | 0.86 (0.33;2.27) | .765 | 8.12 (1.07;61.89) | .043 | |
| <2 years | - | - | |||
| Microscopic resection margins | .654 | .380 | |||
| At least one positive | 1.06 (0.37;3.05) | .907 | 2.01 (0.69;5.83) | .197 | |
| Not reported | 0.491 (0.10;2.45) | .385 | 0.72 (0.09;5.81) | .761 | |
| Both Negative | - | - | |||
| Prophylactic medical treatment | |||||
| Yes | 0.33 (0.13;0.85) | .021 | 0.63 (0.21;1.85) | .399 | |
| No | - | - | |||
| . | Endoscopic recurrencea . | Clinical recurrenceb . | |||
|---|---|---|---|---|---|
| Odds ratio (95%CI) . | P . | Hazard ratio . | P . | ||
| Length of resected bowel segment | (cm) | 1.02 (0.99;1.04) | .203 | 1.00 (0.98;1.03) | .905 |
| Thickness of the mesentery | .923 | .071 | |||
| 5-10 mm | 0.98 (0.30;3.18) | .972 | 6.32 (0.81;49.43) | .079 | |
| <5 mm | 0.81 (0.24;2.78) | .738 | 3.11 (0.35;27.93) | .310 | |
| >10 mm | - | - | |||
| Serosal fat infiltration proximal to resected bowel | |||||
| Yes | 1.13 (0.45;2.83) | .799 | 0.90 (0.32;2.45) | .833 | |
| No | - | - | |||
| Abnormal serosal vasodilation proximal to resected bowel | |||||
| Yes | 1.38 (0.55;3.48) | .484 | 1.15 (0.42;3.19) | .784 | |
| No | - | - | |||
| Montreal (Behaviour) | .661 | .026 | |||
| B1 | 1.17 (0.22;6.13) | .861 | 2.13 (0.19;23.58) | .537 | |
| B2 | 1.56 (0.59;4.12) | .367 | 5.542 (1.24;24.84) | .025 | |
| B3 | - | - | |||
| Concomitant peri-anal disease | |||||
| Yes | 2.02 (0.49;8.31) | .331 | 1.86 (0.52;6.61) | .340 | |
| No | - | - | |||
| Smoking | .823 | .286 | |||
| Ex | 1.06 (0.29;3.88) | .925 | 0.97 (0.20;4.67) | .969 | |
| Yes | 1.41 (0.47;4.22) | .538 | 2.37 (0.79;7.09) | .122 | |
| No | - | - | |||
| BMI (Kg/m2) | 1.01 (0.92;1.11) | .892 | 0.99 (0.88;1.12) | .930 | |
| Type of anastomosis | SS | 1.03 (0.41;2.58) | .955 | 1.29 (0.45;3.74) | .634 |
| Kono-S | |||||
| Years from diagnosis to surgery | 0.99 (0.95;1.04) | .681 | 1.05 (1.00;1.10) | .031 | |
| >2 years | 0.86 (0.33;2.27) | .765 | 8.12 (1.07;61.89) | .043 | |
| <2 years | - | - | |||
| Microscopic resection margins | .654 | .380 | |||
| At least one positive | 1.06 (0.37;3.05) | .907 | 2.01 (0.69;5.83) | .197 | |
| Not reported | 0.491 (0.10;2.45) | .385 | 0.72 (0.09;5.81) | .761 | |
| Both Negative | - | - | |||
| Prophylactic medical treatment | |||||
| Yes | 0.33 (0.13;0.85) | .021 | 0.63 (0.21;1.85) | .399 | |
| No | - | - | |||
Abbreviations: BMI, Body Mass Index; SS: side-to-side. - sign means “blanco”. The analysis is reported a row higher.
aUnivariable logistic regression.
bUnivariable Cox regression.
No association between type of ileocolic anastomosis (side-to-side vs Kono-S) with endoscopic and clinical POR was observed (P = .634). Longer disease duration was associated to a higher rate of clinical recurrence (hazard ratio [HR], 1.05; 95% CI, 1.00-1.10; P = .031). In particular, patients with a disease duration longer than 2 years (n = 15) had a risk of developing clinical POR 8.12 higher (95% CI, 1.07-61.89, P = .043) than patients with disease duration <2 years.
After multivariable analysis limited to the 4 investigated intraoperative factors, no association with endoscopic POR was observed. When applying forward selection using P < .10 as threshold for the multivariable analysis, only postoperative prophylactic treatment resulted significant for endoscopic POR (Supplementary Table 4). When the same was done for clinical POR, only the disease phenotype showed association with recurrence, as observed after univariable analysis (Supplementary Table 5).
When looking at the different modified Rutgeerts scores, no trend was observed between higher scores and the presence of serosal fat infiltration and abnormal serosal vasodilation proximal to the macroscopic-affected and resected bowel (Table 6).
Presence of serosal fat infiltration and abnormal serosal vasodilation according to modified Rutgeerts Score.
| Modified Rutgeerts Score . | i0 8/76 (10.5%) . | i1 10/76 (13.2%) . | i2a 13/76 (17.1%) . | i2b 31/76 (40.8%) . | i3 9/76 (11.8%) . | i4 5/76 (6.6%) . |
|---|---|---|---|---|---|---|
| Areas of serosal fat infiltration 43/76 (56.6%) | 4/8 (50.0%) | 5/10 (50.0%) | 8/13 (61.5%) | 20/31 (64.5%) | 4/9 (44.4%) | 2/5 (40.0%) |
| Areas of abnormal serosal vasodilation 38/76 (50.0%) | 3/8 (37.5%) | 4/10 (40.4%) | 7/13 (53.9%) | 17/31 (54.8%) | 3/9 (33.3%) | 4/5 (80.0%) |
| Modified Rutgeerts Score . | i0 8/76 (10.5%) . | i1 10/76 (13.2%) . | i2a 13/76 (17.1%) . | i2b 31/76 (40.8%) . | i3 9/76 (11.8%) . | i4 5/76 (6.6%) . |
|---|---|---|---|---|---|---|
| Areas of serosal fat infiltration 43/76 (56.6%) | 4/8 (50.0%) | 5/10 (50.0%) | 8/13 (61.5%) | 20/31 (64.5%) | 4/9 (44.4%) | 2/5 (40.0%) |
| Areas of abnormal serosal vasodilation 38/76 (50.0%) | 3/8 (37.5%) | 4/10 (40.4%) | 7/13 (53.9%) | 17/31 (54.8%) | 3/9 (33.3%) | 4/5 (80.0%) |
Presence of serosal fat infiltration and abnormal serosal vasodilation according to modified Rutgeerts Score.
| Modified Rutgeerts Score . | i0 8/76 (10.5%) . | i1 10/76 (13.2%) . | i2a 13/76 (17.1%) . | i2b 31/76 (40.8%) . | i3 9/76 (11.8%) . | i4 5/76 (6.6%) . |
|---|---|---|---|---|---|---|
| Areas of serosal fat infiltration 43/76 (56.6%) | 4/8 (50.0%) | 5/10 (50.0%) | 8/13 (61.5%) | 20/31 (64.5%) | 4/9 (44.4%) | 2/5 (40.0%) |
| Areas of abnormal serosal vasodilation 38/76 (50.0%) | 3/8 (37.5%) | 4/10 (40.4%) | 7/13 (53.9%) | 17/31 (54.8%) | 3/9 (33.3%) | 4/5 (80.0%) |
| Modified Rutgeerts Score . | i0 8/76 (10.5%) . | i1 10/76 (13.2%) . | i2a 13/76 (17.1%) . | i2b 31/76 (40.8%) . | i3 9/76 (11.8%) . | i4 5/76 (6.6%) . |
|---|---|---|---|---|---|---|
| Areas of serosal fat infiltration 43/76 (56.6%) | 4/8 (50.0%) | 5/10 (50.0%) | 8/13 (61.5%) | 20/31 (64.5%) | 4/9 (44.4%) | 2/5 (40.0%) |
| Areas of abnormal serosal vasodilation 38/76 (50.0%) | 3/8 (37.5%) | 4/10 (40.4%) | 7/13 (53.9%) | 17/31 (54.8%) | 3/9 (33.3%) | 4/5 (80.0%) |
Discussion
Debate about the optimal postoperative management to prevent POR in CD patients undergoing ileocolic resection is still ongoing, as reflected by different recommendations on the use of prophylactic medical therapy in international guidelines.16–18 Different strategies can be adopted: systematic initiation of immunosuppressive therapy in the immediate postoperative phase (systematic postoperative prophylactic therapy)19 or initiation of the medical therapy after assessment of endoscopic POR 6 to 12 months after surgery (endoscopy-driven postoperative prophylactic therapy).20 On this point, clinical risk stratification could appropriately select patients who might benefit from immediate prophylactic therapy in the attempt to limit disease progression and irreversible bowel damage, avoiding at the same time overtreatment of those other patients who reach stable clinical remission after surgery.4 Recently, algorithms have been proposed integrating risk stratification for immediate prophylactic treatment after surgery and endoscopy at 6 months for further treatment escalation.11,21
Several risk factors for POR have already been identified including active smoking,22 age 30 years and younger,23 concomitant perianal disease,24 prior intestinal resections,25 disease behavior,26 microscopic resection margin positivity,27 and granulomas/myoenteric plexitis in the resection specimen.27,28 However, the relative contribution of each to the risk of POR is unclear,29 and except for smoking, none of them has been prospectively validated. This results in discrepancy in the definition of patients at high risk in the current international guidelines.16,18 From a surgical perspective, the focus has been mainly set on the role of the surgical technique in influencing POR. Configuration and technique of the ileocolic anastomosis and type of mesenteric dissection have been investigated, and results of prospective randomized trials are currently awaited.30
The macroscopic appearance of the bowel and the associated mesentery in CD can vary exceptionally from patient to patient, even in absence of complications such as stenoses, abscesses, and fistulas. This is particularly true for the amount of creeping fat surrounding the diseased bowel, the thickness of the mesentery, and the length of the bowel macroscopically involved by the disease.
So far, the possible association between these macroscopic intraoperative findings and POR have been poorly investigated. Literature provides conflicting data on the association between length of resected bowel and recurrence. Although in several studies the length of bowel resected was not associated to POR,31 a retrospective historical study on 23 patients showed that the length of intestinal resection was correlated with a shorter interval to POR and with the extent of recurrent disease.32 Similarly, a retrospective large study on more than 900 patients observed a significant higher relative risk for POR in those patients undergoing extensive resections.33 In the present study, the limited median length of resected bowel and the fact that only primary ileocecal resections were included were probably responsible for the lack of association with POR. At partial confirmation of this, only extensive resections of more than 50 cm are currently recognized by the European Crohn’s and Colitis Organization (ECCO) as risk factor of POR.16 Similarly, the results of the present study failed to show any association between mesentery thickness and POR. Although the amount of mesenteric fat has already been correlated with the severity of local inflammation, no direct association with POR has been demonstrated so far in the published literature.34 On the other hand, a satellite study of the POCER trial and another retrospective study indicated that the total amount of visceral adipose tissue could have a possible influence on POR.35,36
In CD, the bowel has unique features due to the presence of creeping fat. Its role in the disease pathogenesis is unclear and is the focus of current research. It has been shown that the mesenteric creeping fat contains viable bacteria and secretes pro-inflammatory cytokines, indicating that the mesentery could be the main trigger of the inflammatory process related to CD.37,38 Therefore, early alterations of the mesentery—serosal fat infiltration and abnormal serosal vasodilation—which are frequently observed during surgery on normal bowel proximal to the resected segment, can be viewed as initial forms of creeping fat and can be indicative of early CD recurrence. Interestingly, endoluminal assessment and full thickness biopsies taken at these sites revealed a normal mucosa and no histological signs of chronic inflammation related to CD, potentially indicating that POR is triggered by the mesentery and tends to progress according to an outer-inner gradient involving the mesentery and the serosa first and the inner layers of the bowel at a later phase.37 Nonetheless, the present study could not detect any association between these findings and POR, even when the analysis was restricted to those patients who did not receive immediate medical (prophylactic) therapy after surgery. A clear association between BMI and the presence of fat infiltration on normal bowel proximal to the resected segment and mesentery thickness was observed, suggesting a possible role of visceral obesity in influencing the intraoperative characteristic of the surgical specimen. Despite this, no association between BMI and POR was shown.
The lack of association between the investigated intraoperative factors and CD recurrence could have been influenced by several factors. First, the choice of using the mRS to assess endoscopic POR. Endoscopic lesions located at the level of anastomosis were therefore interpreted as a result of postsurgical ischemia rather than as CD lesions.39 At present, it is unclear what the origin and prognostic role is of ulcerations confined to the anastomosis. A second limitation is the relative limited number of patients included and the choice of focusing only on primary ileocecal resection, potentially selecting a pool of patients at lower risk for POR. The last limitation is the choice of allowing prophylactic medical therapy for those patients deemed at high risk of developing POR according to current guidelines.16,17
Although a high inter-rater reliability was observed for all intraoperative findings, the same findings lacked perhaps sufficient granularity to characterize appropriately the variability of the macroscopic appearance of the bowel and the mesentery and, eventually, to answer the research question of the study. For instance, the presence and the number of enlarged lymph nodes at preoperative cross-sectional imaging and the number of visible and palpable lymph nodes during surgery could be included in future studies. The present study also has strengths. This is the first prospective study looking at intraoperative findings relating this to postoperative recurrence. A recent consensus statement of expert IBD surgeons recommended that photo documentation during surgery for CD should be used, allowing a standardized and reproducible assessment of fixed portions of the bowel and mesentery.40 Since video documentation was included in the present study, these data could offer the opportunity to develop artificial intelligence technologies to enhance accuracy of POR prediction from merely the analysis of intraoperative pictures.41
To conclude, results of the present study seem to indicate that the appearance of the bowel and corresponding mesentery during ileocecal resection for CD surgery are not predictive of POR. In particular, the length of resected ileum and the thickness of the mesentery did not show any association with endoscopic, clinical, and biological POR. Serosal fat infiltration and abnormal serosal vasodilation located proximal to the macroscopic-affected ileum should probably not be interpreted as prodromes of CD recurrence arising from the mesentery.
Supplementary Data
Supplementary data is available at Inflammatory Bowel Diseases online.
Funding
Crohn & Colitis Ulcerosa Vereniging (CCV) Grant for the year 2020.
Conflicts of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this article. Further, all the possible conflicts of interest are now disclosed:
G.B.
Speaker’s fee from Janssen, Galapagos.
Albert Wolthuis, Julie Van Den Bossch, Steffen Fieuws
No conflicts of interest to be declared.
M.F.
Research grants from AbbVie, Amgen, Biogen, EG, Janssen, Pfizer, Takeda and Viatris
Consultancy fees from AbbVie, AgomAb Therapeutics, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Janssen-Cilag, Medtronic, MSD, Pfizer, Regeneron, Samsung Bioepis, Sandoz, Takeda and ThermoFisher
Speakers’ fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Falk, Ferring, Janssen-Cilag, Lamepro, MSD, Pfizer, Sandoz, Takeda, Truvion Healthcare and Viatris
S.,V.
Research grants from AbbVie, J&J, Pfizer, Takeda and Galapagos
speakers’ and/or consultancy fees from AbbVie, Abivax, AbolerISPharma, AgomAb, Alimentiv, Arena Pharmaceuticals, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Cytoki Pharma, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, GSK, Hospira, Imidomics, Janssen, J&J, Lilly, Materia Prima, Mestag Therapeutics, MiroBio, Morphic, MrMHealth, Mundipharma, MSD, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Surrozen, Takeda, Theravance, Tillots Pharma AG, VectivBio, Ventyx, Zealand Pharma.
G.dH.
His employer KULeuven receives fees for his activities as central pathology reader in clinical trials of Centocor J&J.
Data Availability
Institutional database. The data underlying this article will be shared on reasonable request to the corresponding author.



