-
PDF
- Split View
-
Views
-
Cite
Cite
Ruben J Colman, Stephanie A Vuijk, Ron A A Mathôt, Johan Van Limbergen, Maria M E Jongsma, Marco W J Schreurs, Phillip Minar, Lissy de Ridder, Geert R A M D’Haens, Infliximab Monotherapy vs Combination Therapy for Pediatric Crohn’s Disease Exhibit Similar Pharmacokinetics, Inflammatory Bowel Diseases, Volume 30, Issue 10, October 2024, Pages 1678–1685, https://doi.org/10.1093/ibd/izad307
Close - Share Icon Share
Abstract
The use of concomitant azathioprine may improve efficacy and pharmacokinetic (PK) properties of infliximab (IFX) but is also associated with an increased risk of adverse events. Proactive therapeutic drug monitoring (pTDM) of IFX monotherapy is an alternative strategy to improve PK. The aim of this study was to evaluate whether IFX with an immunomodulator (combo) has PK benefits over IFX-pTDM (mono) in pediatric Crohn’s disease (CD).
This PK analysis included pediatric CD patients who started either IFX combo (TISKids study) or IFX mono with pTDM (REFINE cohort). Combo and mono IFX trough levels (TLs) and antibodies-to-infliximab were assessed at infusion 3, 4, and 5. A population PK model was built to compare IFX PK outcomes (clearance [CL], TLs and cumulative exposure) between combo and mono groups at infusion 4 and 5. Clinical response and steroid-free clinical remission (SFCR) was assessed at infusion 4 and 5.
This study included 128 pediatric CD patients (66 mono and 62 combo). At infusion 5, there was no significant difference between mono and combo median TLs 4.1 µg/mL (2.1, 7.8) vs 5.9 µg/mL (3.2, 9.4; P = .14) or median CL 0.26 L/d (0.21, 0.32) vs 0.26 L/d (0.21, 0.33; P = .81). Mono patients had a lower SFCR rate at infusion 5 (53% [31 of 59] vs 80% [32 of 40]; P = .01). Clinical response rates were significantly higher among combo than mono patients at both infusion 4 and 5.
This study suggests that there are no PK differences (TLs and CL) between combo and mono therapy in pediatric CD patients who started IFX.
Lay Summary
This study compared the pharmacokinetics of infliximab combination therapy with azathioprine vs infliximab with proactive therapeutic drug monitoring as monotherapy among pediatric patients with Crohn’s disease within the first 22 weeks. No pharmacokinetic differences were found between monotherapy and combination therapy.
Among pediatric patients with Crohn’s disease, it is unclear whether the use of infliximab combined with concomitant immunomodulator (combo) leads to improved pharmacokinetic outcomes compared with infliximab combined with proactive therapeutic drug monitoring (pTDM; Mono).
This study found no pharmacokinetic differences in drug clearance or trough levels between combo and mono therapy for pediatric Crohn’s disease.
This study suggests that if patients are treated with infliximab monotherapy while pTDM is practiced, they could achieve similar pharmacokinetics compared with patients treated with infliximab and azathioprine combination therapy.
Introduction
Infliximab (IFX) has been one of the most efficacious therapies for Crohn’s disease (CD) to date.1,2 However, large variability in IFX pharmacokinetics (PK) among CD patients may hamper optimal clinical efficacy.3 Antibodies-to-infliximab (ATIs), fecal drug loss, high tumor necrosis factor (TNF) burden, younger age, and lower body weight have been associated with accelerated IFX clearance (CL) leading to lower IFX trough levels (TLs).4–8 Standard (labeled) IFX therapy resulting in subtherapeutic drug exposure may contribute to the relatively high loss of response rate in children.9,10
The addition of an immunomodulator (IMM) to IFX therapy reduces the development of ATI, resulting in improved drug exposure and therefore improved therapy durability and disease outcomes.11,12 The landmark study of biologic and immunomodulator-naïve patients in Crohn’s disease (SONIC) showed that patients who started combination of IFX (combo) with azathioprine (AZA) had superior efficacy at week 26 compared with patients who received IFX monotherapy (mono).13 Yet, results from a post hoc analysis of SONIC suggest that superior efficacy of combo could be solely related to higher TLs, as there were more combo patients with high IFX serum TLs compared with mono patients at week 30.11 Despite these results, the American Gastroenterological Association (AGA) guideline for managing adults with CD recommends combo for biologic-naïve patients starting IFX.14
However, among pediatric inflammatory bowel disease (IBD) patients, it is unclear if the concomitant use of IMM leads to improved outcomes or has any effect on IFX TLs.8 As a result, the decision to use combo or mono therapy alone at the start of IFX therapy varies widely among pediatric practitioners.15 In the current pediatric European guidelines, concomitant IMM is recommended when starting IFX treatment.2 However, the use of combo remains controversial, as AZA is associated with potentially toxic side effects, including an increased risk of highly aggressive cancer particularly in younger male patients.16,17 As a result, thiopurines are used sparingly among pediatric institutions and practitioners in the United States. In line with the post hoc analysis of SONIC, there are alternative strategies to optimize the IFX PK in young patients, such as with proactive therapeutic drug monitoring (pTDM).2,18
It remains unclear if there are PK differences among children receiving mono pTDM vs combo IFX therapy. The primary objective of this study was to evaluate whether combo has PK benefits (IFX TLs, CL, and drug exposure) compared with treatment with mono guided by pTDM.
Materials and Methods
Study Population
A head-to-head PK analysis was conducted comparing mono pTDM and combo among pediatric CD patients enrolled in 2 separate prospective studies. Inclusion criteria for this PK analysis were CD diagnosis (as per the revised Porto criteria),19 age 3 to 17 years, body weight >10 kg, and naïve to biologics prior to starting IFX.
Mono Cohort
Patients for the mono pTDM cohort were selected from the US-based Multicenter Observational Study Targeting the Inflammatory Signature to Personalize Biologics in Pediatric IBD (REFINE) if they met the aforementioned inclusion criteria for the PK analysis and started on IFX monotherapy.4,6 The IFX induction was not limited to time after diagnosis. Infliximab dose modification was allowed at the discretion of the clinician and pTDM was recommended based on institutional guidelines, which stipulated that TLs should initially be assessed at the fourth IFX infusion and that IFX should be escalated if infusion 4 TLs were <5 µg/mL.18
Combo Cohort
Combo patients were selected from the multicenter European open-label top-down infliximab study in kids with Crohn’s disease (TISKids) randomized controlled trial.20,21 Combo patients received IFX immediately after diagnosis or following failed induction therapy with exclusive enteral nutrition (EEN)/prednisolone. Five IFX infusions were given at weeks 0, 2, 6, 14, and 22. For all combo patients, IFX treatment was combined with AZA maintenance therapy (2-3 mg/kg, once daily). An overview of the 2 different studies is given in Table S1.
Data Collection and PK Assessments
Baseline demographic, clinical, and laboratory data were collected at IFX induction. For PK modeling, IFX dose, IFX infusions date and time, height, and body weight were collected at every infusion. Laboratory results including serum albumin and inflammatory markers (such as erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) were recorded if available. Disease activity was tabulated at the fourth and fifth infusion using the weighted Pediatric CD Activity Index (wPCDAI).
In both cohorts, IFX TLs were measured before infusion 3, 4, and 5. In the REFINE cohort, ATIs were determined at each infusion. In the TISKids study, ATIs were determined if the TL was <3 µg/mL. Infliximab concentrations and ATIs of the REFINE cohort were measured with electrochemiluminescence immunoassay (ECLIA; Esoterix, LabCorp Specialty lab, Calabasas, CA), which includes a drug-tolerant ATI assay. More detailed PK and assay characteristics for this cohort are published elsewhere.4,6 Within the TISKids study, IFX concentrations were determined by the Sanquin MabTrack level IFX enzyme-linked immunosorbent assay (ELISA; M2920) procedure, which has a lower limit of quantification of 0.08 mcg/L and an upper limit of quantification of 46 µg/L using a 1:2000 serum dilution. Antibodies-to-infliximab were determined by the drug-sensitive Sanquin MabTrack ADA Infliximab ELISA (M2960).
In both cohorts, TLs and ATIs were measured in batched analysis after study completion. As research TLs were not yet available during treatment, a post hoc sensitivity analysis was conducted to determine the prevalence of treatment intensification (interval shortened by >1 week, or dose increased >1mg/kg) if the research TL was <5 ug/mL at infusion 4.
Study Outcomes
The primary aim of the study was to investigate PK outcomes between patients on IFX mono vs combo therapy. Pharmacokinetic outcomes included three endpoints: (1) TLs, (2) drug CL, and (3) cumulative IFX exposure (measured as area under the curve [AUC]) at end-of-induction (infusion 4; AUCinfusion1-4) and up to the fifth maintenance dose (infusion 5; AUCinfusion1-5). Secondary aims included steroid-free clinical remission (SFCR, defined as a wPCDAI <12.5 without corticosteroid use at time of infusion) and clinical response (defined as a decrease >17.5 in wPCDAI from baseline or a wPCDAI <12.5) at infusion 4 and 5.
Pharmacokinetic and Statistical Analysis
We fitted a population-PK model to calculate CL and AUC with Bayesian estimation based on previously published models.3,6 Potential PK covariates that were evaluated included age, sex, weight, BMI, BSA, concomitant medication (including IMM and prednisone use), serum biomarkers (including albumin, CRP, ESR), and immunogenicity status. Detailed pharmacometric methods are described in the Supplemental Methods. Descriptive statistics between the 2 subcohorts were documented and analyzed with the Wilcoxon rank sum test for continuous variables. Each of the continuous PK end points between the 2 groups were compared with the Wilcoxon rank sum test at infusion 4 and 5. The χ2 test was used to analyze the categorical variables. In addition, the change in wPCDAI over time as a continuous variable between the 2 groups was compared with the Wilcoxon rank sum test. Multivariable logistic regression was conducted to further assess if clinical baseline variables significantly impacted clinical outcomes. Statistical analysis was conducted in R version 4.0.3. (Vienna, Austria).
Results
This study included 128 (66 mono and 62 combo) patients, with a median age of 14 years (interquartile range [IQR], 11, 16), of which 56 (44%) were female patients. Time to start IFX treatment after diagnosis was 5.4 weeks (2.3, 42.4) for mono patients compared with 1.6 weeks (1.0, 6.1) among combo patients (P < .01). At baseline, mono patients had a lower albumin level and higher fecal calprotectin, while combo patients had higher ESR, CRP, and wPCDAI scores. Additional baseline characteristics by cohort are described in Table 1.
Baseline characteristics of proactive TDM Monotherapy vs labeled Combination therapy.
| Variable | Overall (n = 128) | Mono (n = 66) | Combo (n = 62) | P |
| Age, years (median [IQR]) | 14.0 (11.1, 16.0) | 13.1 (10.6, 15.6) | 15.0 (12.0, 16.0) | 0.08 |
| Sex (F), (%) | 56 (43.8) | 24 (36.4) | 32 (51.6) | 0.12 |
| Starting dose, mg (median [IQR]) | 265.0 (200.0, 300.0) | 300.0 (200.0, 300.0) | 260.0 (200.0, 300.0] | 0.11 |
| Weight-based starting dose (mg/kg) | 5.2 (5.0, 6.2) | 6.0 (5.2, 7.1) | 5.1 (4.9, 5.2) | <0.01 |
| Time from diagnosis to start IFX, weeks (median [IQR]) | 2.6 (1.3, 11.3) | 5.4 (2.3, 42.4) | 1.6 (1.0, 6.1) | <0.01 |
| Weight, kg (median [IQR]) | 45.5 (33.2, 55.8) | 41.6 (28.1, 54.1) | 47.2 (37.4, 56.8) | 0.08 |
| BMI (median [IQR]) | 17.1 (15.1, 19.8) | 17.1 (15.2, 20.6) | 17.2 (15.0, 19.4) | 0.72 |
| BSA (median [IQR]) | 1.5 (1.3, 1.8) | 1.6 (1.3, 1.8) | 1.5 (1.3, 1.7) | 0.03 |
| Prednisone use n (%) | 46 (35.9) | 43 (65.2) | 3 (4.8) | <0.01 |
| Albumin, g/dl (median [IQR]) | 3.4 (3.0, 4.0) | 3.3 (2.9, 3.6) | 3.8 (3.3, 4.0) | <0.01 |
| ESR, mm/hr (median [IQR]) | 25.5 (10.0, 41.0) | 15.0 (8.5, 38.0) | 30.0 (20.0, 47.0) | <0.01 |
| CRP, mg/dl, (median [IQR]) | 1.7 (0.4, 3.5) | 1.1 (0.3, 2.3) | 2.4 (0.7, 3.8) | <0.01 |
| Fecal calprotectin, μg/g (median [IQR]) | 1191.0 (850.0, 1890.6) | 1677.2 (1126.1, 2501.0) | 1032.2 (693.5, 1241.4) | <0.01 |
| wPCDAI (median [IQR]) | 48.8 (25.0, 65.6) | 36.2 (20.0, 65.0) | 52.5 (40.0, 66.9) | 0.02 |
| Variable | Overall (n = 128) | Mono (n = 66) | Combo (n = 62) | P |
| Age, years (median [IQR]) | 14.0 (11.1, 16.0) | 13.1 (10.6, 15.6) | 15.0 (12.0, 16.0) | 0.08 |
| Sex (F), (%) | 56 (43.8) | 24 (36.4) | 32 (51.6) | 0.12 |
| Starting dose, mg (median [IQR]) | 265.0 (200.0, 300.0) | 300.0 (200.0, 300.0) | 260.0 (200.0, 300.0] | 0.11 |
| Weight-based starting dose (mg/kg) | 5.2 (5.0, 6.2) | 6.0 (5.2, 7.1) | 5.1 (4.9, 5.2) | <0.01 |
| Time from diagnosis to start IFX, weeks (median [IQR]) | 2.6 (1.3, 11.3) | 5.4 (2.3, 42.4) | 1.6 (1.0, 6.1) | <0.01 |
| Weight, kg (median [IQR]) | 45.5 (33.2, 55.8) | 41.6 (28.1, 54.1) | 47.2 (37.4, 56.8) | 0.08 |
| BMI (median [IQR]) | 17.1 (15.1, 19.8) | 17.1 (15.2, 20.6) | 17.2 (15.0, 19.4) | 0.72 |
| BSA (median [IQR]) | 1.5 (1.3, 1.8) | 1.6 (1.3, 1.8) | 1.5 (1.3, 1.7) | 0.03 |
| Prednisone use n (%) | 46 (35.9) | 43 (65.2) | 3 (4.8) | <0.01 |
| Albumin, g/dl (median [IQR]) | 3.4 (3.0, 4.0) | 3.3 (2.9, 3.6) | 3.8 (3.3, 4.0) | <0.01 |
| ESR, mm/hr (median [IQR]) | 25.5 (10.0, 41.0) | 15.0 (8.5, 38.0) | 30.0 (20.0, 47.0) | <0.01 |
| CRP, mg/dl, (median [IQR]) | 1.7 (0.4, 3.5) | 1.1 (0.3, 2.3) | 2.4 (0.7, 3.8) | <0.01 |
| Fecal calprotectin, μg/g (median [IQR]) | 1191.0 (850.0, 1890.6) | 1677.2 (1126.1, 2501.0) | 1032.2 (693.5, 1241.4) | <0.01 |
| wPCDAI (median [IQR]) | 48.8 (25.0, 65.6) | 36.2 (20.0, 65.0) | 52.5 (40.0, 66.9) | 0.02 |
Abbreviations: Numbers are No. (%) unless otherwise noted. IQR, interquartile range. TDM; Therapeutic Drug Monitoring, IFX, infliximab; BMI; Body Mass Index, BSA; Body Surface Area, ESR; erythrocyte sedimentation rate, CRP; C-reactive protein, wPCDAI; weighted pediatric Crohn’s Disease activity index.
Baseline characteristics of proactive TDM Monotherapy vs labeled Combination therapy.
| Variable | Overall (n = 128) | Mono (n = 66) | Combo (n = 62) | P |
| Age, years (median [IQR]) | 14.0 (11.1, 16.0) | 13.1 (10.6, 15.6) | 15.0 (12.0, 16.0) | 0.08 |
| Sex (F), (%) | 56 (43.8) | 24 (36.4) | 32 (51.6) | 0.12 |
| Starting dose, mg (median [IQR]) | 265.0 (200.0, 300.0) | 300.0 (200.0, 300.0) | 260.0 (200.0, 300.0] | 0.11 |
| Weight-based starting dose (mg/kg) | 5.2 (5.0, 6.2) | 6.0 (5.2, 7.1) | 5.1 (4.9, 5.2) | <0.01 |
| Time from diagnosis to start IFX, weeks (median [IQR]) | 2.6 (1.3, 11.3) | 5.4 (2.3, 42.4) | 1.6 (1.0, 6.1) | <0.01 |
| Weight, kg (median [IQR]) | 45.5 (33.2, 55.8) | 41.6 (28.1, 54.1) | 47.2 (37.4, 56.8) | 0.08 |
| BMI (median [IQR]) | 17.1 (15.1, 19.8) | 17.1 (15.2, 20.6) | 17.2 (15.0, 19.4) | 0.72 |
| BSA (median [IQR]) | 1.5 (1.3, 1.8) | 1.6 (1.3, 1.8) | 1.5 (1.3, 1.7) | 0.03 |
| Prednisone use n (%) | 46 (35.9) | 43 (65.2) | 3 (4.8) | <0.01 |
| Albumin, g/dl (median [IQR]) | 3.4 (3.0, 4.0) | 3.3 (2.9, 3.6) | 3.8 (3.3, 4.0) | <0.01 |
| ESR, mm/hr (median [IQR]) | 25.5 (10.0, 41.0) | 15.0 (8.5, 38.0) | 30.0 (20.0, 47.0) | <0.01 |
| CRP, mg/dl, (median [IQR]) | 1.7 (0.4, 3.5) | 1.1 (0.3, 2.3) | 2.4 (0.7, 3.8) | <0.01 |
| Fecal calprotectin, μg/g (median [IQR]) | 1191.0 (850.0, 1890.6) | 1677.2 (1126.1, 2501.0) | 1032.2 (693.5, 1241.4) | <0.01 |
| wPCDAI (median [IQR]) | 48.8 (25.0, 65.6) | 36.2 (20.0, 65.0) | 52.5 (40.0, 66.9) | 0.02 |
| Variable | Overall (n = 128) | Mono (n = 66) | Combo (n = 62) | P |
| Age, years (median [IQR]) | 14.0 (11.1, 16.0) | 13.1 (10.6, 15.6) | 15.0 (12.0, 16.0) | 0.08 |
| Sex (F), (%) | 56 (43.8) | 24 (36.4) | 32 (51.6) | 0.12 |
| Starting dose, mg (median [IQR]) | 265.0 (200.0, 300.0) | 300.0 (200.0, 300.0) | 260.0 (200.0, 300.0] | 0.11 |
| Weight-based starting dose (mg/kg) | 5.2 (5.0, 6.2) | 6.0 (5.2, 7.1) | 5.1 (4.9, 5.2) | <0.01 |
| Time from diagnosis to start IFX, weeks (median [IQR]) | 2.6 (1.3, 11.3) | 5.4 (2.3, 42.4) | 1.6 (1.0, 6.1) | <0.01 |
| Weight, kg (median [IQR]) | 45.5 (33.2, 55.8) | 41.6 (28.1, 54.1) | 47.2 (37.4, 56.8) | 0.08 |
| BMI (median [IQR]) | 17.1 (15.1, 19.8) | 17.1 (15.2, 20.6) | 17.2 (15.0, 19.4) | 0.72 |
| BSA (median [IQR]) | 1.5 (1.3, 1.8) | 1.6 (1.3, 1.8) | 1.5 (1.3, 1.7) | 0.03 |
| Prednisone use n (%) | 46 (35.9) | 43 (65.2) | 3 (4.8) | <0.01 |
| Albumin, g/dl (median [IQR]) | 3.4 (3.0, 4.0) | 3.3 (2.9, 3.6) | 3.8 (3.3, 4.0) | <0.01 |
| ESR, mm/hr (median [IQR]) | 25.5 (10.0, 41.0) | 15.0 (8.5, 38.0) | 30.0 (20.0, 47.0) | <0.01 |
| CRP, mg/dl, (median [IQR]) | 1.7 (0.4, 3.5) | 1.1 (0.3, 2.3) | 2.4 (0.7, 3.8) | <0.01 |
| Fecal calprotectin, μg/g (median [IQR]) | 1191.0 (850.0, 1890.6) | 1677.2 (1126.1, 2501.0) | 1032.2 (693.5, 1241.4) | <0.01 |
| wPCDAI (median [IQR]) | 48.8 (25.0, 65.6) | 36.2 (20.0, 65.0) | 52.5 (40.0, 66.9) | 0.02 |
Abbreviations: Numbers are No. (%) unless otherwise noted. IQR, interquartile range. TDM; Therapeutic Drug Monitoring, IFX, infliximab; BMI; Body Mass Index, BSA; Body Surface Area, ESR; erythrocyte sedimentation rate, CRP; C-reactive protein, wPCDAI; weighted pediatric Crohn’s Disease activity index.
When all 5 IFX infusions were evaluated, mono patients received a higher absolute and weight-based dose 6.2 mg/kg (5.2, 7.7) compared with combo patients 4.9 mg/kg (4.8, 5.1; P < .001). Median IFX dose and interval for each infusion are summarized in Table S1. Post hoc analysis identified no significant differences in rates of subtherapeutic (<5 ug/mL) TL at infusion 4 and 5 between both groups. However, among the patients with an infusion 4 TL <5 ug/mL, IFX was intensified in 54% (14 of 26) of the mono patients and only 7% (2 of 29) of the combo patients (P < .001).
Concomitant Therapy
All combo patients started on concomitant therapy with either AZA (n = 61) or methotrexate (MTX, n = 1). By infusion 5, 92% (57 of 62) remained on IMM for the entire 5 infusions. Sixty-five percent (n = 43 of 66) of mono patients also received a course of prednisone at time of IFX initiation compared with 4.8% (n = 3 of 62) among combo patients (P < .001). At infusion 5, 8% (5 of 59) of mono patients and none of the combo patients received prednisone (Table S2).
Pharmacokinetic Outcomes
Using an unbiased pharmacokinetic modeling approach to identify covariates of drug CL, IMM use did not significantly improve the prediction of IFX CL in the model on univariable or multivariable analysis. Furthermore, age, sex, BMI, BSA, prednisone use, CRP, and immunogenicity status were not found to have a significant effect on the PK model. However, ESR, albumin, and weight were found to have a significant effect on IFX CL in the model. A lower albumin, a higher ESR, and a lower body weight were associated with accelerated CL (expressed as L/day/kg; Table S3). If albumin decreased by 1 point from 3.5 to 2.5 mg/dL, there was a relative change with a 28% increase in CL on average. Similarly, if ESR increased from 10 to 30 mm/hr, there was about an 18% increase in CL on average. In addition, a lower weight particularly under 30 kg was associated with a higher CL (L/day/kg), which is illustrated in Figure 1. Additional details about the final PK model are documented in the supplemental results.

Distribution of weight (kg) vs clearance (L/day/kg) demonstrates that lower weight is associated with higher clearance.
At infusion 4, the median TL was 5.1 µg/mL (2.4, 13.0) for mono patients compared with a median of 4.4 µg/mL (2.3, 7.7) for combo patients (P = .24). Likewise, we found a similar median IFX CL of 0.27 L/d (0.21, 0.36) compared with 0.26 L/d (0.22, 0.33) at infusion 4, respectively (P = .77; (Table 2, Figure 2). As TL and CL only reflect a single time point, we calculated the cumulative exposure AUCinfusion1-4 for the 2 groups. The median mono AUCinfusion1-4 was 89, 700 µg/mL*h (69 000, 125 500) compared with a median of 81 700 µg/mL*h (66, 000, 101, 800) in the combo cohort (P = .06). At infusion 5, the median TL was 4.1 µg/mL (2.1, 7.8) in the mono cohort compared with a median of 5.9 µg/mL (3.2, 9.4) in the combo cohort (P = .14). Similarly, the median IFX CL was 0.26 L/d (0.21, 0.32) compared with a median of 0.26 L/d (0.21, 0.33) in the combo cohort (P = .81; (Table 2, Figure 2). In contrast, the cumulative exposure AUCinfusion1-5 for mono 119 200 µg/mL*h (95, 000, 158, 400) was higher than combo 108, 100 µg/mL*h (83, 600, 132, 500) patients (P = .01). However, as drug exposure is dependent on dose, additional sensitivity analysis was conducted which showed that there were no differences in AUCinfusion1-5 between mono and combo when both groups were stratified by standard dose and/or interval (Table S4). In contrast, when only including patients with IFX 5 mg/kg and labeled intervals, combo patients (n = 30 of 62) had higher TL at infusion 5 and lower CL at infusion 4 and 5 compared with mono patients (n = 16 of 66; Table S4).
| Variable | Overall (n = 128) | Mono (n = 66) | Combo (n = 62) | P |
| Trough level infusion 4 (median [IQR]) | 4.85 (2.30, 10.00) | 5.10 (2.40, 13.00) | 4.40 (2.30, 7.70) | 0.24 |
| Clearance infusion4 (L/d, median [IQR]) | 0.27 (0.22, 0.33) | 0.27 (0.21, 0.36) | 0.26 (0.22, 0.33) | 0.77 |
| AUC infusion1-4 (µg*h/mL; median [IQR]) | 85, 500 (67, 400; 107, 400) | 89, 700 (69, 000; 125, 500) | 81, 695.5 (66, 000; 101, 800) | 0.06 |
| Trough level infusion 5 (median [IQR]) | 4.80 (2.10, 8.80) | 4.05 (2.05, 7.75) | 5.85 (3.23, 9.40) | 0.14 |
| Clearance infusion 5 (L/d, median [IQR]) | 0.26 (0.21, 0.33) | 0.26 (0.21, 0.32) | 0.26 (0.22, 0.33) | 0.81 |
| AUC infusion1-5 (µg*h/mL; median [IQR]) | 113, 000 (89, 000; 137, 600) | 119, 200 (95, 000; 158, 400) | 108, 100 (83, 600; 132, 500) | 0.01 |
| Variable | Overall (n = 128) | Mono (n = 66) | Combo (n = 62) | P |
| Trough level infusion 4 (median [IQR]) | 4.85 (2.30, 10.00) | 5.10 (2.40, 13.00) | 4.40 (2.30, 7.70) | 0.24 |
| Clearance infusion4 (L/d, median [IQR]) | 0.27 (0.22, 0.33) | 0.27 (0.21, 0.36) | 0.26 (0.22, 0.33) | 0.77 |
| AUC infusion1-4 (µg*h/mL; median [IQR]) | 85, 500 (67, 400; 107, 400) | 89, 700 (69, 000; 125, 500) | 81, 695.5 (66, 000; 101, 800) | 0.06 |
| Trough level infusion 5 (median [IQR]) | 4.80 (2.10, 8.80) | 4.05 (2.05, 7.75) | 5.85 (3.23, 9.40) | 0.14 |
| Clearance infusion 5 (L/d, median [IQR]) | 0.26 (0.21, 0.33) | 0.26 (0.21, 0.32) | 0.26 (0.22, 0.33) | 0.81 |
| AUC infusion1-5 (µg*h/mL; median [IQR]) | 113, 000 (89, 000; 137, 600) | 119, 200 (95, 000; 158, 400) | 108, 100 (83, 600; 132, 500) | 0.01 |
Abbreviations: IQR, interquartile range. AUC; area under the curve PK; pharmacokinetic.
| Variable | Overall (n = 128) | Mono (n = 66) | Combo (n = 62) | P |
| Trough level infusion 4 (median [IQR]) | 4.85 (2.30, 10.00) | 5.10 (2.40, 13.00) | 4.40 (2.30, 7.70) | 0.24 |
| Clearance infusion4 (L/d, median [IQR]) | 0.27 (0.22, 0.33) | 0.27 (0.21, 0.36) | 0.26 (0.22, 0.33) | 0.77 |
| AUC infusion1-4 (µg*h/mL; median [IQR]) | 85, 500 (67, 400; 107, 400) | 89, 700 (69, 000; 125, 500) | 81, 695.5 (66, 000; 101, 800) | 0.06 |
| Trough level infusion 5 (median [IQR]) | 4.80 (2.10, 8.80) | 4.05 (2.05, 7.75) | 5.85 (3.23, 9.40) | 0.14 |
| Clearance infusion 5 (L/d, median [IQR]) | 0.26 (0.21, 0.33) | 0.26 (0.21, 0.32) | 0.26 (0.22, 0.33) | 0.81 |
| AUC infusion1-5 (µg*h/mL; median [IQR]) | 113, 000 (89, 000; 137, 600) | 119, 200 (95, 000; 158, 400) | 108, 100 (83, 600; 132, 500) | 0.01 |
| Variable | Overall (n = 128) | Mono (n = 66) | Combo (n = 62) | P |
| Trough level infusion 4 (median [IQR]) | 4.85 (2.30, 10.00) | 5.10 (2.40, 13.00) | 4.40 (2.30, 7.70) | 0.24 |
| Clearance infusion4 (L/d, median [IQR]) | 0.27 (0.22, 0.33) | 0.27 (0.21, 0.36) | 0.26 (0.22, 0.33) | 0.77 |
| AUC infusion1-4 (µg*h/mL; median [IQR]) | 85, 500 (67, 400; 107, 400) | 89, 700 (69, 000; 125, 500) | 81, 695.5 (66, 000; 101, 800) | 0.06 |
| Trough level infusion 5 (median [IQR]) | 4.80 (2.10, 8.80) | 4.05 (2.05, 7.75) | 5.85 (3.23, 9.40) | 0.14 |
| Clearance infusion 5 (L/d, median [IQR]) | 0.26 (0.21, 0.33) | 0.26 (0.21, 0.32) | 0.26 (0.22, 0.33) | 0.81 |
| AUC infusion1-5 (µg*h/mL; median [IQR]) | 113, 000 (89, 000; 137, 600) | 119, 200 (95, 000; 158, 400) | 108, 100 (83, 600; 132, 500) | 0.01 |
Abbreviations: IQR, interquartile range. AUC; area under the curve PK; pharmacokinetic.
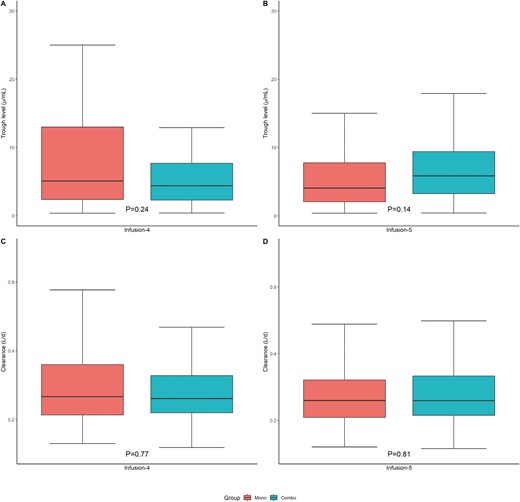
No difference between mono and combo therapy in median trough levels (μg/mL) (A) at infusion 4 and (B) infusion 5 or clearance (L/d) at (C) infusion 4 and (D) infusion 5.
Immunogenicity
Among mono patients, there were 29 patients with a detectable ATI level, of which 5 were above 200 ng/mL (the clinically relevant cutoff for this assay). Antibodies-to-infliximab of 3 patients increased to >200 ng/mL at infusion 4, while in 2 of the other patients, the ATI level increased to >200 ng/mL at infusion 5. No patients had discontinued IFX because of ATIs by infusion 5. Among combo patients, ATIs were measured in 26 of 29 with TL below ≤3 ug/mL. None were found to have detectable ATIs.
Clinical Outcomes
At the end of induction (infusion 4), there was no difference in SFCR between mono 59% (38 of 65) or combo 71% (32 of 45, P = .25). However, the mono cohort had a lower rate of SFCR rate at infusion 5 (53% [31 of 59] vs 80% [32 of 40]; P = .01). Of note, 33% (20 of 60) of combo patients did not have wPCDAI assessments available at infusion 5 compared with none that were missing in the mono cohort, but there were no appreciable significant differences between patients with and without missing wPCDAI scores (Table S5). Clinical response rates were higher among combo than mono patients at both infusion 4 and 5 (Table 3a). As not every patient in the mono cohort underwent pTDM, we conducted a sensitivity analysis in which we excluded mono patients who were not optimized despite an infusion 4 TL of <5µg/mL (Table S6). The differences in clinical outcomes remained the same between both groups. In contrast, a sensitivity analysis that included only moderately/severely active CD patients (wPCDAI >40; mono n = 29; combo n = 42) showed that there were no differences in clinical response between combo and mono patients at both infusion 4 and 5, as well as SFCR at infusion 4; but combo patients had significantly higher SFCR rates at infusion 5. Alternatively, in an additional sensitivity analysis that only included patients who reached a threshold of IFX exposure (infusion 4 or infusion 5 >5µg/mL), there were no differences in SFCR between combo and mono patients at either infusion 4 or infusion 5 (Table S7).
A, Clinical outcomes by infliximab regimen. B, Predictors of clinical outcomes based on multivariable logistic regression.
| Variable | Mono (n = 66) | Combo (n = 62) | P | |
| SFCR at infusion 4 | 59% (38/65) | 71% (32/45) | 0.25 | |
| Clinical response by infusion 4 | 52 % (31/65) | 84% (38/45) | <0.01 | |
| SFCR at infusion 5 | 53% (31/59) | 80% (32/40) | 0.01 | |
| Clinical response by infusion 5 | 59% (35/59) | 95% (38/40) | <0.01 | |
| Variable | SFCR at infusion 4 | Clinical response by infusion 4 | SFCR at infusion 5 | Clinical response by infusion 5 |
| Mono vs. Combo therapy | OR 0.99 (95% CI 0.29; 3.14) | OR 4.93 (95% CI 1.54; 17.00) | OR 2.24 (95% CI 0.59; 8.65) | OR 12 (95% CI 2.48; 89.84) |
| Prednisone use at baseline | OR 0.28 (95% CI 0.82; 0.88) | OR 1.49 (95% CI 0.48-4.73) | OR 0.38 (95% CI 0.11; 1.28) | OR 2.56 (95% CI 0.71; 9.80) |
| CRP at baseline | OR 0.99 (95% CI 0.82; 1.20) | OR 1.18 (95% CI 0.93; 1.58) | OR 0.91 (95% CI 0.74; 1.10) | OR 1.55 (95% CI 1.04; 2.68)* |
| Variable | Mono (n = 66) | Combo (n = 62) | P | |
| SFCR at infusion 4 | 59% (38/65) | 71% (32/45) | 0.25 | |
| Clinical response by infusion 4 | 52 % (31/65) | 84% (38/45) | <0.01 | |
| SFCR at infusion 5 | 53% (31/59) | 80% (32/40) | 0.01 | |
| Clinical response by infusion 5 | 59% (35/59) | 95% (38/40) | <0.01 | |
| Variable | SFCR at infusion 4 | Clinical response by infusion 4 | SFCR at infusion 5 | Clinical response by infusion 5 |
| Mono vs. Combo therapy | OR 0.99 (95% CI 0.29; 3.14) | OR 4.93 (95% CI 1.54; 17.00) | OR 2.24 (95% CI 0.59; 8.65) | OR 12 (95% CI 2.48; 89.84) |
| Prednisone use at baseline | OR 0.28 (95% CI 0.82; 0.88) | OR 1.49 (95% CI 0.48-4.73) | OR 0.38 (95% CI 0.11; 1.28) | OR 2.56 (95% CI 0.71; 9.80) |
| CRP at baseline | OR 0.99 (95% CI 0.82; 1.20) | OR 1.18 (95% CI 0.93; 1.58) | OR 0.91 (95% CI 0.74; 1.10) | OR 1.55 (95% CI 1.04; 2.68)* |
Abbreviations: SFCR, steroid-free clinical remission.
Reference category for categorical variables are mono therapy and no prednisone use at baseline, SFCR, steroid-free clinical remission; CRP, C-reactive protein. Significant odds ratios (OR) noted in bold. *P = .07.
A, Clinical outcomes by infliximab regimen. B, Predictors of clinical outcomes based on multivariable logistic regression.
| Variable | Mono (n = 66) | Combo (n = 62) | P | |
| SFCR at infusion 4 | 59% (38/65) | 71% (32/45) | 0.25 | |
| Clinical response by infusion 4 | 52 % (31/65) | 84% (38/45) | <0.01 | |
| SFCR at infusion 5 | 53% (31/59) | 80% (32/40) | 0.01 | |
| Clinical response by infusion 5 | 59% (35/59) | 95% (38/40) | <0.01 | |
| Variable | SFCR at infusion 4 | Clinical response by infusion 4 | SFCR at infusion 5 | Clinical response by infusion 5 |
| Mono vs. Combo therapy | OR 0.99 (95% CI 0.29; 3.14) | OR 4.93 (95% CI 1.54; 17.00) | OR 2.24 (95% CI 0.59; 8.65) | OR 12 (95% CI 2.48; 89.84) |
| Prednisone use at baseline | OR 0.28 (95% CI 0.82; 0.88) | OR 1.49 (95% CI 0.48-4.73) | OR 0.38 (95% CI 0.11; 1.28) | OR 2.56 (95% CI 0.71; 9.80) |
| CRP at baseline | OR 0.99 (95% CI 0.82; 1.20) | OR 1.18 (95% CI 0.93; 1.58) | OR 0.91 (95% CI 0.74; 1.10) | OR 1.55 (95% CI 1.04; 2.68)* |
| Variable | Mono (n = 66) | Combo (n = 62) | P | |
| SFCR at infusion 4 | 59% (38/65) | 71% (32/45) | 0.25 | |
| Clinical response by infusion 4 | 52 % (31/65) | 84% (38/45) | <0.01 | |
| SFCR at infusion 5 | 53% (31/59) | 80% (32/40) | 0.01 | |
| Clinical response by infusion 5 | 59% (35/59) | 95% (38/40) | <0.01 | |
| Variable | SFCR at infusion 4 | Clinical response by infusion 4 | SFCR at infusion 5 | Clinical response by infusion 5 |
| Mono vs. Combo therapy | OR 0.99 (95% CI 0.29; 3.14) | OR 4.93 (95% CI 1.54; 17.00) | OR 2.24 (95% CI 0.59; 8.65) | OR 12 (95% CI 2.48; 89.84) |
| Prednisone use at baseline | OR 0.28 (95% CI 0.82; 0.88) | OR 1.49 (95% CI 0.48-4.73) | OR 0.38 (95% CI 0.11; 1.28) | OR 2.56 (95% CI 0.71; 9.80) |
| CRP at baseline | OR 0.99 (95% CI 0.82; 1.20) | OR 1.18 (95% CI 0.93; 1.58) | OR 0.91 (95% CI 0.74; 1.10) | OR 1.55 (95% CI 1.04; 2.68)* |
Abbreviations: SFCR, steroid-free clinical remission.
Reference category for categorical variables are mono therapy and no prednisone use at baseline, SFCR, steroid-free clinical remission; CRP, C-reactive protein. Significant odds ratios (OR) noted in bold. *P = .07.
Lastly, multivariable logistic regression identified that patients who used prednisone at baseline were less likely to achieve SFCR at infusion 4 (OR, 0.28; 95% CI, 0.82-0.88). No other factors impacted SFCR at infusion 4 or infusion 5. However, combination therapy but no other baseline factors was the only predictor for clinical response at infusion 4 (OR, 4.93; 95% CI, 1.54-17.00) and at infusion 5 (OR, 12; 95% CI, 2.48-89.84; Table 3b).
Discussion
To our knowledge, this is the first study that compared IFX mono therapy combined with pTDM vs combo therapy using a PK modeling approach during the first 22 weeks of IFX treatment. This analysis did not demonstrate differences with respect to IFX CL and TLs between these cohorts. In addition, there was no difference in SFCR rates at infusion 4, while more combo patients were in SFCR at infusion 5.
While several studies have evaluated the addition of IMM to IFX therapy, studies conducting formal PK analysis (including CL and exposure) beyond TLs are limited. Including more advanced PK parameters is important, as several studies found that CL is a better predictor of clinical outcomes than TLs itself.22 A post hoc analysis of SONIC found that combo patients had higher TLs than mono patients, but no differences were found in clinical or endoscopic healing outcomes among patients with similar TLs.11 Most pediatric studies found no significant difference in IFX TLs with or without concomitant IMM, which is in line with our results.8,10,23–27 Interestingly, as our study compared observational dose-adjusted cohort to clinical trial dosing, sensitivity analysis did find some PK benefit (lower CL and higher TLs at infusion 4 and 5) of combo dosing, among a subset of patients who received standard labeled IFX regimens.
Similarly, most pediatric studies do not show an IMM effect on IFX CL, though most of the CL estimation was derived from cohorts with skewed distribution of IMM use towards almost all or almost no patients receiving combination therapy.3,6,28 In fact, Fasanmade et al found a 14% decrease on IFX CL of concomitant IMM use, which was only apparent after a mixed adult cohort was added to the PK analysis.3 While IMM use had no significant impact on IFX CL in our study (48% combo patients), we did find several other factors influencing IFX CL. Markers of more active disease, including lower albumin and higher ESR, resulted in accelerated CL. This is consistent with prior IFX PK models.3,6,8,28 These factors are hypothesized to accelerate CL via a higher TNF-alpha load, IFX neutralization, and more IFX excretion through a leaky gut, thereby increasing CL.5,29 Furthermore, we found that there was a positive but nonlinear association between body weight and CL. This is consistent with prior literature that patients with a lower body weight, particularly under 30 kg, have a higher IFX CL if evaluated as L/d/kg and, therefore, likely require higher doses to achieve similar targeted TL.6,7 Furthermore, IMMs may have a protective effect on CL by reduction of immunogenicity, but ATIs did not significantly influence CL in our study.4,12 However, absolute ATI incidence was low, and it is uncertain if no effect of ATI on CL was observed due to a relatively small sample size.
For the secondary outcome measure, there was no difference in SFCR at infusion 4 between mono and combo patients. However, we did find a higher percentage of SFCR in combo patients compared with mono patients at infusion 5. While combination therapy has been largely standard of care in adults with CD,13,14 data and practice among pediatric populations are more mixed.8 As our study showed no significant impact of concomitant IMMs on IFX CL, the higher clinical response and remission rates of combo therapy may reflect the additive immunosuppressive effect of AZA to IFX. These results should be taken with caution, as data was compiled from 2 different study designs. As early anti-TNF is associated with better disease outcomes,30 earlier start of anti-TNF in combo patients might have influenced the SFCR rates. However, this appears less likely as the median difference in time since diagnosis was less than 1 month. Additionally, a smaller proportion of combo patients had clinical disease assessments available at infusion 4 and 5, as not all study visits were scheduled at infusion visits for combo patients who received IFX as step-up treatment. Furthermore, the original clinical trial of the combo cohort was partially restricted to moderately/severely active disease at baseline. In fact, in a sensitivity analysis among moderately/severely active patients, combo and mono patients had similar clinical response rates at infusion 4 and infusion 5 and SFCR at infusion 4, but combo patients had higher SFCR rates at infusion 5. Unfortunately, more objective biochemical, endoscopic, or histologic outcomes were not uniformly available to compare between both cohorts.
This study had some limitations. While we compared 2 prospective induction studies that both had robust longitudinal collection of PK data, there was some heterogeneity in the study designs. First, IFX CL and ATI were measured with different assays. While the Sanquin ELISA and Labcorp ECLIA were not compared directly, it has been reported that these assays both have excellent intraclass correlation in comparison with the Janssen method for IFX TLs.31 Secondly, several covariates were different between mono and combo patients at baseline, such as CRP, ESR, and proportion of patients with prednisone use. Besides ESR, weight, and albumin, none of these covariates were found to have a significant impact on the PK model. As ESR, albumin, and weight were included as covariates, the PK model takes these covariates into account while calculating baseline (and subsequent) IFX CL. Thus, it is unlikely that differences in these covariates at baseline between mono and combo confounded our PK outcomes. Furthermore, while the mono cohort was recruited from a practice where pTDM was endorsed, about 50% of patients with subtherapeutic infusion 4 TLs (<5 µg/mL) had no change in dose or interval. However, among patients with subtherapeutic TLs within the mono group itself, no differences in PK outcomes were seen between pTDM vs no-pTDM (data not shown). Another limitation of the study was the fact that the follow-up time was limited to infusion 5. There is a numeric TL increase in the combo cohort and a numeric TL decrease in the mono cohort between infusion 4 and 5. While these differences were not statistically significant, we cannot exclude that this was a type 2 error. It is unknown whether IMM use would impact IFX CL and IFX TL for longer follow-up by preventing additional ATI formation and/or a synergistic effect, as optimal therapeutic effect of AZA is often reached in 2 to 4 months.2 Alternatively, as the effect of mono IFX regimen adjustments take time as well, an argument could be made that both groups could have improved their PK at a hypothetical infusion 6.
In conclusion, this study identified no immediate PK differences (TL and CL) between combo and pTDM mono therapy in pediatric CD patients newly started on IFX up to infusion 5. While there may be some clinical efficacy and immunogenicity benefits of combo therapy for select patients, this study design was unable to reach firm conclusions. The potential benefits of combo therapy need to be balanced against potential toxicity risks of concomitant IMM exposure, and it is recommended to be used for a limited time.2 Given the small but real absolute risk of serious adverse events with the use of combination therapy with AZA, this choice varies widely among pediatric gastroenterology practices worldwide, and the use of AZA is actively debated.2,32 Further information is necessary to balance the benefits and disadvantages of concomitant IMM use. Therefore, in addition to intercontinental research efforts such as this study, pediatric head-to-head clinical trials of early optimized mono IFX dosing vs combo therapy with longer follow-up are needed to further evaluate the clinical efficacy and potential immunogenicity benefits of concomitant IMM in the modern era.
Supplementary Data
Supplementary data is available at Inflammatory Bowel Diseases online.
Ethical Considerations
Both the TISKids study (Trial number: NCT02517684) and REFINE cohort received local ethics approval. Consent and/or assent were obtained for each patient prior to study enrolment as per local regulations.
Funding
R.C.: NIH: T32DK007727. S.V.: TISKids study was supported by ZonMw (The Netherlands Organisation for Health Research and Development) under project number 113202001, Crocokids (a Dutch fundraising organization to support research on IBD in children), and an investigator-sponsored research award from Pfizer (Study ID WI213008) PM NIH: K23DK105229 and R03DK118314, Crohn’s Colitis Foundation PRO-KIIDS award. L.R.: ZonMw, ECCO, Pfizer.
Conflicts of Interest
L.R.: Collaboration with Abbvie, Lilly, Takeda, Janssen, and Pfizer. R.A.A.M. has received grants from governmental and societal research institutes such as NWO, ZonMW, Dutch Kidney Foundation, and Innovation Fund and unrestricted investigator research grants from Baxter/Baxalta/Shire/Takeda, Bayer, CSL Behring, Sobi, and CelltrionHC. He has served as advisor for Bayer, CSL Behring, Merck Sharp & Dohme, Baxter/Baxalta/Shire/Takeda. All grants and fees paid to the institution. R.C., S.V., J.vL., M.J., M.S., P.M., and G.H. have nothing to disclose.
References
Author notes
Ruben J Colman and Stephanie A Vuijk shared first-authorship.



