-
PDF
- Split View
-
Views
-
Cite
Cite
Mark M Gallagher, Gang Yi, Hanney Gonna, Lisa W M Leung, Idris Harding, Banu Evranos, Rachel Bastiaenen, Rajan Sharma, Sue Wright, Mark Norman, Zia Zuberi, A John Camm, Multi-catheter cryotherapy compared with radiofrequency ablation in long-standing persistent atrial fibrillation: a randomized clinical trial, EP Europace, Volume 23, Issue 3, March 2021, Pages 370–379, https://doi.org/10.1093/europace/euaa289
Close - Share Icon Share
Abstract
Restoring sinus rhythm (SR) by ablation alone is an endpoint used in radiofrequency (RF) ablation for long-standing persistent atrial fibrillation (AF) but not with cryotherapy. The simultaneous use of two cryotherapy catheters can improve ablation efficiency; we compared this with RF ablation in chronic persistent AF aiming for termination to SR by ablation alone.
Consecutive patients undergoing their first ablation for persistent AF of >6 months duration were screened. A total of 100 participants were randomized 1:1 to multi-catheter cryotherapy or RF. For cryotherapy, a 28-mm Arctic Front Advance was used in tandem with focal cryoablation catheters. Open-irrigated, non-force sensing catheters were used in the RF group with a 3D mapping system. Pulmonary vein (PV) isolation and non-PV triggers were targeted. Participants were followed up at 6 and 12 months, then yearly. Acute PVI was achieved in all cases. More patients in the multi-catheter cryotherapy group were restored to SR by ablation alone, with a shorter procedure duration. Sinus rhythm continued to the last available follow-up in 16/49 patients (33%) in the multi-catheter at 3.0 ± 1.6 years post-ablation and in 12/50 patients (24%) in the RF group at 4.0 ± 1.2 years post-ablation. The yearly rate of arrhythmia recurrence was similar.
Multi-catheter cryotherapy can restore SR by ablation alone in more cases and more quickly than RF ablation. Long-term success is difficult to achieve by either methods and is similar with both.
Ablation that continues in a stepwise manner until sinus rhythm is restored is a common approach when radiofrequency energy is used in longstanding persistent atrial fibrillation (AF) but has not been used with cryotherapy.
We designed a method using multiple cryotherapy catheters to create an extensive lesion set efficiently and used it in a stepwise manner.
Standard radiofrequency ablation was compared with multi-catheter cryotherapy in a 1:1 randomized trial.
Multi-catheter cryotherapy proved quicker than radiofrequency ablation at restoring sinus rhythm, but long-term maintenance of sinus rhythm was similar with both methods.
Introduction
Catheter ablation for persistent atrial fibrillation (AF) results in long-term restoration of sinus rhythm (SR) in most cases, but usually requires more than one procedure.1 Pulmonary vein (PV) isolation is a requirement for success2 but most case series suggest that the rate of long-term success is greater if additional ablation is performed.3–5 Some of the best results have been achieved by stepwise ablation, with the goal of converting AF to SR.6–8 Despite the positive results of these nonrandomized studies, the randomized STAR AF II study suggested that strategies involving extensive ablation are counterproductive compared with PVI alone in this patient population.9 Because of doubt about the applicability of these results, ablation to SR is still used in many centres.
Cryotherapy using the arctic front cryoballoon is safe and effective in PV isolation.10–12 The cryoballoon is suitable for isolation of the superior vena cava (SVC)13,14 and for ablation of left atrial sites other than the PVs including the left atrial appendage and the posterior wall of the left atrium.15–18 The simultaneous use of a cryoballoon and a focal cryocatheter for ablation of AF and coexisting atrial flutter has been shown.19 The use of simultaneous multi-catheter cryotherapy decreased the fluoroscopy and procedure duration compared with standard methods.19 These findings raise the possibility of multi-catheter cryotherapy in conditions where standard methods struggle, such as in persistent AF. This trial aimed to compare multi-catheter cryotherapy with standard radiofrequency (RF) ablation methods for persistent AF.
Methods
All patients with persistent AF scheduled for a first left atrial ablation by our group, were considered. Patients were considered if they had continuous AF throughout the 6 months before the procedure and were excluded if AF terminated or organized to typical atrial flutter at any time between listing for ablation and admission for the procedure. Decisions about the mode of anaesthesia to be used were made at the time of listing for ablation, typically 4 months before the performance of the procedure. Patients were randomly assigned 1:1 to either a standard RF method or to dual catheter cryotherapy; randomization occurred on the day of the procedure.
This study complies with the Declaration of Helsinki; the research protocol was approved by the Research Ethics Committee of London, and all participants had given written informed consent before the procedure.
Ablation protocol
The desired procedural objective in trial participants was to restore SR by ablation alone. All procedures in the trial followed a stepwise approach aiming to organize AF to atrial tachycardia (AT) or SR. If AF terminated to SR, the operator continued with ablation aiming to achieve isolation of all veins and block at the roof line, mitral line, and cavotricuspid isthmus (CTI) if possible.
When AF organized to AT, the AT was mapped and if possible, terminated by ablation. If AF was still present when we considered that time had run out, electrical cardioversion was performed. After restoration of SR, whether by ablation or electrical cardioversion, we re-mapped the atria to confirm isolation of all PVs in SR and to determine whether bidirectional block was present across all lines.
Logistical constraints made it impossible to persevere for longer than 4 h in many cases. For safety reasons, we imposed a maximum procedure duration of 6 h in any patient from vessel puncture to removal of sheaths. The stepwise sequence was followed until all steps had been exhausted, time ran out or SR was achieved by ablation alone. The minimum endpoint that was considered acceptable in trial participants was isolation of all PVs and achievement of block of the CTI.
Multi-catheter cryoablation
In the multi-catheter cryotherapy group, a Freezor™ MAX Cardiac Cryoablation Catheter (Medtronic PLC, Dublin, Ireland) was used to ablate the CTI during preparation for and performance of trans septal puncture and imaging of the PVs. Pulmonary vein isolation was accomplished using a 28 mm Arctic Front Advance™ Cardiac CryoAblation Catheter (Medtronic) delivered through a 12-Fr FlexCath Advance™ Steerable Sheath (Medtronic) and guided by an Achieve™ Mapping Catheter (Medtronic).11 We aimed to complete ablation of the CTI before or during the treatment of the left superior PV (Figure 1). The Freezor™ MAX catheter was then moved to the coronary sinus (CS) and used to deliver therapy at the point closest the left inferior PV while this vein was being treated. During therapy of the right PVs, the diagnostic catheter from the CS was moved to the right subclavian vein and used to stimulate the right phrenic nerve, and the Freezor™ MAX was used to ablate the right atrial surface of the interatrial septum adjacent to the right PVs.
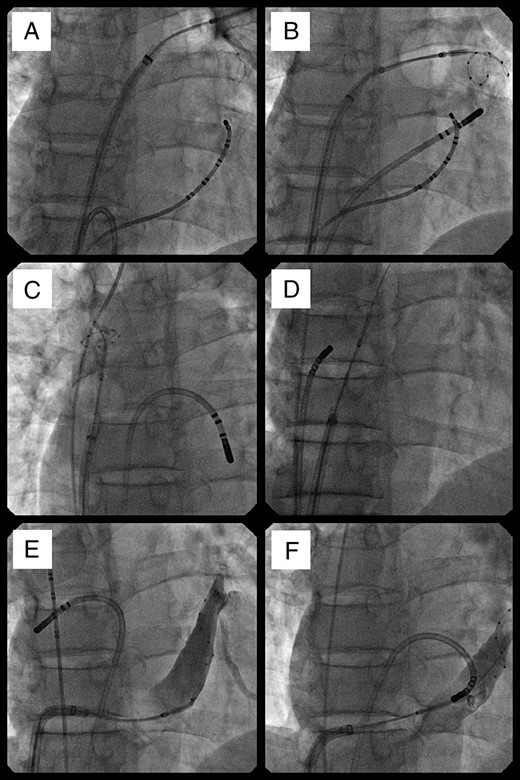
Radiographic images of the simultaneous use of a cryoballoon and a focal cryotherapy catheter. Al views are in an anteroposterior projection. Starting before transseptal puncture and continuing during treatment of the right superior vein, a focal catheter was used to ablate the cavotricuspid isthmus (A). It was moved to the coronary sinus during cryoballoon treatment of the left inferior pulmonary vein (B), then to the inter-atrial septum as the balloon treated the ostium of the superior vena cava (C), and the right atrial aspect of the inter-atrial septum (D). It was moved back to the left atrium as the balloon was used to treat the ostium of the coronary sinus (E).
After administration of two therapies of 240 s each to each PV, the cryoballoon was moved to the right atrium and used to treat the right atrial aspect of the interatrial septum (two deliveries of 240 s each), then used to treat the SVC-right atrial junction (two therapies of 90–180 s) then the ostium of the CS (two therapies of 240 s). The cryoballoon was then removed, and in its place a second Freezor™ MAX catheter was introduced.
Using the two Freezor™ MAX catheters in concert, we treated the endocardial and epicardial aspects of the CS, the isthmus between the mitral annulus and the left inferior PV and then the roof of the left atrium (Figure 2). The catheters were then used separately to map and ablate areas of fractionation.
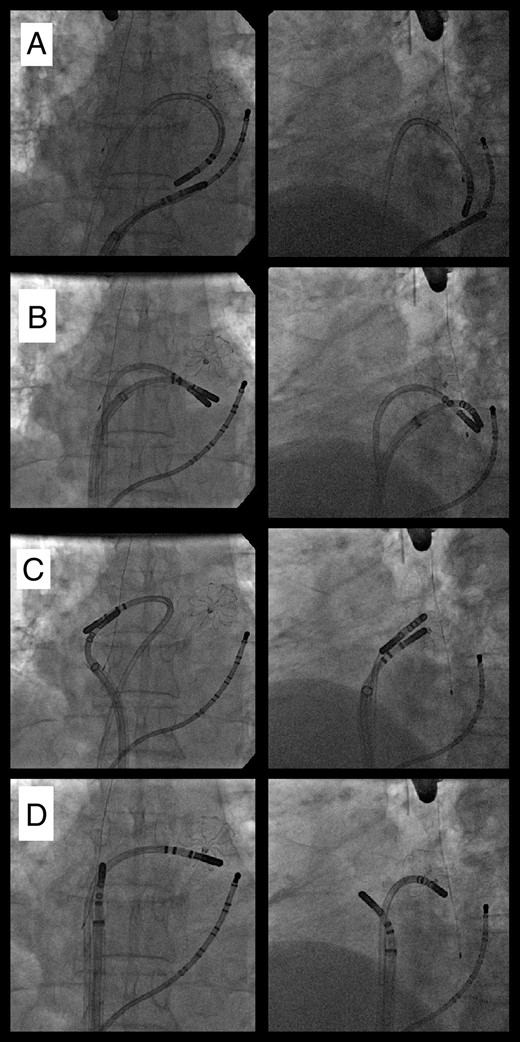
Radiographic images of the simultaneous use of two focal cryocatheters. Viewed in an anteroposterior projection (left panels) and a left anterior oblique projection (right panels) a patient with closure device in the left atrial appendage has had the pulmonary veins isolated. After removal of the balloon, one focal catheter was used to treat the epicardial myocardium through the coronary sinus as other was used to create an endocardial coronary sinus line (A). Both catheters were than used together to treat the mitral isthmus (B) then the left atrial roof (C), then used separately to treat areas of fractionation (D).
Radiofrequency ablation
In the RF group, the Carto® mapping system and a Lasso®-NAV circular mapping catheter (Johnson & Johnson, New Brunswick, NJ, USA) were used to guide the creation of RF lesions with a Biosense Webster ThermoCool® SmartTouch® Surround flow® ablation catheter (STSF, Johnson & Johnson). The stepwise approach consisted of isolation of the PVs by encirclement in the antrum, then performance of a line of lesions from the mitral annulus to the left inferior PV, a line across the roof of the left atrium, then across the floor of the left atrium, a line along the endocardial aspect of the left atrium adjacent to CS, a line across CTI, and then ablation of fractionated electrograms in the left atrium and then the right.
Follow-up
All patients were scheduled to be discharged from hospital the day after the procedure. Every participant was interviewed at their bedside the morning after ablation and a telephone interview was conducted at 2 months after the procedure by a dedicated researcher. Further follow-ups were scheduled at 3-, 6-, and 12-month post-ablation, then at 3 and 5 years, with attendance at an arrhythmia clinic, including a 48-h Holter monitor at each follow-up point. Patients were instructed to contact us for follow-up outside these time-windows if there was any recurrence of symptoms, in which case 12-lead and ambulatory electrocardiograms (ECGs) were recorded. If an arrhythmia was documented outside the blanking period, patients were offered additional ablation and data were collected on the nature of the recurrent arrhythmia and the findings on the repeat procedure.
Reference group
Following the release of the STAR AF II trial, we collected data retrospectively on a reference group of patients who had received PVI only using cryotherapy. We reviewed all patients who had undergone ablation for persistent AF at our centre during the recruitment period of this trial who would have met the inclusion criteria for the study but were not recruited to it. Within this group, we identified those whose ablation was performed using cryoballoon PVI with or without CTI ablation but no ablation at other sites. This was defined as the reference group. Because these patients were not participating in the research protocol, follow-up was not formally structured.
Definitions
Technical success for all procedures was defined as the attainment of isolation of all PVs that persisted to the end of the procedure. Achievement of bidirectional block at the CTI was also required for trial participants but not in the reference group.
AF recurrence was defined as any episode of AF/AT >30 s, either symptomatic or asymptomatic, documented on a Holter ECG and/or 12-lead resting ECG or other ECG recording devices during follow-up. Calculations of freedom from AF/AT were at 6 months post-ablation procedure. At the time that the study was designed in 2011, a blanking period of 6 months was commonly used. Clinical success was defined as freedom from AF for >5 years after the index procedure.
Power calculation
Due to the difference in clinical experience between the two ablation modalities and methodologies in ablation to SR in persistent AF, sampling size calculation was limited by anticipated incidence. The pre-trial estimate to power the study (alpha = 0.80) was a sampling population between n = 88–99, and the upper limit was chosen for the study, giving at least 15% clinical difference between the two groups in the anticipated incidence of the study outcome-ablation to SR.
Statistical analysis
Continuous variables are expressed as mean (SD), other variables as median (inter-quartile range). Rates of occurrence of events were compared by the log-rank method. The differences between variables were analysed by χ2, Fisher’s exact test, or T-test as appropriate. Analysis was in SPSS.
Results
We recruited 100 patients from 3 October 2012 to 16 March 2016, with 1:1 randomization to each modality of ablation. Patients were well matched for clinical characteristics (Table 1). At the time of the procedure, all patients had been in AF persistently for ≥6 months. All patients underwent their ablation according to protocol. One patient in the cryotherapy group converted to SR during induction of anaesthesia; two patients in the cryotherapy group and one in the RF group were found to have isthmus dependent flutter on placement of electrophysiological catheters and were converted to SR by ablation of the CTI before any left atrial ablation. These patients were not considered in the analysis of ablation to SR.
| . | Multi-catheter cryotherapy group . | RF group . | P (between trial groups) . | Reference group . | P (reference group vs. trial population) . |
|---|---|---|---|---|---|
| n | 50 | 50 | – | 27 | – |
| Age (years) | 63 ± 9 | 63 ± 10 | NS | 65 ± 10 | NS |
| Sex (male) | 40 (80%) | 38 (76%) | NS | 21 (77%) | NS |
| Duration of continuous AF at time of ablation (months) | 33 ± 34 | 26 ± 19 | NS | 10.5 ± 6.9 | 0.012 |
| Body mass index (kg/m2) | 31 ± 6 | 30 ± 5 | NS | 30 ± 5 | NS |
| Left atrial diameter (mm) | 45 ± 5 | 45 ± 7 | NS | 39 ± 6 | 0.0034 |
| LV ejection fraction (%) | 51 ± 12 | 54 ± 10 | NS | 54 8 | NS |
| Use of general anaesthesia | 30 (60%) | 34 (68%) | NS | 23 (85%) | NS |
| Medical history | |||||
| Diabetes | 7 (14%) | 6 (12%) | NS | 2 (7%) | NS |
| Coronary artery disease | 6 (12%) | 8 (16%) | NS | 5 (19%) | NS |
| Hypertension | 19 (38%) | 19 (38%) | NS | 6 (22%) | NS |
| LV systolic dysfunction | 13 (26%) | 9 (18%) | NS | 5 (19%) | NS |
| Prior ablation for atrial flutter | 4 (8%) | 4 (8%) | NS | 2 (7%) | NS |
| CHADSVASC score | |||||
| 0 | 11 (22%) | 11 (22%) | NS | 8 (30%) | NS |
| 1 | 15 (30%) | 13 (26%) | NS | 7 (26%) | NS |
| 2 | 10 (20%) | 16 (32%) | NS | 6 (22%) | NS |
| >2 | 14 (28%) | 10 (20%) | NS | 6 (22%) | NS |
| Previous anti-arrhythmia drugs failed | |||||
| 0 | 7 (14%) | 7 (14%) | NS | Unknown | – |
| 1 | 23 (46%) | 24 (48%) | NS | Unknown | – |
| 2 | 14 (28%) | 12 (24%) | NS | Unknown | – |
| >2 | 4 (8%) | 5 (10%) | NS | Unknown | – |
| Anti-arrhythmic medications on discharge | |||||
| Flecainide | 0 | 0 | NS | 7 | <0.001 |
| Amiodarone | 0 | 0 | NS | 6 | <0.001 |
| Sotalol | 0 | 1 (2%) | NS | 0 | NS |
| Other anti-arrhythmic | 0 | 0 | NS | 0 | NS |
| . | Multi-catheter cryotherapy group . | RF group . | P (between trial groups) . | Reference group . | P (reference group vs. trial population) . |
|---|---|---|---|---|---|
| n | 50 | 50 | – | 27 | – |
| Age (years) | 63 ± 9 | 63 ± 10 | NS | 65 ± 10 | NS |
| Sex (male) | 40 (80%) | 38 (76%) | NS | 21 (77%) | NS |
| Duration of continuous AF at time of ablation (months) | 33 ± 34 | 26 ± 19 | NS | 10.5 ± 6.9 | 0.012 |
| Body mass index (kg/m2) | 31 ± 6 | 30 ± 5 | NS | 30 ± 5 | NS |
| Left atrial diameter (mm) | 45 ± 5 | 45 ± 7 | NS | 39 ± 6 | 0.0034 |
| LV ejection fraction (%) | 51 ± 12 | 54 ± 10 | NS | 54 8 | NS |
| Use of general anaesthesia | 30 (60%) | 34 (68%) | NS | 23 (85%) | NS |
| Medical history | |||||
| Diabetes | 7 (14%) | 6 (12%) | NS | 2 (7%) | NS |
| Coronary artery disease | 6 (12%) | 8 (16%) | NS | 5 (19%) | NS |
| Hypertension | 19 (38%) | 19 (38%) | NS | 6 (22%) | NS |
| LV systolic dysfunction | 13 (26%) | 9 (18%) | NS | 5 (19%) | NS |
| Prior ablation for atrial flutter | 4 (8%) | 4 (8%) | NS | 2 (7%) | NS |
| CHADSVASC score | |||||
| 0 | 11 (22%) | 11 (22%) | NS | 8 (30%) | NS |
| 1 | 15 (30%) | 13 (26%) | NS | 7 (26%) | NS |
| 2 | 10 (20%) | 16 (32%) | NS | 6 (22%) | NS |
| >2 | 14 (28%) | 10 (20%) | NS | 6 (22%) | NS |
| Previous anti-arrhythmia drugs failed | |||||
| 0 | 7 (14%) | 7 (14%) | NS | Unknown | – |
| 1 | 23 (46%) | 24 (48%) | NS | Unknown | – |
| 2 | 14 (28%) | 12 (24%) | NS | Unknown | – |
| >2 | 4 (8%) | 5 (10%) | NS | Unknown | – |
| Anti-arrhythmic medications on discharge | |||||
| Flecainide | 0 | 0 | NS | 7 | <0.001 |
| Amiodarone | 0 | 0 | NS | 6 | <0.001 |
| Sotalol | 0 | 1 (2%) | NS | 0 | NS |
| Other anti-arrhythmic | 0 | 0 | NS | 0 | NS |
AF, atrial fibrillation; LV, left ventricular; RF, radiofrequency.
| . | Multi-catheter cryotherapy group . | RF group . | P (between trial groups) . | Reference group . | P (reference group vs. trial population) . |
|---|---|---|---|---|---|
| n | 50 | 50 | – | 27 | – |
| Age (years) | 63 ± 9 | 63 ± 10 | NS | 65 ± 10 | NS |
| Sex (male) | 40 (80%) | 38 (76%) | NS | 21 (77%) | NS |
| Duration of continuous AF at time of ablation (months) | 33 ± 34 | 26 ± 19 | NS | 10.5 ± 6.9 | 0.012 |
| Body mass index (kg/m2) | 31 ± 6 | 30 ± 5 | NS | 30 ± 5 | NS |
| Left atrial diameter (mm) | 45 ± 5 | 45 ± 7 | NS | 39 ± 6 | 0.0034 |
| LV ejection fraction (%) | 51 ± 12 | 54 ± 10 | NS | 54 8 | NS |
| Use of general anaesthesia | 30 (60%) | 34 (68%) | NS | 23 (85%) | NS |
| Medical history | |||||
| Diabetes | 7 (14%) | 6 (12%) | NS | 2 (7%) | NS |
| Coronary artery disease | 6 (12%) | 8 (16%) | NS | 5 (19%) | NS |
| Hypertension | 19 (38%) | 19 (38%) | NS | 6 (22%) | NS |
| LV systolic dysfunction | 13 (26%) | 9 (18%) | NS | 5 (19%) | NS |
| Prior ablation for atrial flutter | 4 (8%) | 4 (8%) | NS | 2 (7%) | NS |
| CHADSVASC score | |||||
| 0 | 11 (22%) | 11 (22%) | NS | 8 (30%) | NS |
| 1 | 15 (30%) | 13 (26%) | NS | 7 (26%) | NS |
| 2 | 10 (20%) | 16 (32%) | NS | 6 (22%) | NS |
| >2 | 14 (28%) | 10 (20%) | NS | 6 (22%) | NS |
| Previous anti-arrhythmia drugs failed | |||||
| 0 | 7 (14%) | 7 (14%) | NS | Unknown | – |
| 1 | 23 (46%) | 24 (48%) | NS | Unknown | – |
| 2 | 14 (28%) | 12 (24%) | NS | Unknown | – |
| >2 | 4 (8%) | 5 (10%) | NS | Unknown | – |
| Anti-arrhythmic medications on discharge | |||||
| Flecainide | 0 | 0 | NS | 7 | <0.001 |
| Amiodarone | 0 | 0 | NS | 6 | <0.001 |
| Sotalol | 0 | 1 (2%) | NS | 0 | NS |
| Other anti-arrhythmic | 0 | 0 | NS | 0 | NS |
| . | Multi-catheter cryotherapy group . | RF group . | P (between trial groups) . | Reference group . | P (reference group vs. trial population) . |
|---|---|---|---|---|---|
| n | 50 | 50 | – | 27 | – |
| Age (years) | 63 ± 9 | 63 ± 10 | NS | 65 ± 10 | NS |
| Sex (male) | 40 (80%) | 38 (76%) | NS | 21 (77%) | NS |
| Duration of continuous AF at time of ablation (months) | 33 ± 34 | 26 ± 19 | NS | 10.5 ± 6.9 | 0.012 |
| Body mass index (kg/m2) | 31 ± 6 | 30 ± 5 | NS | 30 ± 5 | NS |
| Left atrial diameter (mm) | 45 ± 5 | 45 ± 7 | NS | 39 ± 6 | 0.0034 |
| LV ejection fraction (%) | 51 ± 12 | 54 ± 10 | NS | 54 8 | NS |
| Use of general anaesthesia | 30 (60%) | 34 (68%) | NS | 23 (85%) | NS |
| Medical history | |||||
| Diabetes | 7 (14%) | 6 (12%) | NS | 2 (7%) | NS |
| Coronary artery disease | 6 (12%) | 8 (16%) | NS | 5 (19%) | NS |
| Hypertension | 19 (38%) | 19 (38%) | NS | 6 (22%) | NS |
| LV systolic dysfunction | 13 (26%) | 9 (18%) | NS | 5 (19%) | NS |
| Prior ablation for atrial flutter | 4 (8%) | 4 (8%) | NS | 2 (7%) | NS |
| CHADSVASC score | |||||
| 0 | 11 (22%) | 11 (22%) | NS | 8 (30%) | NS |
| 1 | 15 (30%) | 13 (26%) | NS | 7 (26%) | NS |
| 2 | 10 (20%) | 16 (32%) | NS | 6 (22%) | NS |
| >2 | 14 (28%) | 10 (20%) | NS | 6 (22%) | NS |
| Previous anti-arrhythmia drugs failed | |||||
| 0 | 7 (14%) | 7 (14%) | NS | Unknown | – |
| 1 | 23 (46%) | 24 (48%) | NS | Unknown | – |
| 2 | 14 (28%) | 12 (24%) | NS | Unknown | – |
| >2 | 4 (8%) | 5 (10%) | NS | Unknown | – |
| Anti-arrhythmic medications on discharge | |||||
| Flecainide | 0 | 0 | NS | 7 | <0.001 |
| Amiodarone | 0 | 0 | NS | 6 | <0.001 |
| Sotalol | 0 | 1 (2%) | NS | 0 | NS |
| Other anti-arrhythmic | 0 | 0 | NS | 0 | NS |
AF, atrial fibrillation; LV, left ventricular; RF, radiofrequency.
For the reference group, we identified 27 patients who had received cryoballoon PVI for persistent AF that would have met trial entry criteria but were not considered by their treating physician for trial recruitment. Their baseline criteria differed from those of the randomized study in having a younger mean age, and shorter AF duration.
Safety
Procedural complications occurred in four patients in the multi-catheter cryotherapy group: two cases of profound hypotension occurred after administration of adenosine. In both cases, this progressed to cardiac arrest but recovered completely after resuscitation including the administration of adrenaline and fluid. These cases and a similar case that occurred after RF ablation unrelated to this study were investigated and judged to have been related to adverse reaction to protamine.20 One patient experienced a pseudoaneurysm of the right femoral artery that required prothrombin injection, one had a pericardial effusion that required drainage at 3 h post-ablation.
In the RF group, there were two complications: a skin burn that required repeated dressing for 3 months and a respiratory infection that led to a pleural effusion that required drainage at 1 week post-ablation. In the reference group, one patient suffered a phrenic nerve palsy that persisted to hospital discharge.
Lesion sets
All patients achieved PVI that lasted to the end of the procedure. Bidirectional block of the CTI was achieved for all patients in whom it was attempted and tested. Other lesion sets were attempted in all patients and were acutely successful in most (Tables 2 and 3).
| . | Multi-catheter cryotherapy group (n = 50) . | RF group (n = 50) . | P . |
|---|---|---|---|
| AF terminated or organized before ablation | 3 (4%) | 1 (2%) | NS |
| Ablated to sinus rhythm | 24/47 (51%) | 11/49 (22%) | 0.004 |
| All pulmonary veins isolated until end of procedure | 50/50 (100%) | 50/50 (100%) | NS |
| Remapping of lines after ablation | |||
| Performed | 44 | 44 | NS |
| Impossible due to incessant arrhythmia | 0 | 2 | NS |
| Omitted because of patient intolerance/instabilitya | 3 | 2 | NS |
| Omitted due to time constrainta | 2 | 2 | NS |
| Cavotricuspid Isthmus ablation | |||
| Previously ablated, block verified | 4 | 4 | NS |
| Attempted on this procedure | 46 | 46 | NS |
| Success undetermined because inadequate mapping | 1/46 (2%) | 2/46 (4%) | NS |
| Verified success | 45/46 (98%) | 44/46 (96%) | NS |
| Failed | 0 | 0 | NS |
| Roof line ablation | |||
| Attempted | 42 | 49 | |
| Success undetermined because inadequate mapping | 5/42 (12%) | 6/49 (12%) | NS |
| Verified success | 33/42 (79%) | 42/49 (86%) | NS |
| Failed | 4/42 (10%) | 1/49 (2%) | NS |
| Mitral line ablation | |||
| Attempted | 45 | 49 | |
| Success undetermined because inadequate mapping | 5/45 (9%) | 6/49 (12%) | NS |
| Undetermined due to absence of CS electrograms | 6/45 (13%) | 0/49 (0%) | 0.01 |
| Verified success | 29/45 (64%) | 30/49 (61%) | NS |
| Failed | 5/45 (9%) | 13/49 (27%) | NS |
| Ablation of fragmented potentials | |||
| Left atrium | 24 (48%) | 36 (72%) | 0.02 |
| Right atrium | 12 (24%) | 2 (4%) | 0.008 |
| Procedure duration (min) | 227±54 | 268±54 | <0.001 |
| Fluoroscopy duration (min) | 48±16 | 44±13 | NS |
| . | Multi-catheter cryotherapy group (n = 50) . | RF group (n = 50) . | P . |
|---|---|---|---|
| AF terminated or organized before ablation | 3 (4%) | 1 (2%) | NS |
| Ablated to sinus rhythm | 24/47 (51%) | 11/49 (22%) | 0.004 |
| All pulmonary veins isolated until end of procedure | 50/50 (100%) | 50/50 (100%) | NS |
| Remapping of lines after ablation | |||
| Performed | 44 | 44 | NS |
| Impossible due to incessant arrhythmia | 0 | 2 | NS |
| Omitted because of patient intolerance/instabilitya | 3 | 2 | NS |
| Omitted due to time constrainta | 2 | 2 | NS |
| Cavotricuspid Isthmus ablation | |||
| Previously ablated, block verified | 4 | 4 | NS |
| Attempted on this procedure | 46 | 46 | NS |
| Success undetermined because inadequate mapping | 1/46 (2%) | 2/46 (4%) | NS |
| Verified success | 45/46 (98%) | 44/46 (96%) | NS |
| Failed | 0 | 0 | NS |
| Roof line ablation | |||
| Attempted | 42 | 49 | |
| Success undetermined because inadequate mapping | 5/42 (12%) | 6/49 (12%) | NS |
| Verified success | 33/42 (79%) | 42/49 (86%) | NS |
| Failed | 4/42 (10%) | 1/49 (2%) | NS |
| Mitral line ablation | |||
| Attempted | 45 | 49 | |
| Success undetermined because inadequate mapping | 5/45 (9%) | 6/49 (12%) | NS |
| Undetermined due to absence of CS electrograms | 6/45 (13%) | 0/49 (0%) | 0.01 |
| Verified success | 29/45 (64%) | 30/49 (61%) | NS |
| Failed | 5/45 (9%) | 13/49 (27%) | NS |
| Ablation of fragmented potentials | |||
| Left atrium | 24 (48%) | 36 (72%) | 0.02 |
| Right atrium | 12 (24%) | 2 (4%) | 0.008 |
| Procedure duration (min) | 227±54 | 268±54 | <0.001 |
| Fluoroscopy duration (min) | 48±16 | 44±13 | NS |
AF, atrial fibrillation; CTI, cavotricuspid isthmus; CS, coronary sinus.
In cases in which remapping was impossible due to patient agitation or clinical instability and in cases in which full re-mapping was impossible due to time constraints, the CTI was always mapped.
| . | Multi-catheter cryotherapy group (n = 50) . | RF group (n = 50) . | P . |
|---|---|---|---|
| AF terminated or organized before ablation | 3 (4%) | 1 (2%) | NS |
| Ablated to sinus rhythm | 24/47 (51%) | 11/49 (22%) | 0.004 |
| All pulmonary veins isolated until end of procedure | 50/50 (100%) | 50/50 (100%) | NS |
| Remapping of lines after ablation | |||
| Performed | 44 | 44 | NS |
| Impossible due to incessant arrhythmia | 0 | 2 | NS |
| Omitted because of patient intolerance/instabilitya | 3 | 2 | NS |
| Omitted due to time constrainta | 2 | 2 | NS |
| Cavotricuspid Isthmus ablation | |||
| Previously ablated, block verified | 4 | 4 | NS |
| Attempted on this procedure | 46 | 46 | NS |
| Success undetermined because inadequate mapping | 1/46 (2%) | 2/46 (4%) | NS |
| Verified success | 45/46 (98%) | 44/46 (96%) | NS |
| Failed | 0 | 0 | NS |
| Roof line ablation | |||
| Attempted | 42 | 49 | |
| Success undetermined because inadequate mapping | 5/42 (12%) | 6/49 (12%) | NS |
| Verified success | 33/42 (79%) | 42/49 (86%) | NS |
| Failed | 4/42 (10%) | 1/49 (2%) | NS |
| Mitral line ablation | |||
| Attempted | 45 | 49 | |
| Success undetermined because inadequate mapping | 5/45 (9%) | 6/49 (12%) | NS |
| Undetermined due to absence of CS electrograms | 6/45 (13%) | 0/49 (0%) | 0.01 |
| Verified success | 29/45 (64%) | 30/49 (61%) | NS |
| Failed | 5/45 (9%) | 13/49 (27%) | NS |
| Ablation of fragmented potentials | |||
| Left atrium | 24 (48%) | 36 (72%) | 0.02 |
| Right atrium | 12 (24%) | 2 (4%) | 0.008 |
| Procedure duration (min) | 227±54 | 268±54 | <0.001 |
| Fluoroscopy duration (min) | 48±16 | 44±13 | NS |
| . | Multi-catheter cryotherapy group (n = 50) . | RF group (n = 50) . | P . |
|---|---|---|---|
| AF terminated or organized before ablation | 3 (4%) | 1 (2%) | NS |
| Ablated to sinus rhythm | 24/47 (51%) | 11/49 (22%) | 0.004 |
| All pulmonary veins isolated until end of procedure | 50/50 (100%) | 50/50 (100%) | NS |
| Remapping of lines after ablation | |||
| Performed | 44 | 44 | NS |
| Impossible due to incessant arrhythmia | 0 | 2 | NS |
| Omitted because of patient intolerance/instabilitya | 3 | 2 | NS |
| Omitted due to time constrainta | 2 | 2 | NS |
| Cavotricuspid Isthmus ablation | |||
| Previously ablated, block verified | 4 | 4 | NS |
| Attempted on this procedure | 46 | 46 | NS |
| Success undetermined because inadequate mapping | 1/46 (2%) | 2/46 (4%) | NS |
| Verified success | 45/46 (98%) | 44/46 (96%) | NS |
| Failed | 0 | 0 | NS |
| Roof line ablation | |||
| Attempted | 42 | 49 | |
| Success undetermined because inadequate mapping | 5/42 (12%) | 6/49 (12%) | NS |
| Verified success | 33/42 (79%) | 42/49 (86%) | NS |
| Failed | 4/42 (10%) | 1/49 (2%) | NS |
| Mitral line ablation | |||
| Attempted | 45 | 49 | |
| Success undetermined because inadequate mapping | 5/45 (9%) | 6/49 (12%) | NS |
| Undetermined due to absence of CS electrograms | 6/45 (13%) | 0/49 (0%) | 0.01 |
| Verified success | 29/45 (64%) | 30/49 (61%) | NS |
| Failed | 5/45 (9%) | 13/49 (27%) | NS |
| Ablation of fragmented potentials | |||
| Left atrium | 24 (48%) | 36 (72%) | 0.02 |
| Right atrium | 12 (24%) | 2 (4%) | 0.008 |
| Procedure duration (min) | 227±54 | 268±54 | <0.001 |
| Fluoroscopy duration (min) | 48±16 | 44±13 | NS |
AF, atrial fibrillation; CTI, cavotricuspid isthmus; CS, coronary sinus.
In cases in which remapping was impossible due to patient agitation or clinical instability and in cases in which full re-mapping was impossible due to time constraints, the CTI was always mapped.
| Target . | Number of procedures attempted . | Intended freeze duration (s) . | Effects on electrograms . | Events requiring termination of freeze . |
|---|---|---|---|---|
| Inter-atrial septum | 43 | 240s × 2 | AF termination × 1 | None |
| Coronary sinus ostium | 30 | 240s × 2 | AF organized to AT × 1. Elimination of coronary sinus electrograms in 4 (but also seen in 2 patients with use of focal catheter only in coronary sinus) | Transient drop-in ventricular response rate to <45/min in 5 cases at 60–180 s from onset of freeze. Full resolution within 1 min in all cases |
| SVC-right atrial junction | 25 | 120s × 2 | SVC isolation in 2 of 4 cases with clear electrograms | Transient patient discomfort × 1 at 60 s Transient weakening of phrenic response × 1 at 74 s on second freeze, resolved within 5 min |
| Target . | Number of procedures attempted . | Intended freeze duration (s) . | Effects on electrograms . | Events requiring termination of freeze . |
|---|---|---|---|---|
| Inter-atrial septum | 43 | 240s × 2 | AF termination × 1 | None |
| Coronary sinus ostium | 30 | 240s × 2 | AF organized to AT × 1. Elimination of coronary sinus electrograms in 4 (but also seen in 2 patients with use of focal catheter only in coronary sinus) | Transient drop-in ventricular response rate to <45/min in 5 cases at 60–180 s from onset of freeze. Full resolution within 1 min in all cases |
| SVC-right atrial junction | 25 | 120s × 2 | SVC isolation in 2 of 4 cases with clear electrograms | Transient patient discomfort × 1 at 60 s Transient weakening of phrenic response × 1 at 74 s on second freeze, resolved within 5 min |
AF, atrial fibrillation; AT, atrial tachycardia; SVC, superior vena cava.
| Target . | Number of procedures attempted . | Intended freeze duration (s) . | Effects on electrograms . | Events requiring termination of freeze . |
|---|---|---|---|---|
| Inter-atrial septum | 43 | 240s × 2 | AF termination × 1 | None |
| Coronary sinus ostium | 30 | 240s × 2 | AF organized to AT × 1. Elimination of coronary sinus electrograms in 4 (but also seen in 2 patients with use of focal catheter only in coronary sinus) | Transient drop-in ventricular response rate to <45/min in 5 cases at 60–180 s from onset of freeze. Full resolution within 1 min in all cases |
| SVC-right atrial junction | 25 | 120s × 2 | SVC isolation in 2 of 4 cases with clear electrograms | Transient patient discomfort × 1 at 60 s Transient weakening of phrenic response × 1 at 74 s on second freeze, resolved within 5 min |
| Target . | Number of procedures attempted . | Intended freeze duration (s) . | Effects on electrograms . | Events requiring termination of freeze . |
|---|---|---|---|---|
| Inter-atrial septum | 43 | 240s × 2 | AF termination × 1 | None |
| Coronary sinus ostium | 30 | 240s × 2 | AF organized to AT × 1. Elimination of coronary sinus electrograms in 4 (but also seen in 2 patients with use of focal catheter only in coronary sinus) | Transient drop-in ventricular response rate to <45/min in 5 cases at 60–180 s from onset of freeze. Full resolution within 1 min in all cases |
| SVC-right atrial junction | 25 | 120s × 2 | SVC isolation in 2 of 4 cases with clear electrograms | Transient patient discomfort × 1 at 60 s Transient weakening of phrenic response × 1 at 74 s on second freeze, resolved within 5 min |
AF, atrial fibrillation; AT, atrial tachycardia; SVC, superior vena cava.
| . | Multi-catheter cryotherapy group (n = 50) . | RF group (n = 50) . | P . |
|---|---|---|---|
| Recurrence with AF | 17 | 18 | NS |
| Recurrence with regular arrhythmia | 16 | 20 | NS |
| Re-study refused by patient | 0 | 1 | NS |
| Currently awaiting re-mapping and ablation | 0 | 2 | NS |
| Rhythm disorganized or terminated before re-map | 8 | 7 | NS |
| Remapping and ablation performed | 8 | 11a | NS |
| Characteristics of re-mapped clinical tachyarrhythmias | |||
| Typical cavotricuspid dependent atrial flutter | 1 | 2 | NS |
| Mitral annular circuit | 3 | 3 | NS |
| Roof-dependent tachycardia | 1 | 1 | NS |
| Micro-reentrant circuit involving inter-atrial septum | 3 | 2 | NS |
| Micro-reentrant or focal in other site | 0 | 3 | NS |
| . | Multi-catheter cryotherapy group (n = 50) . | RF group (n = 50) . | P . |
|---|---|---|---|
| Recurrence with AF | 17 | 18 | NS |
| Recurrence with regular arrhythmia | 16 | 20 | NS |
| Re-study refused by patient | 0 | 1 | NS |
| Currently awaiting re-mapping and ablation | 0 | 2 | NS |
| Rhythm disorganized or terminated before re-map | 8 | 7 | NS |
| Remapping and ablation performed | 8 | 11a | NS |
| Characteristics of re-mapped clinical tachyarrhythmias | |||
| Typical cavotricuspid dependent atrial flutter | 1 | 2 | NS |
| Mitral annular circuit | 3 | 3 | NS |
| Roof-dependent tachycardia | 1 | 1 | NS |
| Micro-reentrant circuit involving inter-atrial septum | 3 | 2 | NS |
| Micro-reentrant or focal in other site | 0 | 3 | NS |
AF, atrial fibrillation.
Includes one case that re-presented as AF but had organized to a regular tachycardia by the time of admission for re-ablation.
| . | Multi-catheter cryotherapy group (n = 50) . | RF group (n = 50) . | P . |
|---|---|---|---|
| Recurrence with AF | 17 | 18 | NS |
| Recurrence with regular arrhythmia | 16 | 20 | NS |
| Re-study refused by patient | 0 | 1 | NS |
| Currently awaiting re-mapping and ablation | 0 | 2 | NS |
| Rhythm disorganized or terminated before re-map | 8 | 7 | NS |
| Remapping and ablation performed | 8 | 11a | NS |
| Characteristics of re-mapped clinical tachyarrhythmias | |||
| Typical cavotricuspid dependent atrial flutter | 1 | 2 | NS |
| Mitral annular circuit | 3 | 3 | NS |
| Roof-dependent tachycardia | 1 | 1 | NS |
| Micro-reentrant circuit involving inter-atrial septum | 3 | 2 | NS |
| Micro-reentrant or focal in other site | 0 | 3 | NS |
| . | Multi-catheter cryotherapy group (n = 50) . | RF group (n = 50) . | P . |
|---|---|---|---|
| Recurrence with AF | 17 | 18 | NS |
| Recurrence with regular arrhythmia | 16 | 20 | NS |
| Re-study refused by patient | 0 | 1 | NS |
| Currently awaiting re-mapping and ablation | 0 | 2 | NS |
| Rhythm disorganized or terminated before re-map | 8 | 7 | NS |
| Remapping and ablation performed | 8 | 11a | NS |
| Characteristics of re-mapped clinical tachyarrhythmias | |||
| Typical cavotricuspid dependent atrial flutter | 1 | 2 | NS |
| Mitral annular circuit | 3 | 3 | NS |
| Roof-dependent tachycardia | 1 | 1 | NS |
| Micro-reentrant circuit involving inter-atrial septum | 3 | 2 | NS |
| Micro-reentrant or focal in other site | 0 | 3 | NS |
AF, atrial fibrillation.
Includes one case that re-presented as AF but had organized to a regular tachycardia by the time of admission for re-ablation.
End points
The procedural target of ablation to SR was achieved more frequently and more quickly in the multi-catheter cryotherapy group than in the RF group (Table 2 and Figure 3). Procedure duration was shorter in the multi-catheter cryotherapy group but was associated with similar fluoroscopy duration (Table 2).
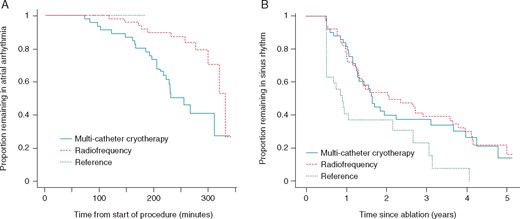
Kaplan–Meier plot of arrhythmia resolution and recurrence. During ablation, an increasing proportion of trial patients return to sinus rhythm in response to ablation as the procedure progresses, with a higher rate of conversion in the multi-catheter cryotherapy group than in the radiofrequency group (A). In the reference group, spontaneous conversion to sinus rhythm did not occur. In the years after ablation, after a 6-month blanking period, an increasing proportion of patients developed atrial fibrillation, atrial tachycardia or atrial flutter (B) with a similar rate in both of the treatment groups, a higher rate in the reference group.
In the reference group, one patient received electrical cardioversion at the start of the procedure to facilitate the interpretation of PV electrograms. All other patients remained in AF throughout the procedure and received electrical cardioversion at the end. Procedures in the reference group were substantially shorter than in the trial patients (132 ± 32 min, P < 0.001 for both trial groups), as was the fluoroscopy duration (27 ± 13 min, P < 0.001 for both trial groups).
Long-term clinical outcome
One patient in the cryotherapy group was lost to follow-up within the blanking period and was not considered in the analysis of arrhythmia recurrence. One died of progressive respiratory disease at 9 months. All others remained under follow-up to at least 2 years, but a further 11 were lost to follow-up between 2 and 5 years. Continued SR was documented to the last available follow-up in 16/49 patients (33%) in the multi-catheter cryotherapy group to 3.0 ± 1.6 years post-ablation and in 12/50 patients (24%) in the RF group at 4.0 ± 1.2 years post-ablation. The yearly rate of arrhythmia recurrence was similar (P = NS, Figure 3).
In the reference group, follow-up information was less complete. Only 5/27 patients (19%) had sustained clinical success to the last available follow-up visit which was 1.7 ± 0.4 years post-ablation, a yearly recurrence rate that was significantly higher than in either trial group (P = 0.007 for multi-catheter cryotherapy, P = 0.0017 for RF, Figure 3).
Mode of arrhythmia recurrence
Of the patients who had a recurrent atrial tachyarrhythmia outside the blanking period, regular atrial tachyarrhythmias accounted for approximately half in both trial groups [16/33 (48%) after multi-catheter cryotherapy and 20/38 (53%) in the RF group (P = NS (Non statistically significant))]. The atrial tachyarrhythmia was mapped in most cases and the distribution of tachycardia circuits was similar in both groups (Table 4).
In the reference group, atrial tachycardia accounted for only 2/22 (9%) of the documented recurrences (P = 0.003 and P < 0.001 for comparison with the trial groups). Neither of these had the electrocardiographic features of typical atrial flutter; both were treated pharmacologically.
Lesion set integrity on follow-up procedures
Additional ablation was performed in 27/50 patients in the multi-catheter cryotherapy group at 2.3 ± 1.5 years after the index procedure, 26/50 patients in the RF group at 2.0 ± 1.3 years. In one patient in each group, the presenting arrhythmia was CTI-dependent atrial flutter, so mapping was performed in the right atrium only. In one patient in the RF group, there was incessant arrhythmia that precluded any mapping of lines. All other redo procedures involved detailed 3D-mapping to verify the lesion sets created at the index procedure (Figure 4).
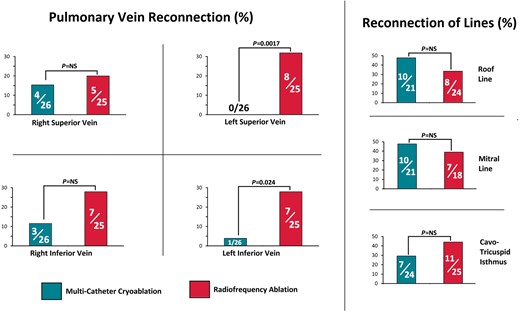
Findings on re-mapping. The left pulmonary veins were more frequently found to be reconnected in the radiofrequency group than in the multi-catheter cryotherapy group. Other lesion sets had a similar rate of failure in both groups. Vein reconnection was ascertained in all patients who had a repeat left atrial procedure. Reconnection of each line was ascertained in patients who had verified block or undeterminable conduction of that line created at the index procedure and whose second procedure permitted remapping of that line.
Discussion
In this randomized trial, we found that multi-catheter cryotherapy could achieve our procedural endpoint of ablation to SR more quickly and in more patients than a standard method using RF energy. This was achieved without an excess of procedural risk despite the unfamiliarity of the methods used. This is the most extensive cryotherapy research protocol investigated in a randomized trial, described in literature.
This trial seemed dated before recruitment ended. The release of the results of STAR AF II at the European Society of Cardiology meeting in September 2014 undermined the rationale for performing the study.9 At the time we initiated this work, ablation to SR was the most widely accepted approach in ablation for long-standing persistent AF7; by the time recruitment ended, the approach had almost been abandoned in favour of a PV isolation only approach or PV isolation plus a variety of adjunctive lesion sets less aggressive than the step-wise approach. In the years since then, the limitations of PV isolation alone have become clear: success rate on a single procedure has remained low, despite advances in technology.
Technology
The equipment used in the RF arm of this study has been superseded by force sensing catheters that give superior results21 and by technology to quantify lesion formation that augments the effectiveness of these tools.22 The equipment used for cryoablation used in the trial has also been superseded by new models of balloon and mapping catheter but there has not been a comparable conceptual leap.
Linear ablation
The long-term clinical outcome from a single procedure in our series was disappointing but in keeping with previous studies allowing for the duration of follow-up. In STAR AF II, the rate of recurrent arrhythmias was already above 50% by 18 months, similar to ours.9 Our study examined a different time window: We used a blanking period of 6 months rather than 3 months and a follow-up duration >5 years.
We had a rate of failure in linear ablation similar to that of STAR AF II, but also an unexpectedly high rate of recurrent conduction at the lines of ablation, particularly at the CTI. When CTI ablation is done as a stand-alone procedure at our centre, we achieve long-term clinical success in 98%.23 The CTI ablations in this study occurred at the end of a procedure of ∼4 h. We did not use a waiting period and may have been rushed in this last part of the ablation. With the technology used, the procedure that we planned was probably too ambitious for the duration available.
Cryotherapy for non-PV targets in AF
The cryoballoon was designed for the specific task of achieving PVI. Our data confirm those of many prior studies in verifying its effectiveness in this task. We have shown that the balloon can be used in non-PV targets, specifically the SVC, the right atrial aspect of the inter-atrial septum and the ostium of the CS. Others have applied it to isolate the posterior wall of the left atrium.15 In all of these, it is effective and appears to be reasonably safe. Although our trial shows a high rate of success in achieving procedural endpoints, this has not translated into any enhancement of clinical success.
Reference group: PVI only
After the results of STAR AF II were published, there was a shift towards minimalization of ablation protocols and PVI only for first time persistent AF ablation, some of which were delivered by cryoballoon. The low rate of success of the reference group, who would have met criteria for the study but were not recruited had cryoballoon-delivered PVI only, confirming that a PVI only strategy is rarely sufficient for this cohort of patients. The recurrence rate was higher in the reference group compared with the RF or multi-catheter groups in this study.
Tolerability, applicability, and cost-effectiveness
The multi-catheter cryoablation procedures were physically demanding for the operator because of the need to track two catheters. But once cryoablation commences, the stability of the catheters to tissue was an advantage unlike with RF. Manoeuvring the Freezor™ MAX catheters into correct position were not particularly difficult due to simplicity of design.
Multi-catheter procedures were unpopular with the electrophysiology lab team because of the multiple initiations and terminations of therapy and because of the need for vigilance for orders to cease therapy whenever it was close to the phrenic nerve or the conduction system. For patients, there was little pain, and no hypothermia, but they did feel unpleasantly cold, particularly on waking after general anaesthetic procedures. We now have better tools for controlling patient temperature,24 but in the absence of a clearly superior clinical outcome, we will not be resuming the use of this technique.
Another factor against the use of multi-catheter cryotherapy is the lack of cost-effectiveness. The multi-catheter cryotherapy protocol required the use of two consoles and two Freezor™ MAX cryocatheters in addition to the standard set of cryoballoon equipment. At list prices, multi-catheter cryotherapy was more expensive than RF.25
Current strategies: left atrial appendage isolation
In this cohort of patients, it remains challenging to improve on clinical outcomes after AF ablation, with neither ablation modality nor methodology being able to achieve more favourable long-term freedom from AF/AT. A recent study, the BELIEF trial,26 investigated the effect of empirical electrical isolation of the left atrial appendage alongside extensive ablation on clinical outcomes and found that at a follow-up duration of 24 months there was improved clinical success (76% vs. 56%, P = 0.003) without significant increase in complications. However, there is evidence from other studies that left atrial appendage isolation (LAAI) is associated with increased thromboembolic risk and therefore patient morbidity.27 Heeger et al.28 reported on the benefits and risks of this approach. In this study, 23.2% of the LAAI patients were found to have post-procedural LAA thrombus compared with propensity-matched controls (2.2%) and this was confirmed by a much higher rate of clinically relevant cerebrovascular events after a 4 year follow-up period (14.7% vs. 2.6%, P = 0.002). This occurred despite adequate anticoagulation.
Limitations
This study was completed in 2012–16; the ablation technology used in this period has been superseded by more advanced 3D mapping systems, ablation catheters and methods in RF delivery, though the cryotherapy tools remain in use. The multi-catheter cryotherapy method was used by an experienced operator with support from an experienced catheter lab team.
Conclusions
Successful ablation for long-standing persistent AF is difficult to achieve on a single procedure by any current methodology. Cryoablation using multiple catheters simultaneously is no more successful than traditional RF methods.
Funding
G.Y.’s salary was supported by an unrestricted educational grant from Medtronic AF Solutions, Medtronic Limited. L.W.L.’s salary was supported by a fellowship from the charity ‘Cardiac Risk in the Young’.
Conflict of interest: M.M.G. has received research funding from Medtronic Inc. and has acted as a consultant for Medtronic, Cook Medical, Biosense Webster, and Attune Medical.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.



