-
PDF
- Split View
-
Views
-
Cite
Cite
Lucas Boersma, Edward Koźluk, Giampiero Maglia, João de Sousa, Olaf Grebe, Lars Eckardt, Robert B Hokanson, Lauren A Hemingway, Ekaterina Ostern, Hyoung-Seob Park, Giovanni Rovaris, Fernando Arribas, Christoph Scharf, Zoltán Csanádi, Ángel Arenal, Francesco Laurenzi, Martijn Klaver, Andreas Goette, Paroxysmal and persistent atrial fibrillation ablation outcomes with the pulmonary vein ablation catheter GOLD duty-cycled phased radiofrequency ablation catheter: quality of life and 12-month efficacy results from the GOLD Atrial Fibrillation Registry, EP Europace, Volume 22, Issue 6, June 2020, Pages 888–896, https://doi.org/10.1093/europace/euaa042
Close - Share Icon Share
Abstract
The GOLD AF Registry has been designed to prospectively assess the population, indications, and outcomes using second-generation phased radiofrequency (RF) ablation (pulmonary vein ablation catheter GOLD) in a global examination of standard-of-care use for the treatment of paroxysmal and persistent atrial fibrillation (AF).
GOLD AF (NCT02433613) is a prospective, observational, multi-centre registry designed to characterize efficacy and safety of phased RF ablation in patients with AF. The primary endpoint was freedom from AF recurrence at 12-month follow-up after a 90-day blanking period. Ancillary objectives include safety, procedural efficiency, and quality of life (QoL). The QoL assessment using the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) and the European Heart Rhythm Association (EHRA) Score of AF-related symptoms was collected at baseline and 12 months. In total, 1054 patients were included in this analysis (age 60.6, 67.6% male, 26.5% PersAF). Kaplan–Meier estimate of freedom from AF recurrence was 77.7% at 12 months. Peri-procedural device or procedure-related complications were observed in 26 (2.5%) patients, with a low stroke rate of 0.3%. One-year post-ablation, the EHRA AF Symptom score decreased in 68% of patients. The AFEQT score improvement was observed in 88.4% and 90.4% of patients who completed the questionnaire in-person or interviewed by phone at 12 month follow-up, respectively.
Phased RF ablation for the treatment of paroxysmal and persistent AF demonstrated a 77.7% freedom from AF recurrence at 12 months in addition to a significant reduction in arrhythmia symptoms and clinically meaningful improved QoL. Low peri-procedural complication rate of <3% was reported.
GOLD AF is the largest and most diverse multi-centre cohort of patients treated with the second-generation phased RF system, pulmonary vein ablation catheter GOLD.
Results demonstrated that phased RF procedures appear safe and predictable, with relatively short procedure times, and safety outcomes that are in line with previously published literature.
Results also demonstrated that phased RF procedures are effective, with high-acute procedural success rates (96.1%), sustained 12-month freedom from recurrence in 77.7% of patients, reductions in arrhythmia-related symptoms, and improved quality of life.
Introduction
International guidelines concur that pulmonary vein isolation (PVI) by catheter ablation is a class I level A treatment for drug-refractory paroxysmal atrial fibrillation (PAF).1–3 Historically, point-by-point RF has been the standard ablation tool, requiring multiple consecutive ablation applications for PVI (and any additional targeted substrate modification). Although upgrades of single-tip ablation catheters with irrigation and contact force sensing in combination with advanced 3D mapping systems have increased efficiency, point-by-point ablation remains laborious and is more dependent on operator experience, particularly at low-volume institutions.4,5
Technological advancements to overcome the challenges of point-by-point ablation, without compromising safety and efficacy, resulted in the development of so-called ‘single-shot’ devices that eliminate the need for 3D mapping and result in a relatively straight-forwarded procedure, among them the circular multi-electrode pulmonary vein ablation catheter (PVAC; Medtronic, Minneapolis, MN, USA) using duty-cycled bipolar/unipolar phased radiofrequency (RF). The phased RF system was designed with an anatomical shape for isolation of the pulmonary veins. Furthermore, the duty-cycled phased RF technology with passive cooling does not require irrigation and delivers temperature-controlled energy with the objective of delivering a transmural lesion while reducing collateral damage and complications.6
The first-generation PVAC successfully achieved shorter procedures, shorter learning curves, and reduced fluoroscopy times compared with point-by-point RF without detrimental effects on procedural success.7–10 However, concern for the risk of asymptomatic cerebral embolism (ACE) emerged following procedures with the first generation, PVAC. To address these findings, a redesign of the PVAC platform (PVAC GOLD; Medtronic, Minneapolis, MN, USA), including the generator (GENius Contact IQ; Medtronic, Minneapolis, MN, USA) was engineered to mitigate the risk of ACE while maintaining the technological and practical advances of the first-generation PVAC.
Pulmonary vein ablation catheter GOLD is the second generation, over-the-wire, circular mapping, and RF catheter with a 25-mm diameter array at the distal tip (canted forward by 20°) for optimal contact force with cardiac tissue along the entire length of the array. The PVAC GOLD array was reduced to 9 electrodes (vs. 10 electrodes in PVAC) to eliminate electrode interference and reduce the risk of overheating observed between electrodes 1 and 10 (Figure 1). Furthermore, the platinum-iridium electrodes (PVAC) were replaced with gold (PVAC GOLD) to enhance thermal conductivity and power output with greater passive cooling capacity for better thermodynamic control.11 The physical changes to PVAC GOLD were accompanied by software enhancements including improved algorithms through the generator. Following this catheter redesign and procedural optimization (e.g. peri-procedural anticoagulation), a reduction in ACE lesions and maintenance of clinical safety and efficacy were confirmed post-ablation.12,13
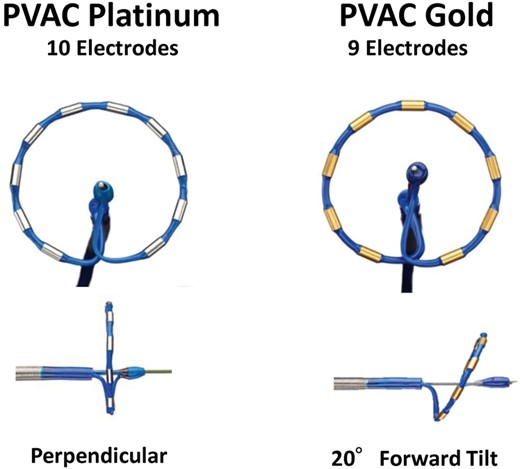
First generation (PVAC) comparison to second generation (PVAC GOLD). PVAC, pulmonary vein ablation catheter.
The GOLD AF registry was designed to evaluate PVAC GOLD in a real-world clinical setting with subjects treated per standard of care. The purpose of this prospective, observational study is to provide insight into daily use of the phased RF system in a robust and diverse number of patients and evaluate procedural efficiency, efficacy, and safety outcomes.
Methods
Study design and governance
The GOLD AF Registry (NCT02433613) was a prospective, observational, multi-centre, registry designed to characterize procedural outcomes, effectiveness, and safety of phased RF in a representative AF patient population. A total of 40 worldwide sites in France, Germany, Greece, Hungary, Italy, the Netherlands, Poland, Portugal, Spain, Switzerland, UK, Georgia, Israel, and South Korea participated in this study (Supplementary material online, Table S1). The registry included adults (≥18 years old) with PAF, persistent AF (PersAF), or LS PersAF who underwent a phased RF ablation in accordance with the current ACC/AHA/HRS and/or ESC guidelines for the management of AF.1,3 All study participants were followed per standard of care up to 12 (+2 months) post-ablation.
The registry was conducted in accordance with the Declaration of Helsinki and approved by local ethics committees and competent authorities. Patients were required to provide written informed consent to participate. This registry was governed by a Steering Committee with representatives from each geography. In addition, an independent adverse event advisory board is responsible for the adjudication of all adverse events.
Phased radiofrequency ablation procedure
Transoesophageal echocardiogram was performed according to institutional requirements prior to an index or repeat ablation procedure to exclude left atrial (LA) thrombus, and an magnetic resonance imaging/computed tomography was utilized at operator discretion to image the left atrium and pulmonary veins (PVs). Heparin was administered prior to or immediately after the trans-septal puncture and maintained at activated clotting time (ACT) levels prescribed by the operator. Trans-septal catheterization was performed and the trans-septal sheath was exchanged for a ≥10-Fr steerable sheath.
The procedural techniques for ablation using the phased RF system have been previously described.8,10 In brief, PVAC GOLD uses an over-the-wire design to facilitate placement at the antrum of the pulmonary veins. Multi-array septal catheter and MAAC do not use an over-the-wire design as the steering or sliding mechanisms within these catheters allow for placement on the septum or in the LA body, respectively. Once the physician has positioned the catheter over the target site, intra-cardiac signal mapping, pacing, and ablation are undertaken. As previously described, phased RF energy is delivered in five modes: bipolar, unipolar, and ratios of 1:1, 2:1, and 4:1, respectively, resulting in consistent depth and continuous lesions.6
Each of the electrodes on the phased RF catheters has one thermocouple located on the tissue side of the electrode to allow for regulation of the energy based on tissue temperature. These thermocouples allow the user to ensure that the electrode/tissue interface maintains the target temperature, with generator feedback, to create an adequate lesion without excessive power that could create charring or thrombosis.
The pulmonary veins were isolated using phased RF according to the PVAC GOLD labelling in the respective countries. If additional cardiac substrate modification was deemed necessary by the operator, two accessory phased RF catheters were employed including the Multi-Array Ablation Catheter (MAAC; Medtronic, Minneapolis, MN, USA) and the Multi-Array Septal Catheter (MASC; Medtronic, Minneapolis, MN, USA). These multi-electrode accessory catheters were designed to map and ablate complex fractionated atrial electrograms in the LA body and along the LA septum, respectively. The protocol did not specify mapping practices, use of medication, or additional electrophysiology test requirements. Accessory equipment and consumables (e.g. mapping/diagnostic catheters, sheaths, and trans-septal puncture needles) were not specified but required to have market approval in the respective country.
Patient follow-up and study endpoints
Post-ablation, patients were followed according to standard-of-care for up to 12 (+2) months. During each follow-up visit, patients were assessed for relevant arrhythmia status, complications, previous cardioversions, recent hospitalizations, and cardiovascular medications. Quality of life (QoL) was assessed using two validated tools, Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) questionnaire14 and the European Heart Rhythm Association (EHRA) Score of AF-related symptoms; prior to the ablation and at the end of the follow-up period. All arrhythmia monitoring was performed according to local practice. Follow-ups were intended to be performed in person. However, if an in-person visit was not standard-of-care or the patient was unavailable in the defined time window, a telephone interview was permitted. Patients were exited from the study upon follow-up completion, patient death, or subject withdrawal (Figure 2).
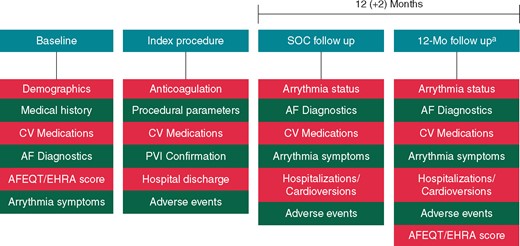
Study design. aIn-hospital or by phone. AF, atrial fibrillation; AFEQT, Atrial Fibrillation Effect on QualitTy-of-Life; CV, cardiovascular; EHRA, European Heart Rhythm Association; Mo, month; PVI, pulmonary vein isolation; SOC, standard of care.
The primary study endpoint was freedom from AF recurrence at 12 months following a blanking period of 90 days. Recurrences were defined as AF ≥30 s, electrical or pharmacological cardioversions, or re-ablations outside of the blanking period. Ancillary objectives included safety, acute procedural success, efficiency, and QoL.
Statistical analysis
Data from all patients who met the eligibility criteria and underwent a phased RF procedure were included in the analysis. Binary, categorical, and ordinal parameters were summarized by the means of absolute and percentage numbers within the various categories. Numerical data were summarized by mean and standard deviation statistics. Kaplan–Meier analyses were conducted to assess freedom from AF recurrence at 12 months.
Results
Patient characteristics
Enrolment of patients began in 2015 and was completed in 2017. A total of 1071 patients were enrolled; 17 patients were excluded as a result of incomplete consent and procedural data, while 1054 patients were included in the baseline and the primary endpoint analysis cohort with 943 patients in the QoL analysis (Figure 3). Among the baseline cohort, 713 (67.6%) were male, the average age was 60.6 ± 10.9 years. Time from first diagnosed AF episode to enrolment was 4.3 ± 5.2 years. Patients were predominately paroxysmal (70.2%) while 26.5% and 3.3% had PersAF and LS PersAF, respectively. The mean LA dimension was 42.0 ± 8.1 mm and the mean LA volume was 49.0 ± 27.4. Comorbidity prevalence included hypertension 561 (53.2%), history of smoking 359 (34.1%), dyslipidaemia 304 (28.8%), obesity 286 (27.1%), and diabetes 102 (9.7%). Most patients had failed an anti-arrhythmic drug (80.3%), a cardioversion was completed in 38.4% of patients, and 31.5% were hospitalized for AF within the 12 months preceding enrolment. At the time of enrolment, 947 patients (89.8%) were on anticoagulation therapy. Most patients (669, 63.5%) were on a novel oral anticoagulant; rivaroxaban (294, 27.9%), apixaban (199, 18.9%), dabigatran (142, 13.5%), and edoxaban (34, 3.2%), while 245 patients (23.2%) were prescribed a vitamin K antagonist. The mean HAS-BLED Score was 1.02 ± 0.85, and CHA2DS2-VASc Score was 1.79 ± 1.43. All patient characteristics are detailed in Table 1.
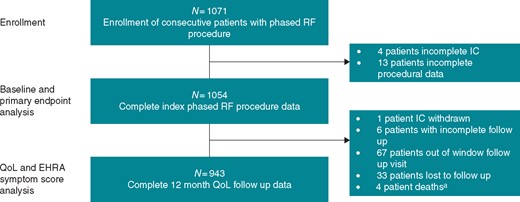
Patient selection and disposition. aNo device- or procedural-related deaths reported. EHRA, European Heart Rhythm Association; IC, informed consent; RF, radiofrequency.
| Characteristics . | Patients (n = 1054) . |
|---|---|
| Age (years) | 60.6 ± 10.9 |
| Gender, male (%, N) | 67.6% (713) |
| AF type (%, N) | |
| Paroxysmal | 70.2% (740) |
| Persistent | 26.5% (279) |
| Long-standing persistent | 3.3% (35) |
| Years since first AF diagnosis (years) | 4.3 ± 5.2 |
| Symptomatic AF (%, N) | 95.5 % (1007) |
| Patient Failed AAD (%, N) | 80.3% (846) |
| Patients on AAD (%, N) | 88.0% (927) |
| Cardioversions <12 months (%, N) | 38.4% (405) |
| Arrhythmia-related symptoms (%, N) | 95.5% (1007) |
| AF hospitalizations <12 months (%, N) | 31.5% (332) |
| First AF ablation | 94.2% (993) |
| Patients on anticoagulants (%, N) | 89.8% (947) |
| Type of anticoagulant not available | 3.1% (33) |
| Vitamin K antagonist | 23.2% (245) |
| Novel oral anticoagulants | 63.5% (669) |
| Rivaroxaban | 27.9% (294) |
| Apixaban | 18.9% (199) |
| Dabigatran | 13.5% (142) |
| Edoxaban | 3.2% (34) |
| BMI | 27.8 ± 4.5 |
| LVEF (N) | 59.4 ± 7.6 |
| LAD (mm) | 42.0 ± 8.1 |
| LA volume | 49.0 ± 27.4 |
| NYHA classification (%, N) | |
| No heart failure | 96.2% (1014) |
| Class I or II | 3.1% (33) |
| Class III or IV | 0.7% (7) |
| CHA2DS2-VASc score | 1.79 ± 1.43 |
| HAS-BLED score | 1.02 ± 0.85 |
| Medical history (%, N) | |
| Previous MI | 4.3% (45) |
| Coronary PCI/stent | 6.5% (68) |
| CABG | 1.1% (12) |
| Peripheral artery disease | 2.3% (24) |
| Valvular disease | 5.4% (57) |
| Stroke/TIA | 5.8% (61) |
| Obesity | 27.1% (286) |
| Cardiomyopathy | 7.1% (75) |
| Diabetes | 9.7% (102) |
| Smoking history | 34.1% (359) |
| Hypertension | 53.2% (561) |
| Sleep apnoea | 4.5% (47) |
| Hyperthyroidism | 5.5% (58) |
| Dyslipidaemia | 28.8% (304) |
| Characteristics . | Patients (n = 1054) . |
|---|---|
| Age (years) | 60.6 ± 10.9 |
| Gender, male (%, N) | 67.6% (713) |
| AF type (%, N) | |
| Paroxysmal | 70.2% (740) |
| Persistent | 26.5% (279) |
| Long-standing persistent | 3.3% (35) |
| Years since first AF diagnosis (years) | 4.3 ± 5.2 |
| Symptomatic AF (%, N) | 95.5 % (1007) |
| Patient Failed AAD (%, N) | 80.3% (846) |
| Patients on AAD (%, N) | 88.0% (927) |
| Cardioversions <12 months (%, N) | 38.4% (405) |
| Arrhythmia-related symptoms (%, N) | 95.5% (1007) |
| AF hospitalizations <12 months (%, N) | 31.5% (332) |
| First AF ablation | 94.2% (993) |
| Patients on anticoagulants (%, N) | 89.8% (947) |
| Type of anticoagulant not available | 3.1% (33) |
| Vitamin K antagonist | 23.2% (245) |
| Novel oral anticoagulants | 63.5% (669) |
| Rivaroxaban | 27.9% (294) |
| Apixaban | 18.9% (199) |
| Dabigatran | 13.5% (142) |
| Edoxaban | 3.2% (34) |
| BMI | 27.8 ± 4.5 |
| LVEF (N) | 59.4 ± 7.6 |
| LAD (mm) | 42.0 ± 8.1 |
| LA volume | 49.0 ± 27.4 |
| NYHA classification (%, N) | |
| No heart failure | 96.2% (1014) |
| Class I or II | 3.1% (33) |
| Class III or IV | 0.7% (7) |
| CHA2DS2-VASc score | 1.79 ± 1.43 |
| HAS-BLED score | 1.02 ± 0.85 |
| Medical history (%, N) | |
| Previous MI | 4.3% (45) |
| Coronary PCI/stent | 6.5% (68) |
| CABG | 1.1% (12) |
| Peripheral artery disease | 2.3% (24) |
| Valvular disease | 5.4% (57) |
| Stroke/TIA | 5.8% (61) |
| Obesity | 27.1% (286) |
| Cardiomyopathy | 7.1% (75) |
| Diabetes | 9.7% (102) |
| Smoking history | 34.1% (359) |
| Hypertension | 53.2% (561) |
| Sleep apnoea | 4.5% (47) |
| Hyperthyroidism | 5.5% (58) |
| Dyslipidaemia | 28.8% (304) |
AAD, anti-arrhythmic drug; AF, atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass graft; LA, left atrial/atrium; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
| Characteristics . | Patients (n = 1054) . |
|---|---|
| Age (years) | 60.6 ± 10.9 |
| Gender, male (%, N) | 67.6% (713) |
| AF type (%, N) | |
| Paroxysmal | 70.2% (740) |
| Persistent | 26.5% (279) |
| Long-standing persistent | 3.3% (35) |
| Years since first AF diagnosis (years) | 4.3 ± 5.2 |
| Symptomatic AF (%, N) | 95.5 % (1007) |
| Patient Failed AAD (%, N) | 80.3% (846) |
| Patients on AAD (%, N) | 88.0% (927) |
| Cardioversions <12 months (%, N) | 38.4% (405) |
| Arrhythmia-related symptoms (%, N) | 95.5% (1007) |
| AF hospitalizations <12 months (%, N) | 31.5% (332) |
| First AF ablation | 94.2% (993) |
| Patients on anticoagulants (%, N) | 89.8% (947) |
| Type of anticoagulant not available | 3.1% (33) |
| Vitamin K antagonist | 23.2% (245) |
| Novel oral anticoagulants | 63.5% (669) |
| Rivaroxaban | 27.9% (294) |
| Apixaban | 18.9% (199) |
| Dabigatran | 13.5% (142) |
| Edoxaban | 3.2% (34) |
| BMI | 27.8 ± 4.5 |
| LVEF (N) | 59.4 ± 7.6 |
| LAD (mm) | 42.0 ± 8.1 |
| LA volume | 49.0 ± 27.4 |
| NYHA classification (%, N) | |
| No heart failure | 96.2% (1014) |
| Class I or II | 3.1% (33) |
| Class III or IV | 0.7% (7) |
| CHA2DS2-VASc score | 1.79 ± 1.43 |
| HAS-BLED score | 1.02 ± 0.85 |
| Medical history (%, N) | |
| Previous MI | 4.3% (45) |
| Coronary PCI/stent | 6.5% (68) |
| CABG | 1.1% (12) |
| Peripheral artery disease | 2.3% (24) |
| Valvular disease | 5.4% (57) |
| Stroke/TIA | 5.8% (61) |
| Obesity | 27.1% (286) |
| Cardiomyopathy | 7.1% (75) |
| Diabetes | 9.7% (102) |
| Smoking history | 34.1% (359) |
| Hypertension | 53.2% (561) |
| Sleep apnoea | 4.5% (47) |
| Hyperthyroidism | 5.5% (58) |
| Dyslipidaemia | 28.8% (304) |
| Characteristics . | Patients (n = 1054) . |
|---|---|
| Age (years) | 60.6 ± 10.9 |
| Gender, male (%, N) | 67.6% (713) |
| AF type (%, N) | |
| Paroxysmal | 70.2% (740) |
| Persistent | 26.5% (279) |
| Long-standing persistent | 3.3% (35) |
| Years since first AF diagnosis (years) | 4.3 ± 5.2 |
| Symptomatic AF (%, N) | 95.5 % (1007) |
| Patient Failed AAD (%, N) | 80.3% (846) |
| Patients on AAD (%, N) | 88.0% (927) |
| Cardioversions <12 months (%, N) | 38.4% (405) |
| Arrhythmia-related symptoms (%, N) | 95.5% (1007) |
| AF hospitalizations <12 months (%, N) | 31.5% (332) |
| First AF ablation | 94.2% (993) |
| Patients on anticoagulants (%, N) | 89.8% (947) |
| Type of anticoagulant not available | 3.1% (33) |
| Vitamin K antagonist | 23.2% (245) |
| Novel oral anticoagulants | 63.5% (669) |
| Rivaroxaban | 27.9% (294) |
| Apixaban | 18.9% (199) |
| Dabigatran | 13.5% (142) |
| Edoxaban | 3.2% (34) |
| BMI | 27.8 ± 4.5 |
| LVEF (N) | 59.4 ± 7.6 |
| LAD (mm) | 42.0 ± 8.1 |
| LA volume | 49.0 ± 27.4 |
| NYHA classification (%, N) | |
| No heart failure | 96.2% (1014) |
| Class I or II | 3.1% (33) |
| Class III or IV | 0.7% (7) |
| CHA2DS2-VASc score | 1.79 ± 1.43 |
| HAS-BLED score | 1.02 ± 0.85 |
| Medical history (%, N) | |
| Previous MI | 4.3% (45) |
| Coronary PCI/stent | 6.5% (68) |
| CABG | 1.1% (12) |
| Peripheral artery disease | 2.3% (24) |
| Valvular disease | 5.4% (57) |
| Stroke/TIA | 5.8% (61) |
| Obesity | 27.1% (286) |
| Cardiomyopathy | 7.1% (75) |
| Diabetes | 9.7% (102) |
| Smoking history | 34.1% (359) |
| Hypertension | 53.2% (561) |
| Sleep apnoea | 4.5% (47) |
| Hyperthyroidism | 5.5% (58) |
| Dyslipidaemia | 28.8% (304) |
AAD, anti-arrhythmic drug; AF, atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass graft; LA, left atrial/atrium; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
Procedural parameters and site experience
Acute procedural success, as determined by the operator, was achieved in 1008/1049 (96%) of patients. At the end of the procedure, 992/1049 (95%) patients were in sinus rhythm. Acute success and sinus rhythm was not operator reported for five patients. Pulmonary vein isolation was confirmed via entrance/exit block in all PVs in 977/1036 (94.3%) patients. Standard PV anatomy was observed in 84% of patients, a left common PV was observed in 9% of patients, and a right middle PV was noted in 5% of patients. The highest procedural ACT was 357 and lowest ACT was 274. The mean number of phased RF applications was 25.8 ± 13 per patient. A PVI-only ablation strategy was used in 907/1054 (86%) of ablation procedures. Pulmonary vein ablation catheter GOLD was used in 100% of procedures. At the time of study initiation, among the 40 sites participating in the registry, 7 (17.5%) had completed ≤25 phased RF procedures, 11 (27.5%) had completed ≤100 procedures, 8 (20.0%) had completed ≤200 procedures, and 14 (35%) had completed ≥200 procedures. The mean lab occupancy, procedure, fluoroscopy, and LA dwell times were 154.7 ± 49.3, 107.0 ± 39.5, 25.0 ± 15.5, and 79.6 ± 32.5 min, respectively. Mean procedure and fluoroscopy times were 106 and 25 min in PAF and 110 and 26 min in PersAF patients. Procedural parameters are summarized in Table 2.
| Procedure parameter . | Index procedures (N = 1054) . |
|---|---|
| Patient anatomy | |
| Normal PV anatomy (%, N) | 84.4% (890) |
| Left common PV (%, N) | 9.4% (99) |
| Right middle PV (%, N) | 5.4% (57) |
| Procedural ACT | |
| Highest procedural ACT (s) | 356.9 ± 115.7 |
| Lowest procedural ACT (s) | 274.4 ± 95.3 |
| Procedural data | |
| PV isolation confirmed (exit/entrance in all PVs) | 94.3% (977/1036) |
| Anticoagulation discontinued prior to procedure (%, N) | 53.8% (567) |
| General anaesthesia (%, N) | 13.6% (143) |
| Dormant conduction check | 15.6% (163/1042) |
| Phased RF applications (No.) | 25.8 ± 13.0 |
| LSPV applications | 7.1 ± 5.0 |
| LIPV applications | 5.9 ± 4.6 |
| RSPV applications | 5.9 ± 3.8 |
| RIPV applications | 5.4 ± 3.7 |
| Catheters used | 2.2 ± 1.3 |
| PVAC GOLD | 100.0% (1054) |
| MAAC | 1.5% (16) |
| MASC | 1.3% (14) |
| PVI—only strategy | 86.0% (907) |
| Non-PVI lesions | 13.9% (147) |
| Lines | 83.6% (122/146) |
| CFAE | 11.0% (16/146) |
| LA roof | 13.6% (20/147) |
| LA posterior wall | 4.8% (7/147) |
| LA isthmus | 9.5% (14/147) |
| RA isthmus | 51.7% (76/147) |
| LA septum | 18.4% (27/147) |
| SVC line | 13.6% (20/147) |
| 3D mapping/navigation | 13.6% (143) |
| Duration of hospital stay (days) | 2.4 ± 1.9 |
| Procedure times | |
| Total lab time | 154.7 ± 49.3 |
| Total procedure time | 107.0 ± 39.5 |
| LA Dwell time | 79.6 ± 32.5 |
| PVAC ablation time | 58.0 ± 28.8 |
| Total fluoroscopy time | 25.0 ± 15.5 |
| Procedure parameter . | Index procedures (N = 1054) . |
|---|---|
| Patient anatomy | |
| Normal PV anatomy (%, N) | 84.4% (890) |
| Left common PV (%, N) | 9.4% (99) |
| Right middle PV (%, N) | 5.4% (57) |
| Procedural ACT | |
| Highest procedural ACT (s) | 356.9 ± 115.7 |
| Lowest procedural ACT (s) | 274.4 ± 95.3 |
| Procedural data | |
| PV isolation confirmed (exit/entrance in all PVs) | 94.3% (977/1036) |
| Anticoagulation discontinued prior to procedure (%, N) | 53.8% (567) |
| General anaesthesia (%, N) | 13.6% (143) |
| Dormant conduction check | 15.6% (163/1042) |
| Phased RF applications (No.) | 25.8 ± 13.0 |
| LSPV applications | 7.1 ± 5.0 |
| LIPV applications | 5.9 ± 4.6 |
| RSPV applications | 5.9 ± 3.8 |
| RIPV applications | 5.4 ± 3.7 |
| Catheters used | 2.2 ± 1.3 |
| PVAC GOLD | 100.0% (1054) |
| MAAC | 1.5% (16) |
| MASC | 1.3% (14) |
| PVI—only strategy | 86.0% (907) |
| Non-PVI lesions | 13.9% (147) |
| Lines | 83.6% (122/146) |
| CFAE | 11.0% (16/146) |
| LA roof | 13.6% (20/147) |
| LA posterior wall | 4.8% (7/147) |
| LA isthmus | 9.5% (14/147) |
| RA isthmus | 51.7% (76/147) |
| LA septum | 18.4% (27/147) |
| SVC line | 13.6% (20/147) |
| 3D mapping/navigation | 13.6% (143) |
| Duration of hospital stay (days) | 2.4 ± 1.9 |
| Procedure times | |
| Total lab time | 154.7 ± 49.3 |
| Total procedure time | 107.0 ± 39.5 |
| LA Dwell time | 79.6 ± 32.5 |
| PVAC ablation time | 58.0 ± 28.8 |
| Total fluoroscopy time | 25.0 ± 15.5 |
ACT, activated clotting time; CFAE, complex fractionated atrial electrogram; LA, left atrial/atrium; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MAAC, multi-array ablation catheter; MASC, multi-array septal catheter; No., Number; PV, pulmonary vein; PVAC, pulmonary vein ablation catheter; RA, right atrial/atrium; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; RF, radiofrequency; SVC, superior vena cava.
| Procedure parameter . | Index procedures (N = 1054) . |
|---|---|
| Patient anatomy | |
| Normal PV anatomy (%, N) | 84.4% (890) |
| Left common PV (%, N) | 9.4% (99) |
| Right middle PV (%, N) | 5.4% (57) |
| Procedural ACT | |
| Highest procedural ACT (s) | 356.9 ± 115.7 |
| Lowest procedural ACT (s) | 274.4 ± 95.3 |
| Procedural data | |
| PV isolation confirmed (exit/entrance in all PVs) | 94.3% (977/1036) |
| Anticoagulation discontinued prior to procedure (%, N) | 53.8% (567) |
| General anaesthesia (%, N) | 13.6% (143) |
| Dormant conduction check | 15.6% (163/1042) |
| Phased RF applications (No.) | 25.8 ± 13.0 |
| LSPV applications | 7.1 ± 5.0 |
| LIPV applications | 5.9 ± 4.6 |
| RSPV applications | 5.9 ± 3.8 |
| RIPV applications | 5.4 ± 3.7 |
| Catheters used | 2.2 ± 1.3 |
| PVAC GOLD | 100.0% (1054) |
| MAAC | 1.5% (16) |
| MASC | 1.3% (14) |
| PVI—only strategy | 86.0% (907) |
| Non-PVI lesions | 13.9% (147) |
| Lines | 83.6% (122/146) |
| CFAE | 11.0% (16/146) |
| LA roof | 13.6% (20/147) |
| LA posterior wall | 4.8% (7/147) |
| LA isthmus | 9.5% (14/147) |
| RA isthmus | 51.7% (76/147) |
| LA septum | 18.4% (27/147) |
| SVC line | 13.6% (20/147) |
| 3D mapping/navigation | 13.6% (143) |
| Duration of hospital stay (days) | 2.4 ± 1.9 |
| Procedure times | |
| Total lab time | 154.7 ± 49.3 |
| Total procedure time | 107.0 ± 39.5 |
| LA Dwell time | 79.6 ± 32.5 |
| PVAC ablation time | 58.0 ± 28.8 |
| Total fluoroscopy time | 25.0 ± 15.5 |
| Procedure parameter . | Index procedures (N = 1054) . |
|---|---|
| Patient anatomy | |
| Normal PV anatomy (%, N) | 84.4% (890) |
| Left common PV (%, N) | 9.4% (99) |
| Right middle PV (%, N) | 5.4% (57) |
| Procedural ACT | |
| Highest procedural ACT (s) | 356.9 ± 115.7 |
| Lowest procedural ACT (s) | 274.4 ± 95.3 |
| Procedural data | |
| PV isolation confirmed (exit/entrance in all PVs) | 94.3% (977/1036) |
| Anticoagulation discontinued prior to procedure (%, N) | 53.8% (567) |
| General anaesthesia (%, N) | 13.6% (143) |
| Dormant conduction check | 15.6% (163/1042) |
| Phased RF applications (No.) | 25.8 ± 13.0 |
| LSPV applications | 7.1 ± 5.0 |
| LIPV applications | 5.9 ± 4.6 |
| RSPV applications | 5.9 ± 3.8 |
| RIPV applications | 5.4 ± 3.7 |
| Catheters used | 2.2 ± 1.3 |
| PVAC GOLD | 100.0% (1054) |
| MAAC | 1.5% (16) |
| MASC | 1.3% (14) |
| PVI—only strategy | 86.0% (907) |
| Non-PVI lesions | 13.9% (147) |
| Lines | 83.6% (122/146) |
| CFAE | 11.0% (16/146) |
| LA roof | 13.6% (20/147) |
| LA posterior wall | 4.8% (7/147) |
| LA isthmus | 9.5% (14/147) |
| RA isthmus | 51.7% (76/147) |
| LA septum | 18.4% (27/147) |
| SVC line | 13.6% (20/147) |
| 3D mapping/navigation | 13.6% (143) |
| Duration of hospital stay (days) | 2.4 ± 1.9 |
| Procedure times | |
| Total lab time | 154.7 ± 49.3 |
| Total procedure time | 107.0 ± 39.5 |
| LA Dwell time | 79.6 ± 32.5 |
| PVAC ablation time | 58.0 ± 28.8 |
| Total fluoroscopy time | 25.0 ± 15.5 |
ACT, activated clotting time; CFAE, complex fractionated atrial electrogram; LA, left atrial/atrium; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MAAC, multi-array ablation catheter; MASC, multi-array septal catheter; No., Number; PV, pulmonary vein; PVAC, pulmonary vein ablation catheter; RA, right atrial/atrium; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; RF, radiofrequency; SVC, superior vena cava.
Procedural safety, subject follow-up, and 12-month freedom from atrial fibrillation
In total, 26 (2.5%) procedural complications were observed within 30 days of the index ablation and are listed in Table 3. No pulmonary vein stenosis, atrioesophageal fistulae, or deaths were observed. The mean time to first event was 2.3 ± 3.3 days. There were 18 access site complications (1.7%), 3 strokes (0.3%), 2 pericardial effusions without tamponade (0.2%), 2 transient ST-segment elevations (0.2%), and 1 phrenic nerve injury (0.1%) observed.
| . | Events (subjects, %), N = 1054 . |
|---|---|
| Access site complications | 18 (1.7) |
| Ischaemic stroke | 3 (0.3) |
| Pericardial effusion | 2 (0.2) |
| Transient ST-segment elevation | 2 (0.2) |
| Phrenic nerve injury | 1 (0.1) |
| Total | 26 (2.5) |
| . | Events (subjects, %), N = 1054 . |
|---|---|
| Access site complications | 18 (1.7) |
| Ischaemic stroke | 3 (0.3) |
| Pericardial effusion | 2 (0.2) |
| Transient ST-segment elevation | 2 (0.2) |
| Phrenic nerve injury | 1 (0.1) |
| Total | 26 (2.5) |
| . | Events (subjects, %), N = 1054 . |
|---|---|
| Access site complications | 18 (1.7) |
| Ischaemic stroke | 3 (0.3) |
| Pericardial effusion | 2 (0.2) |
| Transient ST-segment elevation | 2 (0.2) |
| Phrenic nerve injury | 1 (0.1) |
| Total | 26 (2.5) |
| . | Events (subjects, %), N = 1054 . |
|---|---|
| Access site complications | 18 (1.7) |
| Ischaemic stroke | 3 (0.3) |
| Pericardial effusion | 2 (0.2) |
| Transient ST-segment elevation | 2 (0.2) |
| Phrenic nerve injury | 1 (0.1) |
| Total | 26 (2.5) |
Patients had a mean of 2.3 ± 1.7 follow-up visits. The mean time from index ablation procedure to first follow-up visit was 42.7 ± 29.4 days. The mean time from first procedure to 12 MFU was 395 ± 20 days. At 12 months, the Kaplan–Meier estimate of freedom from AF was 77.7% (Figure 4A). The mean time to recurrence was 185.1 ± 76.6 days. When reported per AF indication, freedom from AF was 82% and 68% in PAF and PersAF, respectively (Figure 4B). Recurrence of AF was documented predominately using either an electrocardiogram (64%), Holter monitor (23%), or implantable loop recorder/pacemaker/internal cardioverter-defibrillator (11%). A total of 213 patients had an AF recurrence event and among these patients, 106 required a repeat procedure, while 28 received a cardioversion. Presence of atrial flutter and tachycardia recurrence was reported in 0.4% and 0.2% of patients, respectively.
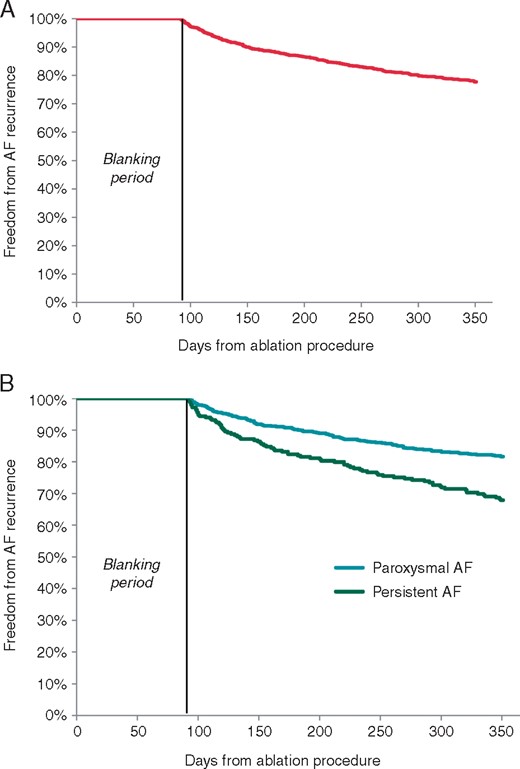
(A) Freedom from AF recurrence at 12 months and (B) 12 months in paroxysmal and persistent cohorts. AF, atrial fibrillation.
Quality of life outcomes (Atrial Fibrillation Effect on QualiTy-of-Life, arrhythmia-related symptoms, European Heart Rhythm Association symptom score)
One-year post-ablation, the overall AFEQT score improvement was observed in 88.4% of patients who completed the questionnaire during in-hospital visits, and in 90.4% of patients who were interviewed by phone at 12 months follow-up (Figure 5A). In patients with and without a recurrence, a statistically significant improvement in the AFEQT score was observed in both groups (Figure 5B). The EHRA AF Symptom score decreased in 68% of patients with a mean score improvement (baseline to 12 months) of 2.3–1.5 (Figure 5C). The improvement in QoL measures was reinforced by a reduction in arrhythmia-related symptoms at baseline to 12 months post-ablation: palpitations (82% to 30%; P < 0.0001), syncope (4% to 1%; P = 0.20), dizziness (20% to 5%; P < 0.0001), irregular heartbeats (32% to 16%; P = 0.27), shortness of breath (58% to 9%; P = 0.50), and fatigue (34% to 14%; P < 0.0001) (Figure 5D).
Quality of life, EHRA AF symptom score, arrhythmia-related symptoms (baseline—12 months). (A) AFEQT score improvement. (B) AFEQT score improvement with and without recurrence. (C) EHRA symptom score reduction. (D) Arrhythmia-related symptom reduction. aChange ≥19, meaningful clinical improvement.20 *P < 0.0001; **Kruskal–Wallis test. AFEQT , Atrial Fibrillation Effect on QualiTy-of-Life; FU, follow-up; Mo, month.AQ16
Discussion
To our knowledge, this is the largest and first prospective, international, multi-centre evaluation using PVAC GOLD in PAF and PersAF patients. The present analysis reports 78% efficacy at 12 months, and a low 2.5% adverse event rate among 1000 patients treated primarily using a PVI-only ablation strategy and followed per routine clinical practice. More specifically, the present multi-centre evaluation of PVAC GOLD reports 12-month freedom from arrhythmia recurrence of 82% in PAF patients and 68% among PersAF patients.
These efficacy results are consistent with those reported by Scharf et al.15 in their evaluation of 3748 patients using the first-generation PVAC which demonstrated 82%, and 70% freedom from arrhythmia recurrence in PAF and PersAF patients, respectively. Similarly, Spitzer et al.16 reported on outcomes among 348 patients at two high-volume centres in Germany using the second-generation PVAC GOLD. Their research demonstrated 71% and 62% freedom from AF in PAF and PersAF.
Among the low rate (2.5%) of procedure and/or device-related adverse events reported in the GOLD AF registry, only three (0.3%) neurological events (strokes) were observed. These safety results for stroke from the GOLD AF registry are similar to other predominant AF ablation energy sources, such as cryoballoon (0.5%) and radiofrequency catheter ablation (0.5%) as reported in the randomized FIRE and ICE Trial,17 and consistent with previous reports (0.3%) using PVAC GOLD.16 It is important to note that the stroke rate in GOLD AF is lower than published reports using the first-generation PVAC (1.1%).15 The GOLD AF registry did not proactively evaluate ACE as this is not part of normal clinical practice for AF ablation. Also, the prior studies ERACE and PRECISION GOLD have already demonstrated that ACE rate is as low as other technologies by eliminating the interaction of a 10th proximal electrode on the array. The present data reinforce safety of phased RF technology as summarized in the 2017 Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation1 which references the ERACE12 and PRECISION GOLD trial,13 concluding there is considerable evidence to support that PVAC GOLD has achieved a similar safety profile when compared with other technologies.
Additional procedural characteristics were evaluated to confirm the efficiency of the second-generation PVAC GOLD. Among patients treated in the study, comparable procedure times were observed between PAF and PersAF patients, 106 and 110 min, respectively, and reflective of adherence to a PVI-only ablation strategy. These results are favourable to the first-generation data from Scharf et al.15 reporting procedure times of 122 (PAF) and 145 min (PersAF), however, marginally higher than the procedure times with two experienced users reported by Spitzer et al.,16 which demonstrated PAF and PersAF procedure times of 94 and 92 min, respectively. Furthermore, these results are favourable to the procedure times from the FIRE and ICE Trial which reported mean procedure times of 141 and 124 min in the radiofrequency and cryoballoon arms, respectively.17 However, the fluoroscopy time observed in the GOLD AF registry was greater than the fluoroscopy times of 17 and 22 min in the radiofrequency and cryoballoon arms, respectively.17 Phased RF procedures in the GOLD AF registry were performed predominantly using conscious sedation (86%) and with limited use of 3D mapping and navigation in only 14% of procedures, which may require more fluoroscopy while reducing healthcare system burden and use of adjunctive tools.
While there is no agreement on the ideal treatment strategy for PersAF, contemporary evidence has not yet been able to demonstrate consistent benefit of ablation outside of the pulmonary veins, particularly at the time of the index procedure.18,19 As such, the guidelines currently recommend a PVI-only approach with additional substrate modification at the time of a repeat ablation and only after verification of PVI durability.2,3 Among a diverse group of operators at 40 sites in 14 countries, with no pre-defined ablation protocol, the consistency in outcomes observed in GOLD AF, support adherence to the 2016 European Society of Cardiology Guidelines and 2017 Heart Rhythm Society/EHRA Consensus Statement1,3 which recommend PVI-only as a reasonable ablation strategy, with nearly 90% of this PAF and PersAF patient cohort undergoing PVI alone. A PVI-only ablation strategy was utilized in 648/740 (87.6%) of PAF ablation procedures, and in 227/279 (81.4%) PersAF procedures.
The clinically meaningful improvement20 in QoL measures and correlation with reduction in patient symptoms was notable. Among all patients, >88% reported an improvement in AFEQT score and 68% of patients demonstrated an improvement in EHRA score. It was particularly interesting to note that improvement was observed in both patients with AF recurrence and patients that remained atrial arrhythmia free. While this improvement could reflect a placebo effect, they may also reflect a true change in AF burden, and these data support the growing body of evidence that challenge, or at least warrant further debate on the definition of ‘clinical trial failure’ for a symptom-based indication.19
Based on our results, and previous reports13,21,22 it is reasonable to suggest that the procedural benefits of phased RF technology have been retained, in the improved second generation, PVAC GOLD. Similarly, the results of the present analysis suggest these improvements, have not compromised the efficacy, and have likely improved the safety profile of the technology.
Limitations
The GOLD AF Registry results are evaluated from an observational, post-market study with inherent disadvantages in such a design, including selection bias and variable procedural (ACT levels, ablation mode), operator, and follow-up protocols at each institution.
Conclusion
Phased RF ablation for the treatment of paroxysmal and persistent AF demonstrated a 77.7% freedom from symptomatic AF recurrence at 12 months in addition to a significant reduction in arrhythmia symptoms and clinically meaningful improved QoL. Low peri-procedural complication rate of 2.5% was reported.
Acknowledgements
The authors would like to thank (i) the GOLD AF sites and staff for their valuable contributions to this study, (ii) Ralf Meyer from Medtronic for support with the study and the generation of this manuscript, and (iii) Christiane Lober for statistical support.
Funding
Medtronic International Trading Sàrl (Tolochenaz, Switzerland).
Conflict of interest: L.B. consultancy fees and grants to R&D Cardiology for Medtronic, Boston Scientific, Abbott, Member advisory boards Medtronic, Member steering committees Medtronic, Boston Scientific; E.K. proctor for Abbott, Johnson&Johnson, Medtronic. G.M none; J.S. none; Olaf Grebe, none; L.E speaker fees from Medtronic; R.B.H. Medtronic employee; L.A.H. Medtronic employee; E.O. Medtronic employee; H.S.B. none; G.R. none; F.A. compensation for participation in advisory boards from MEDTRONIC, BOSTON SCIENTIFIC and MICROPORT. Grants to the institution for investigation or fellowships from MEDTRONIC and BOSTON SCIENTIFIC. Compensation for educational activities or sponsored talks from ABBOTT (SAINT JUDE MEDICAL) and MEDTRONIC; C.S. founder/stockholder ACUTUS Medical; Z.C. none; A.A. none; F.L. none; M.K. none; A.G. speaker fees from Medtronic, Boston Scientific, Abbott, Biotronik.
References
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace 2018;20:157-208





