-
PDF
- Split View
-
Views
-
Cite
Cite
Aiman Alak, Stefan H Hohnloser, Mandy Fräßdorf, Paul Reilly, Michael Ezekowitz, Jeff S Healey, Martina Brueckmann, Salim Yusuf, Stuart J Connolly, Reasons for hospitalization and risk of mortality in patients with atrial fibrillation treated with dabigatran or warfarin in the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial, EP Europace, Volume 21, Issue 7, July 2019, Pages 1023–1030, https://doi.org/10.1093/europace/euz021
Close - Share Icon Share
Hospitalizations are common among patients with atrial fibrillation. This article aimed to analyse the causes and consequences of hospitalizations occurring during the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial.
The RE-LY database was used to evaluate predictors of hospitalization using multivariate regression modelling. The relationship between hospitalization and subsequent major adverse cardiac events was evaluated in a time dependent Cox proportional-hazard modelling. Of the 18 113 patients in RE-LY, 7200 (39.8%) were hospitalized at least once during a mean follow-up of 2 years. First hospitalization rates were 2312 (39.5%) for dabigatran etexilate (DE) 110, 2430 (41.6%) for DE 150, and 42.6% (N = 2458) for warfarin. Hospitalization was associated with post-discharge death [absolute event rate 9.1% vs. 2.2%; adjusted hazard ratio (HR) 3.6, 95% confidence interval (CI) 3.2–4.0, P < 0.0001], vascular death (adjusted HR 2.9, 95% CI 2.5–3.3, P < 0.0001), and sudden cardiac death (adjusted HR 2.3; 95% CI 1.8–2.9, P < 0.0001). Cardiovascular hospitalization was also associated with an increased risk of post-discharge death (adjusted HR 2.8, 95% CI 2.5–3.2, P < 0.0001), vascular death (adjusted HR 2.8, 95% CI 2.4–3.2, P < 0.0001), and sudden cardiac death (adjusted HR 2.1, 95% CI 1.6–2.7, P < 0.0001) compared with patients not hospitalized for any cardiovascular reason.
Hospitalizations are associated an increased risk of with death and cardiovascular death in patients with atrial fibrillation.
Of the patients in the RE-LY study, at least 40% were hospitalized during a mean follow-up of 2 years.
Hospitalization was associated with a high risk of post-discharge death [absolute event rate 9.1% vs. 2.2%; adjusted hazard ratio (HR) 3.6, 95% confidence interval (CI) 3.2–4.0, P < 0.0001], vascular death (adjusted HR 2.9, 95% CI 2.5–3.3, P < 0.0001), and sudden cardiac death (adjusted HR 2.3; 95% CI 1.8–2.9, P < 0.0001).
Hospitalizations or cardiovascular hospitalization may be considered as surrogate outcomes in future atrial fibrillation studies; analogous to the use of hospitalizations in heart failure studies.
Introduction
The prevalence of atrial fibrillation has increased with a global prevalence estimated to be 596 (per 100 000) in males and 373 (per 100 000) in females.1 Atrial fibrillation is associated with an increased risk of thromboembolic events, congestive heart failure, and mortality. Atrial fibrillation is the arrhythmia most commonly associated with hospitalization, and hospitalizations represent the largest part of the total cost of atrial fibrillation treatment.2
The Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial evaluated two different doses of dabigatran or warfarin in patients with atrial fibrillation and an increased risk of stroke.3 In RE-LY, hospitalization occurred in approximately 40% of patients with more than 14 000 total hospitalizations overall.
While randomized controlled trials in congestive heart failure have widely used hospitalization as part of the primary composite outcome,4 randomized controlled trials in atrial fibrillation have used stroke, mortality, and arrhythmia recurrence, as the main endpoints.5 Hospitalization has the potential to serve as a meaningful outcome in atrial fibrillation trials, which is much more common than mortality.6 The objectives of this post hoc analysis of the RE-LY trial were to determine the precise incidence and causes of hospitalizations in patients receiving dabigatran vs. warfarin. Furthermore, we aimed to examine the association between hospitalization and subsequent mortality.
Methods
Patients in RE-LY
The RE-LY trial was a non-inferiority trial of 18 113 patients with atrial fibrillation and an increased risk of stroke. Patients were randomly assigned to receive dabigatran 150 mg bid, dabigatran 110 mg bid (the two dabigatran dosages were blinded), or unblinded warfarin. Patients with documented atrial fibrillation were included if they had one of the following stroke risk factors: history of transient ischaemic attack, stroke, or systemic embolism; ejection fraction less than 40%; symptomatic New York Heart Association (NYHA) Class 2 or higher; age greater than 75; if patients were 65 then they had either diabetes mellitus on treatment, documented coronary artery disease, or hypertension requiring treatment. Patients were excluded if they had severe valvular disease, recent stroke, increased risk of bleeding, estimated glomerular filtration rate less than 30 mL/min, active liver disease, or if they were pregnant (Supplementary material online, Methods). The major efficacy outcome was stroke or systemic embolism. The primary safety outcome was major bleeding. The detailed study and findings have been previously published.3,7
Endpoint assessment
All primary and secondary outcomes including death, stroke, systemic embolism, major bleed, myocardial infarction, transient ischaemic attack, pulmonary embolism, and hospitalization were adjudicated by two blinded investigators unaware of treatment assignment. Details on cardiovascular and non-cardiovascular reasons for hospitalizations were identified on a case report form with investigators allowed to select multiple reasons for a particular hospitalization but the primary reason for hospitalization was specified. Reasons for admission were categorized as related to either a primary cardiovascular outcome event (death, stroke, systemic embolism, major bleed, myocardial infarction, transient ischaemic attack, pulmonary embolism); other cardiovascular event (new angina, atrial fibrillation/flutter, supraventricular arrhythmia, non-fatal cardiac arrest, or ventricular arrhythmia); surgery (valve surgery, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, carotid endarterectomy, peripheral angioplasty or surgery, limb amputation, or other); other non-cardiovascular cause (cancer, injury/fall, fracture, psychiatric, haematologic, genitourinary, gastrointestinal, diabetic, or other). Hospitalizations for congestive heart failure, and other subcategories of cardiovascular hospitalizations (syncope, blood pressure related, cardiovascular infections) were manually searched in the ‘other’ categories above.
Major bleeding events were reported on a specific case report form, which identified whether a patient was hospitalized for this event. Major bleeding was defined as a reduction in the haemoglobin level of at least 2 g per litre, transfusion of at least two units of blood, or symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding). For any bleeding hospitalization, events were derived from the major bleeding case report form, or from the general hospitalization case report form when the reason for admission contained variants of the terms: bleed, epistaxis, haematuria, melena, haematoma, or bruise.
Statistical analysis
The first part of this analysis included assessing the effect of the RE-LY treatment arms [dabigatran etexilate (DE) 110, DE 150, and warfarin] on first hospitalizations since randomization including total hospitalizations, cardiovascular hospitalizations, subcategories of cardiovascular hospitalizations, and non-cardiovascular hospitalizations. The Fisher’s exact tests, χ2 tests, or t-tests were used to compare the groups of interest with a P < 0.05 considered statistically significant. Analyses were conducted with the intention-to-treat principle (i.e. in randomized patients).
Univariate and multivariate hazard models were used to assess the association between hospitalizations with baseline characteristics including treatment assignment.
The second aim of the analysis was to investigate the association of between hospitalizations and adverse events. The relationship between hospitalization and subsequent death was evaluated in a ‘time-updated’ Cox proportional-hazard model providing hazard ratios (HRs) and 95% confidence intervals (CIs). For this analysis, each patient would potentially contribute follow-up years to both groups: patients hospitalized and not hospitalized. At baseline of randomization, patients would all start in the non-hospitalized group. If a patient is admitted to hospital and discharged alive, they would change status to the hospitalized group. Events that occurred during hospital admission were assigned to the non-hospitalized group.
Major safety and efficacy outcomes evaluated included death, vascular death, sudden cardiac death, stroke or systemic embolism, major bleeding and the composite of stroke, systemic embolism, pulmonary embolism, and all-cause mortality. These endpoints were analysed in a non-adjusted model, and a covariate-adjusted model. Relevant covariates in this model included: age, gender, renal function, history of heart failure, hypertension, diabetes, prior stroke or transient ischaemic attack (TIA) or embolic event, coronary artery disease or prior myocardial infarction, peripheral vascular disease, cancer at baseline, valvular heart disease, amiodarone use, other antiarrhythmics use, atrial fibrillation type, and vitamin K antagonist use at baseline.
This analysis was repeated for the first cardiovascular hospitalization. If a patient had multiple cardiovascular hospitalizations, only the first cardiovascular hospitalization was considered.
All analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Cary, NC, USA). No informed consent was required for this post hoc study. The original RE-LY study was approved by all appropriate national regulatory authorities and ethics committees of the participating centres.3
Results
Treatment arms and hospitalization
Of the 18 113 patients in RE-LY, 7200 (39.8%) were hospitalized at least once during a mean follow-up of 2 years, with 14 025 total hospitalizations. Of the hospitalized patients, 3936 (54.7%) were hospitalized once and 3264 (45.3%) had more than one hospitalization. First hospitalization rates were 2312 (39.5%) for DE 110, 2430 (41.6%) for DE 150, and 42.6% (N = 2458) for warfarin.
Characteristics of hospitalized vs. non-hospitalized patients
Table 1 shows that patients with a hospitalization were older and were more likely to have comorbidities such as previous stroke or TIA, prior myocardial infarction or coronary artery disease, heart failure, diabetes mellitus, hypertension, valvular heart disease, chronic kidney disease, and cancer at baseline. Patients with a hospitalizations were more likely to have paroxysmal atrial fibrillation but less likely to have permanent atrial fibrillation. Multivariate predictors of hospitalization included: age; coronary artery disease, atrial fibrillation type especially paroxysmal type; chronic kidney disease; peripheral arterial disease; heart failure; obesity; diabetes; valvular heart disease; cancer and certain medications use at baseline (diuretic, amiodarone, and other antiarrhythmics) (Supplementary material online, Table S1).
| Characteristics . | Hospitalized (N = 7200) . | Not hospitalized (N = 10 913) . | P-valuea . | Cardiovascular hospitalized (N = 2920) . |
|---|---|---|---|---|
| Age (years) (SD) | 72.5 (8.4) | 70.8 (8.8) | <0.0001 | 71.8 (8.7) |
| BMI (kg/m2), N (%) | <0.0001 | |||
| <25 | 1801 (25.0) | 2896 (26.6) | 675 (23.1) | |
| 25–30 | 2757 (38.3) | 4354 (40.0) | 1153 (39.5) | |
| 30–35 | 1657 (23.0) | 2381 (21.9) | 689 (23.6) | |
| ≥35 | 976 (13.6) | 1265 (11.6) | 400 (13.7) | |
| Male gender, N (%) | 4517 (62.7) | 6997 (64.1) | 0.06 | 1804 (61.8) |
| Type of atrial fibrillation, N (%) | <0.0001 | |||
| Paroxysmal | 2602 (36.1) | 3341 (30.6) | 1173 (40.2) | |
| Persistent | 2271 (31.5) | 3518 (32.3) | 907 (31.1) | |
| Permanent | 2326 (32.3) | 4049 (37.1) | 840 (28.8) | |
| CHADS2 score, N (%) | <0.0001 | |||
| 0 or 1 | 1966 (27.3) | 3817 (35.0) | 754 (25.8) | |
| 2 | 2549 (35.4) | 3904 (35.8) | 1059 (36.3) | |
| ≥3 | 2685 (37.3) | 3191 (29.2) | 1107 (37.9) | |
| Previous stroke or TIA or systemic embolic event, N (%) | 1636 (22.7) | 2317 (21.2) | 0.02 | 646 (22.1) |
| Prior myocardial infarction or CAD, N (%) | 2673 (37.1) | 2977 (27.3) | <0.0001 | 1218 (41.7) |
| History of heart failure, N (%) | 2494 (34.6) | 3299 (30.2) | <0.0001 | 1137 (38.9) |
| Diabetes mellitus, N (%) | 1940 (26.9) | 2281 (20.9) | <0.0001 | 832 (28.5) |
| Hypertension, N (%) | 5763 (80.0) | 8520 (78.1) | 0.001 | 2363 (80.9) |
| Valvular heart disease, N (%) | 1728 (24.0) | 2216 (20.3) | <0.0001 | 719 (24.6) |
| CrCl ≤50 mL/min, N (%) | 1658 (23.2) | 1896 (17.5) | <0.0001 | 654 (22.6) |
| Cancer at baseline, N (%) | 889 (12.3) | 996 (9.1) | <0.0001 | 348 (11.9) |
| Characteristics . | Hospitalized (N = 7200) . | Not hospitalized (N = 10 913) . | P-valuea . | Cardiovascular hospitalized (N = 2920) . |
|---|---|---|---|---|
| Age (years) (SD) | 72.5 (8.4) | 70.8 (8.8) | <0.0001 | 71.8 (8.7) |
| BMI (kg/m2), N (%) | <0.0001 | |||
| <25 | 1801 (25.0) | 2896 (26.6) | 675 (23.1) | |
| 25–30 | 2757 (38.3) | 4354 (40.0) | 1153 (39.5) | |
| 30–35 | 1657 (23.0) | 2381 (21.9) | 689 (23.6) | |
| ≥35 | 976 (13.6) | 1265 (11.6) | 400 (13.7) | |
| Male gender, N (%) | 4517 (62.7) | 6997 (64.1) | 0.06 | 1804 (61.8) |
| Type of atrial fibrillation, N (%) | <0.0001 | |||
| Paroxysmal | 2602 (36.1) | 3341 (30.6) | 1173 (40.2) | |
| Persistent | 2271 (31.5) | 3518 (32.3) | 907 (31.1) | |
| Permanent | 2326 (32.3) | 4049 (37.1) | 840 (28.8) | |
| CHADS2 score, N (%) | <0.0001 | |||
| 0 or 1 | 1966 (27.3) | 3817 (35.0) | 754 (25.8) | |
| 2 | 2549 (35.4) | 3904 (35.8) | 1059 (36.3) | |
| ≥3 | 2685 (37.3) | 3191 (29.2) | 1107 (37.9) | |
| Previous stroke or TIA or systemic embolic event, N (%) | 1636 (22.7) | 2317 (21.2) | 0.02 | 646 (22.1) |
| Prior myocardial infarction or CAD, N (%) | 2673 (37.1) | 2977 (27.3) | <0.0001 | 1218 (41.7) |
| History of heart failure, N (%) | 2494 (34.6) | 3299 (30.2) | <0.0001 | 1137 (38.9) |
| Diabetes mellitus, N (%) | 1940 (26.9) | 2281 (20.9) | <0.0001 | 832 (28.5) |
| Hypertension, N (%) | 5763 (80.0) | 8520 (78.1) | 0.001 | 2363 (80.9) |
| Valvular heart disease, N (%) | 1728 (24.0) | 2216 (20.3) | <0.0001 | 719 (24.6) |
| CrCl ≤50 mL/min, N (%) | 1658 (23.2) | 1896 (17.5) | <0.0001 | 654 (22.6) |
| Cancer at baseline, N (%) | 889 (12.3) | 996 (9.1) | <0.0001 | 348 (11.9) |
Missing data: CHADS2 score data missing in one patient and CrCl <50 data missing in 162 patients.
BMI, body mass index; CAD, coronary artery disease; CrCl, creatinine clearance; SD, standard deviation; TIA, transient ischaemic attack.
P-value (from Fisher’s exact test for binary data, t-test for continuous data, or χ2 test for categorical data) reported compares patients hospitalized compared with non-hospitalized patients.
| Characteristics . | Hospitalized (N = 7200) . | Not hospitalized (N = 10 913) . | P-valuea . | Cardiovascular hospitalized (N = 2920) . |
|---|---|---|---|---|
| Age (years) (SD) | 72.5 (8.4) | 70.8 (8.8) | <0.0001 | 71.8 (8.7) |
| BMI (kg/m2), N (%) | <0.0001 | |||
| <25 | 1801 (25.0) | 2896 (26.6) | 675 (23.1) | |
| 25–30 | 2757 (38.3) | 4354 (40.0) | 1153 (39.5) | |
| 30–35 | 1657 (23.0) | 2381 (21.9) | 689 (23.6) | |
| ≥35 | 976 (13.6) | 1265 (11.6) | 400 (13.7) | |
| Male gender, N (%) | 4517 (62.7) | 6997 (64.1) | 0.06 | 1804 (61.8) |
| Type of atrial fibrillation, N (%) | <0.0001 | |||
| Paroxysmal | 2602 (36.1) | 3341 (30.6) | 1173 (40.2) | |
| Persistent | 2271 (31.5) | 3518 (32.3) | 907 (31.1) | |
| Permanent | 2326 (32.3) | 4049 (37.1) | 840 (28.8) | |
| CHADS2 score, N (%) | <0.0001 | |||
| 0 or 1 | 1966 (27.3) | 3817 (35.0) | 754 (25.8) | |
| 2 | 2549 (35.4) | 3904 (35.8) | 1059 (36.3) | |
| ≥3 | 2685 (37.3) | 3191 (29.2) | 1107 (37.9) | |
| Previous stroke or TIA or systemic embolic event, N (%) | 1636 (22.7) | 2317 (21.2) | 0.02 | 646 (22.1) |
| Prior myocardial infarction or CAD, N (%) | 2673 (37.1) | 2977 (27.3) | <0.0001 | 1218 (41.7) |
| History of heart failure, N (%) | 2494 (34.6) | 3299 (30.2) | <0.0001 | 1137 (38.9) |
| Diabetes mellitus, N (%) | 1940 (26.9) | 2281 (20.9) | <0.0001 | 832 (28.5) |
| Hypertension, N (%) | 5763 (80.0) | 8520 (78.1) | 0.001 | 2363 (80.9) |
| Valvular heart disease, N (%) | 1728 (24.0) | 2216 (20.3) | <0.0001 | 719 (24.6) |
| CrCl ≤50 mL/min, N (%) | 1658 (23.2) | 1896 (17.5) | <0.0001 | 654 (22.6) |
| Cancer at baseline, N (%) | 889 (12.3) | 996 (9.1) | <0.0001 | 348 (11.9) |
| Characteristics . | Hospitalized (N = 7200) . | Not hospitalized (N = 10 913) . | P-valuea . | Cardiovascular hospitalized (N = 2920) . |
|---|---|---|---|---|
| Age (years) (SD) | 72.5 (8.4) | 70.8 (8.8) | <0.0001 | 71.8 (8.7) |
| BMI (kg/m2), N (%) | <0.0001 | |||
| <25 | 1801 (25.0) | 2896 (26.6) | 675 (23.1) | |
| 25–30 | 2757 (38.3) | 4354 (40.0) | 1153 (39.5) | |
| 30–35 | 1657 (23.0) | 2381 (21.9) | 689 (23.6) | |
| ≥35 | 976 (13.6) | 1265 (11.6) | 400 (13.7) | |
| Male gender, N (%) | 4517 (62.7) | 6997 (64.1) | 0.06 | 1804 (61.8) |
| Type of atrial fibrillation, N (%) | <0.0001 | |||
| Paroxysmal | 2602 (36.1) | 3341 (30.6) | 1173 (40.2) | |
| Persistent | 2271 (31.5) | 3518 (32.3) | 907 (31.1) | |
| Permanent | 2326 (32.3) | 4049 (37.1) | 840 (28.8) | |
| CHADS2 score, N (%) | <0.0001 | |||
| 0 or 1 | 1966 (27.3) | 3817 (35.0) | 754 (25.8) | |
| 2 | 2549 (35.4) | 3904 (35.8) | 1059 (36.3) | |
| ≥3 | 2685 (37.3) | 3191 (29.2) | 1107 (37.9) | |
| Previous stroke or TIA or systemic embolic event, N (%) | 1636 (22.7) | 2317 (21.2) | 0.02 | 646 (22.1) |
| Prior myocardial infarction or CAD, N (%) | 2673 (37.1) | 2977 (27.3) | <0.0001 | 1218 (41.7) |
| History of heart failure, N (%) | 2494 (34.6) | 3299 (30.2) | <0.0001 | 1137 (38.9) |
| Diabetes mellitus, N (%) | 1940 (26.9) | 2281 (20.9) | <0.0001 | 832 (28.5) |
| Hypertension, N (%) | 5763 (80.0) | 8520 (78.1) | 0.001 | 2363 (80.9) |
| Valvular heart disease, N (%) | 1728 (24.0) | 2216 (20.3) | <0.0001 | 719 (24.6) |
| CrCl ≤50 mL/min, N (%) | 1658 (23.2) | 1896 (17.5) | <0.0001 | 654 (22.6) |
| Cancer at baseline, N (%) | 889 (12.3) | 996 (9.1) | <0.0001 | 348 (11.9) |
Missing data: CHADS2 score data missing in one patient and CrCl <50 data missing in 162 patients.
BMI, body mass index; CAD, coronary artery disease; CrCl, creatinine clearance; SD, standard deviation; TIA, transient ischaemic attack.
P-value (from Fisher’s exact test for binary data, t-test for continuous data, or χ2 test for categorical data) reported compares patients hospitalized compared with non-hospitalized patients.
Reasons for first cardiovascular hospitalization
Patients in the DE 110 arm were hospitalized least often compared with other arms due to atrial fibrillation and other supraventricular rhythm disorders (Table 2). Patients were hospitalized least often due to myocardial infarction in the warfarin group, but there were no differences in hospitalizations for new angina or atherosclerosis related admissions. There was a statistical trend for lower hospitalizations for TIA or ischaemic stroke, and cardiovascular surgery in the DE 150 arm. Among the three treatment arms, there were no statistically significant differences in hospitalizations due congestive heart failure, ventricular arrhythmias, heart failure, syncope, pacemaker or implantable cardioverter-defibrillator implantation, pulmonary embolism, hypotension or hypertension, major bleeding, cardiovascular infection, or non-cardiovascular hospitalizations.
Subcategories of cardiovascular hospitalization among different treatment arms
| . | DE 110 . | DE 150 . | Warfarin . | P-value from Fisher’s exact test . |
|---|---|---|---|---|
| Patients hospitalized | 2312 | 2430 | 2458 | |
| Patients dead or hospitalized | 2436 | 2569 | 2599 | |
| Patients with a cardiovascular hospitalization, N (%) | 952 (100%) | 982 (100%) | 986 (100%) | 0.74 |
| Myocardial infarction | 35 (3.7%) | 47 (4.8%) | 26 (2.6%) | 0.04 |
| New angina | 98 (10.3%) | 105 (10.7%) | 113 (11.5%) | 0.82 |
| Atherosclerosis relateda | 171 (18.0%) | 174 (17.7%) | 181 (18.4%) | 0.95 |
| Transcutaneous coronary, cerebrovascular, or peripheral procedureb | 65 (6.8%) | 69 (7.0%) | 68 (6.9%) | 0.99 |
| Valve or coronary artery bypass grafting surgery | 37 (3.9%) | 20 (2.0%) | 27 (2.7%) | 0.05 |
| AF and other supraventricular rhythm disorders | 287 (30.1%) | 358 (36.5%) | 357 (36.2%) | 0.04 |
| Ventricular arrhythmia or non-fatal cardiac arrest | 34 (3.6%) | 37 (3.8%) | 40 (4.1%) | 0.91 |
| Worsening congestive heart failure | 199 (20.9%) | 176 (17.9%) | 176 (17.8%) | 0.12 |
| Syncope | 29 (3.0%) | 45 (4.6%) | 40 (4.1%) | 0.25 |
| Implantation of a pacemaker, or a ICD | 118 (12.4%) | 107 (10.9%) | 91 (9.2%) | 0.06 |
| TIA or ischaemic stroke | 100 (10.5%) | 74 (7.5%) | 93 (9.4%) | 0.06 |
| Pulmonary embolism | 4 (0.4%) | 3 (0.3%) | 2 (0.2%) | 0.59 |
| Hypotension or hypertension | 30 (3.2%) | 28 (2.9%) | 32 (3.2%) | 0.87 |
| Major bleedingc | 26 (2.7%) | 45 (4.6%) | 40 (4.1%) | 0.10 |
| Cardiovascular infection | 2 (0.2%) | 3 (0.3%) | 1 (0.1%) | 0.62 |
| Patients with hospitalization due to any bleeding | 178 | 261 | 256 | 0.0004 |
| Patients hospitalized for non-cardiovascular reason | 1360 | 1448 | 1472 | 0.74 |
| . | DE 110 . | DE 150 . | Warfarin . | P-value from Fisher’s exact test . |
|---|---|---|---|---|
| Patients hospitalized | 2312 | 2430 | 2458 | |
| Patients dead or hospitalized | 2436 | 2569 | 2599 | |
| Patients with a cardiovascular hospitalization, N (%) | 952 (100%) | 982 (100%) | 986 (100%) | 0.74 |
| Myocardial infarction | 35 (3.7%) | 47 (4.8%) | 26 (2.6%) | 0.04 |
| New angina | 98 (10.3%) | 105 (10.7%) | 113 (11.5%) | 0.82 |
| Atherosclerosis relateda | 171 (18.0%) | 174 (17.7%) | 181 (18.4%) | 0.95 |
| Transcutaneous coronary, cerebrovascular, or peripheral procedureb | 65 (6.8%) | 69 (7.0%) | 68 (6.9%) | 0.99 |
| Valve or coronary artery bypass grafting surgery | 37 (3.9%) | 20 (2.0%) | 27 (2.7%) | 0.05 |
| AF and other supraventricular rhythm disorders | 287 (30.1%) | 358 (36.5%) | 357 (36.2%) | 0.04 |
| Ventricular arrhythmia or non-fatal cardiac arrest | 34 (3.6%) | 37 (3.8%) | 40 (4.1%) | 0.91 |
| Worsening congestive heart failure | 199 (20.9%) | 176 (17.9%) | 176 (17.8%) | 0.12 |
| Syncope | 29 (3.0%) | 45 (4.6%) | 40 (4.1%) | 0.25 |
| Implantation of a pacemaker, or a ICD | 118 (12.4%) | 107 (10.9%) | 91 (9.2%) | 0.06 |
| TIA or ischaemic stroke | 100 (10.5%) | 74 (7.5%) | 93 (9.4%) | 0.06 |
| Pulmonary embolism | 4 (0.4%) | 3 (0.3%) | 2 (0.2%) | 0.59 |
| Hypotension or hypertension | 30 (3.2%) | 28 (2.9%) | 32 (3.2%) | 0.87 |
| Major bleedingc | 26 (2.7%) | 45 (4.6%) | 40 (4.1%) | 0.10 |
| Cardiovascular infection | 2 (0.2%) | 3 (0.3%) | 1 (0.1%) | 0.62 |
| Patients with hospitalization due to any bleeding | 178 | 261 | 256 | 0.0004 |
| Patients hospitalized for non-cardiovascular reason | 1360 | 1448 | 1472 | 0.74 |
Only first hospitalization is considered. Multiple reasons are allowed for a single patient.
AF, atrial fibrillation; CABG, coronary artery bypass grafting; DE, dabigatran etexilate; ICD, implantable cardioverter-defibrillator; MBE, major bleeding event; PTCA, percutaneous transluminal coronary angioplasty; TIA, transient ischaemic attack.
New angina, or CABG surgery, or PTCA surgery, or carotid endarterectomy, or peripheral angioplasty/surgery, or limb amputation.
PTCA surgery, or carotid endarterectomy, or peripheral angioplasty/surgery, or limb amputation.
Major bleeding: hospitalization with admission due to outcome event MBE requiring two or more units of blood or any intracranial haemorrhage.
Subcategories of cardiovascular hospitalization among different treatment arms
| . | DE 110 . | DE 150 . | Warfarin . | P-value from Fisher’s exact test . |
|---|---|---|---|---|
| Patients hospitalized | 2312 | 2430 | 2458 | |
| Patients dead or hospitalized | 2436 | 2569 | 2599 | |
| Patients with a cardiovascular hospitalization, N (%) | 952 (100%) | 982 (100%) | 986 (100%) | 0.74 |
| Myocardial infarction | 35 (3.7%) | 47 (4.8%) | 26 (2.6%) | 0.04 |
| New angina | 98 (10.3%) | 105 (10.7%) | 113 (11.5%) | 0.82 |
| Atherosclerosis relateda | 171 (18.0%) | 174 (17.7%) | 181 (18.4%) | 0.95 |
| Transcutaneous coronary, cerebrovascular, or peripheral procedureb | 65 (6.8%) | 69 (7.0%) | 68 (6.9%) | 0.99 |
| Valve or coronary artery bypass grafting surgery | 37 (3.9%) | 20 (2.0%) | 27 (2.7%) | 0.05 |
| AF and other supraventricular rhythm disorders | 287 (30.1%) | 358 (36.5%) | 357 (36.2%) | 0.04 |
| Ventricular arrhythmia or non-fatal cardiac arrest | 34 (3.6%) | 37 (3.8%) | 40 (4.1%) | 0.91 |
| Worsening congestive heart failure | 199 (20.9%) | 176 (17.9%) | 176 (17.8%) | 0.12 |
| Syncope | 29 (3.0%) | 45 (4.6%) | 40 (4.1%) | 0.25 |
| Implantation of a pacemaker, or a ICD | 118 (12.4%) | 107 (10.9%) | 91 (9.2%) | 0.06 |
| TIA or ischaemic stroke | 100 (10.5%) | 74 (7.5%) | 93 (9.4%) | 0.06 |
| Pulmonary embolism | 4 (0.4%) | 3 (0.3%) | 2 (0.2%) | 0.59 |
| Hypotension or hypertension | 30 (3.2%) | 28 (2.9%) | 32 (3.2%) | 0.87 |
| Major bleedingc | 26 (2.7%) | 45 (4.6%) | 40 (4.1%) | 0.10 |
| Cardiovascular infection | 2 (0.2%) | 3 (0.3%) | 1 (0.1%) | 0.62 |
| Patients with hospitalization due to any bleeding | 178 | 261 | 256 | 0.0004 |
| Patients hospitalized for non-cardiovascular reason | 1360 | 1448 | 1472 | 0.74 |
| . | DE 110 . | DE 150 . | Warfarin . | P-value from Fisher’s exact test . |
|---|---|---|---|---|
| Patients hospitalized | 2312 | 2430 | 2458 | |
| Patients dead or hospitalized | 2436 | 2569 | 2599 | |
| Patients with a cardiovascular hospitalization, N (%) | 952 (100%) | 982 (100%) | 986 (100%) | 0.74 |
| Myocardial infarction | 35 (3.7%) | 47 (4.8%) | 26 (2.6%) | 0.04 |
| New angina | 98 (10.3%) | 105 (10.7%) | 113 (11.5%) | 0.82 |
| Atherosclerosis relateda | 171 (18.0%) | 174 (17.7%) | 181 (18.4%) | 0.95 |
| Transcutaneous coronary, cerebrovascular, or peripheral procedureb | 65 (6.8%) | 69 (7.0%) | 68 (6.9%) | 0.99 |
| Valve or coronary artery bypass grafting surgery | 37 (3.9%) | 20 (2.0%) | 27 (2.7%) | 0.05 |
| AF and other supraventricular rhythm disorders | 287 (30.1%) | 358 (36.5%) | 357 (36.2%) | 0.04 |
| Ventricular arrhythmia or non-fatal cardiac arrest | 34 (3.6%) | 37 (3.8%) | 40 (4.1%) | 0.91 |
| Worsening congestive heart failure | 199 (20.9%) | 176 (17.9%) | 176 (17.8%) | 0.12 |
| Syncope | 29 (3.0%) | 45 (4.6%) | 40 (4.1%) | 0.25 |
| Implantation of a pacemaker, or a ICD | 118 (12.4%) | 107 (10.9%) | 91 (9.2%) | 0.06 |
| TIA or ischaemic stroke | 100 (10.5%) | 74 (7.5%) | 93 (9.4%) | 0.06 |
| Pulmonary embolism | 4 (0.4%) | 3 (0.3%) | 2 (0.2%) | 0.59 |
| Hypotension or hypertension | 30 (3.2%) | 28 (2.9%) | 32 (3.2%) | 0.87 |
| Major bleedingc | 26 (2.7%) | 45 (4.6%) | 40 (4.1%) | 0.10 |
| Cardiovascular infection | 2 (0.2%) | 3 (0.3%) | 1 (0.1%) | 0.62 |
| Patients with hospitalization due to any bleeding | 178 | 261 | 256 | 0.0004 |
| Patients hospitalized for non-cardiovascular reason | 1360 | 1448 | 1472 | 0.74 |
Only first hospitalization is considered. Multiple reasons are allowed for a single patient.
AF, atrial fibrillation; CABG, coronary artery bypass grafting; DE, dabigatran etexilate; ICD, implantable cardioverter-defibrillator; MBE, major bleeding event; PTCA, percutaneous transluminal coronary angioplasty; TIA, transient ischaemic attack.
New angina, or CABG surgery, or PTCA surgery, or carotid endarterectomy, or peripheral angioplasty/surgery, or limb amputation.
PTCA surgery, or carotid endarterectomy, or peripheral angioplasty/surgery, or limb amputation.
Major bleeding: hospitalization with admission due to outcome event MBE requiring two or more units of blood or any intracranial haemorrhage.
There was a difference in the rates for all-cause bleeding (Supplementary material online, Table S2) with events occurring at a rate of 3.0% (N = 178) for DE 110 mg compared with both warfarin 4.3% (N = 256, P = 0.0012), and to DE 150 at 4.3% (N = 261, P = 0.0003). Rates were not statistically different between DE 150 and warfarin (P = 0.75).
Hospitalizations and subsequent adverse events
There were 8325 follow-up patient years post hospital discharge for hospitalized patients, and 27 340 follow-up patient years until hospitalization for hospitalized patients or for non-hospitalized patients. The yearly mortality rate was 9.1% following hospitalization in the hospitalized patients compared with 2.2% in the non-hospitalized patients (adjusted HR 3.6, 95% CI 3.2–4.0, P < 0.0001; Table 3 and Figure 1). The yearly event rate of vascular death was 5.3% in the post-hospitalized patients compared with 1.6% in the non-hospitalized patients (adjusted HR 2.9, 95% CI 2.5–3.3, P < 0.0001). Hospitalization was also associated with an increased risk of sudden cardiac death (adjusted HR 2.3, 95% CI 1.8–2.9, P < 0.0001) compared with patients not hospitalized for any reason. Patients in the hospitalized group were 70% more likely to have a stroke and systemic embolism compared with the non-hospitalized group (adjusted HR 1.7, 95% CI 1.4–2.0, P < 0.0001), and they were 90% more likely to have a major bleed (adjusted HR 1.9, 95% CI 1.7–2.2, P < 0.0001). Overall, hospitalization was associated with an increased hazard of the composite of stroke, systemic embolism, pulmonary embolism, myocardial infarction, and all-cause death compared with the non-hospitalized patients (adjusted HR 2.8, 95% CI 2.5–3.0, P < 0.0001).
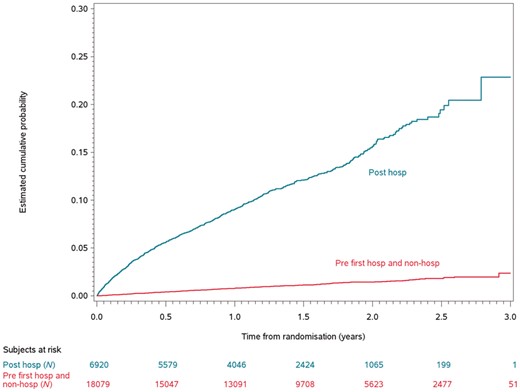
The Kaplan–Meier curves of time to death post first hospitalization. hosp, hospitalization.
| Event\N patient years . | Post-hospitalization (8325 patient years) . | No hospitalization (27 340 patient years) . | Hospitalized vs. non-hospitalized patients . | |||||
|---|---|---|---|---|---|---|---|---|
| N . | Rate per patient years (%) . | N . | Rate per patient years (%) . | Adjustedb hazard ratioc . | Unadjusted hazard ratioc . | |||
| HR . | 95% CI . | HR . | 95% CI . | |||||
| Death | 755 | 9.1 | 610 | 2.2 | 3.6 | 3.2–4.0 | 4.0 | 3.6–4.5 |
| Vascular death | 443 | 5.3 | 434 | 1.6 | 2.9 | 2.5–3.3 | 3.3 | 2.9–3.7 |
| Sudden cardiac death | 136 | 1.6 | 169 | 0.6 | 2.3 | 1.8–2.9 | 2.5 | 2.0–3.1 |
| Stroke/SEE/PE/MI/all-cause death | 948 | 11.4 | 1017 | 3.7 | 2.8 | 2.5–3.0 | 3.1 | 2.8–3.3 |
| Stroke/SEE | 194 | 2.3 | 341 | 1.3 | 1.7 | 1.4–2.0 | 1.8 | 1.5–2.2 |
| Major bleeding | 510 | 6.1 | 757 | 2.8 | 1.9 | 1.7–2.2 | 2.1 | 1.9–2.4 |
| Event\N patient years . | Post-hospitalization (8325 patient years) . | No hospitalization (27 340 patient years) . | Hospitalized vs. non-hospitalized patients . | |||||
|---|---|---|---|---|---|---|---|---|
| N . | Rate per patient years (%) . | N . | Rate per patient years (%) . | Adjustedb hazard ratioc . | Unadjusted hazard ratioc . | |||
| HR . | 95% CI . | HR . | 95% CI . | |||||
| Death | 755 | 9.1 | 610 | 2.2 | 3.6 | 3.2–4.0 | 4.0 | 3.6–4.5 |
| Vascular death | 443 | 5.3 | 434 | 1.6 | 2.9 | 2.5–3.3 | 3.3 | 2.9–3.7 |
| Sudden cardiac death | 136 | 1.6 | 169 | 0.6 | 2.3 | 1.8–2.9 | 2.5 | 2.0–3.1 |
| Stroke/SEE/PE/MI/all-cause death | 948 | 11.4 | 1017 | 3.7 | 2.8 | 2.5–3.0 | 3.1 | 2.8–3.3 |
| Stroke/SEE | 194 | 2.3 | 341 | 1.3 | 1.7 | 1.4–2.0 | 1.8 | 1.5–2.2 |
| Major bleeding | 510 | 6.1 | 757 | 2.8 | 1.9 | 1.7–2.2 | 2.1 | 1.9–2.4 |
CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; PE, pulmonary embolism; SEE, systemic embolism; TIA, transient ischaemic attack.
Each patient would potentially contribute to follow-up years to both groups: patients hospitalized and not hospitalized. All patients were in the not hospitalized group at the beginning of the trial. Patients changed status to hospitalized if they are discharged alive from hospital. Therefore, some patients contributed to follow-up years to both groups: the post-hospitalized and to the non-hospitalized groups. Events that occurred during admission to hospital were assigned to the non-hospitalized group.
Adjusted for baseline covariates: age, gender, renal function, history of heart failure, hypertension, diabetes, prior stroke or TIA or embolic event, coronary artery disease, amiodarone, antiarrhytmics, atrial fibrillation type, and vitamin K antagonist use at baseline.
For all comparisons, P < 0.0001.
| Event\N patient years . | Post-hospitalization (8325 patient years) . | No hospitalization (27 340 patient years) . | Hospitalized vs. non-hospitalized patients . | |||||
|---|---|---|---|---|---|---|---|---|
| N . | Rate per patient years (%) . | N . | Rate per patient years (%) . | Adjustedb hazard ratioc . | Unadjusted hazard ratioc . | |||
| HR . | 95% CI . | HR . | 95% CI . | |||||
| Death | 755 | 9.1 | 610 | 2.2 | 3.6 | 3.2–4.0 | 4.0 | 3.6–4.5 |
| Vascular death | 443 | 5.3 | 434 | 1.6 | 2.9 | 2.5–3.3 | 3.3 | 2.9–3.7 |
| Sudden cardiac death | 136 | 1.6 | 169 | 0.6 | 2.3 | 1.8–2.9 | 2.5 | 2.0–3.1 |
| Stroke/SEE/PE/MI/all-cause death | 948 | 11.4 | 1017 | 3.7 | 2.8 | 2.5–3.0 | 3.1 | 2.8–3.3 |
| Stroke/SEE | 194 | 2.3 | 341 | 1.3 | 1.7 | 1.4–2.0 | 1.8 | 1.5–2.2 |
| Major bleeding | 510 | 6.1 | 757 | 2.8 | 1.9 | 1.7–2.2 | 2.1 | 1.9–2.4 |
| Event\N patient years . | Post-hospitalization (8325 patient years) . | No hospitalization (27 340 patient years) . | Hospitalized vs. non-hospitalized patients . | |||||
|---|---|---|---|---|---|---|---|---|
| N . | Rate per patient years (%) . | N . | Rate per patient years (%) . | Adjustedb hazard ratioc . | Unadjusted hazard ratioc . | |||
| HR . | 95% CI . | HR . | 95% CI . | |||||
| Death | 755 | 9.1 | 610 | 2.2 | 3.6 | 3.2–4.0 | 4.0 | 3.6–4.5 |
| Vascular death | 443 | 5.3 | 434 | 1.6 | 2.9 | 2.5–3.3 | 3.3 | 2.9–3.7 |
| Sudden cardiac death | 136 | 1.6 | 169 | 0.6 | 2.3 | 1.8–2.9 | 2.5 | 2.0–3.1 |
| Stroke/SEE/PE/MI/all-cause death | 948 | 11.4 | 1017 | 3.7 | 2.8 | 2.5–3.0 | 3.1 | 2.8–3.3 |
| Stroke/SEE | 194 | 2.3 | 341 | 1.3 | 1.7 | 1.4–2.0 | 1.8 | 1.5–2.2 |
| Major bleeding | 510 | 6.1 | 757 | 2.8 | 1.9 | 1.7–2.2 | 2.1 | 1.9–2.4 |
CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; PE, pulmonary embolism; SEE, systemic embolism; TIA, transient ischaemic attack.
Each patient would potentially contribute to follow-up years to both groups: patients hospitalized and not hospitalized. All patients were in the not hospitalized group at the beginning of the trial. Patients changed status to hospitalized if they are discharged alive from hospital. Therefore, some patients contributed to follow-up years to both groups: the post-hospitalized and to the non-hospitalized groups. Events that occurred during admission to hospital were assigned to the non-hospitalized group.
Adjusted for baseline covariates: age, gender, renal function, history of heart failure, hypertension, diabetes, prior stroke or TIA or embolic event, coronary artery disease, amiodarone, antiarrhytmics, atrial fibrillation type, and vitamin K antagonist use at baseline.
For all comparisons, P < 0.0001.
There were 4051 patient follow-up patient years for post-discharge for patients for a cardiovascular cause, and 31 643 follow-up patient years for patient not hospitalized for a cardiovascular cause. The yearly event rate of death was 9.9% in the cardiovascular hospitalized patients compared with 3.1% in the non-cardiovascular hospitalized patients (adjusted HR 2.8, 95% CI 2.5–3.2, P < 0.0001; Table 4 and Figure 2). The yearly event rate of vascular death was 6.6% in the cardiovascular hospitalized patients compared with 1.9% in the non-cardiovascular hospitalized patients (adjusted HR 2.8, 95% CI 2.4–3.2, P < 0.0001). Cardiovascular hospitalization was also associated with an increased risk of sudden cardiac death (adjusted HR 2.1, 95% CI 1.6–2.7, P < 0.0001) compared with patients not hospitalized for any cardiovascular reason. Patients in the cardiovascular hospitalized group were 60% more likely to have a stroke and systemic embolism compared with the non-hospitalized group (adjusted HR 1.6, 95% CI 1.3–2.0, P < 0.0001), and they were 80% more likely to have a major bleed (adjusted HR 1.8, 95% CI 1.6–2.1, P < 0.0001). Overall, cardiovascular hospitalization was associated with an increased hazard of the composite of stroke, systemic embolism, pulmonary embolism, myocardial infarction, and all-cause death compared with the non-cardiovascular hospitalized patients (adjusted HR 2.4, 95% CI 2.2–2.7, P < 0.0001).
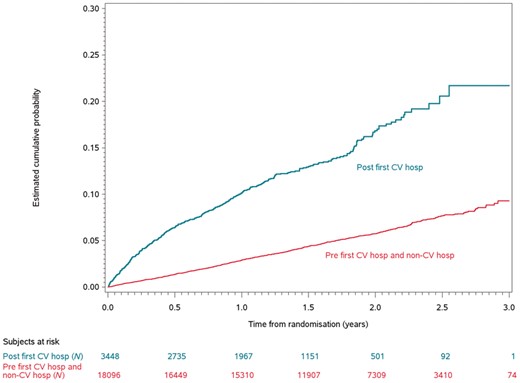
The Kaplan–Meier curves of time to death post first cardiovascular hospitalization. CV hosp, cardiovascular hospitalization.
Relationship between cardiovascular hospitalization and major adverse cardiac eventsa
| Event\N patient years . | Post any CV hospitalization (4051 patient years) . | No CV hospitalization (31 643 patient years) . | CV hospitalized vs. non-CV hospitalized patients . | |||||
|---|---|---|---|---|---|---|---|---|
| N . | Rate per patient years (%) . | N . | Rate per patient years (%) . | Adjustedb hazard ratioc . | Unadjusted hazard ratioc . | |||
| HR . | 95% CI . | HR . | 95% CI . | |||||
| Death | 401 | 9.9 | 967 | 3.1 | 2.8 | 2.5–3.2 | 3.3 | 2.9–3.7 |
| Vascular death | 267 | 6.6 | 610 | 1.9 | 2.8 | 2.4–3.2 | 3.3 | 2.9–3.9 |
| Sudden cardiac death | 78 | 1.9 | 227 | 0.7 | 2.1 | 1.6–2.7 | 2.5 | 1.9–3.2 |
| Stroke/SEE/PE/MI/all-cause death | 506 | 12.5 | 1468 | 4.6 | 2.4 | 2.2–2.7 | 2.7 | 2.4–3.0 |
| Stroke/SEE | 103 | 2.5 | 431 | 1.4 | 1.6 | 1.3–2.0 | 1.8 | 1.5–2.3 |
| Major bleeding | 262 | 6.5 | 969 | 3.1 | 1.8 | 1.6–2.1 | 2.0 | 1.7–2.3 |
| Event\N patient years . | Post any CV hospitalization (4051 patient years) . | No CV hospitalization (31 643 patient years) . | CV hospitalized vs. non-CV hospitalized patients . | |||||
|---|---|---|---|---|---|---|---|---|
| N . | Rate per patient years (%) . | N . | Rate per patient years (%) . | Adjustedb hazard ratioc . | Unadjusted hazard ratioc . | |||
| HR . | 95% CI . | HR . | 95% CI . | |||||
| Death | 401 | 9.9 | 967 | 3.1 | 2.8 | 2.5–3.2 | 3.3 | 2.9–3.7 |
| Vascular death | 267 | 6.6 | 610 | 1.9 | 2.8 | 2.4–3.2 | 3.3 | 2.9–3.9 |
| Sudden cardiac death | 78 | 1.9 | 227 | 0.7 | 2.1 | 1.6–2.7 | 2.5 | 1.9–3.2 |
| Stroke/SEE/PE/MI/all-cause death | 506 | 12.5 | 1468 | 4.6 | 2.4 | 2.2–2.7 | 2.7 | 2.4–3.0 |
| Stroke/SEE | 103 | 2.5 | 431 | 1.4 | 1.6 | 1.3–2.0 | 1.8 | 1.5–2.3 |
| Major bleeding | 262 | 6.5 | 969 | 3.1 | 1.8 | 1.6–2.1 | 2.0 | 1.7–2.3 |
CI, confidence interval; CV, cardiovascular; HR, hazard ratio; MI, myocardial infarction; PE, pulmonary embolism; SEE, systemic embolism; TIA, transient ischaemic attack.
Each patient would potentially contribute to follow-up years to both groups: patients hospitalized and not hospitalized. All patients were in the not hospitalized group at the beginning of the trial. Patients changed status to post-hospitalized if they are discharged alive from hospital after a cardiovascular hospitalization (not necessarily the first hospitalization). Therefore, some patients contributed to follow-up years to both groups: the post any CV-hospitalized and to the non CV-hospitalized groups. Events that occurred during admission to hospital were assigned to the non CV-hospitalized group.
Adjusted for baseline covariates: age, gender, renal function, history of heart failure, hypertension, diabetes, prior stroke or TIA or embolic event, coronary artery disease, amiodarone, antiarrhytmics, atrial fibrillation type, and vitamin K antagonist use at baseline.
For all comparisons, P < 0.0001.
Relationship between cardiovascular hospitalization and major adverse cardiac eventsa
| Event\N patient years . | Post any CV hospitalization (4051 patient years) . | No CV hospitalization (31 643 patient years) . | CV hospitalized vs. non-CV hospitalized patients . | |||||
|---|---|---|---|---|---|---|---|---|
| N . | Rate per patient years (%) . | N . | Rate per patient years (%) . | Adjustedb hazard ratioc . | Unadjusted hazard ratioc . | |||
| HR . | 95% CI . | HR . | 95% CI . | |||||
| Death | 401 | 9.9 | 967 | 3.1 | 2.8 | 2.5–3.2 | 3.3 | 2.9–3.7 |
| Vascular death | 267 | 6.6 | 610 | 1.9 | 2.8 | 2.4–3.2 | 3.3 | 2.9–3.9 |
| Sudden cardiac death | 78 | 1.9 | 227 | 0.7 | 2.1 | 1.6–2.7 | 2.5 | 1.9–3.2 |
| Stroke/SEE/PE/MI/all-cause death | 506 | 12.5 | 1468 | 4.6 | 2.4 | 2.2–2.7 | 2.7 | 2.4–3.0 |
| Stroke/SEE | 103 | 2.5 | 431 | 1.4 | 1.6 | 1.3–2.0 | 1.8 | 1.5–2.3 |
| Major bleeding | 262 | 6.5 | 969 | 3.1 | 1.8 | 1.6–2.1 | 2.0 | 1.7–2.3 |
| Event\N patient years . | Post any CV hospitalization (4051 patient years) . | No CV hospitalization (31 643 patient years) . | CV hospitalized vs. non-CV hospitalized patients . | |||||
|---|---|---|---|---|---|---|---|---|
| N . | Rate per patient years (%) . | N . | Rate per patient years (%) . | Adjustedb hazard ratioc . | Unadjusted hazard ratioc . | |||
| HR . | 95% CI . | HR . | 95% CI . | |||||
| Death | 401 | 9.9 | 967 | 3.1 | 2.8 | 2.5–3.2 | 3.3 | 2.9–3.7 |
| Vascular death | 267 | 6.6 | 610 | 1.9 | 2.8 | 2.4–3.2 | 3.3 | 2.9–3.9 |
| Sudden cardiac death | 78 | 1.9 | 227 | 0.7 | 2.1 | 1.6–2.7 | 2.5 | 1.9–3.2 |
| Stroke/SEE/PE/MI/all-cause death | 506 | 12.5 | 1468 | 4.6 | 2.4 | 2.2–2.7 | 2.7 | 2.4–3.0 |
| Stroke/SEE | 103 | 2.5 | 431 | 1.4 | 1.6 | 1.3–2.0 | 1.8 | 1.5–2.3 |
| Major bleeding | 262 | 6.5 | 969 | 3.1 | 1.8 | 1.6–2.1 | 2.0 | 1.7–2.3 |
CI, confidence interval; CV, cardiovascular; HR, hazard ratio; MI, myocardial infarction; PE, pulmonary embolism; SEE, systemic embolism; TIA, transient ischaemic attack.
Each patient would potentially contribute to follow-up years to both groups: patients hospitalized and not hospitalized. All patients were in the not hospitalized group at the beginning of the trial. Patients changed status to post-hospitalized if they are discharged alive from hospital after a cardiovascular hospitalization (not necessarily the first hospitalization). Therefore, some patients contributed to follow-up years to both groups: the post any CV-hospitalized and to the non CV-hospitalized groups. Events that occurred during admission to hospital were assigned to the non CV-hospitalized group.
Adjusted for baseline covariates: age, gender, renal function, history of heart failure, hypertension, diabetes, prior stroke or TIA or embolic event, coronary artery disease, amiodarone, antiarrhytmics, atrial fibrillation type, and vitamin K antagonist use at baseline.
For all comparisons, P < 0.0001.
Discussion
Main findings
First hospitalization occurred five times more than all-cause death, and eight times more than death from vascular causes.3 We found that, despite adjustment for covariate predictors, hospitalizations were strongly associated with subsequent post-discharge death with an adjusted HR of 3.6, and a HR of 2.9 for vascular death. Cardiovascular hospitalizations were also associated with an adjusted HR 2.8 for both death and vascular death. Hospitalizations and cardiovascular hospitalizations were moderately associated with stroke and major bleeding. Analogous to heart failure clinical trials where hospitalization is a widely accepted outcome, our analysis suggests that hospitalization and cardiovascular hospitalization, if clearly pre-specified and defined, may serve as a surrogate for all-cause mortality in atrial fibrillation trials. This finding requires validation in multiple studies and varying clinical settings.
The burden of hospitalization
Atrial fibrillation is the most common arrhythmia associated with hospitalizations and hospitalizations represent the largest part of the total cost of treatment of atrial fibrillation treatment with costs significantly increasing over time.2 Hospitalizations are also associated with a decrease in patients’ quality of life.8 Hospitalization is then an important patient-oriented and economic burden outcome.
Naccarelli et al.9 used Medicare claims to classify cardiovascular and non-cardiovascular causes among elderly patients with atrial fibrillation and Torp-Pedersen et al.10 classified the causes of cardiovascular hospitalization in the ATHENA study (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter). Similar to our report, common reasons for cardiovascular hospitalizations included atrial fibrillation or supraventricular rhythm disorders, congestive heart failure, atherosclerotic disease, and stroke or TIA. Major bleeding was the 9th9 and 10th10 most common cause of cardiovascular hospitalization. In the ORBIT-AF registry, bleeding accounted for 3.3/100 hospitalizations.11 Our report is unique in that identifies that bleeding events were the most common cause of hospitalization other than atrial fibrillation, and that most of these bleeding events were not major bleeds. Consistent with previous reports, patients were hospitalized least often due to myocardial infarction in the warfarin group, but there were no differences in hospitalizations for new angina or atherosclerosis related admissions.12 Aside from any bleeding, atrial fibrillation or embolic events related admissions, patients were commonly admitted for coronary events and congestive heart failure highlighting the need for optimizing the treatment of these common comorbid conditions in patients with atrial fibrillation.
Previous analyses have identified similar characteristics associated with hospitalization: age, coronary disease, baseline diuretic use, baseline vitamin K antagonist use, chronic kidney disease, diabetes, obesity, and peripheral arterial or carotid artery disease.11,13 DeVore et al. highlight geographic variation and lung disease, whereas our report identified categories of body mass index, congestive heart failure, peripheral arterial disease, paroxysmal atrial fibrillation, and cancer as important associations with hospitalization. From an analysis of the ORBIT-AF registry, additional factors associated with hospitalization include: NYHA class, obstructive sleep apnoea, left atrial size, frailty, and not living independently. In an analysis of the AVERROES trial, paroxysmal atrial fibrillation was significantly associated with more hospitalizations for atrial fibrillation treatment, whereas permanent atrial fibrillation was significantly more associated with hospitalizations for congestive heart failure.14 The differences in the predictors identified are due to differences in populations, timelines and cointerventions in the studies, and different variables being incorporated into the multivariate analysis.
Hospitalization as a possible surrogate outcome
For an outcome to be a valid surrogate, it must meet certain criteria (i) it must occur more frequently and earlier than the primary endpoint; (ii) it must accurately predict the event of interest; and (iii) the strength of the association with the primary endpoint should not vary based on the treatment assignment.
Previous reports have explored the relationship between hospitalization and death in patients with atrial fibrillation. In the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study, which evaluated rhythm vs. rate control, cardiovascular hospitalization was associated with an increased hazard of death (HR range 1.7–2.1) regardless of treatment assignment.6 In the Stockholm Cohort Study of Atrial Fibrillation cardiovascular hospitalizations were associated with an increased hazard of mortality (HR 1.4, 95% CI 1.2–1.6).15 Both the Stockholm Cohort and AFFIRM study did not assess planned and unplanned cardiovascular hospitalizations separately. This may have decreased the hazards associated with hospitalization since many of the hospitalizations may have been due to planned cardioversions or initiation of rhythm control medications. In the ORBIT-AF registry, patients with and without any hospitalization died at a rate of 10.81 events per 100 patient-years compared with 1.92 deaths per 100 patient-years.11 In an analysis of the AVERROES trial, Hohnloser et al.14 reported that cardiovascular hospitalization was the strongest predictor of subsequent mortality (HR 3.95, 95% CI 3.06–5.09, P < 0.001).
Cardiovascular hospitalization has been used rarely as an outcome in atrial fibrillation trials, for example, as part of the primary composite outcome in the ATHENA trial.16 However, hospitalization is not widely accepted in atrial fibrillation trials due to concerns over variability in hospitalization practices and due to a significant number of hospitalizations being unrelated to atrial fibrillation, which may dilute the effect of a treatment.5
Despite adjustment for covariate predictors in our study, hospitalizations were strongly associated with death with an adjusted HR of 3.6, and a HR of 2.9 for vascular death. Cardiovascular hospitalizations were associated with the same adjusted HR 2.8 for both death and vascular death. In congestive heart failure trials where hospitalizations for congestive heart failure is widely accepted as part of the primary composite outcomes,4 the adjusted hazard of death associated with heart failure hospitalization ranged from 2.5 in the Digitalis Investigation Group (DIG) trial to 3.1 in the Candesartan in Heart failure: Reduction in Mortality and morbidity (CHARM) trials.17,18
Hospitalization then marks a dramatic change in a patient’s health status that is not predicted by baseline comorbidities. In addition, the period after hospitalization represents a period of increased vulnerability due to the toxic combination of the acute illness and comorbidities, immobility and deconditioning, sleep interruption, poor nutrition, secondary illness, iatrogenic complications, and social isolation.19 Iatrogenic complications may include polypharmacy, management decisions, orders of bed rest, overuse of diapers, and unnecessary urinary catheterizations.20 These complications are particularly relevant given that elderly patients are most predisposed to these complications, and atrial fibrillation patients are mostly elderly.
Strengths of this study
To our knowledge, our study is the first to investigate the risk of hospitalizations generally and cardiovascular hospitalizations specifically, among atrial fibrillation patients, for death, vascular death, stroke, and major bleeding. Our analysis indicates that hospitalization and cardiovascular hospitalization may be useful surrogates for all-cause mortality.
Compared with an administrative database which may suffer from coding inaccuracies, RE-LY has strict criteria of documented atrial fibrillation for entry along with a prospective evaluation of comorbidities. Since atrial fibrillation accompanies other serious conditions such as cerebrovascular disease, heart failure, or sepsis, databases may be predisposed to underestimating the risk associated with hospitalization and hospitalization burden. In contrast to an administrative database, RE-LY included a detailed assessment of clinical variables, which allowed a more robust covariate analysis. RE-LY also allowed the examination of hospitalization with various outcomes, which were evaluated by an adjudication committee. RE-LY was a multinational trial compared with usual single country administrative databases.
Limitations
This was a post hoc analysis of a randomized controlled trial with specific inclusion and exclusion criteria, so the findings may not be generalizable. While the HRs reported were adjusted for important covariates, unmeasured confounding is always possible. We did not capture patterns of prior hospitalizations, which may have impacted our analysis. An alternative model could have attempted to readjust the comorbidities of patients after discharge; however, this could not be incorporated into our models as changes of patient characteristics were not captured in RE-LY. Changing the comorbidity status of patients at the time of hospitalization may impact the calculated HRs. We did not perform a recurrent event analysis of hospitalization; however, this would have been difficult given that recurrent hospitalizations would not be statistically independent events. The duration of hospitalization and time course of complications were not analysed. Finally, reasons and thresholds for hospitalizations may have geographic variations; however, RE-LY was a multinational trial representing 951 clinical centres in 44 different countries representing a broad patient population.
Conclusions
In conclusion, we found that hospitalizations and cardiovascular hospitalizations are associated with death and cardiovascular death in patients with atrial fibrillation. These findings may support the use of hospitalization and/or cardiovascular hospitalization as surrogate outcomes in future atrial fibrillation studies. They also emphasize the importance of preventing hospitalizations among atrial fibrillation patients and adequate surveillance post-discharge.
Funding
The study was funded by Boehringer Ingelheim.
Conflict of interest: A.A. reports no conflict of interest. S.H.H. reports personal fees from Boehringer Ingelheim, personal fees from Bayer, personal fees from Bristol-Myers Squibb, personal fees from Daiichi Sankyo, personal fees from Pfizer, personal fees from Medtronic, outside the submitted work. M.F., M.B., and P.R. are employees of Boehringer Ingelheim. M.E. reports personal fees from Bristol-Myers Squibb; Pfizer, grants from Pfizer, during the conduct of the study; personal fees from Boehringer Ingelheim, Armetheon, Pfizer, Sanofi, Bristol-Myers Squibb, Portola, Daiichi-Sankyo, Medtronics, Johnson & Johnson, and Janssen Scientific Affairs, grants from Boehringer Ingelheim, outside the submitted work. He is the co-principal investigator X-VERT and sits on the executive committee of ENSURE-AF. J.S.H. reports grants from Medtronic, grants from Bristol-Meyers-Squibb/Pfizer, outside the submitted work; S.Y. reports grants, personal fees and other fees from Boehringer-Ingelheim, during the conduct of the study; grants from Boehringer-Ingelheim, outside the submitted work. S.J.C. reports grants from Boehringer Ingelheim, during the conduct of the study.



