-
PDF
- Split View
-
Views
-
Cite
Cite
Michelle M Monasky, Emanuele Micaglio, Gabriele Vicedomini, Emanuela T Locati, Giuseppe Ciconte, Luigi Giannelli, Federica Giordano, Simonetta Crisà, Mattia Vecchi, Valeria Borrelli, Andrea Ghiroldi, Sara D'Imperio, Chiara Di Resta, Sara Benedetti, Maurizio Ferrari, Vincenzo Santinelli, Luigi Anastasia, Carlo Pappone, Comparable clinical characteristics in Brugada syndrome patients harboring SCN5A or novel SCN10A variants, EP Europace, Volume 21, Issue 10, October 2019, Pages 1550–1558, https://doi.org/10.1093/europace/euz186
Close - Share Icon Share
Abstract
The Brugada syndrome (BrS) is an inherited disease associated with an increased risk of sudden cardiac death. Often, the genetic cause remains undetected. Perhaps due at least in part because the NaV1.8 protein is expressed more in both the central and peripheral nervous systems than in the heart, the SCN10A gene is not included in diagnostic arrhythmia/sudden death panels in the vast majority of cardiogenetics centres.
Clinical characteristics were assessed in patients harboring either SCN5A or novel SCN10A variants. Genetic testing was performed using Next Generation Sequencing on genomic DNA. Clinical characteristics, including the arrhythmogenic substrate, in BrS patients harboring novel SCN10A variants and SCN5A variants are comparable. Clinical characteristics, including gender, age, personal history of cardiac arrest/syncope, spontaneous BrS electrocardiogram pattern, family history of sudden death, and arrhythmic substrate are not significantly different between probands harboring SCN10A or SCN5A variants.
Future studies are warranted to further characterize the role of these specific SCN10A variants.
Genetic testing for the Brugada syndrome (BrS) is negative in about half of BrS patients and the SCN10A gene is not included in diagnostic arrhythmia/sudden death panels in the vast majority of cardiogenetics centres.
Herein described are nine novel variants in the SCN10A gene with clinical characteristics similar to BrS patients harboring SCN5A variants.
Clinical characteristics, including gender, age, personal history of cardiac arrest/syncope, spontaneous BrS electrocardiogram pattern, family history of sudden death, and arrhythmic substrate are not significantly different between probands harboring SCN10A or SCN5A variants.
Future studies are warranted to further characterize the role of these specific SCN10A variants.
Introduction
The Brugada syndrome (BrS) is a genetic disease associated with an increased risk of syncope and sudden cardiac death (SCD).1 Brugada syndrome can affect people of every age, and many patients are asymptomatic and not aware they are affected. The first occurrence of symptoms is often in young adulthood, and SCD or aborted SCD, due to ventricular tachycardia/fibrillation (VT/VF), can be the first clinical manifestation of the syndrome. Although BrS seems to be equally transmitted to males and females, males are more often symptomatic.2 Patients are more likely to be symptomatic while feverish or when engaging in an activity that leads to an increase in vagal tone, including sleep.3
Diagnosis is achieved when the typical ST-segment elevation with a type 1 BrS pattern on the electrocardiogram (ECG), as defined in the current guidelines,1 is identified, either occurring spontaneously or provoked by the administration of a sodium channel blocking agent, such as ajmaline.1 Since inducibility during electrophysiological study (EPS) is known to be associated with the risk of future ventricular arrhythmias, BrS patients may undergo an EPS, and if VT/VF is induced, an implantable cardioverter-defibrillator (ICD) is implanted.4
The occurrence of arrhythmias in BrS patients have been linked to an arrhythmogenic substrate (AS), usually located in the epicardium of the right ventricular outflow tract. In our experience,5 the inducibility for VT/VF during EPS is correlated with the amplitude of the AS area, which can be visualized by epicardial potential duration maps (PDM). Transcatheter radiofrequency (RF) ablation of the entire AS, guided by PDM obtained during ajmaline administration, has been shown to normalize the ECG pattern and prevent the recurrence of VT/VF.6
The most commonly associated mutated gene in BrS is SCN5A, which accounts for 15–30% of molecularly confirmed cases of BrS,7 and encodes for the α-subunit of the Nav1.5 cardiac sodium channel. However, other genes encoding for subunits of cardiac sodium, potassium, and calcium channels have also been associated with BrS,1 including SCN10A, which encodes for the Nav1.8 voltage-gated sodium channel alpha subunit. SCN10A mutations may reduce sodium channel currents, potentially favouring arrhythmogenesis.8 However, the functional significance of mutations in the SCN10A gene remains unclear.8
Molecular confirmation of BrS is complicated by extreme clinical variability, including different phenotypes between members of the same family carrying the same mutation,3 and negative genetic testing for known mutations in the majority of BrS cases. These challenges highlight the need for a better understanding of the molecular causes of BrS, particularly the identification of further genetic mutations associated with BrS and a more accurate genotype–phenotype correlation.
In the present study, we describe nine novel variants in the SCN10A gene, as well as their association with BrS and the characterization of the AS associated with these genotypes, which has implications for future risk stratification. Furthermore, we compare the clinical characteristics and substrate area between patients harboring SCN5A and SCN10A heterozygous variants.
Methods
Patient characteristics
The Brugada syndrome patients referred to the Clinical Arrhythmology and Electrophysiology Department of Policlinico San Donato, Milan, Italy, who underwent BrS genotyping and substrate ablation were included in this study.
All patients signed an informed consent to participate in this study, and the study was approved by the Local Ethics Committee.
Genetic testing
To determine the genotype, at least 15 BrS-associated genes (CACNA1C, CACNA2D1, CACNB2, GPD1L, HCN4, KCND2, KCND3, PKP2, RANGRF, SCN10A, SCN1B, SCN2B, SCN3B, SCN5A, and TRPM4) were analysed from genomic DNA and processed with Next Generation Sequencing (TruSight One sequencing kit with NextSeq platform).
VarSome9 genetic database was used to search for the significance of each of the SCN10A variants, as well as the prediction results of SIFT tool and Provean. The Genomic Evolutionary Rate Profiling (GERP) value was noted, which is defined by VarSome as ‘a conservation score calculated by quantifying substitution deficits across multiple alignments of orthologues using the genomes of 35 mammals. It ranges from −12.3 to 6.17, with 6.17 being the most conserved’.10
Electrophysiological study procedure
All patients with variants in SCN10A or SCN5A were further analysed for arrhythmic risk both by EPS and by investigating the location and extent of the AS responsible for the arrhythmias.
Programmed electrical stimulation was performed according to the standard protocol as described elsewhere.5 If sustained VT or VF lasting >30 s or requiring electrical cardioversion was induced, the patient was categorized as having inducible arrhythmia.
Arrhythmic substrate characterization
Combined epi–endocardial mapping to determine the extent and location of the AS was performed as previously described.2 All the PDM were obtained by collecting the duration of each bipolar electrogram (EGM). Abnormal EGMs were identified if they met at least 1 of the following characteristics: (i) a wide duration (>110 ms) with fragmented component (>3 distinct peaks); (ii) late component of low-voltage amplitude ranging from 0.05 to 1.5 mV; (iii) distinct and delayed component exceeding the end of the QRS complex; or (iv) discrete double activity. Measurements were interpreted and validated online by two expert electrophysiologists using CARTO3 system electronic callipers. Electrogram acquisition was performed only if the multipolar catheter was stable in each epicardial position. As a result, a colour-coded map was obtained showing the regions displaying the shortest (<110 ms cut-off, red) and longest (>160 ms cut-off, purple) durations.5
Ethical approval and informed consent
Written informed consent of human subjects included in this case series report was obtained for their participation in the study and for publication. The procedures employed were reviewed and approved by the local Ethical Committee.
Statistical analysis
Data are expressed as mean ± standard deviation and were analysed by χ2 test or Mann–Whitney U test, where appropriate. Values of P < 0.05 were considered statistically significant.
Results
Study population characteristics
A total of 297 patients were screened for this study. Eighty-four (28.3%) patients from 57 families were found to harbor SCN5A variants, and 8 patients from 8 families were found to harbor SCN10A variants (2.7%). Noteworthy, in our BrS population, SCN10A was the second most frequent gene after SCN5A. Clinical characteristics of probands harboring SCN10A or SCN5A variants are summarized in Table 1. Clinical characteristics, including gender, age, personal history of cardiac arrest/syncope, spontaneous BrS ECG pattern, family history of sudden death, and arrhythmic substrate are not significantly different between probands harboring SCN10A or SCN5A variants.
| . | SCN10A . | SCN5A . | P-value . |
|---|---|---|---|
| Probands, n (%) | 8 | 57 | |
| Male, n (%) | 6 (75.0%) | 38 (66.7%) | 0.051 |
| Age (years) | 0.074 | ||
| Mean ± SD | 49.7 ± 13.0 | 40.6 ± 11.4 | |
| Min–Max | 34.1–72.5 | 16.6–67.5 | |
| Cardiac arrest/syncope, n (%) | 3 (37.5%) | 18 (31.6%) | 0.469 |
| Spontaneous BrS ECG pattern | 0.244 | ||
| Type 1, n (%) | 4 (50.0%) | 16 (28.1%) | |
| Type 2, n (%) | 1 (12.5%) | 10 (17.5%) | |
| Type 3, n (%) | 3 (37.5%) | 31 (54.4%) | |
| Family history of sudden death, n (%) | 3 (37.5%) | 17 (29.8%) | 0.982 |
| Baseline substrate size (cm2) | 0.486 | ||
| Mean ± SD | 1.91 ± 4.54 | 3.11 ± 5.46 | |
| Min–Max | 0.00–13.10 | 0.00–24.60 | |
| Substrate size after ajmaline (cm2) | 0.118 | ||
| Mean ± SD | 15.68 ± 4.42 | 20.36 ± 9.26 | |
| Min–Max | 9.90–24.20 | 2.20–44.10 | |
| Baseline potential duration (ms) | 0.352 | ||
| Mean ± SD | 106.11 ± 37.07 | 113.54 ± 33.57 | |
| Min–Max | 74.00–180.10 | 64.40–187.40 | |
| Potential duration after ajmaline (ms) | 0.251 | ||
| Mean ± SD | 236.19 ± 10.76 | 226.48 ± 20.85 | |
| Min–Max | 210.20–241.20 | 205.60–288.90 |
| . | SCN10A . | SCN5A . | P-value . |
|---|---|---|---|
| Probands, n (%) | 8 | 57 | |
| Male, n (%) | 6 (75.0%) | 38 (66.7%) | 0.051 |
| Age (years) | 0.074 | ||
| Mean ± SD | 49.7 ± 13.0 | 40.6 ± 11.4 | |
| Min–Max | 34.1–72.5 | 16.6–67.5 | |
| Cardiac arrest/syncope, n (%) | 3 (37.5%) | 18 (31.6%) | 0.469 |
| Spontaneous BrS ECG pattern | 0.244 | ||
| Type 1, n (%) | 4 (50.0%) | 16 (28.1%) | |
| Type 2, n (%) | 1 (12.5%) | 10 (17.5%) | |
| Type 3, n (%) | 3 (37.5%) | 31 (54.4%) | |
| Family history of sudden death, n (%) | 3 (37.5%) | 17 (29.8%) | 0.982 |
| Baseline substrate size (cm2) | 0.486 | ||
| Mean ± SD | 1.91 ± 4.54 | 3.11 ± 5.46 | |
| Min–Max | 0.00–13.10 | 0.00–24.60 | |
| Substrate size after ajmaline (cm2) | 0.118 | ||
| Mean ± SD | 15.68 ± 4.42 | 20.36 ± 9.26 | |
| Min–Max | 9.90–24.20 | 2.20–44.10 | |
| Baseline potential duration (ms) | 0.352 | ||
| Mean ± SD | 106.11 ± 37.07 | 113.54 ± 33.57 | |
| Min–Max | 74.00–180.10 | 64.40–187.40 | |
| Potential duration after ajmaline (ms) | 0.251 | ||
| Mean ± SD | 236.19 ± 10.76 | 226.48 ± 20.85 | |
| Min–Max | 210.20–241.20 | 205.60–288.90 |
Data were analysed by χ2 test or Mann–Whitney U test, where appropriate.
BrS, Brugada syndrome; ECG, electrocardiogram; SD, standard deviation.
| . | SCN10A . | SCN5A . | P-value . |
|---|---|---|---|
| Probands, n (%) | 8 | 57 | |
| Male, n (%) | 6 (75.0%) | 38 (66.7%) | 0.051 |
| Age (years) | 0.074 | ||
| Mean ± SD | 49.7 ± 13.0 | 40.6 ± 11.4 | |
| Min–Max | 34.1–72.5 | 16.6–67.5 | |
| Cardiac arrest/syncope, n (%) | 3 (37.5%) | 18 (31.6%) | 0.469 |
| Spontaneous BrS ECG pattern | 0.244 | ||
| Type 1, n (%) | 4 (50.0%) | 16 (28.1%) | |
| Type 2, n (%) | 1 (12.5%) | 10 (17.5%) | |
| Type 3, n (%) | 3 (37.5%) | 31 (54.4%) | |
| Family history of sudden death, n (%) | 3 (37.5%) | 17 (29.8%) | 0.982 |
| Baseline substrate size (cm2) | 0.486 | ||
| Mean ± SD | 1.91 ± 4.54 | 3.11 ± 5.46 | |
| Min–Max | 0.00–13.10 | 0.00–24.60 | |
| Substrate size after ajmaline (cm2) | 0.118 | ||
| Mean ± SD | 15.68 ± 4.42 | 20.36 ± 9.26 | |
| Min–Max | 9.90–24.20 | 2.20–44.10 | |
| Baseline potential duration (ms) | 0.352 | ||
| Mean ± SD | 106.11 ± 37.07 | 113.54 ± 33.57 | |
| Min–Max | 74.00–180.10 | 64.40–187.40 | |
| Potential duration after ajmaline (ms) | 0.251 | ||
| Mean ± SD | 236.19 ± 10.76 | 226.48 ± 20.85 | |
| Min–Max | 210.20–241.20 | 205.60–288.90 |
| . | SCN10A . | SCN5A . | P-value . |
|---|---|---|---|
| Probands, n (%) | 8 | 57 | |
| Male, n (%) | 6 (75.0%) | 38 (66.7%) | 0.051 |
| Age (years) | 0.074 | ||
| Mean ± SD | 49.7 ± 13.0 | 40.6 ± 11.4 | |
| Min–Max | 34.1–72.5 | 16.6–67.5 | |
| Cardiac arrest/syncope, n (%) | 3 (37.5%) | 18 (31.6%) | 0.469 |
| Spontaneous BrS ECG pattern | 0.244 | ||
| Type 1, n (%) | 4 (50.0%) | 16 (28.1%) | |
| Type 2, n (%) | 1 (12.5%) | 10 (17.5%) | |
| Type 3, n (%) | 3 (37.5%) | 31 (54.4%) | |
| Family history of sudden death, n (%) | 3 (37.5%) | 17 (29.8%) | 0.982 |
| Baseline substrate size (cm2) | 0.486 | ||
| Mean ± SD | 1.91 ± 4.54 | 3.11 ± 5.46 | |
| Min–Max | 0.00–13.10 | 0.00–24.60 | |
| Substrate size after ajmaline (cm2) | 0.118 | ||
| Mean ± SD | 15.68 ± 4.42 | 20.36 ± 9.26 | |
| Min–Max | 9.90–24.20 | 2.20–44.10 | |
| Baseline potential duration (ms) | 0.352 | ||
| Mean ± SD | 106.11 ± 37.07 | 113.54 ± 33.57 | |
| Min–Max | 74.00–180.10 | 64.40–187.40 | |
| Potential duration after ajmaline (ms) | 0.251 | ||
| Mean ± SD | 236.19 ± 10.76 | 226.48 ± 20.85 | |
| Min–Max | 210.20–241.20 | 205.60–288.90 |
Data were analysed by χ2 test or Mann–Whitney U test, where appropriate.
BrS, Brugada syndrome; ECG, electrocardiogram; SD, standard deviation.
Clinical presentations of patients with SCN10A variants
The clinical characteristics of SCN10A probands are summarized in Table 2.
| Proband . | Gender . | Age (years) . | History of syncope . | Spontaneous ECG pattern . | Family history of sudden death . | Result of EPS . | Results of ajmaline infusion . | Substrate size (cm2) . |
|---|---|---|---|---|---|---|---|---|
| I | M | 50 | Y | 3 | N | Inducible | Positive | 13.9 |
| II | M | 49 | N | 2 | N | Inducible | Positive | 9.9 |
| III | F | 63 | Y | 1 | Y | Inducible | Positive | 11.7 |
| IV | M | 36 | N | 1 | N | Inducible | Positive | 24.2 |
| V | M | 33 | N | 1 | N | Inducible | Not performed | 17.2 |
| VI | F | 72 | Y | 3 | Y | Not Inducible | Positive | 15.5 |
| VII | M | 41 | N | 3 | Y | Inducible | Positive | 18.5 |
| VIII | M | 48 | N | 1 | N | Inducible | Positive | 14.5 |
| Proband . | Gender . | Age (years) . | History of syncope . | Spontaneous ECG pattern . | Family history of sudden death . | Result of EPS . | Results of ajmaline infusion . | Substrate size (cm2) . |
|---|---|---|---|---|---|---|---|---|
| I | M | 50 | Y | 3 | N | Inducible | Positive | 13.9 |
| II | M | 49 | N | 2 | N | Inducible | Positive | 9.9 |
| III | F | 63 | Y | 1 | Y | Inducible | Positive | 11.7 |
| IV | M | 36 | N | 1 | N | Inducible | Positive | 24.2 |
| V | M | 33 | N | 1 | N | Inducible | Not performed | 17.2 |
| VI | F | 72 | Y | 3 | Y | Not Inducible | Positive | 15.5 |
| VII | M | 41 | N | 3 | Y | Inducible | Positive | 18.5 |
| VIII | M | 48 | N | 1 | N | Inducible | Positive | 14.5 |
ECG, electrocardiogram; EPS, electrophysiological study.
| Proband . | Gender . | Age (years) . | History of syncope . | Spontaneous ECG pattern . | Family history of sudden death . | Result of EPS . | Results of ajmaline infusion . | Substrate size (cm2) . |
|---|---|---|---|---|---|---|---|---|
| I | M | 50 | Y | 3 | N | Inducible | Positive | 13.9 |
| II | M | 49 | N | 2 | N | Inducible | Positive | 9.9 |
| III | F | 63 | Y | 1 | Y | Inducible | Positive | 11.7 |
| IV | M | 36 | N | 1 | N | Inducible | Positive | 24.2 |
| V | M | 33 | N | 1 | N | Inducible | Not performed | 17.2 |
| VI | F | 72 | Y | 3 | Y | Not Inducible | Positive | 15.5 |
| VII | M | 41 | N | 3 | Y | Inducible | Positive | 18.5 |
| VIII | M | 48 | N | 1 | N | Inducible | Positive | 14.5 |
| Proband . | Gender . | Age (years) . | History of syncope . | Spontaneous ECG pattern . | Family history of sudden death . | Result of EPS . | Results of ajmaline infusion . | Substrate size (cm2) . |
|---|---|---|---|---|---|---|---|---|
| I | M | 50 | Y | 3 | N | Inducible | Positive | 13.9 |
| II | M | 49 | N | 2 | N | Inducible | Positive | 9.9 |
| III | F | 63 | Y | 1 | Y | Inducible | Positive | 11.7 |
| IV | M | 36 | N | 1 | N | Inducible | Positive | 24.2 |
| V | M | 33 | N | 1 | N | Inducible | Not performed | 17.2 |
| VI | F | 72 | Y | 3 | Y | Not Inducible | Positive | 15.5 |
| VII | M | 41 | N | 3 | Y | Inducible | Positive | 18.5 |
| VIII | M | 48 | N | 1 | N | Inducible | Positive | 14.5 |
ECG, electrocardiogram; EPS, electrophysiological study.
Proband I is a 50-year-old male who came to our attention for familial history of psychomotor delay (sister), as well as both personal and familial history of heart right bundle branch block (mother) (Family pedigree, Figure 1). One sibling and one descendant child were spontaneously aborted during pregnancy. The proband has a 5-year-old asymptomatic son. The proband experienced syncopal episodes during fever during both paediatric age and puberty. Previous medical examinations were unremarkable with the exception of a history of paroxysmal atrial fibrillation. An ajmaline challenge confirmed a BrS diagnosis. An ICD was implanted after the patient was inducible during EPS.
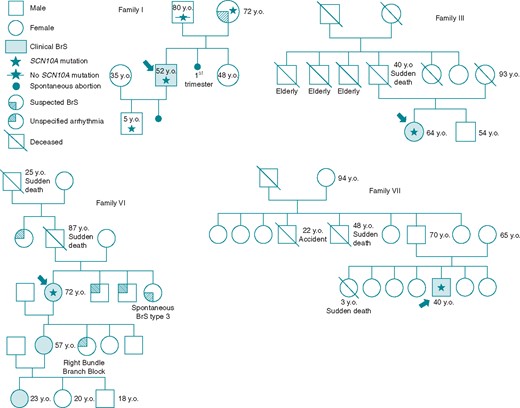
Family pedigrees. Family pedigrees corresponding to Probands I, III, VI, and VII are shown. Probands identified with arrow. Star: molecularly confirmed SCN10A mutation; Star with slash: genetic test for SCN10A mutation performed but negative. BrS, Brugada syndrome; y.o., years old at diagnosis.
Proband II is a 49-year-old male diagnosed with type-2 BrS after performing a routine ECG, and the patient tested positive during a flecainide challenge and EPS performed elsewhere. When he came to our attention, he underwent ajmaline challenge and repeated EPS to confirm the diagnosis. Both tests resulted positive, and an ICD was implanted.
Proband III is a 63-year-old female with a family history of SCD (father at age 40) (Family pedigree, Figure 1). An ECG was performed after she experienced a syncopal episode, revealing a spontaneous type-1 BrS pattern. The patient tested negative in a tilt test and EPS, and genetic testing performed elsewhere was negative, and no arrhythmic events were observed from an implanted internal loop recorder. When she came to our attention, an ajmaline test was performed and the EPS repeated, both of which resulted positive, and an ICD was implanted.
Proband IV is a 36-year-old male who performed a routine ECG, which showed a type-2 BrS pattern. When she came to our attention, we performed an ajmaline challenge and EPS, both of which were positive, and an ICD was implanted.
Proband V is a 33-year-old male with a history of arterial hypertension. The type-1 BrS pattern had been observed in two 12-lead basal ECGs. A 24-h Holter ECG showed isolated ventricular extrasystoles, and confirmed the presence of type-1 BrS pattern. The patient tested positive during EPS and had a spontaneous type-1 pattern during the test. Thus, an ajmaline challenge was not required, and an ICD was implanted.
Proband VI is a 72-year-old female with history of recurrent paroxysmal atrial fibrillation (with onset at about 60 years old), arterial hypertension (onset after menopause, at 50 years old), and family history of sudden death (father and paternal grandfather) (Family pedigree, Figure 1). The patient has experienced several syncopal episodes (at least 4) after menopause. At the age of 60, she underwent EPS and RF ablation for atrial fibrillation by circumferential lesion of the lung veins at another facility. Due to recurrent paroxysmal atrial fibrillation, she repeated the same ablation procedure at our centre (at the age of 73). An ajmaline challenge was performed both because of recurrent atrial fibrillation with recurrent syncope in the proband and a familial history of BrS (diagnosed in her granddaughter). The ajmaline challenge was positive for BrS, but the EPS was negative, and the patient was not implanted with an ICD.
Proband VII is a 41-year-old male professional athlete who presented with a BrS pattern on a 12-lead ECG performed for professional reasons. He has no known family history of BrS, although his paternal uncle died during sleep at the age of 48 (Family pedigree, Figure 1). EPS and ajmaline test were positive, and an ICD was implanted.
Proband VIII is a 48-year-old male without known risk factors for coronary artery disease, who presented with an elevated ST-segment in the anterior precordial leads. Coronary artery disease was excluded following a negative coronary angiography and negative biochemical signs of myocardial infarction (normal curve of troponin T, normal concentrations of both creatine phosphokinase-muscle/brain isoenzyme and plasmatic lactate dehydrogenase), thus the presence of BrS was then suspected. A persistent type-1 BrS pattern was later confirmed, and a loop recorder was implanted in another hospital. When he came to our attention, we performed EPS and ajmaline challenge, which were both positive, and an ICD was implanted.
Genetic testing of patients harboring SCN10A variants
The results of the genetic testing in patients harboring SCN10A variants are illustrated in Table 3 and Figure 2.
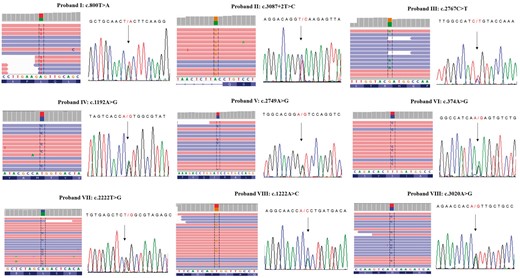
SCN10A variants identified in Brugada syndrome patients. Nine different SCN10A variants have been identified using the Next Generation Sequencing (NGS) approach and confirmed by Sanger sequencing. On the left of each panel, NGS paired-end reads loaded in the IGV genome browser. SCN10A gene is in the reverse orientation on the chromosome, as highlighted in the BAM file. On the right, Sanger sequencing electropherograms are reported. The arrows indicate the position of the single nucleotide variation in the SCN10A gene.
| Proband . | Variant . | Predicted protein change . | GERP . | Significance . | SIFT tool . | Provean . | Frequency . |
|---|---|---|---|---|---|---|---|
| I | NM_006514.2:c.800T>A | p.Leu267His | 4.6599 | Uncertain | Damaging | Damaging | Not found in exome studies with median coverage of 54× |
| II | NM_006514.2:c.3087 + 2T>C | 4.0599 | Uncertain | n/a | n/a | 1:227488 | |
| III | NM_006514.2:c.2767C>T | p.Arg923Cys | 5.4499 | Uncertain | Damaging | Neutral | 1:61541 |
| IV | NM_006514.2:c.1192A>G | p.Met398Val | 5.21 | Uncertain | Damaging | Damaging | 1:123070 |
| V | NM_006514.2:c.2749A>G | p.Ile917Val | 5.03 | Uncertain | Tolerated | Neutral | 1:246169 |
| VI | NM_006514.2:c.374A>G | p.Lys125Arg | 4.3899 | Uncertain | Tolerated | Neutral | 1:245843 |
| VII | NM_006514.2:c.2222T>G | p.Leu741Arg | 4.19 | Uncertain | Damaging | Damaging | 1:41022 |
| VIII | NM_006514.2:c.1222A>C | p.Thr408Pro | 5.21 | Uncertain | Damaging | Neutral | Not found in exome studies with median coverage of 100× |
| NM_006514.2:c.3020A>G | p.Asp1007Gly | 4.78 | Uncertain | Damaging | Damaging | 1:10237 |
| Proband . | Variant . | Predicted protein change . | GERP . | Significance . | SIFT tool . | Provean . | Frequency . |
|---|---|---|---|---|---|---|---|
| I | NM_006514.2:c.800T>A | p.Leu267His | 4.6599 | Uncertain | Damaging | Damaging | Not found in exome studies with median coverage of 54× |
| II | NM_006514.2:c.3087 + 2T>C | 4.0599 | Uncertain | n/a | n/a | 1:227488 | |
| III | NM_006514.2:c.2767C>T | p.Arg923Cys | 5.4499 | Uncertain | Damaging | Neutral | 1:61541 |
| IV | NM_006514.2:c.1192A>G | p.Met398Val | 5.21 | Uncertain | Damaging | Damaging | 1:123070 |
| V | NM_006514.2:c.2749A>G | p.Ile917Val | 5.03 | Uncertain | Tolerated | Neutral | 1:246169 |
| VI | NM_006514.2:c.374A>G | p.Lys125Arg | 4.3899 | Uncertain | Tolerated | Neutral | 1:245843 |
| VII | NM_006514.2:c.2222T>G | p.Leu741Arg | 4.19 | Uncertain | Damaging | Damaging | 1:41022 |
| VIII | NM_006514.2:c.1222A>C | p.Thr408Pro | 5.21 | Uncertain | Damaging | Neutral | Not found in exome studies with median coverage of 100× |
| NM_006514.2:c.3020A>G | p.Asp1007Gly | 4.78 | Uncertain | Damaging | Damaging | 1:10237 |
The GERP score demonstrates the level of conservation during evolution. The overall significance for each of these variants in the VarSome database is still uncertain.
| Proband . | Variant . | Predicted protein change . | GERP . | Significance . | SIFT tool . | Provean . | Frequency . |
|---|---|---|---|---|---|---|---|
| I | NM_006514.2:c.800T>A | p.Leu267His | 4.6599 | Uncertain | Damaging | Damaging | Not found in exome studies with median coverage of 54× |
| II | NM_006514.2:c.3087 + 2T>C | 4.0599 | Uncertain | n/a | n/a | 1:227488 | |
| III | NM_006514.2:c.2767C>T | p.Arg923Cys | 5.4499 | Uncertain | Damaging | Neutral | 1:61541 |
| IV | NM_006514.2:c.1192A>G | p.Met398Val | 5.21 | Uncertain | Damaging | Damaging | 1:123070 |
| V | NM_006514.2:c.2749A>G | p.Ile917Val | 5.03 | Uncertain | Tolerated | Neutral | 1:246169 |
| VI | NM_006514.2:c.374A>G | p.Lys125Arg | 4.3899 | Uncertain | Tolerated | Neutral | 1:245843 |
| VII | NM_006514.2:c.2222T>G | p.Leu741Arg | 4.19 | Uncertain | Damaging | Damaging | 1:41022 |
| VIII | NM_006514.2:c.1222A>C | p.Thr408Pro | 5.21 | Uncertain | Damaging | Neutral | Not found in exome studies with median coverage of 100× |
| NM_006514.2:c.3020A>G | p.Asp1007Gly | 4.78 | Uncertain | Damaging | Damaging | 1:10237 |
| Proband . | Variant . | Predicted protein change . | GERP . | Significance . | SIFT tool . | Provean . | Frequency . |
|---|---|---|---|---|---|---|---|
| I | NM_006514.2:c.800T>A | p.Leu267His | 4.6599 | Uncertain | Damaging | Damaging | Not found in exome studies with median coverage of 54× |
| II | NM_006514.2:c.3087 + 2T>C | 4.0599 | Uncertain | n/a | n/a | 1:227488 | |
| III | NM_006514.2:c.2767C>T | p.Arg923Cys | 5.4499 | Uncertain | Damaging | Neutral | 1:61541 |
| IV | NM_006514.2:c.1192A>G | p.Met398Val | 5.21 | Uncertain | Damaging | Damaging | 1:123070 |
| V | NM_006514.2:c.2749A>G | p.Ile917Val | 5.03 | Uncertain | Tolerated | Neutral | 1:246169 |
| VI | NM_006514.2:c.374A>G | p.Lys125Arg | 4.3899 | Uncertain | Tolerated | Neutral | 1:245843 |
| VII | NM_006514.2:c.2222T>G | p.Leu741Arg | 4.19 | Uncertain | Damaging | Damaging | 1:41022 |
| VIII | NM_006514.2:c.1222A>C | p.Thr408Pro | 5.21 | Uncertain | Damaging | Neutral | Not found in exome studies with median coverage of 100× |
| NM_006514.2:c.3020A>G | p.Asp1007Gly | 4.78 | Uncertain | Damaging | Damaging | 1:10237 |
The GERP score demonstrates the level of conservation during evolution. The overall significance for each of these variants in the VarSome database is still uncertain.
Genetic testing identified the novel missense heterozygous substitution NM_006514.2:c.800T>A (p.Leu267His) in SCN10A [The Leiden Open Variation Database (LOVD): https://databases.lovd.nl/shared/variants/0000406036] in Proband I. The same variant was found in his mother, who refused to undergo ajmaline challenge, and his son.
The novel splice site heterozygous variant NM_006514.2:c.3087+2T>C in SCN10A (LOVD: https://databases.lovd.nl/shared/variants/0000406037) and the novel missense heterozygous variant NM_005751.4:c.1259A>G (p.Gln420Arg) in AKAP9 (LOVD: https://databases.lovd.nl/shared/variants/0000406038) were identified in Proband II.
The novel heterozygous substitution NM_006514.2:c.2767C>T (p.Arg923Cys) in SCN10A (LOVD: https://databases.lovd.nl/shared/variants/0000406039) was discovered in Proband III.
The novel missense variant NM_006514.2:c.1192A>G (p.Met398Val) in SCN10A (LOVD: https://databases.lovd.nl/shared/variants/00184302) was discovered in Proband IV. The same variant was found in his mother, who has not undergone arrhythmogenic evaluation.
The novel missense heterozygous variant NM_006514.2:c.2749A>G (Ile917Val) in SCN10A (LOVD: https://databases.lovd.nl/shared/variants/0000439111) was found in Proband V.
The novel missense heterozygous variant NM_006514.2:c.374A>G (p.Lys125Arg) in SCN10A (LOVD: https://databases.lovd.nl/shared/variants/0000439112) and the missense heterozygous variant NM_172057.2:c.73C>T (p.Arg25Trp) in KCNH2 (LOVD: https://databases.lovd.nl/shared/variants/0000439113) were identified in Proband VI.
The missense variant NM_006514.2:c.2222T>G (p.Leu741Arg) in SCN10A (LOVD: https://databases.lovd.nl/shared/variants/0000439114) was identified in Proband VII.
The novel missense heterozygous variants NM_006514.2:c.1222A>C (p.Thr408Pro) (LOVD: https://databases.lovd.nl/shared/variants/0000439115) and NM_006514.2:c.3020A>G (p.Asp1007Gly) (LOVD: https://databases.lovd.nl/shared/variants/0000439116) in SCN10A were identified in Proband VIII.
No other low frequency variants nor mutations were identified in the analysed genes.
In silico predictions of novel SCN10A variants
The GERP score for each of the SCN10A variants described herein demonstrate that these variants have occurred in highly conserved areas of the genome, suggesting that variants in these regions would be pathogenic. Most of the variants were listed as having an unknown significance, with the exception of the splicing variant NM_006514.2:c.3087+2T>C, which is classified as presumably pathogenic according to The American College of Medical Genetics and Genomics criteria since it affects the consensus splicing sequence and it has never been described before. The variants in Probands I, III, IV, VII, and VIII were predicted to be damaging by SIFT tool, whereas data were unavailable for the variant found in Proband II, and the variants found in Probands V and VI were predicted to be tolerated. The full results of the in silico search results using VarSome are listed in Table 3.
Arrhythmogenic substrate characterization
The ECG and PDM for Probands I–VIII are shown in Figures 3 and 4. The type 1 BrS pattern confirms the diagnosis of BrS. The area of the AS is denoted by the ‘marked area’ in cm2 in each of the figures. The AS areas in patients harboring SCN10A variants ranged from 9.9–24.2 cm2, well above the published cut-off of 4 cm2 that has been generally associated with inducibility for VT/VF during EPS,5 which is associated with future ventricular arrhythmia risk.4 Remarkably, these areas were not statistically significantly different from patients harboring SCN5A variants (Table 1). Thus, the size of the AS in patients harboring SCN10A variants is comparable to the size of the AS in patients harboring SCN5A variants. The duration of fragmented potentials were prolonged after ajmaline administration (Table 1).
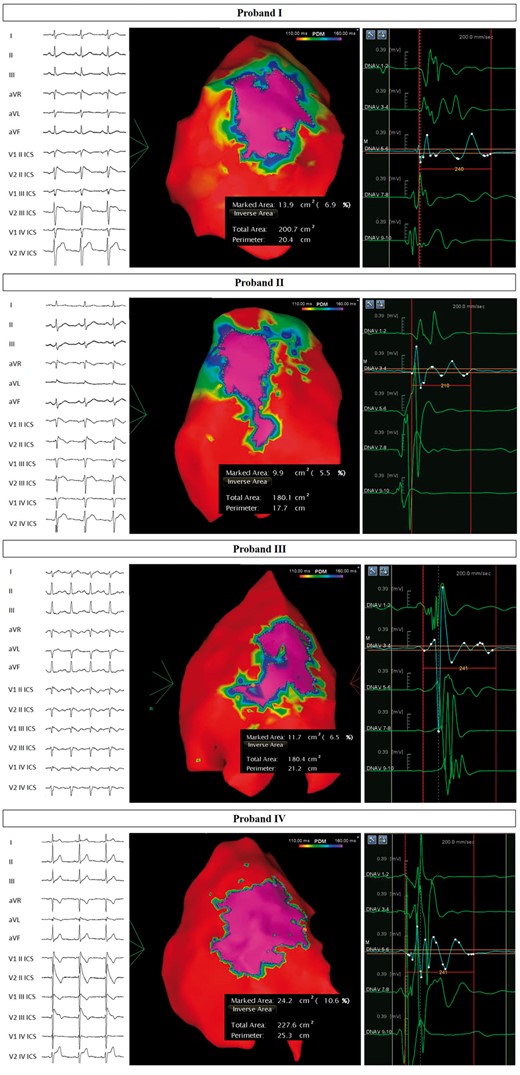
Arrhythmogenic substrate characterization in Probands I–IV. Electrocardiogram (left) demonstrates the type 1 BrS pattern after ajmaline administration, confirming the diagnosis. Potential duration map after ajmaline infusion (centre) reveals the full extent of the arrhythmogenic substrate. BrS, Brugada syndrome.
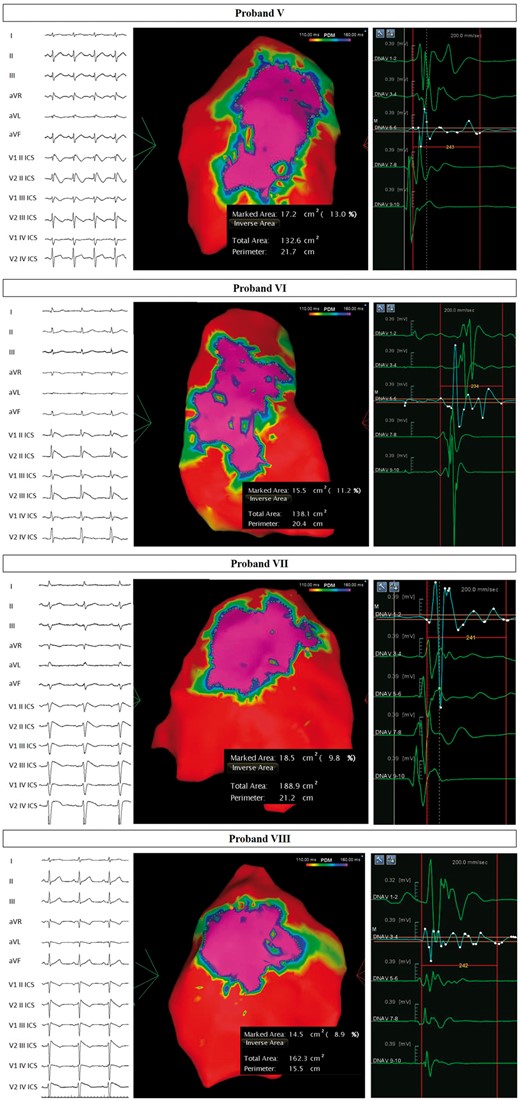
Arrhythmogenic substrate characterization in Probands V–VIII. Electrocardiogram (left) demonstrates the type 1 BrS pattern after ajmaline administration, confirming the diagnosis. Potential duration map after ajmaline infusion (centre) reveals the full extent of the arrhythmogenic substrate. BrS, Brugada syndrome.
Discussion
This study demonstrates that the clinical characteristics and AS in BrS patients harboring novel SCN10A variants and SCN5A variants are comparable. Clinical characteristics, including gender, age, personal history of cardiac arrest/syncope, spontaneous BrS ECG pattern, family history of sudden death, and arrhythmic substrate are not significantly different between probands harboring SCN10A or SCN5A variants.
Noteworthy, in our BrS population, SCN10A was the second most frequent gene after SCN5A, even though it is not listed in the Online Mendelian Inheritance in Man BrS phenotypic series to date (https://www.omim.org/phenotypicSeries/PS601144). This may be due, at least in part, because the NaV1.8 protein is expressed more in both the central and peripheral nervous systems than in the heart. On the other hand, it is remarkable that the most frequently mutated BrS gene (SCN5A) and SCN10A map very closely on the same region of chromosome’s 3 short arm, and they are both regulated by the TBX3/TBX5 pathway.11 While the association between heterozygous SCN10A variants and BrS are currently debated, many studies have suggested a pivotal role of NaV1.8 (encoded by SCN10A) in the regulation of SCN5A gene expression.12 Given the role of a common SCN10A polymorphism on SCN5A function, it is likely that rare heterozygous SCN10A variants, such as those demonstrated in this study, can actually cause an arrhythmic phenotype.
The size of the area of the AS is important in the characterization of these variants. An area larger than 4 cm2 has been generally associated with inducibility for VT/VF during EPS.5 Inducibility during EPS is associated with future ventricular arrhythmia risk.4 The patients harboring SCN10A variants included in this study have an AS area greatly above 4 cm2 (range 9.9–24.2 cm2), suggesting that they have an increased risk of future arrhythmic events compared to non-inducible BrS patients or the general population. Furthermore, the size of the AS in these patients harboring SCN10A mutations is similar to the size of the AS in patients harboring SCN5A mutations.
Important variants in SCN10A are emerging as causative of BrS.8 The SCN10A gene encodes the protein NaV1.8, which plays a key role for many physiological and pathological processes, such as heart conduction regulation,13 neuromuscular disease, and epileptic encephalopathy. Rare SCN10A variants may be responsible for early onset atrial fibrillation as well.14 Among BrS patients with a SCN10A variant, some are affected also by atrioventricular nodal re-entrant tachycardia.15
Because patients with SCN10A variants can exhibit an array of phenotypes, discerning the pathogenic mechanisms resulting from such variants is difficult. Studying the effect of NaV1.8 on normal heart rhythm in a dog model demonstrated the importance of NaV1.8 for cardiac ganglionated plexi,16 suggesting that NaV1.8 is important for the vagus nerve, consistent with the existence of an influence of the autonomic nervous system over the arrhythmic phenotype in general. Heterozygous pathogenic variants in the SCN10A gene have been described in association with sudden unexplained nocturnal death,17 suggesting a role for this gene in the development of fatal arrhythmias. Thus, the SCN10A gene is clearly important for the development of cardiac arrhythmias, although the exact pathogenic mechanisms remain unclear.
The SCN10A gene and the sodium channel for which it encodes have been the subject of much debate in recent years, and SCN10A has recently been described as a possible BrS candidate gene.18 It has also been identified as a target for antiarrhythmic intervention due to its influence over the late sodium current in the heart.19 In the commonly used genetic database VarSome,9 all of the novel SCN10A variants described for the first time in this study were listed as having an uncertain significance. This study is the first to characterize patients exhibiting these nine novel variants, demonstrating that each patient with a SCN10A variant exhibits a typical BrS phenotype associated with a higher future risk of arrhythmic events, not significantly different from the phenotype exhibited by patients with SCN5A variants.
It is remarkable that many variants described in our series occurred in conserved regions. In fact, probands III, IV, and VIII are heterozygous for variants evaluated with a GERP score higher than 5, when 6.17 is the most conserved value. It is worth to specify that SCN10A variants are rare in the general population, to the best of current knowledge, and that many polymorphisms in the SCN10A gene have been related to non-cardiological conditions, such as inflammatory bowel disease.20 This further suggests that variants in important parts of the SCN10A gene may play a more important role than previously described polymorphisms. This suggests that SCN10A heterozygous variants might actually be one of the causes for the arrhythmic phenotype observed in these patients.
Limitations
While genetic testing was performed on nearly 300 cases of confirmed BrS, the genetics of BrS remain complex. Although variants in the SCN10A gene were only found in eight patients, they represented the second largest group after SCN5A in our BrS population. This large sample size is probably what enabled us to identify these nine novel variants in eight patients. However, the number of patients harboring SCN10A variants may limit the statistical analysis. Borderline significant differences in gender and age were observed between the two groups, which could be significant with a larger population sample size. The presence of the BrS pattern (spontaneous or induced by ajmaline) together with the VT/VF inducibility by EPS testing and the large AS, indicate a high risk phenotype that is comparable between patients harboring SCN10A or SCN5A variants. These factors together suggest that further understanding of the role and incidence of SCN10A variants in BrS patients is warranted.
While in silico predictions are useful, there is still an incomplete understanding of the molecular mechanisms involved in BrS pathogenesis. This is particularly true for the SCN10A gene and prevents us from completely relying on in silico predictions. In these cases, functional studies, such as patch and/or voltage clamp could help elucidate the functional consequences of the individual variants. Future studies using such techniques would be useful to understand the functional consequences of each of the novel SCN10A variants described in this study, regardless of the in silico predictions. The BrS phenotype is extremely complex, and in silico predictions can be improved with more experimental data. In summary, in silico predictions cannot be completely relied upon for functional predictions. Further studies are certainly warranted to better understand these nine novel variants in SCN10A and their effect on disease expression.
Conclusions
This study demonstrates that the clinical characteristics and AS in BrS patients harboring novel SCN10A variants and SCN5A variants are comparable. Clinical characteristics, including gender, age, personal history of cardiac arrest/syncope, spontaneous BrS ECG pattern, family history of sudden death, and arrhythmic substrate are not significantly different between probands harboring SCN10A or SCN5A variants. Future studies are warranted to further characterize the role of these specific SCN10A variants.
Acknowledgements
The authors thank the patients and family members for their help and participation in the study.
Funding
Ricerca Corrente funding from Italian Ministry of Health to IRCCS Policlinico San Donato , in part.
Conflict of interest: none declared.
References
VarSome: The Human Genomics Community. https://varsome.com/ (19 November 2018, date last accessed).
Author notes
Michelle M. Monasky and Emanuele Micaglio authors contributed equally to the study.



