-
PDF
- Split View
-
Views
-
Cite
Cite
Ibrahim El-Battrawy, Sebastian Albers, Lukas Cyganek, Zhihan Zhao, Huan Lan, Xin Li, Qiang Xu, Mandy Kleinsorge, Mengying Huang, Zhenxing Liao, Rujia Zhong, Boris Rudic, Jonas Müller, Hendrik Dinkel, Siegfried Lang, Sebastian Diecke, Wolfram-Hubertus Zimmermann, Jochen Utikal, Thomas Wieland, Martin Borggrefe, Xiaobo Zhou, Ibrahim Akin, A cellular model of Brugada syndrome with SCN10A variants using human-induced pluripotent stem cell-derived cardiomyocytes, EP Europace, Volume 21, Issue 9, September 2019, Pages 1410–1421, https://doi.org/10.1093/europace/euz122
Close - Share Icon Share
Abstract
Brugada syndrome (BrS) is associated with a pronounced risk to develop sudden cardiac death (SCD). Up to 21% of patients are related to mutations in SCN5A. Studies identified SCN10A as a contributor of BrS. However, the investigation of the human cellular phenotype of BrS in the presence of SCN10A mutations remains lacking. The objective of this study was to establish a cellular model of BrS in presence of SCN10A mutations using human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs).
Dermal fibroblasts obtained from a BrS patient suffering from SCD harbouring the SCN10A double variants (c.3803G>A and c.3749G>A) and three independent healthy control subjects were reprogrammed to hiPSCs. Human-induced pluripotent stem cells were differentiated into cardiomyocytes (hiPSC-CMs).The hiPSC-CMs from the BrS patient showed a significantly reduced peak sodium channel current (INa) and a significantly reduced ATX II (sea anemone toxin, an enhancer of late INa) sensitive as well as A-887826 (a blocker of SCN10A channel) sensitive late sodium channel current (INa) when compared with the healthy control hiPSC-CMs, indicating loss-of-function of sodium channels. Consistent with reduced INa the action potential amplitude and upstroke velocity (Vmax) were significantly reduced, which may contribute to arrhythmogenesis of BrS. Moreover, Ajmaline effects on action potentials were stronger in BrS-hiPSC-CMs than in healthy control cells. This is in agreement with the higher susceptibility of patients to sodium channel blocking drugs in unmasking BrS.
Patient-specific hiPSC-CMs are able to recapitulate single-cell phenotype features of BrS with SCN10A mutations and may provide novel opportunities to further elucidate the cellular disease mechanism.
The cellular phenotype of Brugada syndrome (BrS) in presence of SCN10A mutation could be for the first time modelled using human cardiomyocytes from induced pluripotent stem cells (hiPSC-CMs).
A reduction of peak and late sodium channel current is detected.
Brugada syndrome cardiomyocytes show a reduction of action potential amplitude and upstroke velocity.
Ajmaline effects on action potential were enhanced in in BrS-hiPSC-CMs.
Introduction
Ventricular tachyarrhythmias are a known cause of sudden cardiac death (SCD). Brugada syndrome (BrS) is characterized by right bundle branch block and coved ST-segment elevation in precordial leads (V1 to V3) and these patients have a pronounced risk to develop malignant tachyarrhythmias.1 The prevalence of the disease is estimated to be 5/10 000 inhabitants with a higher prevalence in Japan and Philippines compared with western countries. Apart from accidents, BrS is the leading cause of death in men <40 years old, particularly in countries in which the syndrome is endemic. Patients with BrS can experience other arrhythmias, such as atrial fibrillation and atrial flutter. The disease is associated with up to 300 mutations in the cardiac sodium channel gene SCN5A (encoding the ion channel Nav 1.5) in 21% of patients, however, the contribution of other genes has also been reported.2 Fever and sodium-channel blockers could potentially unmask BrS, leading to an expert consensus advising patients with BrS to avoid these drugs and express caution during clinical states such as fever and infections.3
In spite of rapid advances in understanding the mechanisms and genetic bases of BrS, much less is known about the genotype–phenotype association and appropriate treatments for BrS patients. Recently, Liang et al.4 generated successfully human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) from two BrS patients carrying SCN5A mutations, revealing the first single-cell phenotype of BrS. More recently, Ma et al.5 reported that hiPSC-CMs generated from a BrS1 patient with a compound SCN5A mutation (p. A226V and p. R1629X) displayed reduced sodium currents. However, cellular models of BrS with SCN10A variants are still lacking.
Due to the high risk of SCD, it has been recommended to implantable cardioverter-defibrillator (ICD) in BrS patients with a previous episode of SCD and inducibility of a sustained ventricular arrhythmia during electrophysiological study.2 However, ICD is not always feasible or adequate for every patient. Established cellular models may help search for appropriate drug therapy for BrS.
For studies on cardiac functions, especially the cardiac ion channel functions, hiPSC-CMs have important advantages over heterologous expression systems such as Xenopus oocytes, human embryonic kidney (HEK) cells, and Chinese Hamster Ovary (CHO) cells lacking important constituents of cardiac ion channel macromolecular complexes that might be necessary for the normal electrophysiological characteristics. Animals possess cardiac electrophysiological properties crucially different from that in humans. Thus, taking into account the hurdle for obtaining human ventricular cardiomyocytes, hiPSC-CMs could be a valuable alternative for heart disease studies, either mechanistic or therapeutic. Indeed, hiPSC-CMs have been successfully used to recapitulate the phenotype of some genetic or non-genetic heart diseases such as long QT syndromes, BrS, arrhythmogenetic right ventricular cardiomyopathy, catecholaminergic polymorphic ventricular tachycardia, hypertrophic cardiomyopathy, dilated cardiomyopathy, takotsubo syndrome, sepsis cardiomyopathy, and short QT syndrome.4–9 Here, we report a cellular model of BrS in presence of SCN10A double variants established by using hiPSC-CMs.
Methods
Ethics statement
The skin biopsies from three healthy donors and one BrS patient were obtained with written informed consent. The study was approved by the Ethical Committee of the Medical Faculty Mannheim, University of Heidelberg (approval number: 2009-350N-MA) and by the Ethical Committee of University Medical Center Göttingen (approval number: 10/9/15). The study was carried out in accordance with the approved guidelines and conducted in accordance with the Helsinki Declaration of 1975, as revised in 1983.
DNA-sequencing analysis
DNA was isolated from blood lymphocytes. Ninety-eight of 107 coding exons of the genes CACNA1C, CACNB2, GPD1L, KCNE3, SCN1B, SCN3B, SCN10A, and SCN5A including exon/intron boundaries were amplified by PCR and subjected to bidirectional Sanger sequencing. Sequences were mapped against hg19 references (NM_015141.3, NM_000719.5, NM_201590.2, NM_001037.4, NM_005472.4, NM_018400.3, NM_006514.3, and NM_198056.2) via JSI Sequence Pilot for further analysis.
Generation of human induced pluripotent stem cells
Human induced pluripotent stem cells (hiPSCs) were generated from primary human fibroblasts derived from skin biopsies. The BrS cell lines isBrSd1.9 (GOEi098-A.9), isBrSd1.12 (GOEi098-A.12), and isBrSd1.23 (GOEi098-A.23) were generated in feeder free culture conditions using the integration-free CytoTune-iPS 2.0 Sendai Reprogramming Kit (Thermo Fisher Scientific, #A16517) with the reprogramming factors OCT4, KLF4, SOX2, c-MYC according to the manufacturer’s instructions with modifications. The generated hiPSCs were characterized for their pluripotency and their in vitro differentiation potential as described.6,7,10,11 The cell line from the first healthy donor (D1) was generated using lentiviral particles carrying the transactivator rtTA and an inducible polycistronic cassette containing the reprogramming factors OCT4, SOX2, KLF4, and c-MYC. The cell lines from the 2nd and 3rd healthy donor (GOEi014-B and GOEi094-A, D2 and D3) were generated in feeder free culture conditions using the integration-free episomal 4-in-1 CoMiP reprogramming plasmid (Addgene, #63726) with the reprogramming factors OCT4, KLF4, SOX2, c-MYC, and short hairpin RNA against p53 or the integration-free CytoTune-iPS 2.0 Sendai Reprogramming Kit, respectively and were described previously.
Generation of human-induced pluripotent stem cell-derived cardiomyocytes
Frozen aliquots of hiPSCs were thawed, cultured without feeder cells and differentiated into hiPSC-CMs as described with some modifications.12 In our lab, the differentiation of hiPSC-CMs is regularly performed every 2 to 3 weeks. The beating hiPSC-CMs from different independent differentiations were used for studies and the data were combined. At 40–60 days of differentiation, cardiomyocytes were dissociated from 24 well plates and plated as single cells on Matrigel-coated 3.5 cm petri dishes for patch-clamp measurements.
Polymerase chain reaction assays
To quantify the steady-state mRNA expression of ion channels from the hiPSC-CMs, RNA was reverse transcribed and qPCR was performed as described.7 Gene symbols, RefSeq No., and Cat. No. of the primers used for qPCR analyses in hiPSC-CMs characterization were listed in the Supplementary material online, Table S1. For evaluation of the characteristics of the used hiPSC lines, RT-PCR was performed as follows: Total RNA was isolated using the SV Total RNA Isolation System (Promega, #Z3105) according to manufacturer’s instructions. A 100 ng RNA was used for the first-strand cDNA synthesis by using MULV Reverse Transcriptase (Thermo Fisher Scientific, #N8080018) and Oligo d(T)16 (Thermo Fisher Scientific, #N8080128). One-tenth of cDNA was used as PCR template and amplified using the GoTaq G2 DNA polymerase (Promega, #M7845) according to manufacturer’s instructions. Primer sequences, annealing temperatures and cycles used for RT-PCR analyses of the hiPSC lines are listed in the Supplementary material online, Table S2.
Immunofluorescence staining
Immunofluorescence staining was performed using appropriate primary antibodies and AlexaFluor conjugated secondary antibodies (ThermoFisher). Cells were fixed with Roti-Histofix 4% (Carl Roth, #P087) at room temperature (RT) for 10 min and blocked with 1% bovine serum albumin (BSA; Sigma Aldrich, #F7524) in PBS at RT for 30 min. Primary antibodies were applied in 1% BSA overnight at 4°C. Secondary antibodies with minimal cross reactivity were administered in 1% BSA for 1 h at RT. For nuclear or cytosolic proteins (α-actinin, MLC2V) cells were permeabilized with 0.1% Triton-X100 (Carl Roth, #3051) in staining solution. Nuclei were stained with 4.8 µM DAPI (Thermo Fisher Scientific, #D1306) for 10 min at RT according to the manufacturer’s instructions. The primary antibodies used in hiPSC-CMs were α-actinin (Sigma Aldrich), MLC2V (Protein tech), Connexin 43 (NovusBio), SCN5A (Alomone labs), and SCN10A (Abcam). All the other antibodies used for characterization of hiPSC lines are listed in Supplementary material online, Table S3.
Western blot
The hiPSC-CMs were lysed using RIPA-buffer (Sigma Aldrich) and Protease- and Phosphatase-Inhibitors (Sigma Aldrich). The protein concentration was measured with the BCA-method (Thermo Scientific). For the western blot a 10% (1 mm) SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and PVDF (polyvinylidene difluoride) membrane were used. As protein markers Precision Plus (BioRad) and LMW (GE Healthcare/Amersham) were utilized. The membrane was blocked with a solution of 5% non-fat milk powder in 0.1% TBS-T buffer. For primary antibodies we used SCN5A (Sigma Aldrich), SCN10A (Abcam), GAPDH (Hy test Ltd), and conjugated secondary antibody (Sigma Aldrich). Before taking pictures with the LAS 1000, the immunoblots were enhanced with West Femto maximum sensitivity substrate (SuperSignal®, Thermo Scientific). The analysis of density was performed with AIDA image analyzer (v4.25).
Patch-clamp
Standard patch-clamp recording techniques were used to measure the transient outward potassium (Ito), slowly delayed rectifier potassium current (Iks), rapidly delayed rectifier potassium current (Iks), ATP-sensitive potassium current (IKATP), Na/Ca exchanger current (INCX), inward rectifier potassium current (IK1), peak and late sodium (INa), and L-type calcium (ICaL) channel currents as well as action potential (AP) in the whole-cell configuration at room temperature.
Measurement of intracellular calcium concentration
To measure spontaneous Ca2+ transients, cells were loaded with the fluorescent Ca2+-indicator Fluo-3 AM. The fluorescence of the cells was measured by using Cairn Optoscan calcium imaging system (Cairn Research, UK). Fluorescence is excited by 488 nm and emitted at 520 nm.
Statistical analysis
If not otherwise indicated data are shown as mean ± SEM and were analysed using InStat© (GraphPad, San Diego, USA) and SigmaPlot 11.0 (Systat GmbH, Germany). By analysing the data with the Kolmogorov–Smirnov test it was decided whether parametric or non-parametric tests were used for analysis. The Student’s t-test and the Mann–Whitney U test were used to compare continuous variables with normal and non-normal distributions, respectively. For parametric data, one-way ANOVA with Bonferroni post-test for multiple comparisons was performed. For non-parametric data, the Kruskal–Wallis test with Dunn’s multiple comparisons post-test was used. Paired t-test was used for comparisons of data before and after application of a drug. P-value <0.05 (two-tailed) was considered significant.
Results
Clinical data
Skin biopsy was performed in a 52-year-old male patient with BrS carrying identified variants (c.3803G>A and c.3749G>A) in the SCN10A gene (Figure 1A). The BrS was diagnosed after suffering from SCD by using a sodium channel blocker, ajmaline (70 mg), which unmasked a BrS type I electrocardiogram (ECG) (Figure 1B). Within ajmaline infusion the patient suffered from increased premature ventricular beats and subsequently ventricular fibrillation, which were terminated by appropriate ICD shock. Extensive clinical and instrumental evaluation, including physical examination, echocardiogram, and coronary angiography excluded a structural cardiac disease and abnormal cardiac function. The family pedigree is demonstrated in Figure 1C. The sibling (only one brother) of the patient was checked by ajmaline test. Brugada syndrome was only diagnosed in our patient. In addition, there are no cases of SCD in his family. Screening of parents was not possible because they are very old and refused the gene analysis. Furthermore, the patient did not have a contact to his relatives except to his brother. Therefore, more information is not available. Brugada syndrome was excluded in the son of the patient.
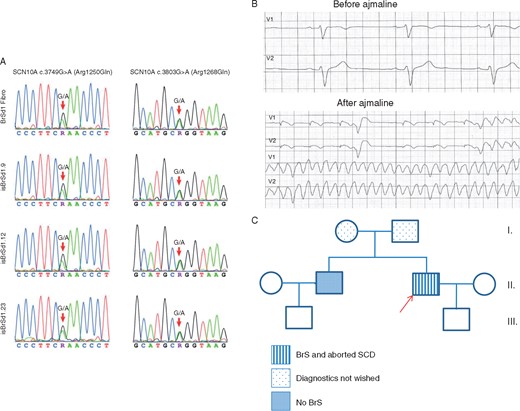
(A) Presence of the SCN10A variants c.3749G>A (p.Arg1250Gln) and c.3803G>A (p.Arg1268Gln) is confirmed by bidirectional Sanger sequencing in the patient’s fibroblasts and the three generated hiPSCs of the BrS patient. (B) The ECG of the BrS patient shows a normal ECG at baseline (before ajmaline), 50 mm/s. After a dose of 70 mg ajmaline, ventricular fibrillation was documented (before dose: upper panels, after dose: lower panels) and terminated with an ICD shock. (C) The pedigree of the family with BrS. The patient recruited for this study is marked by the arrow. Squares: men, circles: women. BrS, Brugada syndrome; ECG, electrocardiogram; hiPSC, human-induced pluripotent stem cell; ICD, implantable cardioverter-defibrillator; SCD, sudden cardiac death.
The variant, c.3803G>A (p.R1268Q) is evaluated as ‘benign’ (by gnomAD) or ‘conflicting of interpretation of pathogenicity’ (by ClinVar). Its MAF is 0.001862 (T) and ExAC is 0.00176 (T) (Link: http://gnomad.broadinstitute.org/variant/3-38755450-C-T). The other variant, c.3749G>A (p.R1250Q), is ‘probably damaging’ (by gnomAD) and ‘uncertain significance’ (ClinVar). Its MAF is 0.00004951(T) and ExAC is 0.00004 (T) (Link: http://gnomad.broadinstitute.org/variant/3-38755504-C-T).
Characterization of patient-specific human-induced pluripotent stem cell and human-induced pluripotent stem cell-derived cardiomyocytes
To confirm the successful generation of hiPSCs reprogrammed from skin fibroblasts of the patient with BrS, three independent iPSC lines of the patient isBrSd1.9, isBrSd1.12, and isBrSd1.23 were first characterized for pluripotency. The pluripotent characterization of iPSC lines from the healthy donors D1, D2, and D3 have been shown in our previous studies.6 Similarly, generated iPSCs from the BrS patient displayed characteristic human embryonic stem cell morphology (Figure 2A) and showed expression of pluripotency markers at mRNA and protein level (Figure 2B–D). Spontaneous differentiation of generated iPSC lines via embryoid bodies confirmed expression of germ layer-specific genes from all three germ layers (Figure 2E).
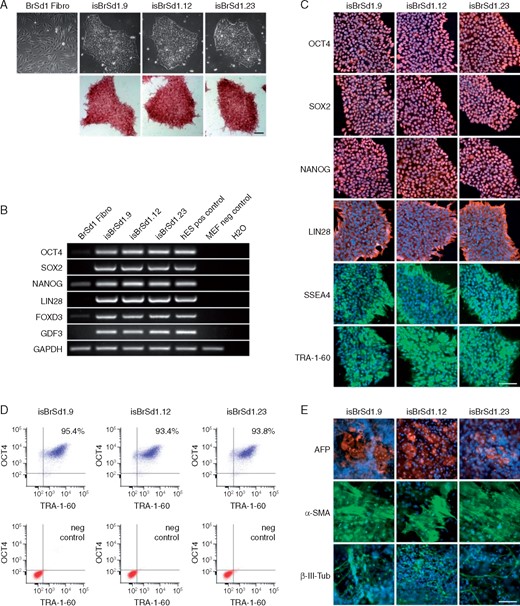
(A) The generated iPSC lines isBrSd1.9, isBrSd1.12, and isBrSd1.23 generated from skin fibroblasts of the BrS patient display a typical morphology for human pluripotent stem cells (upper panel) and are positive for alkaline phosphatase (lower panel). (B) In comparison to donor’s fibroblasts, generated iPSC lines show expression of endogenous pluripotency markers OCT4, SOX2, NANOG, LIN28, FOXD3, and GDF3 at mRNA level proven by RT-PCR. hESCs were used as positive control, MEFs were used as negative control. (C) Generated iPSC lines express pluripotency markers OCT4, SOX2, NANOG, LIN28, SSEA4, and TRA-1-60 as shown by immunofluorescence staining. Nuclei are co-stained with DAPI. Scale bar: 100 µm. (D) Flow cytometry analysis of pluripotency markers OCT4 and TRA-1-60 reveals a homogeneous population of pluripotent cells in generated iPSC lines. (E) Spontaneous differentiation potential of generated iPSC lines was analysed by embryoid body (EB) formation. Immunocytochemical staining of spontaneously differentiated iPSC lines shows expression of endodermal marker AFP, mesodermal-specific α-SMA, and ectodermal βIII-tubulin. Nuclei are co-stained with DAPI. Scale bar: 100 µm. BrS, Brugada syndrome; hESCs, human embryonic stem cells; iPSC, induced pluripotent stem cell; MEFs, mouse embryonic fibroblasts.
Induced pluripotent stem cells (three clones) from the BrS patient and control donors (D1, D2, and D3) were differentiated into functional iPSC-CMs in feeder-free culture conditions for subsequent functional analyses. Beating cardiomyocytes were observed 8–12 days after starting the differentiation. No differences in differentiation efficiency were observed between control and BrS-iPSCs. Typical cardiac markers such as α-actinin, MLC2V, connexin 43, and TNNT2 were expressed as protein and mRNA (Supplementary material online, Figures S1 and S5B). Beating cardiomyocytes at 40–60 days after differentiation were used for patch-clamp and calcium transient imaging.
Changes in ion channel expression in human-induced pluripotent stem cell-derived cardiomyocytes from the Brugada syndrome patient
qPCR analysis showed that after start of differentiation the mRNA level of the ion channel SCN5A was about four-fold, SCN10A about two-fold increased in BrS cells, whereas KNCJ2 level was reduced to about half. Just as with mRNA expression, western blot analysis confirmed an about five-fold increased protein level of SCN5A in BrS cells (Supplementary material online, Figure S5C and D).
Changes of INa in human-induced pluripotent stem cell-derived cardiomyocytes from the Brugada syndrome patient
We employed the patch-clamp technique and specific ion channel blockers to measure and compare the Na currents in BrS- and donor cells. The peak INa (Figure 3A–F) was significantly reduced when compared with healthy donor cardiomyocytes (values of peak INa at −40 mV are: BrS, −54.7 ± 9.4pA/pF; D1, −116.9 ± 28.7pA/pF; D2, −95.4 ± 41.6pA/pF; D, −94.7 ± 28.3pA/pF). To identify selectively the component of late INa that related to SCN10A, a selective blocker (A-887826, 0.1 µM) of SCN10A channel was used. The basal late INa (Figure 3G) and the A-887826-inhibited late currents (Figure 3J, Supplementary material online, Figure S2G) were significantly reduced in BrS when compared with healthy donors (basal late INa: BrS, −1.1 ± 0.1 pA/pF; D1, −2.6 ± 0.3; D2, −2.8 ± 0.4, D3, −3.2 ± 0.8. A-887826 inhibited currents: BrS, −0.6 ± 0.1; D1, −1.1 ± 0.1; D2, −0.7 ± 0.2; D3, −1.0 ± 0.2). These data suggest a loss of function of Na channels in BrS-hiPSC-CMs carrying the double variants in the SCN10A gene. Additionally, to further check the contribution of SCN10A channel to peak and late INa, an enhancer of INa, ATX-II, was used. It enhances the sodium current generated by multiple voltage gated sodium channels including SCN5A and SCN10A channels. ATX-II exerted no significant effect on peak INa but enhanced late INa (Figure 3H and I). These data suggest that SCN10A channel currents contribute mainly to late INa. The activation and inactivation of peak INa were not significantly changed, whereas the recovery from inactivation in BrS cells was accelerated (Supplementary material online, Figure S2A–F).
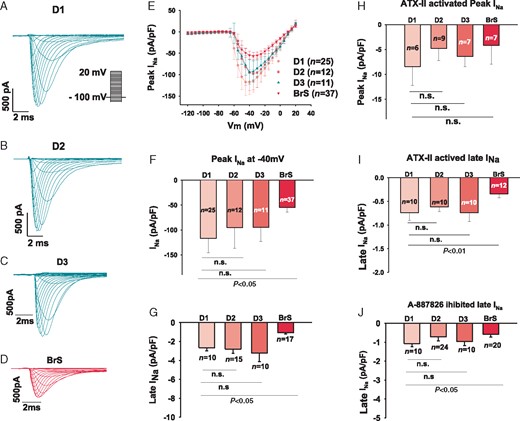
Sodium channel currents were reduced in hiPSC-CMs from the BrS patient. The sodium channel currents (INa) were evoked by pulses of 400 ms from −120 mV to 20 mV with a holding potential of −100 mV (inset in A). Peak INa was normalized to cell capacitance and plotted against the test potentials to obtain I–V curves. Late INa was measured at the time point of 300 ms of the pulses. (A–D) Representative traces of INa in donor (D1, D2, and D3) and BrS cells. (E) I–V curves of peak INa. (F) Mean values of peak INa at −40 mV. (G) Mean values of late INa at −40 mV. (H) Mean values of peak INa at −40 mV enhanced by 3 µM ATX-II, an enhancer of INa. (I) Mean values of late INa at −40 mV enhanced by 10 µM ATX-II. (J) Mean values of late INa at −40 mV inhibited by 10 µM A-887826, a blocker of SCN10A channels. BrS, Brugada syndrome; hiPSC-CMs, human-induced pluripotent stem cell-derived cardiomyocytes; n, number of cell; n.s., not statistically significant.
Changes of L-type calcium channel current (Ica-L) and Na/Ca exchanger current (INCX) in human-induced pluripotent stem cell-derived cardiomyocytes from the Brugada syndrome patient
The peak ICa-L was reduced in BrS cardiomyocytes (BrS, −3.4 ± 1.5 pA; D1, −9.9 pA ± 1.7; D2, −10.1 ± 2.8; D3, −6.5 ± 1.3; Figure 4A–C). While the activation curves of ICa-L were significantly shifted to more positive potentials (V0.5: BrS, 12.3 ± 4.1; D1, −7 ± 1.8; D2, −6.6 ± 1.3; D3, −5.5 ± 1.5, Figure 4D–E), the inactivation curve was significantly shifted to more negative potentials (V0.5: BrS, −47.1 ± 3.2 mV; D1, −36 ± 2.1; D2, −36.2 ± 2.2; D3, −34.6 ± 1.3 mV; Figure 4F–G). Although the recovery of ICa-L from inactivation seems to be accelerated in BrS cardiomyocytes, this difference was not statistically significant (Figure 4H and I). INCX in hiPSC-CMs was also smaller than that in donor cells (Supplementary material online, Figure S3A, B, E).
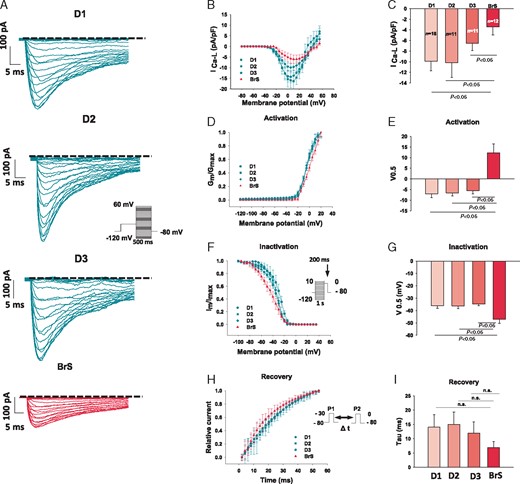
Calcium channel currents were reduced in hiPSC-CMs from the BrS patient. The L-type calcium channel currents (ICa) were evoked by the protocol shown in B (inset) and were plotted against the test potentials to obtain I–V curves. ICa was divided by voltage (the driving force for ICa) to obtain the conductance (Gm). Gm was normalized to Gmax and plotted against the voltages to get the activation curves. For assessing the inactivation of Ca channels, the currents measured with the protocol shown in F (inset) was plotted against voltages to get the inactivation curves. The activation and inactivation curves were fitted by Boltzmann equation and the voltages of half maximal (V0.5) activation or inactivation were obtained. For assessing the recovery from inactivation, double pulses (inset in H) were used. The currents induced by the second pulse were normalized to that induced by the first pulse and plotted against the time intervals between both pulses. The time constants were obtained by exponential fitting. (A) Representative traces of ICa in donor (D1, D2, and D3) and BrS cells. (B) I–V curves of peak ICa. (C) Mean values of peak ICa at −10 mV. (D) Activation curves of peak ICa. (E) Mean values of V0.5 of activation. (F) Inactivation curves of peak ICa. (G) Mean values of V0.5 of inactivation. (H) Time course curves of recovery from inactivation of peak ICa. (I) Mean values of time constants (tau) of recovery of ICa. BrS, Brugada syndrome; hiPSC-CMs, human-induced pluripotent stem cell-derived cardiomyocytes; n, number of cells; n.s., not statistically significant.
Changes of K channel currents in human-induced pluripotent stem cell-derived cardiomyocytes from the Brugada syndrome patient
Some K+ channel currents are supposed to contribute to arrhythmogenesis in BrS. Therefore, we studied the possible changes in some K+ currents including the transient outward current (Ito), the rapidly delayed rectifier potassium current (IKr), slowly delayed rectifier potassium current (IKs), inward rectifier potassium current (IK1), and ATP-sensitive K+ channel current (IKATP) in hiPSC-CMs from the BrS patient. We employed patch-clamp technique and specific ion channel blockers for the measurements and comparisons of the different K+ currents in BrS- and donor cells. 4-AP, E-4031, chromanol 293B, BaCl2, and glibenclamide were used to isolate Ito, IKr, IKs, IK1, and IKATP, respectively. The IKs was significantly reduced in BrS cells (Figure 5), whereas the Ito, IKr, IK1, and IKATP were not significantly altered (Supplementary material online, Figures S3C, D, F and S4).
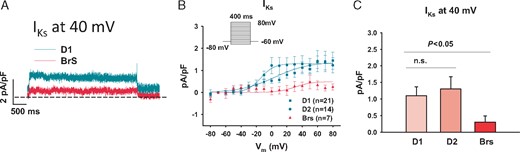
Slowly activating delayed rectifier potassium channel currents (IKs) were reduced in hiPSC-CMs from the BrS patient. The IKs was recorded with the protocol indicated in B (inset). Chromanol 293B (10 µM) was used to separate IKs from other currents. (A) Representative traces of Iks at 40 mV. (B) I–V curves of Iks. (C) Mean values of Iks at 40 mV show a reduced current in BrS when compared with healthy donors. The numbers of measured cells were indicated in B. BrS, Brugada syndrome; hiPSC-CMs, human-induced pluripotent stem cell-derived cardiomyocytes; n.s., not statistically significant.
Action potential in human-induced pluripotent stem cell-derived cardiomyocytes
Action potential characterizations are illustrated in Figure 6. Though the resting potential (RP), the action potential duration at 50% repolarization (APD 50), and action potential duration at 90% repolarization (APD 90) were similar in the measured hiPSC-CMs from the patient and healthy donors, the action potential amplitude (APA) and maximum depolarization velocity (Vmax) were significantly reduced in BrS when compared with healthy donors (Figure 6B–C), being consistent with the reduction of peak INa.
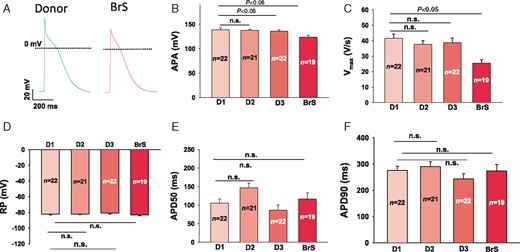
Changes of action potential in hiPSC-CMs from the BrS patient. APs were evoked by pulses of 1 nA for 3 ms at 1 Hz in current clamp mode. When APs reached steady state, 10 sequential APs were recorded and mean values were calculated for each cell. The AP parameters were analysed by computer using an ISO-3 multitasking patch-clamp programme (MFK M. Friedrich, Niedernhausen, Germany). (A) Representative traces of APs in donor (D1, D2, and D3) and BrS cells. (B) Mean values of APA. (C) Mean values of maximal depolarization velocity (Vmax) of APs. (D) Mean values of RPs. (E) Mean values of AP duration at 50% repolarization (APD50). (F) Mean values of AP duration at 90% repolarization (APD90). AP, action potential; APA, action potential amplitude; BrS, Brugada syndrome; hiPSC-CMs, human-induced pluripotent stem cell-derived cardiomyocytes; n, number of cell; n.s., not statistically significant; RP, resting potential.
Differential effects of ajmaline on action potentials in control and Brugada syndrome cells
To check whether ajmaline, a sodium channel current blocker that is frequently used to unmask the phenotypic changes in ECG of BrS patients, implicates any different changes in action potential characterization, 3 µM, 10 µM, and 30 µM ajmaline were used. The same cells were measured after sequentially adding the three concentrations of ajmaline from low to high. In donor cells, 3 µM and 10 µM showed no effect on the APA and Vmax. Only at 30 µM, APA and Vmax were reduced significantly. In BrS cells, however, 3 µM and 10 µM reduced APA and Vmax significantly, indicating that the responses of diseased cells to ajmaline were enhanced (Figure 7). In addition, ajmaline showed differential effects on APDs. It shortened APD90 slightly and APD50 significantly in donor cells, whereas in BrS cells it slightly prolonged APD50 and APD90 when the concentration was increased to 30 µM (Figure 7).
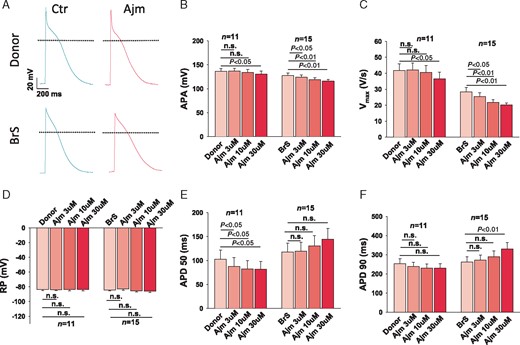
Enhanced effects of ajmaline on action potential in hiPSC-CMs from the BrS patient. The same cells were measured after sequentially adding the three concentrations of ajmaline (3 µM, 10 µM, and 30 µM) from low to high concentrations. (A) Representative traces of action potentials in donor (D1) and BrS cells. (B) Mean values of APA in absence (Donor/BrS) and presence of ajmaline in different concentrations. (C) Mean values of maximal depolarization velocity (Vmax) of action potentials in absence (Donor/BrS) and presence of ajmaline in different concentrations. (D) Mean values of RPs in absence (Donor/BrS) and presence of ajmaline in different concentrations. (E) Mean values of action potential duration at 50% repolarization (APD50) in absence (Donor/BrS) and presence of ajmaline in different concentrations. (F) Mean values of action potential duration at 90% repolarization (APD90) in absence (Donor/BrS) and presence of ajmaline in different concentrations. APA, action potential amplitude; BrS, Brugada syndrome; hiPSC-CMs, human-induced pluripotent stem cell-derived cardiomyocytes; n, number of cells; n.s., not statistically significant; RP, resting potential.
Arrhythmic events were increased in human-induced pluripotent stem cell-derived cardiomyocytes from the Brugada syndrome patient
Liang et al. demonstrated that hiPSC-CMs from BrS patients with SCN5A variants displayed trigger activity, abnormal Ca2+ transients and beating interval variation. To check the possible arrhythmic events in our hiPSC-CMs, we examined the spontaneous Ca2+ transients. The arrhythmic events (EAD- or DAD-like events) and interval variation were enhanced in BrS cells compared with donor cells (Figure 8).
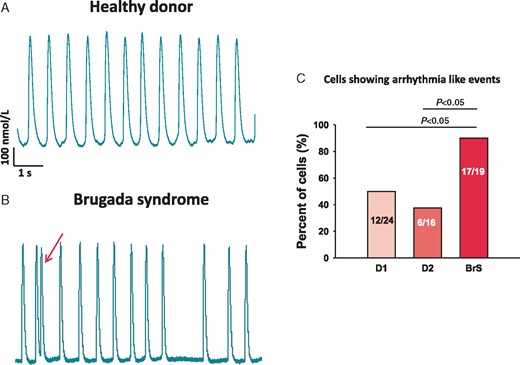
Increased arrhythmic events in BrS-hiPSC-CMs. Spontaneous Ca2+-transients were measured in donor- and BrS-hiPSC-CMs arrhythmic events (EAD- or DAD-like events) and the interval variation were evaluated. (A and B) Representative traces of Ca2+-transients in a donor and a BrS cell. (C) Numbers of cells showing arrhythmic events or enhanced interval variation (irregular transients). BrS, Brugada syndrome; hiPSC-CMs, human-induced pluripotent stem cell-derived cardiomyocytes.
Discussion
We have for the first time generated patient-specific induced pluripotent stem cell-derived cardiomyocytes from patient carrying double variants in the SCN10A gene with BrS diagnosed by ajmaline administration. Their cellular physiological and pharmacological properties were compared with that of healthy cells.
We have found in BrS-hiPSC-CMs: (i) A reduction of peak and late sodium channel current; (ii) Reduced APA and Vmax consistent with reduced peak INa; (iii) Enhanced effects of ajmaline on action potentials in BrS-hiPSC-CMs; (iv) Increased arrhythmia-like events.
It is known that mutations of SCN5A that can cause a reduction of sodium current may lead to BrS. However, recently published data reported that other variants including the neuronal gene SCN10A variants are also associated with cardiovascular diseases such as BrS.13 It has been shown that SCN10A is implicated in the electrical function of the heart.14 The ion channel Nav 1.8 (encoded by SCN10A), like Nav 1.5 (encoded by SCN5A), is a tetrodotoxin-resistant voltage-gated sodium channel and has been detected also in the heart. Several genome-wide association studies and functional studies on Nav 1.8 channels suggested that mutations in SCN10A are related to arrhythmogenesis.15–17 However, the expression level and functional role of Nav 1.8 in the heart remain highly controversial, although significant association of SCN10A with changes of ECG parameters have been reported.18
Here, we report a case of BrS in which the disease phenotype was observed as a result of reduced sodium channel current (INa) in hiPSC-CMs carrying the double variants (c.3749G>A and c.3802G>A) in the SCN10A gene. Additionally, we found that either the peak sodium channel current conducted mainly by SCN5A channels or the late INa conducted by both SCN5A and SCN10A channels were significantly decreased. Hu et al.17 presumed significant Nav 1.8 expression in human ventricular cardiomyocytes and over-expressed it in a heterologous system in a 1:1 ratio with Nav 1.5 to demonstrate its functional effects. In spite of the fact that the coexpression of Nav 1.8 and Nav 1.5 was shown by co-immunoprecipitation studies in HEK cells transfected with both channels, the evidence for the existence of the Nav 1.8 and Nav 1.5 complex in vivo remains lacking. Another interesting study reported that the cardiac enhancer that is located in SCN10A interacts with the promoter of SCN5A and modulates SCN5A gene expression in murine heart.19 These findings together with our data indicate that genetic variations in SCN10A can influence SCN5A channels in cardiomyocytes. A surprising finding is the increased expression levels of both SCN5A and SCN10A mRNA in BrS-hiPSC-CMs, which are not in agreement with the reduced currents. The reason is not known, but probably a compensatory reaction in the ‘diseased’ cells. A strong reduction of INa that is important for the cell excitation may trigger mRNA and protein expression of the ion channels as a compensatory mechanism to counteract the reduction of INa. However, the increased protein amounts were possibly not appropriately transported to the cell membrane (immunostaining did not detect significant differences between BrS and control cells) and hence failed to compensate INa in BrS-hiPS-CMs.
A further novel finding of the present study is a reduction of peak ICa-L and INCX. A loss of function of ICa-L has been reported in BrS implicating mutations in subunits of calcium channels. In our patient, mutations in calcium channel subunits were excluded by gene mutation analysis. A decrease in INa20 or ICa13 or augmentation of outward potassium currents causing preferential abbreviation of the action potential has been debated as the pathophysiological mechanism in BrS. These effects lead to heterogeneities of action potential characteristics and may develop spatial dispersion of repolarization as dominator for polymorphic ventricular tachycardia. In the current study, although we did not observe enhanced outward currents, we detected the reduction of both INa and ICa-L, which may present the important phenotypic changes in BrS. The reduced INa and Vmax can reduce propagation of excitation, in agreement with the conduction defect in BrS. Enhanced arrhythmia-like events and beating interval variation in BrS-hiPSC-CMs were also detected, although the underlying mechanisms are not clear. The APD was not significantly changed in BrS cells probably because of the counteracting effects of reduced inward currents (late INa, INCX, and ICa-L) and outward currents (IKs) on the APD. The decreased ICa-L may reduce Ca2+-transients and intracellular Ca2+ concentration. But the relevance of and reason for the reduction of ICa-L, INCX, and potassium (IKs) channel currents in BrS with SCN10A gene variants remain to be clarified.
Another interesting point of the present study is the effect of ajmaline, a widely used sodium channel blocker for provoking BrS 1 ECG pattern and confirming the diagnosis of BrS. We found that ajmaline exerted stronger inhibitory effects on the APA and Vmax in BrS-hiPSC-CMs than in healthy cells. This might confirm the susceptibility of BrS with SCN10A variants to sodium channel blockers. Of note, our patient developed BrS findings in ECG after infusion of 70 mg ajmaline and also suffered from ventricular flutter that was terminated by appropriate ICD shock.
Limitations
Human-induced pluripotent stem cell-derived cardiomyocytes from three healthy donors and one BrS patient were used for this study. Differences among individuals cannot be ruled out. Human-induced pluripotent stem cell-derived cardiomyocytes present similarities but also distinct differences in their physiological properties when compared with adult human cardiomyocytes. In addition, in the single-cell studies, many factors like cell to cell interaction, nerve and hormone regulations are not involved, which might play a particular role in BrS.
Because the gene analysis was not performed to check all the genes encoding the ion channels in cardiomyocytes, the possibility that additional ion channel variants or SNPs contributed to the changes in multiple ion channels cannot be completely excluded.
Because experiments studying specifically the consequences of these mutations by rescuing or introducing the variants in hiPSC-CMs are lacking in this study, whether the detected loss-of-function of INa is caused by the SCN10A variants (c.3749G>A and c.3802G>A) per se or by the variants plus cofactors remains to be clarified.
Conclusion
Our data demonstrate that the phenotypic features of BrS patients with SCN10A variants were successfully recapitulated in the hiPSC-CMs, including reduced peak and late INa, reduced APA and Vmax as well as increased arrhythmic events. In addition, the possible cause for the higher susceptibility of these patients to sodium channel blocking drugs in unmasking BrS was identified by detecting the enhanced effect of ajmaline on APA and Vmax in BrS hiPSC-CMs. The study supports an application of hiPSC-CMs in future studies on BrS including examination of trigger factors and testing of drug effects.
Acknowledgements
We gratefully thank Laura Cyganek, N. Gotzmann, M. Grohe, L. Krebs, and Y. Wedekind for excellent technical assistance in iPSC generation and characterization. We thank C. Liebetrau for excellent technical assistance. We thank the Chinese Scholarship Council (CSC) for the financial support for Zhihan Zhao, Mengying Huang and Xin Li.
Funding
This study was supported by the DZHK (German Center for Cardiovascular Research).
Conflict of interest: none declared.
References
Author notes
Ibrahim El-Battrawy and Sebastian Albers authors contributed equally to the study.



