-
PDF
- Split View
-
Views
-
Cite
Cite
Bashar Aldhoon, Dan Wichterle, Petr Peichl, Robert Čihák, Josef Kautzner, Complications of catheter ablation for atrial fibrillation in a high-volume centre with the use of intracardiac echocardiography, EP Europace, Volume 15, Issue 1, January 2013, Pages 24–32, https://doi.org/10.1093/europace/eus304
Close - Share Icon Share
Abstract
Catheter ablation (CA) for atrial fibrillation (AF) is a complex procedure that is associated with higher risk of complications. This study aimed at exploring the complication rate and corresponding risk factors in a high-volume centre with routine use of intracardiac echocardiography (ICE).
In total 1192 consecutive AF ablation procedures (100% ICE-guided; 96.4% 3D-navigated; point-by-point radiofrequency ablation with open-irrigated tip catheter; 22.4% robotic navigation; 25.4% repeated ablation) were performed in 959 patients (aged 58 ± 9 years; 70.8% males; 35.9% persistent AF) between March 2006 and December 2010. Ablation endpoint in paroxysmal AF was complete electrical pulmonary vein isolation (PVI). Complex ablation was defined as PVI plus stepwise strategy for left atrial substrate ablation (43.5%) in persistent AF. Forty major complications (3.3%) during the procedure or within the 3 month follow-up were observed. No death or atrioesophageal fistula occurred. Three patients (0.25%) had cardiac tamponade/hemopericardium and five patients (0.42%) had cerebrovascular embolic event. Vascular injury was the most frequent (2.3%) complication. Low body weight was the only significant risk factor with 0.8% increase of complication rate per 10 kg of body weight decrease (P = 0.013). A trend for increase in complication rate was also observed for advanced age, female gender, and complex procedure.
Atrial fibrillation ablation procedures guided by ICE in a high-volume centre are associated with low rate of serious complications. The composite risk score consisting of body weight, age, gender, and complexity of procedure predicted complications.
Introduction
Catheter ablation (CA) has become established treatment modality in atrial fibrillation (AF). As a result, an increasing number of centres with variable experience, case volume, and ablation strategies perform this type of CA. However, it is considered a complex procedure with many steps that is associated with higher incidence of complications in comparison with conventional CA procedures.1
It has been demonstrated that complication rates at high-volume centres are lower than at centres performing fewer procedures.2 Many other factors may be involved in large variability in complication rates among individual centres, including ablation strategy and intra-/peri-procedural anticoagulation regime. The use of imaging, especially on-line intracardiac echocardiography (ICE) may further minimize cardiac complications due to real-time visual guidance during CA.
In this study, 1192 consecutive CA procedures performed at a single, high-volume centre with routine use of ICE were analysed and complication rates were compared with those from other studies. In order to assess risk factors for the development of complications, clinical and procedural variables or their combination were also analysed.
Methods
Consecutive CA procedures for AF performed at our institute between March 2006 and December 2010 were analysed. Specific institutional tracking system was used to identify all complications during the procedure and within the 3 month follow-up. This includes a purposely established central registry for complications of invasive procedures. After admission to the hospital, a special form for complication reporting was assigned to each patient. This form, explicitly listing all possible types of complications associated with invasive procedures, was completed and returned to the central registry at the time of hospital discharge. In addition, the post-discharge complications were assessed during the 3 month outpatient visit (in 0.7% by phone contact) in all patients.
Preprocedural and intraprocedural imaging
Patients with risk factors for thromboembolism [any of the following: persistent AF, left atrium (LA) > 50 mm, and left ventricle dysfunction] underwent transesophageal echocardiography on the day of procedure in order to exclude the presence of LA thrombus. In the large proportion of subjects, preprocedural computed tomography (CT) or magnetic resonance imaging (MRI) angiography were used to assess the anatomy of the LA and of the pulmonary veins.
Intracardiac echocardiography was routinely used throughout different stages of the CA procedure. First, ICE catheter was introduced into the right ventricle to explore possible effusion before transseptal puncture. Subsequently, two separate transseptal punctures were performed under visual control of the tip of the needle engaging the fossa ovalis. Intracardiac echocardiography was then used during LA mapping to visualize and tag ostia of the pulmonary veins, and LA appendage. Subsequently, the course of oesophagus was tagged by ablation catheter under ICE guidance.
During CA itself, ICE was used to monitor the course of radiofrequency (RF) applications, the position and the contact of ablation catheter with the tissue. When sudden tissue whitening or bursts of microbubbles were observed, RF application was switched off. At the end of the procedure, pericardial space was checked again in order to exclude pericardial effusion.
Electroanatomical imaging (CARTO, Biosense Webster, Diamond Bar, CA, USA or NAVx, St Jude Medical, St Paul, MN, USA) was performed alone or in conjunction with preacquired atrial 3D images (CT or MRI).
Ablation procedure
Catheter ablation was performed by one of the four experienced operators. Vascular access was obtained by fellows in training. Four venous accesses were used: two right femoral, one left femoral, and the right subclavian or internal jugular veins. No arterial access was employed. Patients received conscious sedation (continuous drip of midazolam with fentanyl boluses).
Double, two-hole, transseptal puncture was performed under ICE guidance in all cases. Catheter ablation was performed with a 3.5 mm irrigated-tip catheter (Navistar Thermocool or Celsius™; Biosense Webster, Diamond Bar, CA, USA). Rarely (3.6% of procedures) circular ablation catheter (PVAC, Ablation Frontiers, Carlsbad, CA, USA) was used for ablation of paroxysmal AF. Circular linear lesions were placed in the LA around the ostia of the pulmonary veins with an endpoint of complete electrical isolation. In patients with persistent AF after pulmonary vein isolation, stepwise ablation strategy was used that included linear lesions along the LA roof, mitral isthmus, anterior LA wall, coronary sinus ablation, and electrogram-guided LA ablation. In a minority of patients, right atrial sites were targeted except for the cavotricuspid isthmus ablation that was completed in all patients with previously documented or intraprocedurally manifested typical atrial flutter (20.3%).
At repeated ablations, pulmonary veins were re-isolated, the substrate for clinical and/or induced atrial tachycardia was ablated, and all efforts were done to close other incidental gaps in previously created linear lesions.
Radiofrequency energy was applied with a Stockert™ EP Shuttle (Biosense Webster, Diamond Bar, CA, USA) RF generator. Constant irrigation flow of 15 ml/min (30 ml/min inside the coronary sinus) through a Cool Flow pump (Biosense Webster) was employed. The power mode was used with a preset power up to 25–30 W and down-regulation when the tip temperature of 40–42°C was achieved. The duration of each application at one spot varied between 20 and 30 s. Power output was reduced to 20–25 W and 15–20 W at the LA posterior wall and inside the coronary sinus, respectively. Oesophageal temperature probe was not routinely used.
Anticoagulation strategy
Warfarin was stopped in all patients 3–4 days before the procedure to achieve international normalized ratio (INR) value<2.0 (<1.5 in majority of patients) and weight-adjusted doses of low molecular weight heparin (LMWH) were administered. During the procedure, unfractionated heparin was used with a loading dose of 5000 IU prior to the first transseptal puncture. Activated clotting time was measured every 15–30 min, and additional heparin was given to maintain the target value of 350 s throughout the procedure. After the procedure, sheaths were extracted when activated clotting time spontaneously decreased below 170 s. An hour later, a continuous infusion of unfractionated heparin was administered to keep the normalized ratio of activated partial thromboplastin time in the range of 1.6–2.3. In the morning of the first post-operative day, unfractionated heparin was stopped and patients received LMWH in therapeutic dose with a simultaneous initiation of oral anticoagulation therapy. When target INR of 2–3 was achieved, LMWH was discontinued. Anticoagulation was continued for a minimum of 3 months after all ablation procedures.
Definition of complications
All documented complications were reviewed by staff physicians of the arrhythmia service in morbidity and mortality meetings. Major complications were defined as those that results in permanent injury or death, requires intervention for treatment, or prolongs or requires hospitalization for >48 h.3 All major complications documented during the procedure or within the 3 month follow-up using departmental tracking process were considered for the analysis.
Data for comparison purposes
Database MEDLINE was searched for studies, which primarily aimed at complications after AF ablation. For comparison purposes, we included only those studies in which the type and the number of complications had been reported and a minimum of 500 procedures had been performed. The search was structured around the key terms: atrial fibrillation, catheter ablation, complication, and variants of these terms. Search limits were set to English-language publications up to August 2011.
Statistical analysis
Continuous variables were expressed as means with standard deviations after testing for normality of distribution (Shapiro Wilk's test) and compared by the two2-tailed t-test for independent samples. Non-normally distributed variables were expressed as medians and interquartile range and compared by Mann–Whitney U-test. Categorical variables were expressed as percentages and compared by χ2 test with Yates's correction. Patients were categorized by terciles/quartiles of baseline continuous variables and differences between individual subgroups were analysed by analysis of variance with the Newman–Keuls post hoc test. Multivariate regression model including both continuous and categorized variables with stepwise forward analysis was used to test the independent association of baseline factors with complication rates. Composite scoring system was proposed for the prediction of complication risk. A P-value of <0.05 was considered significant. All analyses were performed using the STATISTICA vers.6.1 software (Statsoft, Inc., Tulsa, OK, USA).
Results
The study included 1192 CA procedures in 959 patients. Their baseline clinical characteristics analysed per procedure are shown in Table 1. Forty major complications were recorded in 39 (3.3%) patients/procedures (Table 2). No case of death or atrioesophageal fistula occurred. Three patients (0.25%) experienced permanent disability: mild upper extremity hemiparesis, paresis of the femoral nerve after retroperitoneal bleeding and persistent thoracalgia after surgery for hemothorax.
| . | Mean ± SD or proportion . | Median (IQR) . |
|---|---|---|
| Age (years) | 58 ± 9 | 60 (53–64) |
| Males (%) | 70.8 | – |
| Persistent AF (%) | 35.9 | – |
| Weight (kg) | 91 ± 16 | 90 (80–100) |
| Height (cm) | 177 ± 10 | 178 (170–184) |
| BMI (kg/m2) | 29.0 ± 4.2 | 28.7 (25.9–31.6) |
| BSA (m2) | 2.07 ± 0.21 | 2.07 (1.93–2.23) |
| CHA2DS2VASc score | 1.5 ± 1.3 | 1 (1–2) |
| LV dysfunction (EF < 0.55) (%) | 26.5 | – |
| LA diameter (mm) | 43 ± 5 | 43 (43–46) |
| Procedure time (min) | 259 ± 69 | 252 (205–307) |
| Fluoroscopic time (min) | 21 ± 12 | 19 (12–27) |
| Radiofrequency time (s) | 2699 ± 1359 | 2512 (1755–3459) |
| Complex procedure (%) | 43.5 | – |
| Repeat ablation (%) | 25.4 | – |
| Robotic procedure (%) | 22.4 | – |
| Hypertension (%) | 54.9 | – |
| Heart failure (%) | 1.9 | – |
| Diabetes (%) | 9.8 | – |
| Previous stroke/TIA (%) | 6.3 | – |
| CAD (%) | 10.3 | – |
| PVD (%) | 1.1 | – |
| Warfarin (%) | 69.4 | – |
| Antiplatelet drugs (%) | 22.5 | – |
| Amiodarone (%) | 24.0 | – |
| Beta blockers (%) | 68.3 | – |
| ACEI/ARB (%) | 35.2 | – |
| . | Mean ± SD or proportion . | Median (IQR) . |
|---|---|---|
| Age (years) | 58 ± 9 | 60 (53–64) |
| Males (%) | 70.8 | – |
| Persistent AF (%) | 35.9 | – |
| Weight (kg) | 91 ± 16 | 90 (80–100) |
| Height (cm) | 177 ± 10 | 178 (170–184) |
| BMI (kg/m2) | 29.0 ± 4.2 | 28.7 (25.9–31.6) |
| BSA (m2) | 2.07 ± 0.21 | 2.07 (1.93–2.23) |
| CHA2DS2VASc score | 1.5 ± 1.3 | 1 (1–2) |
| LV dysfunction (EF < 0.55) (%) | 26.5 | – |
| LA diameter (mm) | 43 ± 5 | 43 (43–46) |
| Procedure time (min) | 259 ± 69 | 252 (205–307) |
| Fluoroscopic time (min) | 21 ± 12 | 19 (12–27) |
| Radiofrequency time (s) | 2699 ± 1359 | 2512 (1755–3459) |
| Complex procedure (%) | 43.5 | – |
| Repeat ablation (%) | 25.4 | – |
| Robotic procedure (%) | 22.4 | – |
| Hypertension (%) | 54.9 | – |
| Heart failure (%) | 1.9 | – |
| Diabetes (%) | 9.8 | – |
| Previous stroke/TIA (%) | 6.3 | – |
| CAD (%) | 10.3 | – |
| PVD (%) | 1.1 | – |
| Warfarin (%) | 69.4 | – |
| Antiplatelet drugs (%) | 22.5 | – |
| Amiodarone (%) | 24.0 | – |
| Beta blockers (%) | 68.3 | – |
| ACEI/ARB (%) | 35.2 | – |
BMI, body mass index; BSA, body surface area; LV, left ventricle; EF, ejection fraction; CAD, coronary artery disease; PAD, peripheral artery disease; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
| . | Mean ± SD or proportion . | Median (IQR) . |
|---|---|---|
| Age (years) | 58 ± 9 | 60 (53–64) |
| Males (%) | 70.8 | – |
| Persistent AF (%) | 35.9 | – |
| Weight (kg) | 91 ± 16 | 90 (80–100) |
| Height (cm) | 177 ± 10 | 178 (170–184) |
| BMI (kg/m2) | 29.0 ± 4.2 | 28.7 (25.9–31.6) |
| BSA (m2) | 2.07 ± 0.21 | 2.07 (1.93–2.23) |
| CHA2DS2VASc score | 1.5 ± 1.3 | 1 (1–2) |
| LV dysfunction (EF < 0.55) (%) | 26.5 | – |
| LA diameter (mm) | 43 ± 5 | 43 (43–46) |
| Procedure time (min) | 259 ± 69 | 252 (205–307) |
| Fluoroscopic time (min) | 21 ± 12 | 19 (12–27) |
| Radiofrequency time (s) | 2699 ± 1359 | 2512 (1755–3459) |
| Complex procedure (%) | 43.5 | – |
| Repeat ablation (%) | 25.4 | – |
| Robotic procedure (%) | 22.4 | – |
| Hypertension (%) | 54.9 | – |
| Heart failure (%) | 1.9 | – |
| Diabetes (%) | 9.8 | – |
| Previous stroke/TIA (%) | 6.3 | – |
| CAD (%) | 10.3 | – |
| PVD (%) | 1.1 | – |
| Warfarin (%) | 69.4 | – |
| Antiplatelet drugs (%) | 22.5 | – |
| Amiodarone (%) | 24.0 | – |
| Beta blockers (%) | 68.3 | – |
| ACEI/ARB (%) | 35.2 | – |
| . | Mean ± SD or proportion . | Median (IQR) . |
|---|---|---|
| Age (years) | 58 ± 9 | 60 (53–64) |
| Males (%) | 70.8 | – |
| Persistent AF (%) | 35.9 | – |
| Weight (kg) | 91 ± 16 | 90 (80–100) |
| Height (cm) | 177 ± 10 | 178 (170–184) |
| BMI (kg/m2) | 29.0 ± 4.2 | 28.7 (25.9–31.6) |
| BSA (m2) | 2.07 ± 0.21 | 2.07 (1.93–2.23) |
| CHA2DS2VASc score | 1.5 ± 1.3 | 1 (1–2) |
| LV dysfunction (EF < 0.55) (%) | 26.5 | – |
| LA diameter (mm) | 43 ± 5 | 43 (43–46) |
| Procedure time (min) | 259 ± 69 | 252 (205–307) |
| Fluoroscopic time (min) | 21 ± 12 | 19 (12–27) |
| Radiofrequency time (s) | 2699 ± 1359 | 2512 (1755–3459) |
| Complex procedure (%) | 43.5 | – |
| Repeat ablation (%) | 25.4 | – |
| Robotic procedure (%) | 22.4 | – |
| Hypertension (%) | 54.9 | – |
| Heart failure (%) | 1.9 | – |
| Diabetes (%) | 9.8 | – |
| Previous stroke/TIA (%) | 6.3 | – |
| CAD (%) | 10.3 | – |
| PVD (%) | 1.1 | – |
| Warfarin (%) | 69.4 | – |
| Antiplatelet drugs (%) | 22.5 | – |
| Amiodarone (%) | 24.0 | – |
| Beta blockers (%) | 68.3 | – |
| ACEI/ARB (%) | 35.2 | – |
BMI, body mass index; BSA, body surface area; LV, left ventricle; EF, ejection fraction; CAD, coronary artery disease; PAD, peripheral artery disease; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Major complications in patients undergoing atrial fibrillation ablation: type and frequency
| Complication type . | Frequency . | |
|---|---|---|
| n . | % . | |
| Cardiac tamponade/hemopericardium | 3 | 0.25 |
| Stroke | 2 | 0.17 |
| Transitory ischaemic attack | 3 | 0.25 |
| Atrioventricular block | 1 | 0.08 |
| Pericarditis | 1 | 0.08 |
| Sepsis | 1 | 0.08 |
| Transient phrenic nerve paresis | 1 | 0.08 |
| Vascular complications | ||
| Hemothorax | 2 | 0.17 |
| Retroperitoneal bleeding | 2 | 0.17 |
| Subclavian vein bleeding | 1 | 0.08 |
| Femoral arteriovenous fistula | 7 | 0.59 |
| Femoral pseudoaneurysm | 4 | 0.34 |
| Femoral vein bleeding | 12 | 1.00 |
| Total | 40 | 3.36 |
| Complication type . | Frequency . | |
|---|---|---|
| n . | % . | |
| Cardiac tamponade/hemopericardium | 3 | 0.25 |
| Stroke | 2 | 0.17 |
| Transitory ischaemic attack | 3 | 0.25 |
| Atrioventricular block | 1 | 0.08 |
| Pericarditis | 1 | 0.08 |
| Sepsis | 1 | 0.08 |
| Transient phrenic nerve paresis | 1 | 0.08 |
| Vascular complications | ||
| Hemothorax | 2 | 0.17 |
| Retroperitoneal bleeding | 2 | 0.17 |
| Subclavian vein bleeding | 1 | 0.08 |
| Femoral arteriovenous fistula | 7 | 0.59 |
| Femoral pseudoaneurysm | 4 | 0.34 |
| Femoral vein bleeding | 12 | 1.00 |
| Total | 40 | 3.36 |
In one case, both groin bleeding and transient phrenic nerve injury occurred in the same patient.
Major complications in patients undergoing atrial fibrillation ablation: type and frequency
| Complication type . | Frequency . | |
|---|---|---|
| n . | % . | |
| Cardiac tamponade/hemopericardium | 3 | 0.25 |
| Stroke | 2 | 0.17 |
| Transitory ischaemic attack | 3 | 0.25 |
| Atrioventricular block | 1 | 0.08 |
| Pericarditis | 1 | 0.08 |
| Sepsis | 1 | 0.08 |
| Transient phrenic nerve paresis | 1 | 0.08 |
| Vascular complications | ||
| Hemothorax | 2 | 0.17 |
| Retroperitoneal bleeding | 2 | 0.17 |
| Subclavian vein bleeding | 1 | 0.08 |
| Femoral arteriovenous fistula | 7 | 0.59 |
| Femoral pseudoaneurysm | 4 | 0.34 |
| Femoral vein bleeding | 12 | 1.00 |
| Total | 40 | 3.36 |
| Complication type . | Frequency . | |
|---|---|---|
| n . | % . | |
| Cardiac tamponade/hemopericardium | 3 | 0.25 |
| Stroke | 2 | 0.17 |
| Transitory ischaemic attack | 3 | 0.25 |
| Atrioventricular block | 1 | 0.08 |
| Pericarditis | 1 | 0.08 |
| Sepsis | 1 | 0.08 |
| Transient phrenic nerve paresis | 1 | 0.08 |
| Vascular complications | ||
| Hemothorax | 2 | 0.17 |
| Retroperitoneal bleeding | 2 | 0.17 |
| Subclavian vein bleeding | 1 | 0.08 |
| Femoral arteriovenous fistula | 7 | 0.59 |
| Femoral pseudoaneurysm | 4 | 0.34 |
| Femoral vein bleeding | 12 | 1.00 |
| Total | 40 | 3.36 |
In one case, both groin bleeding and transient phrenic nerve injury occurred in the same patient.
Major complications
Thromboembolic events
Five symptomatic cerebrovascular embolic events (0.42%), two strokes and three transient ischaemic attacks (TIA), occurred during the first 48 h of the post-ablation period. Two patients with paroxysmal AF and sinus rhythm during the ablation procedure were treated by simple pulmonary veins isolation. The remaining three patients presented with persistent AF during the ablation procedure. In all of them an electrogram-guided LA ablation was performed and AF was cardioverted at the end of the procedure. All patients were treated with intravenous heparin and complete resolution of neurological symptoms occurred in all of them except one with permanent mild upper extremity hemiparesis. Two stroke patients had CHA2DS2VASc score of 1 and 3. All TIA patients had CHA2DS2VASc score of 2. Generally, there was a trend for higher incidence of thromboembolic complications in patients with CHA2DS2VASc score≥2 compared with the rest of population (0.75%, n = 533 vs. 0.15%, n = 659; P = 0.11). No significant association between thromboembolism and either presenting rhythm at the time of ablation or intraprocedural electrical cardioversion was found. Ablation of complex fractionated atrial electrograms (n = 227) was the only univariate significant risk factor associated with the incidence of thromboembolic events (1.3% vs. 0.21% in the rest of population; P = 0.02). This association persisted (P = 0.03) even after adjustment for AF type and CHA2DS2VASc score.
Cardiac tamponade or hemopericardium
Two patients suffered from cardiac tamponade (0.16% of all procedures). In the first case, the tamponade occurred during robotic ablation around the right pulmonary veins. Neither excess in indirect contact force assessment, nor audible pop were noticed. The second case was performed manually and the pop phenomenon occurred during RF application at the anterior portion of the left superior pulmonary vein. Both tamponades were successfully treated by immediate percutaneous pericardial drainage. There was another case of hemopericardium that developed after popping during ablation at the anterior LA wall and required no intervention. Nevertheless, the procedure was in this case prematurely terminated and the complication was classified as major.
Vascular complications
There were two cases of hemothorax, likely due to the artery wall injury during jugular vein puncture. One of them associated with life-threatening blood loss and was treated surgically, and the other resolved after transcutaneous pleural drainage. Another procedure was complicated by a large haematoma after subclavian vein puncture requiring surgical intervention with a complete resolution. In two cases, retroperitoneal bleeding was observed. In one of them, surgical intervention was required with a good clinical outcome. The second case was treated conservatively since bleeding stopped spontaneously. However, persistent femoral nerve injury resulted from prolonged compression by haematoma. Other vascular injuries were located in the groin region (n = 23). There were 7 small arteriovenous fistulas, 4 femoral pseudoaneurysms, and 12 local bleedings. Most of them (19 of 23) were managed conservatively. One femoral pseudoaneurysm was treated by ultrasound-guided glue injection and minor surgery was required in the remaining three cases. All groin vascular complications had favourable outcome. Vascular complications in robotic navigated procedures occurred in similar frequencies compared with manually performed procedures.
Other major complications
Less frequent major complications included one case of transient sepsis and one case of pericarditis treated conservatively. There was also a case of advanced atrioventricular block due to an extensive ablation in the right atrium that was treated by pacemaker implantation. In one patient, two simultaneous complications (temporary phrenic nerve injury and groin bleeding) occurred.
No symptomatic pulmonary vein stenoses were suspected clinically and none was diagnosed by echocardiography or cardiac imaging methods. However, CT was not routinely performed in the follow-up period, except of 25.4% patients who had undergone repeated ablation procedure.
Comparison to other studies
We identified five studies from high-volume centres reporting the rates of all-cause complications, tamponade/hemopericardium, and thromboembolic events associated with AF ablation that was not guided by ICE or the ICE usage was very limited (Table 3, Refs. 4–8). We compared our results with pooled data from these studies. In our study, total complication rate was non-significantly lower (3.3% vs. 3.9%, P = 0.30). The incidence of complications of cardiac origin, i.e. thromboembolic events and cardiac tamponades, was significantly lower (0.7% vs. 1.7%, P = 0.007) that was driven by significantly reduced rate of cardiac perforations (0.25% vs. 1.3%, P = 0.002).
| Reference . | Total procedures, n . | Complications, n (%) . | ECHO guidance . | Cardiac complications . | |
|---|---|---|---|---|---|
| Tamponade or hemopericardium, n (%) . | Stroke or TIA, n (%) . | ||||
| Dagres et al.4 | 1000 | 39 (3.9) | No | 13 (1.3) | 4 (0.4) |
| Baman et al.5 | 1642 | 57 (3.5) | No | 20 (1.2) | 4 (0.2) |
| Spragg et al.6 | 641 | 32 (5.0) | No | 8 (1.2) | 7 (1.1) |
| Bertaglia et al.7 | 1011 | 40 (3.9) | ICE (7.8%) | 8/6 (1.4) | 5 (0.5) |
| Lee et al.8 | 500 | 4 (0.8) | TEE (100%) | 0 | 0 |
| Reference . | Total procedures, n . | Complications, n (%) . | ECHO guidance . | Cardiac complications . | |
|---|---|---|---|---|---|
| Tamponade or hemopericardium, n (%) . | Stroke or TIA, n (%) . | ||||
| Dagres et al.4 | 1000 | 39 (3.9) | No | 13 (1.3) | 4 (0.4) |
| Baman et al.5 | 1642 | 57 (3.5) | No | 20 (1.2) | 4 (0.2) |
| Spragg et al.6 | 641 | 32 (5.0) | No | 8 (1.2) | 7 (1.1) |
| Bertaglia et al.7 | 1011 | 40 (3.9) | ICE (7.8%) | 8/6 (1.4) | 5 (0.5) |
| Lee et al.8 | 500 | 4 (0.8) | TEE (100%) | 0 | 0 |
TEE, transesophageal echocardiography.
| Reference . | Total procedures, n . | Complications, n (%) . | ECHO guidance . | Cardiac complications . | |
|---|---|---|---|---|---|
| Tamponade or hemopericardium, n (%) . | Stroke or TIA, n (%) . | ||||
| Dagres et al.4 | 1000 | 39 (3.9) | No | 13 (1.3) | 4 (0.4) |
| Baman et al.5 | 1642 | 57 (3.5) | No | 20 (1.2) | 4 (0.2) |
| Spragg et al.6 | 641 | 32 (5.0) | No | 8 (1.2) | 7 (1.1) |
| Bertaglia et al.7 | 1011 | 40 (3.9) | ICE (7.8%) | 8/6 (1.4) | 5 (0.5) |
| Lee et al.8 | 500 | 4 (0.8) | TEE (100%) | 0 | 0 |
| Reference . | Total procedures, n . | Complications, n (%) . | ECHO guidance . | Cardiac complications . | |
|---|---|---|---|---|---|
| Tamponade or hemopericardium, n (%) . | Stroke or TIA, n (%) . | ||||
| Dagres et al.4 | 1000 | 39 (3.9) | No | 13 (1.3) | 4 (0.4) |
| Baman et al.5 | 1642 | 57 (3.5) | No | 20 (1.2) | 4 (0.2) |
| Spragg et al.6 | 641 | 32 (5.0) | No | 8 (1.2) | 7 (1.1) |
| Bertaglia et al.7 | 1011 | 40 (3.9) | ICE (7.8%) | 8/6 (1.4) | 5 (0.5) |
| Lee et al.8 | 500 | 4 (0.8) | TEE (100%) | 0 | 0 |
TEE, transesophageal echocardiography.
Predictors of complications
Table 4 shows a comparison of baseline characteristics and procedural parameters in patients with and without major complications. Table 5 shows complication rates in subgroups according to clinical categories or when the study population was dichotomized by medians of clinical and procedural variables. It is evident that patients with less robust stature were more prone to complications. In quartile analyses, however, only the body weight showed a consistent trend (Figure 1). When anthropometric indices were analysed multivariately as continuous variables, the body weight remained the only independent factor associated with all-cause complication rates (P = 0.013) with 0.8% increase of complications per each 10 kg of body weight decrease. A trend for an increase in complication rate was also observed for advanced age, female gender, and complex procedure, i.e. that with more than simple pulmonary vein isolation (Figure 2). The composite risk score (ranging 0–8) was calculated as a sum of points for quartiles of body weight (3, 2, 1, 0 points), terciles of age (0, 1, 2 points), female gender (1 point), and complex procedure (2 points). Total complication rate increased by 0.75% per single scoring point (P = 0.004). Not only vascular complication alone but also cerebrovascular embolic event rates were associated with this risk score. In order to simplify the risk stratification, low-, medium- and high-risk range of the risk score was used as shown in Figure 3 with inscribed significant differences in complication rates between these subgroups.
Comparison of baseline characteristics and selected parameters in patients with and without major complications
| . | Without complications (n = 1153) . | With complications (n = 40) . | P value . |
|---|---|---|---|
| Age (years) | 58.2 ± 9.3 | 59.5 ± 8.2 | 0.39 |
| Males (%) | 71.1 | 61.5 | 0.20 |
| Persistent AF (%) | 35.6 | 43.6 | 0.31 |
| Weight (kg) | 90.9 ± 15.8 | 84.5 ± 14.8 | 0.01 |
| Height (cm) | 177 ± 10 | 174 ± 10 | 0.08 |
| BMI (kg/m2) | 29.0 ± 4.2 | 27.8 ± 4.2 | 0.09 |
| BSA (m2) | 2.08 ± 0.21 | 1.99 ± 0.21 | 0.01 |
| CHA2DS2VASc score | 1.52 ± 1.28 | 1.72 ± 1.36 | 0.34 |
| LA diameter (mm) | 43.1 ± 5.3 | 42.1 ± 4.7 | 0.22 |
| Procedure time (min) | 258 ± 69 | 265 ± 71 | 0.57 |
| Radiofrequency time (s) | 2689 ± 1346 | 2988 ± 1695 | 0.19 |
| Complex procedure (%) | 43.1 | 56.4 | 0.10 |
| Repeat ablation (%) | 25.1 | 33.3 | 0.26 |
| Robotic procedure (%) | 22.5 | 17.9 | 0.50 |
| . | Without complications (n = 1153) . | With complications (n = 40) . | P value . |
|---|---|---|---|
| Age (years) | 58.2 ± 9.3 | 59.5 ± 8.2 | 0.39 |
| Males (%) | 71.1 | 61.5 | 0.20 |
| Persistent AF (%) | 35.6 | 43.6 | 0.31 |
| Weight (kg) | 90.9 ± 15.8 | 84.5 ± 14.8 | 0.01 |
| Height (cm) | 177 ± 10 | 174 ± 10 | 0.08 |
| BMI (kg/m2) | 29.0 ± 4.2 | 27.8 ± 4.2 | 0.09 |
| BSA (m2) | 2.08 ± 0.21 | 1.99 ± 0.21 | 0.01 |
| CHA2DS2VASc score | 1.52 ± 1.28 | 1.72 ± 1.36 | 0.34 |
| LA diameter (mm) | 43.1 ± 5.3 | 42.1 ± 4.7 | 0.22 |
| Procedure time (min) | 258 ± 69 | 265 ± 71 | 0.57 |
| Radiofrequency time (s) | 2689 ± 1346 | 2988 ± 1695 | 0.19 |
| Complex procedure (%) | 43.1 | 56.4 | 0.10 |
| Repeat ablation (%) | 25.1 | 33.3 | 0.26 |
| Robotic procedure (%) | 22.5 | 17.9 | 0.50 |
BMI, body mass index; BSA, body surface area.
Comparison of baseline characteristics and selected parameters in patients with and without major complications
| . | Without complications (n = 1153) . | With complications (n = 40) . | P value . |
|---|---|---|---|
| Age (years) | 58.2 ± 9.3 | 59.5 ± 8.2 | 0.39 |
| Males (%) | 71.1 | 61.5 | 0.20 |
| Persistent AF (%) | 35.6 | 43.6 | 0.31 |
| Weight (kg) | 90.9 ± 15.8 | 84.5 ± 14.8 | 0.01 |
| Height (cm) | 177 ± 10 | 174 ± 10 | 0.08 |
| BMI (kg/m2) | 29.0 ± 4.2 | 27.8 ± 4.2 | 0.09 |
| BSA (m2) | 2.08 ± 0.21 | 1.99 ± 0.21 | 0.01 |
| CHA2DS2VASc score | 1.52 ± 1.28 | 1.72 ± 1.36 | 0.34 |
| LA diameter (mm) | 43.1 ± 5.3 | 42.1 ± 4.7 | 0.22 |
| Procedure time (min) | 258 ± 69 | 265 ± 71 | 0.57 |
| Radiofrequency time (s) | 2689 ± 1346 | 2988 ± 1695 | 0.19 |
| Complex procedure (%) | 43.1 | 56.4 | 0.10 |
| Repeat ablation (%) | 25.1 | 33.3 | 0.26 |
| Robotic procedure (%) | 22.5 | 17.9 | 0.50 |
| . | Without complications (n = 1153) . | With complications (n = 40) . | P value . |
|---|---|---|---|
| Age (years) | 58.2 ± 9.3 | 59.5 ± 8.2 | 0.39 |
| Males (%) | 71.1 | 61.5 | 0.20 |
| Persistent AF (%) | 35.6 | 43.6 | 0.31 |
| Weight (kg) | 90.9 ± 15.8 | 84.5 ± 14.8 | 0.01 |
| Height (cm) | 177 ± 10 | 174 ± 10 | 0.08 |
| BMI (kg/m2) | 29.0 ± 4.2 | 27.8 ± 4.2 | 0.09 |
| BSA (m2) | 2.08 ± 0.21 | 1.99 ± 0.21 | 0.01 |
| CHA2DS2VASc score | 1.52 ± 1.28 | 1.72 ± 1.36 | 0.34 |
| LA diameter (mm) | 43.1 ± 5.3 | 42.1 ± 4.7 | 0.22 |
| Procedure time (min) | 258 ± 69 | 265 ± 71 | 0.57 |
| Radiofrequency time (s) | 2689 ± 1346 | 2988 ± 1695 | 0.19 |
| Complex procedure (%) | 43.1 | 56.4 | 0.10 |
| Repeat ablation (%) | 25.1 | 33.3 | 0.26 |
| Robotic procedure (%) | 22.5 | 17.9 | 0.50 |
BMI, body mass index; BSA, body surface area.
Complication rates in a population dichotomomized according to baseline and procedural characteristics
| . | Complication rate (%) . | . | |
|---|---|---|---|
| Subgroup . | Subgroup . | Rest of population . | Chi-square, P . |
| Age > 60 years | 3.9 | 2.8 | 0.30 |
| Males | 2.8 | 4.3 | 0.20 |
| Persistent AF | 4.0 | 2.9 | 0.31 |
| Weight > 90 kg | 2.3 | 4.2 | 0.06 |
| Height > 178 cm | 2.1 | 4.1 | 0.06 |
| BMI > 28.7 kg/m2 | 2.2 | 4.4 | 0.03 |
| BSA > 2.073 m2 | 2.3 | 4.3 | 0.06 |
| CHA2DS2VASc score > 1 | 3.8 | 2.9 | 0.40 |
| LV dysfunction (EF < 0.55) | 3.2 | 3.3 | 0.90 |
| LA diameter > 43 mm | 2.9 | 3.6 | 0.49 |
| Complex ablation | 4.2 | 2.5 | 0.10 |
| Repeat ablation | 2.9 | 4.3 | 0.26 |
| Robotic procedure | 2.6 | 3.5 | 0.50 |
| . | Complication rate (%) . | . | |
|---|---|---|---|
| Subgroup . | Subgroup . | Rest of population . | Chi-square, P . |
| Age > 60 years | 3.9 | 2.8 | 0.30 |
| Males | 2.8 | 4.3 | 0.20 |
| Persistent AF | 4.0 | 2.9 | 0.31 |
| Weight > 90 kg | 2.3 | 4.2 | 0.06 |
| Height > 178 cm | 2.1 | 4.1 | 0.06 |
| BMI > 28.7 kg/m2 | 2.2 | 4.4 | 0.03 |
| BSA > 2.073 m2 | 2.3 | 4.3 | 0.06 |
| CHA2DS2VASc score > 1 | 3.8 | 2.9 | 0.40 |
| LV dysfunction (EF < 0.55) | 3.2 | 3.3 | 0.90 |
| LA diameter > 43 mm | 2.9 | 3.6 | 0.49 |
| Complex ablation | 4.2 | 2.5 | 0.10 |
| Repeat ablation | 2.9 | 4.3 | 0.26 |
| Robotic procedure | 2.6 | 3.5 | 0.50 |
BMI, body mass index; BSA, body surface area; LV, left ventricle; EF, ejection fraction.
Complication rates in a population dichotomomized according to baseline and procedural characteristics
| . | Complication rate (%) . | . | |
|---|---|---|---|
| Subgroup . | Subgroup . | Rest of population . | Chi-square, P . |
| Age > 60 years | 3.9 | 2.8 | 0.30 |
| Males | 2.8 | 4.3 | 0.20 |
| Persistent AF | 4.0 | 2.9 | 0.31 |
| Weight > 90 kg | 2.3 | 4.2 | 0.06 |
| Height > 178 cm | 2.1 | 4.1 | 0.06 |
| BMI > 28.7 kg/m2 | 2.2 | 4.4 | 0.03 |
| BSA > 2.073 m2 | 2.3 | 4.3 | 0.06 |
| CHA2DS2VASc score > 1 | 3.8 | 2.9 | 0.40 |
| LV dysfunction (EF < 0.55) | 3.2 | 3.3 | 0.90 |
| LA diameter > 43 mm | 2.9 | 3.6 | 0.49 |
| Complex ablation | 4.2 | 2.5 | 0.10 |
| Repeat ablation | 2.9 | 4.3 | 0.26 |
| Robotic procedure | 2.6 | 3.5 | 0.50 |
| . | Complication rate (%) . | . | |
|---|---|---|---|
| Subgroup . | Subgroup . | Rest of population . | Chi-square, P . |
| Age > 60 years | 3.9 | 2.8 | 0.30 |
| Males | 2.8 | 4.3 | 0.20 |
| Persistent AF | 4.0 | 2.9 | 0.31 |
| Weight > 90 kg | 2.3 | 4.2 | 0.06 |
| Height > 178 cm | 2.1 | 4.1 | 0.06 |
| BMI > 28.7 kg/m2 | 2.2 | 4.4 | 0.03 |
| BSA > 2.073 m2 | 2.3 | 4.3 | 0.06 |
| CHA2DS2VASc score > 1 | 3.8 | 2.9 | 0.40 |
| LV dysfunction (EF < 0.55) | 3.2 | 3.3 | 0.90 |
| LA diameter > 43 mm | 2.9 | 3.6 | 0.49 |
| Complex ablation | 4.2 | 2.5 | 0.10 |
| Repeat ablation | 2.9 | 4.3 | 0.26 |
| Robotic procedure | 2.6 | 3.5 | 0.50 |
BMI, body mass index; BSA, body surface area; LV, left ventricle; EF, ejection fraction.
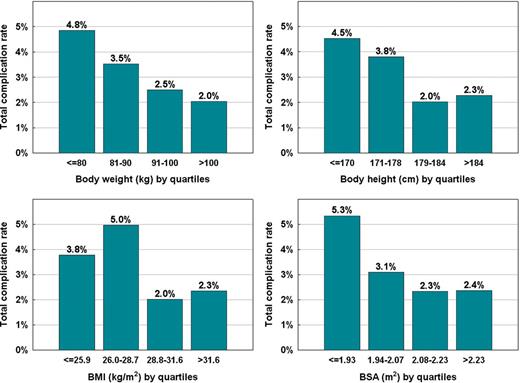
The distribution of all-cause complication rates according to anthropometric indices categorized by quartiles. BMI, body mass index; BSA, body surface area.
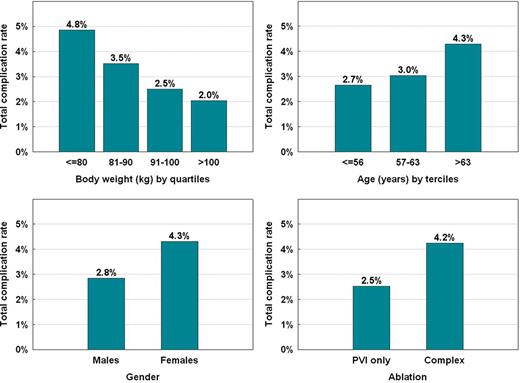
The distribution of all-cause complication rates according to individual categorized variables subsequently used for the composite risk score. PVI, pulmonary vein isolation.
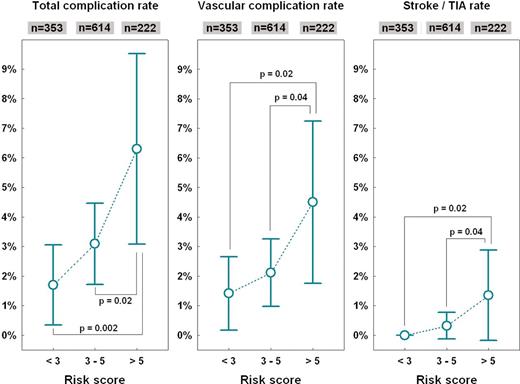
Complication rates in subgroups according to composite risk score based on gender, age, body weight, and complexity of ablation procedure (see the text). Graphs show mean values and 95% confidence intervals compared by analysis of variance with the Newman–Keuls post hoc test.
Discussion
The main findings of this study can be summarized as follows. (1) major complications of CA for AF occurred in 3.3% procedures with a very low incidence of life-threatening complications such as thromboembolism (0.42%) and tamponade/hemopericardium (0.25%). (2) Total complication rate was dominated by vascular complications and was significantly and inversely related to the body weight. (3) The risk of complications during CA of AF can be predicted by the composite risk stratification score that also includes patient body weight.
Thromboembolic events
No thromboembolic event was observed during the CA procedure. We believe that this may reflect our strategy of administering intravenous heparin before the first transseptal puncture. Under this condition, ICE provides guidance for safe transseptal puncture. In accordance with a study of Ren et al.,9 lower level of anticoagulation was shown to be associated with relatively high risk of thrombus formation. All observed thromboembolic events in our cohort occurred during the first 48 h after the ablation procedure. Many factors may predispose to increased risk of thromboembolism in this critical period as shown in varies studies. These include persistent activation of the coagulation cascade due to intravascular placement of catheters10 and endothelial disruption resulting from ablation,11 and finally bridging from warfarin through LMWH to IV heparin and back to oral anticoagulation.12 The latter seems to be an important mechanism in the development of delayed thromboembolic complications. Indeed, recent reports on periprocedural therapeutic anticoagulation with warfarin showed reduced risk of periprocedural stroke without increasing bleeding complications.13,14
We also analysed possible thromboembolism risk predictors. In this respect, we tested the performance of the scoring system CHA2DS2VASc as suggested by Lip et al.15 A recent analysis by Chao et al.16 found that CHA2DS2VASc score could be used to predict adverse events after CA of AF. In our study, we found an association of higher CHA2DS2VASc score with thromboembolic events. Although this relationship did not reach statistical significance, data suggest that CHA2DS2VASc score might be helpful in predicting higher risk of periprocedural thromboembolic events. As a result of low numbers, we also did not find significant association between thromboembolic events and either presenting rhythm at the time of ablation or intraprocedural electrical cardioversion as suggested by Pianelli et al.17 On the other hand, we observed a significant association between increased risk of postprocedural thromboembolism and ablation of complex fractionated atrial electrograms in patients with persistent or long-standing persistent AF that may reflects more extensive ablation in these patients.
Hemopericardium and cardiac tamponade
The rate of cardiac tamponade/hemopericardium in this study was substantially lower than in previously published studies4–7 (Table 3). This could be attributed to the routine use of ICE during the procedure in our centre. There are two main possible explanations. First, all transseptal punctures were done under ICE guidance and no complication occurred during transseptal puncture itself. Taking into account the routine use of two separate transseptal punctures, almost 2400 of punctures were performed without any complication. This finding is in agreement with a study of Daoud et al.18 where ICE was found to be a useful tool for transseptal access guidance. Further, Lee et al.,8 although on smaller cohort of 500 procedures, have recently reported no cardiac tamponade under transoesophageal echocardiography guidance of transseptal puncture. Second, catheter tip–tissue interface was monitored during RF delivery whenever possible. Sudden whitening of the tissue or sudden bursts of microbubbles were used as a sign of tissue overheating, leading to termination of RF delivery as suggested by others.19,20 Nevertheless, it should be noted that all procedures included in our study were performed by experienced operators. This fact could also contribute to low tamponade complication rate.
Vascular complications
In this study, the rate of vascular complications was relatively high (2.3%) in comparison to the other previously published studies in which the vascular complication rates had ranged between 1.1% and 1.9%.4–8 Several factors could contribute to this finding. First, our standard vascular access comprised four venous sheaths with one introduced from the jugular or subclavian vein. Other centres usually use less sheaths and/or femoral access only. Second, the vein access was mainly obtained by fellows in the training. The role of this factor has already been mentioned by others.5 Third, increased risk of vascular complications could be related to our strategy of periprocedural anticoagulation treatment during the study period. The increased risk of vascular complications in case of switching from oral anticoagulation before the procedure to LMWH had previously been reported.21 However, it is important to emphasize that the majority of vascular complications were treated conservatively and only slightly prolonged the hospital stay.
Predictors of complications
Previous studies identified several predictors of complications such as advanced age, female gender, coronary heart disease, congestive heart failure, clopidogrel administration, and CHADS2 score of ≥2.4–7,22,23 In our study, lower body weight was identified as a further predictor of complications, especially of vascular origin. The most plausible explanation of this finding is the periprocedural anticoagulation swing. It appears that the change of periprocedural anticoagulation strategy with uninterrupted oral anticoagulation during the procedure may decrease the risk of vascular complications.12,21 Lean patients are probably more prone to overdosage of anticoagulant drugs. This explanation is supported by a study of Prudente et al.24 who reported a decrease in femoral vascular complications with using low-dose enoxaparine regimen after CA for AF.
The combination of risk factors (low body weight, advanced age, female gender, and complex procedure) proposed in our study predicted not only the total complication rate, but also vascular and thromboembolic complications. We suggest that the use of such a composite scoring scheme may be of clinical value.
Limitations
This study has several potential limitations. First, it is a single centre, retrospective, observational study that did not compare complication rates of ICE-guided procedures with conventionally performed procedures. Comparison to other studies is clearly problematic because of differences in patient populations, experience of operators, ablation strategies and time periods in which the data have been collected because of temporal trends in complications rates.23 However, a randomized study comparing ICE-guided strategy with the conventional approach is unlikely to be performed. It would require very large sample size and might raise some ethical concerns.
Second, despite the existence of institutional tracking system, some postprocedural complications might have been missed, especially when occurring after hospital discharge.
Third, neither CT nor MR angiography of the pulmonary veins were performed routinely during the follow-up. As a result, the rate of pulmonary venous stenosis could have been underestimated.
Fourth, proposed risk stratification score was not prospectively validated and its predictive power may not be reproducible in other centres.
Conclusions
This study showed that CA for AF in a high-volume centre, in which ICE had been routinely used during all stages of procedures, had lower rate of major complications as compared with previous studies. This was due to the substantial reduction of potentially life-threatening complications such as cardiac perforation. The majority of complications observed in this study were of vascular origin and patients with less robust stature were found at higher risk of vascular access complications.
Conflict of interest: J.K. is a member of advisory board for Biosense Webster, Siemens Healthcare, and St Jude Medical and received also speaker's honoraria from these companies.
Funding
This work was supported by a Research Grant No. MZO 00023001 of the Ministry of Health of the Czech Republic (Research in Cardiovascular Diseases, Diabetes Mellitus, and Transplantation of Life-Preserving Organs).



