-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroshi Kato, Shoichi Kubota, Takuya Goto, Koichi Inoue, Naohiko Oku, Toshihiro Haba, Makoto Yamamoto, Transseptal puncture and catheter ablation via the superior vena cava approach for persistent atrial fibrillation in a patient with polysplenia syndrome and interruption of the inferior vena cava: contact force–guided pulmonary vein isolation, EP Europace, Volume 19, Issue 7, July 2017, Pages 1227–1232, https://doi.org/10.1093/europace/euw095
Close - Share Icon Share
Abstract
We sought to establish the technical feasibility of transseptal puncture and left atrial (LA) ablation through the right internal jugular vein via the superior vena cava (SVC) approach in patients with an interrupted inferior vena cava (IVC).
A 34-year-old man with persistent atrial fibrillation (AF) and polysplenia syndrome (hypoplasia of the left kidney, aplasia of the pancreas tail, bilaterally bilobed lungs, and an interrupted IVC) was referred to our hospital for radiofrequency ablation. Because transseptal puncture and LA ablation would be impossible by a standard IVC approach via the femoral vein, we performed transseptal puncture and LA ablation through the right internal jugular vein via the SVC approach using a manually curved Brockenbrough needle and intracardiac echocardiographic guidance. We accomplished pulmonary vein (PV) isolation using a deflectable guiding sheath and a contact force-sensing ablation catheter to monitor the contact force and the force–time integral of the tip of the ablation catheter. No complications occurred during or after the procedure. The patient was discharged home without recurrence of AF 3 days after the procedure. He had no recurrence of AF and was taking no medication 5 months after ablation.
We successfully performed transseptal puncture in a patient with persistent AF, polysplenia syndrome, and complete interruption of the IVC using the superior route through the internal jugular vein. We also accomplished PV isolation using a deflectable guiding sheath and real-time monitoring of the contact force of the ablation catheter.
We successfully performed transseptal puncture in a patient with persistent atrial fibrillation, polysplenia syndrome, and complete interruption of the inferior vena cava using the superior route through the internal jugular vein without any complications.
We performed pulmonary vein isolation from the superior approach using a deflectable guiding sheath and contact force-sensing catheter. This case demonstrates that real-time monitoring of the contact force and force–time integral of the ablation catheter is useful in this setting.
Introduction
Polysplenia is a very rare congenital disease characterized by many organ abnormalities, including left atrial (LA) isomerism and cardiovascular anomalies.1 Congenital heart disease,2 sinus node dysfunction,3–5 supraventricular tachycardia,4 and interruption of the inferior vena cava (IVC)4,5 are reported as possible cardiovascular complications in patients with polysplenia.
We encountered a patient with persistent atrial fibrillation (AF), polysplenia syndrome, and interruption of the IVC. We herein describe our techniques to access the left atrium and pulmonary vein (PV) and accomplish ablation from the superior vena cava (SVC) approach in patients with this congenital venous anomaly.
Patient and history
A 34-year-old man with persistent AF and polysplenia syndrome was referred to our hospital for radiofrequency ablation. He had a medical history of junctional rhythm with bradycardia of 40 bpm on 12-lead electrocardiography (ECG) that had persisted since infancy. In adolescence, he was diagnosed with polysplenia syndrome involving hypoplasia of the left kidney, aplasia of the pancreas tail, and bilaterally bilobed lungs.
Two years previously, at the age of 32 years, ECG during a health checkup revealed AF. He had severe chest discomfort and palpitation during tachycardia due to persistent AF with a rapid ventricular response. A physician in the clinic recommended catheter ablation for treatment of the AF; however, his IVC was interrupted as shown by abdominal computed tomography (CT). He was then referred to our hospital. Contrast-enhanced abdominal CT at our hospital demonstrated that his IVC was completely obstructed at the infra-renal level (Figure 1A), and abdominal venous return occurred via the hemiazygos vein draining into the SVC (Figure 1B); these were the same findings as in the previous clinic. Subsequent abdominal magnetic resonance imaging performed in our hospital also confirmed the diagnosis of an interrupted IVC (Figure 1C). Abdominal venous return occurred via the dilated hemiazygos vein (Figure 1D). Transthoracic and transoesophageal echocardiography showed no shunt disease, including an atrial septal defect or patent foramen ovale.
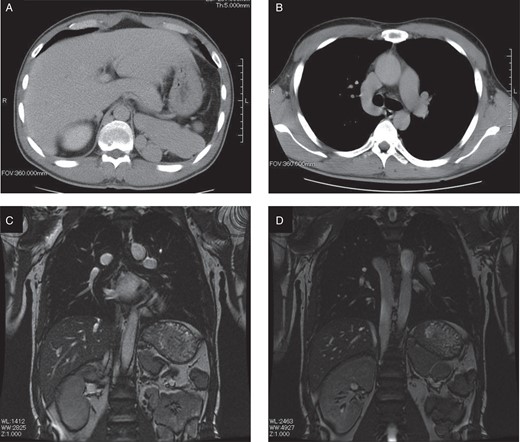
(A) Computed tomography image of the upper abdomen shows the liver, aorta, polysplenia, and absence of the inferior vena cava. (B) Abdominal venous return occurred via the collateral vein (hemiazygos vein draining into the superior vena cava). (C) Magnetic resonance image of the chest and abdomen shows the heart, liver, aorta, polysplenia, and absence of the inferior vena cava. (D) Abdominal venous return occurred via the dilated hemiazygos vein. Hypoplasia of the left kidney was noted.
It was quite evident that transseptal puncture and LA ablation would be impossible by an IVC approach via the femoral vein. Therefore, we decided to perform LA ablation via the SVC approach. Lim et al.6 were the first researchers worldwide to report that transseptal puncture and LA ablation can be safely performed via right internal jugular vein access. Another publication described LA ablation from a superior approach, albeit for LA tachycardia after previous AF ablation.7 We subsequently performed transseptal puncture and LA ablation via right internal jugular vein access in a patient with an interrupted IVC.8 Very recently, Baszko et al.9 reported that successful transseptal puncture and PV isolation can be safely performed using superior access with balloon cryoablation.
However, superior transseptal puncture has evolved over the last 5 years and has been particularly frequently described for left ventricular endocardial pacing. Systems specifically designed for superior transseptal puncture are currently available in Europe and other parts of the world.10
Transseptal puncture from the jugular vein
After written informed consent was obtained, we performed an electrophysiological study and ablation procedure via the right internal jugular vein and the right subclavian vein under conscious sedation. First, a deflectable 10-pole 5-Fr catheter was placed into the coronary sinus from a 6-Fr straight sheath (Terumo Medical, Tokyo, Japan) via the right subclavian vein. An 8-Fr intracardiac echocardiography (ICE) catheter with a single longitudinal plane and linear phased array (AcuNav; Siemens Medical Solutions, Inc., Mountain View, CA, USA) was then placed into the right atrium from the 8-Fr straight sheath (Terumo Medical) via the right subclavian vein. An 8-Fr pre-shaped long sheath (SL3; St Jude Medical, Inc., St Paul, MN, USA) via the right jugular vein was introduced at the posterior portion of the tricuspid annulus, maintaining the relative position of the sheath with the tip oriented in the 10 o'clock position from the operator's view (leftward and anterior). Next, we gently manipulated the SL3 sheath with 60° anticlockwise rotation and 2-cm pull-back, gradually changing the relative position of the sheath with the tip oriented to the 8 o'clock position (leftward and posterior). In this manner, the tip of the SL3 sheath approached the fossa ovalis. We manually curved a Brockenbrough needle with a 120° angle and 7-cm curve to manipulate the tip toward the fossa ovalis horizontally (Figure 2A and B). Using the manually curved Brockenbrough needle under ICE and fluoroscopic guidance, we performed transseptal puncture via the right internal jugular vein through the SL3 sheath. From the operator's view, we maintained the relative position of the Brockenbrough needle; the distal tip and the arrow of the proximal end of the needle were oriented at the 8 o'clock position (leftward and posterior) (Figure 2C and D). Intracardiac echocardiography confirmed that tenting of the fossa ovalis, which demonstrates the tip of the Brockenbrough needle, was located just central to the fossa ovalis (Figure 2E–G). After successful transseptal puncture at the first attempt without any complications, we added heparin intravenously to an activated clotting time of ∼300 s throughout the procedure. Next, the 8-Fr straight sheath and ICE catheter were replaced with another SL3 sheath and a deflectable 7-Fr mapping catheter. The second 8-Fr SL3 sheath was introduced to the left atrium through the same hole made in the fossa ovalis, resulting in placement of double SL3 sheaths in the left atrium. Finally, we replaced the first 8-Fr SL3 sheath via the right jugular vein with a deflectable 8.5-Fr ablation sheath (Destino; Japan Lifeline Co., Tokyo, Japan) in the left atrium.
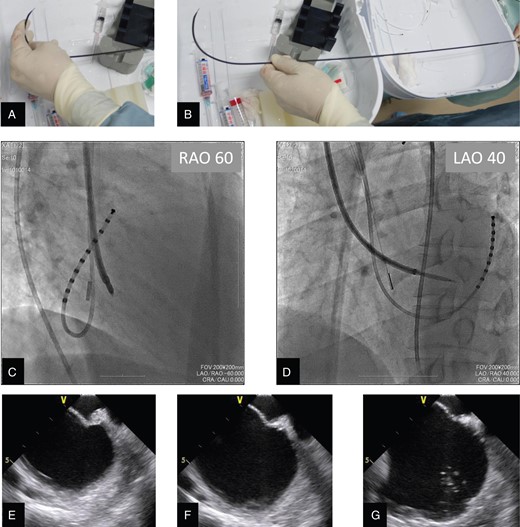
(A) A Brockenbrough needle within the dilator was manually curved to horizontally manipulate the tip toward the fossa ovalis. (B) The tip of the Brockenbrough needle then had a curve of 7 cm and a 120° angle against the proximal shaft. (C and D) Biplane fluoroscopic views during transseptal puncture procedure via the superior vena cava: (C) Images at the right anterior oblique 60° projection and (D) the left anterior oblique 40° projection demonstrate the SL3 dilator and Brockenbrough needle passing through the fossa ovalis with the tip horizontal, maintaining the relative position of the needle with the distal tip and the arrow of the proximal end oriented in the 8 o'clock position from the operator's view (leftward and posterior). To confirm the left atrial access, we injected additional contrast medium through the needle or dilator under intracardiac echocardiography and fluoroscopic guidance. (E–G) Intracardiac echocardiography views during transseptal procedure via the superior vena cava: (E) Tenting of the fossa ovalis is demonstrated by intracardiac echocardiography, which indicates that the tip of the Brockenbrough needle is located just central to the fossa ovalis. (F) Disappearance of tenting of the fossa ovalis is observed just after penetration of the tip of the Brockenbrough needle into the left atrium. (G) Injection of the contrast medium into the left atrium creates contrast echo due to microbubbles in the left atrium, and it is confirmed that the tip of the Brockenbrough needle is located in the left atrium.
Radiofrequency ablation using contact force monitoring
A three-dimensional CT image of the left atrium and PV was merged on the display of an electroanatomical mapping system (CARTO; Biosense Webster, Diamond Bar, CA, USA). Each PV potential at baseline was recorded by a 20-pole 7-Fr circular mapping catheter (Lasso; Biosense Webster) via the right subclavian vein through the 8-Fr SL3 sheath (Figure 3A and B).
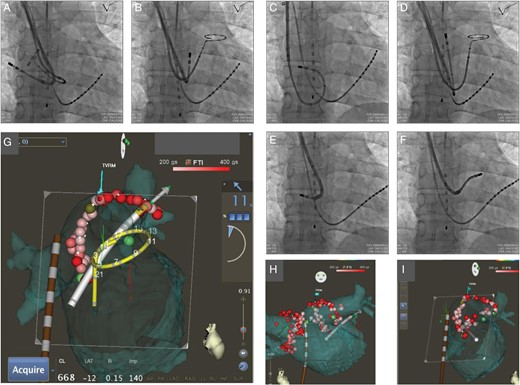
(A and B) Fluoroscopic anteroposterior images showed that the 20-pole circular mapping catheter (Lasso) is located in the right superior pulmonary vein (A) and left superior pulmonary vein (B). An 8-Fr ablation catheter with a contact force sensor (SMARTTOUCH) through the 8.5-Fr deflectable guiding sheath, 10-pole 5-Fr mapping catheter in the coronary sinus vein, and oesophageal temperature probe is also shown. (C–F) Fluoroscopic anteroposterior images showed the ablation catheter in the right superior pulmonary vein antrum (C), left superior pulmonary vein antrum (D), right inferior pulmonary vein antrum (E), and left inferior pulmonary vein antrum (F). Note that the deflectable sheath created variable curves according to each pulmonary vein antrum. (G) Merging of CARTO map with computed tomography and VISITAG (real-time automated visualization of the force–time integral) from the inner view during the ablation procedure for the left pulmonary vein and antrum. The large arrow indicates that the vector of the force at the tip of the catheter is directed perpendicularly to the endocardial tissue as an optimal direction. The real-time contact force of the tip of the ablation catheter (11 g) is indicated in the right portion of the display. VISITAG with a red colour indicates the site of ablation with an force–time integral of >400 gs. VISITAG with a pink colour indicates the site of ablation with an force–time integral of >200 gs. Definition of gradual colouring of the tag (VISITAG) is depicted at the top portion of the display as a colour scale bar ranging from 200 gs (pink) to 400 gs (red). Light green dots indicate CFAE. (H and I) Merging of CARTO map with computed tomography and VISITAG at the end of the procedure: anteroposterior views (H) and inner views (I) show that radiofrequency energy was delivered to encircle the ipsilateral pulmonary vein ostia and left atrial antrum. The oesophageal temperature probe (brown colour) and 20-pole circular mapping catheter (yellow colour) are also depicted by the advanced catheter location system.
An 8-Fr open-irrigated contact force-sensing catheter (SMARTTOUCH Thermocool D-F curve; Biosense Webster) was introduced into the left atrium and PV from the 8.5-Fr deflectable sheath (Destino) via the right internal jugular vein (Figure 3C–F). The contact force of the tip of the ablation catheter and the vector of the force were depicted on the CARTO map in a real-time manner (Figure 3G). During the application of the radiofrequency energy, the force–time integral (FTI) was monitored continuously by gradual colouring of the tag from white to pink (FTI 200 gs) to red (FTI 400 gs) as shown by a VISITAG module (Biosense Webster) (Figure 3G). We targeted an FTI of 400 gs to achieve transmural lesion formation to encircle the ipsilateral PV antrum. However, around the LA posterior wall adjacent to the oesophagus, the duration and power of the RF were limited to 20 s and 20 W, respectively, regardless of the FTI, to avoid oesophageal injury. Circumferential PV ablation was performed with radiofrequency energy (power of 30 W at the anterior wall, 25 W at the posterior wall, and 20 W near the oesophagus) and irrigation flow of 17 mL/m to encircle the ipsilateral PV ostia and LA antrum (Figure 3H). Optimal contact forces of 10–20 g during application of radiofrequency energy were achieved in all segments (roof, anterosuperior, anteroinferior, inferior, posteroinferior, and posterosuperior regions) of the bilateral PV antrum except the limited area at the anterosuperior segment of the right PV ostium. The contact forces were <10 g only in this region, resulting in prolonged and repeated ablation to obtain adequate FTI during the procedure.
At the end of the procedure, AF was terminated by DC cardioversion. No PV potential was recorded in the bilateral PV antrum. No complications occurred during or after the procedure.
The patient was discharged home without recurrence of AF 3 days after the procedure. Brief episodes of paroxysmal AF occurred 17 days after discharge and were terminated by single oral administration of cibenzoline within the same day. Two months after ablation, cibenzoline was discontinued. The patient then maintained sinus rhythm or ectopic atrial rhythm during the follow-up period of 5 months without recurrence of AF. We stopped the oral anticoagulation (rivaroxaban) 5 months after ablation.
Discussion
Interruption of the IVC is a very rare congenital abnormality. Koc and Oguzkurta11 reported that interruption of the IVC was noted in 8 (0.1%) of 7972 patients. Polysplenia is also a very rare congenital abnormality that involves many organ anomalies and cardiovascular complications.1 Polysplenia is frequently associated with interruption of the IVC.1 Furthermore, it has been reported that patients with polysplenia and LA isomerism sometimes have sinus node dysfunction and atrial tachyarrhythmia.2–5
We herein described a patient with polysplenia, interruption of the IVC, and persistent AF with rapid ventricular response.
At the time of consultation, it was quite evident that a standard transseptal approach via the femoral vein was impossible. We decided to perform transseptal puncture from the right internal jugular vein and successfully accomplished PV isolation from the SVC approach.
Special curve and manipulation of the Brockenbrough needle
For successful transseptal puncture from the SVC, it is crucial that the Brockenbrough needle is curved manually so that the angle between the tip and the proximal shaft of the needle becomes 120°. This angle makes it possible to convert the push force of the proximal end of the Brockenbrough needle to a horizontal vector at the fossa ovalis. Furthermore, the Brockenbrough needle with a large curve size of 7 cm provides sufficient back-up force against the lateral wall of the SVC.
Because transseptal puncture via the SVC approach through the right internal jugular vein is a mirror image from the operator's view compared with standard transseptal puncture via the IVC approach through the femoral vein, the relative position of the Brockenbrough needle should be maintained with the distal tip and the arrow of the proximal end of the needle oriented in the 8-o'clock position (leftward and posterior).
Intracardiac echocardiography
As described in our previous case report, the use of ICE from the subclavian vein is helpful to guide the transseptal puncture from the right internal jugular vein via the SVC approach.8 Real-time echocardiographic imaging for tenting of the fossa ovalis enhances the safety of transseptal access into the left atrium because the abrupt leftward movement (snap-in) on the fluoroscopic image, which indicates passage over the limbus into the fossa ovalis, cannot be observed from the SVC approach. Because of the different orientation from the conventional IVC approach, we believe that the use of ICE makes transseptal puncture safer when using the SVC approach.
Deflectable ablation sheath and contact force monitoring in the setting of the superior vena cava approach
As described in our previous report, the deflectable ablation sheath was quite useful when using the SVC approach because manoeuvring of the ablation catheter is frequently needed with the tight curve at the fossa ovalis.8 In particular, the tight curve of the deflectable sheath and ablation catheter was very useful to map and ablate the right and left inferior PV antrum (Figure 3E and F), maintaining a stable position of the tip of the ablation catheter.
In addition, a deflectable ablation sheath supports the contact force of the tip of the ablation catheter as demonstrated by a real-time contact force monitoring system and visualization of the FTI. Neuzil et al.12 reported that a contact force of 10–20 g is needed to create transmural lesions of the left atrium in PVI, and an FTI of >400 gs is associated with no conduction gap between the PV and left atrium.12 In the present case report, we have shown that the SVC approach for PV isolation to cure AF using a deflectable sheath supports a sufficient contact force and FTI around all segments of the bilateral PV antrum except the limited area at the anterosuperior segment of the right PV ostium.
Conclusions
In summary, to the best of our knowledge, this is the first English-language case report of the following: (i) successful transseptal puncture in a patient with polysplenia syndrome and complete interruption of the IVC using the superior route through the internal jugular vein without any complications and (ii) PV isolation from the superior approach using a deflectable guiding sheath and contact force-sensing catheter and demonstration that real-time monitoring of the contact force and FTI of the ablation catheter is useful in this setting. The lack of recurrence of AF in the long term should be confirmed with careful follow-up.
Conflict of interest: none declared.
References
- left atrium
- cardiac ablation
- lung
- superior vena cava
- drug administration routes
- femoral vein
- needles
- pulmonary veins
- inferior vena cava
- catheters
- bilateral left-sidedness sequence
- radiofrequency ablation
- inferior vena cava interruption
- internal jugular vein
- pancreas tail
- kidney, left
- ablation
- pulmonary vein ablation
- intracardiac echocardiography
- right internal jugular vein
- persistent atrial fibrillation



