-
PDF
- Split View
-
Views
-
Cite
Cite
Luc Jordaens, Treatment of atrial fibrillation by catheter-based procedures, EP Europace, Volume 5, Issue s1, 2003, Pages S30–S35, https://doi.org/10.1016/j.eupc.2004.07.005
Close - Share Icon Share
Abstract
Catheter-based procedures have been developed with a view to reproduce or improve upon the excellent results of the Maze procedure in the treatment of atrial fibrillation (AF). Linear epicardial lesions created using minimally invasive techniques, or endocardial lesions to encircle the pulmonary veins (PV) have been associated with restoration of sinus rhythm in high percentages of carefully selected patients. The tricuspid-caval isthmus interruption procedure for atrial flutter is highly successful and, in patients who have both atrial flutter and fibrillation, prevents the development of AF when combined with antiarrhythmic agents. Modification of atrioventricular (AV) nodal conduction by eliminating the posterior atrial inputs to the AV node is performed to decrease the ventricular rate and alleviate symptoms during AF without the need for permanent pacing, though may be complicated by inadvertent AV block. AV junctional ablation and permanent pacing alleviates cardiac symptoms, improves quality-of-life, and reduces the use of health care resources. Its constraints include the inescapable need for anticoagulation, loss of AV synchrony, and life-long pacemaker-dependency. The variety of methods and results among published studies strongly emphasises the importance of patient selection, and the relative importance of substrate versus trigger. Possible complications of catheter ablation for AF include systemic thromboembolism, PV stenosis, pericardial effusion, cardiac tamponade, and phrenic nerve paralysis. These remain a matter of concern and stimulate research toward the development of less complex procedures.
Atrial fibrillation (AF) is the sustained arrhythmia most frequently encountered in clinical practice [1]. The complex arrangement of atrial myocardial cells and fibres, the relation between atria and systemic and pulmonary veins (PV) [2], and the consequences of ageing, including dilation and fibrosis, explain the ubiquity of AF, which can be considered a disease in itself. The acute manifestations of AF differ from those of its chronic forms, which are now generally classified as paroxysmal, when intermittent and self-terminating, persistent, when requiring an intervention for termination, and permanent, when therapeutic attempts to restore sinus rhythm have been abandoned [3]. This classification combined with clinical information based on echocardiographic measurements, is often the only determinant of eligibility for catheter ablation or other non-pharmacological treatments. It is noteworthy, however, that differences in underlying pathophysiology and disease entity, stage or severity may determine different outcomes after therapeutic attempts with drugs, ablation or devices.
Non-pharmacological interventions in perspective
Antiarrhythmic drugs are often ineffective in the management of AF, and may be the source of severe adverse effects, including, in patients with congestive heart failure, a higher mortality [4]. Several alternate, non-pharmacological therapies are in various stages of development, and the first attempts to emulate non-invasively the successful surgical Maze procedure can be scrutinised, though evidence-based data are still missing. The latter remain limited by non-uniform study endpoints, a consequence of a disease which primarily affects quality-of-life, and causes cardiac dysfunction only after prolonged periods of time. In addition, the association of lone AF with stroke is weak, such that large patient populations would be needed to examine the effects of invasive interventions on this complication.
Modifications of substrate and triggers
On the basis of mapping studies in animals and humans, Cox developed the Maze operation, which eliminates AF in >90% of selected patients [5]. The mechanism by which this procedure prevents recurrences of AF was not completely clarified initially, though the barriers to conduction created within the right and left atria limit the amount of myocardium available for the propagation of reentrant wavefronts, thus inhibiting sustained AF [6]. Since the Maze operation isolates the PVs, the subsequent recognition of their role as a source of ectopic activity initiating paroxysms of AF was an important contribution to understanding the mechanisms behind the success of the procedure [7]. The effectiveness of the operation is certainly attributable to a confinement of the triggers of AF, as well as to a block of impulse propagation within the atrial substrate and a modification of the substrate itself.
Non-surgical treatment alternatives
Catheter-based procedures have been developed with a view to reproduce or improve upon these excellent surgical results [8]. Linear epicardial lesions, aimed at isolating the PVs and created with minimally invasive techniques, have been associated with restoration of sinus rhythm in >90% of patients, up to 1 year [9]. These epicardial techniques are particularly promising since they may have a more direct effect on cardiac innervation [10].
Catheter ablation of atrial fibrillation
Focal ablation versus segmental pulmonary vein isolation
The treatment of AF entered a new era with the publication of the landmark observations by Haïssaguerre et al. [7]. The recognition of the role played in the initiation of AF by myocardial extensions within the PV provided a new pathophysiological perspective, changed the therapeutic approach, and explained why surgery was effective. For the first time, one could expect a catheter-based cure of AF, since the presence of ectopic activity was a reliable target to guide ablation. However, this ectopic activity in the PVs is present in no more than one third of patients [11]. Thus, segmental ostial catheter ablation guided by PV potentials may be a better strategy (Fig. 1) than a search for isolated ectopic activity [12]. Even after immediately successful procedures, a high percentage of patients experiences recurrences, and achievable results and endpoints of catheter-based AF ablation have now be redefined, focussing on quality-of-life and reduction of the AF burden. The empirical encircling of the pulmonary venous ostia is a simplified and apparently more effective approach, which, when assisted by detailed mapping (Fig. 2), can be performed expeditiously by experienced operators [13].
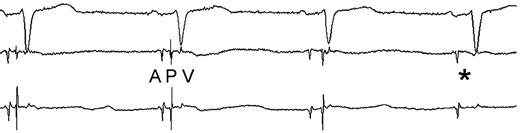
Recording of atrial (A), pulmonary vein (P), and ventricular (V) potentials during catheter ablation. Note the sudden change in the morphology (3rd complex), followed by disappearance (*) of the pulmonary vein potential.
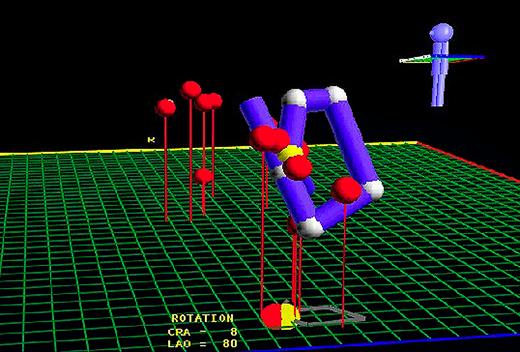
Use of the LocaLisa™ (Medtronic Inc.) mapping system to isolate the venous ostia. Left lateral view showing where ablation lesions were created (red dots) at both superior pulmonary veins, and reconstruction of the position of a circular mapping catheter at the ostium of the left superior PV (blue line).
The original focal procedure was associated with up to 8% incidence of PV stenosis, defined as a ≥50% decrease in luminal diameter, and 16% of patients had a decrease in luminal diameter between 25% and 50% [14]. Since PV stenosis can be life-threatening [15], modifications have been made to the procedure, including ablation encircling the ostia, use of different energy sources, such as cooled tip and cryothermy, and monitoring of radiofrequency heating by intracardiac ultrasound [16–,18].
Other foci
Other arrhythmogenic foci which may trigger AF have been found in the superior vena cava, the right and left atria, the coronary sinus, and the ligament of Marshall [19]. Atrioventricular (AV) nodal reentry and accessory pathway-mediated tachycardias represent other triggers. Isolation of the superior vena cava is a simple procedure and has become part of a systematic strategy in some centres.
Linear ablation
With the anatomic and electrical remodelling associated with prolonged AF, the underlying substrate may play a more prominent role. Attempts to emulate the successful Maze operation with left-sided linear ablation have raised concerns regarding the safety and efficacy of the procedure [20]. Ablation confined to the right atrium is safer, though of limited efficacy [21,,22]. Its highest reported success rate was in selected patients whose AF tended to organise towards a flutter-like appearance [23]. A difficulty inherent in the creation of linear lesions is the need for uninterrupted contact of the catheter with the endocardium, which may be helped by the use of linear catheters (Fig. 3). Intracardiac echography may be of assistance in these procedures. Because of the limited efficacy of focal ablation, linear lesions are now often combined with segmental or focal isolation [20]. A left atrial isthmus line, from the left lower PV to the mitral valve annulus, is also included in some procedural protocols.
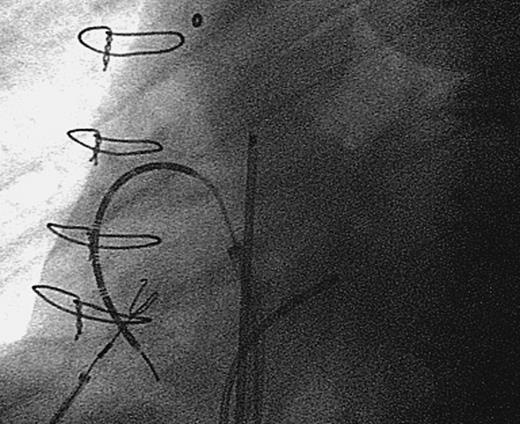
Radiographic appearance of linear radiofrequency catheter (Cardima Inc.) used to create a linear lesion between the inferior and superior venae cavae.
Ablation of atrial flutter
Atrial flutter and AF are closely related arrhythmias, often coexistent in the same patient and evolving from one to the other. Flutter may develop as a consequence of antiarrhythmic drug therapy administered for AF, especially class IC agents or amiodarone. The tricuspid-caval isthmus interruption procedure improves symptoms, and very effectively prevents AF when combined with these antiarrhythmic agents [24]. Its role as a systematic adjunctive intervention remains to be established [25,,26].
AV junctional modification and ablation
Modification of AV junctional conduction by eliminating the posterior atrial inputs to the AV node is a technically challenging procedure performed to decrease the ventricular rate and alleviate symptoms during AF without the need for permanent pacing, though it may be complicated by inadvertent complete AV block [27]. This procedure is especially salutary in patients with high ventricular rates and tachycardia-induced decline in ventricular systolic function despite medical therapy [28].
In a meta-analysis of 1181 patients with highly symptomatic AF, AV junctional ablation and permanent pacemaker implantation significantly alleviated cardiac symptoms, improved quality-of-life, and reduced the consumption of health care resources [29]. Complications of AV junctional ablation include those associated with pacemaker implantation, a higher rate of progression from paroxysmal to persistent AF, and ventricular arrhythmias. Worsening of LV function is not uncommon [30]. The 1-year mortality rate after AV junctional ablation and permanent pacemaker implantation is approximately 6%, and the risk of sudden death approximately 2%. Although the relation between sudden death and this procedure remains controversial, it has been suggested that the risk may be reduced by programming the back up pacing rate between 80 and 90 bpm for the first month after ablation [31,,32].
The constraints of AV junctional ablation include the inescapable need for anticoagulation, loss of AV synchrony, and life-long pacemaker-dependency. Patients with abnormal diastolic ventricular function, who are most dependent on AV synchrony for preservation of cardiac output, may have persistent symptoms after AV junctional ablation. These considerations should be taken into account before proceeding with this irreversible treatment.
Importance of patient selection
The variety of methods and results among published studies strongly emphasises the importance of patient selection. In any given patient, the choice among different procedures depends on the relative importance of substrate versus trigger. Clinical characteristics examined before catheterisation, such as age, arrhythmia pattern, left atrial size, and underlying anatomy, play decisive roles with respect to whether or not to proceed with ablation.
Procedural complications
Possible complications of catheter ablation for AF include systemic thromboembolism, PV stenosis, pericardial effusion, cardiac tamponade, and phrenic nerve paralysis [15,,33,,34]. These remain a matter of concern and stimulate research toward the development of less complex procedures. In the meantime, the decision to proceed with AF ablation needs to be weighed carefully.
Summary
There is growing evidence that a wide, circumferential isolation of the ostia of the pulmonary veins is a more effective catheter-based technique for ablation of atrial fibrillation than a focal approach. This does not prevent ablation of documented foci (e.g. superior vena cava, left atrial free wall…). Additional arrhythmias (flutter, but also AV Needal re-entry tachycardia and accessory pathways), potentially triggering atrial fibrillation should be modified whenever possible. Interpretation of success rates depends on the endpoint used.
References
- anti-arrhythmia agents
- anticoagulation
- artificial cardiac pacemaker
- atrial fibrillation
- atrioventricular block
- pericardial effusion
- atrial flutter
- cardiac tamponade
- atrioventricular node
- thromboembolism
- endocardium
- atrium
- precipitating factors
- pulmonary veins
- heart
- quality of life
- sinus rhythm
- catheters
- maze procedure
- catheter complications
- ablation
- pulmonary vein stenosis
- phrenic nerve paralysis
- ventricular heart rate



