-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew Headrick, Zhining Ou, S Yukiko Asaki, Susan P Etheridge, Benjamin Hammond, Lindsey Gakenheimer-Smith, Thomas Pilcher, Mary Niu, Intracardiac echocardiography in paediatric and congenital cardiac ablation shortens procedure duration and improves success without complications, EP Europace, Volume 26, Issue 2, February 2024, euae047, https://doi.org/10.1093/europace/euae047
Close - Share Icon Share
Abstract
Common to adult electrophysiology studies (EPSs), intracardiac echocardiography (ICE) use in paediatric and congenital heart disease (CHD) EPS is limited. The purpose of this study was to assess the efficacy of ICE use and incidence of associated complications in paediatric and CHD EPS.
This single-centre retrospective matched cohort study reviewed EPS between 2013 and 2022. Demographics, CHD type, and EPS data were collected. Intracardiac echocardiography cases were matched 1:1 to no ICE controls to assess differences in complications, ablation success, fluoroscopy exposure, procedure duration, and arrhythmia recurrence. Cases and controls with preceding EPS within 5 years were excluded. Intracardiac echocardiography cases without an appropriate match were excluded from comparative analyses but included in the descriptive cohort. We performed univariable and multivariable logistic regression to assess associations between variables and outcomes. A total of 335 EPS were reviewed, with ICE used in 196. The median age of ICE cases was 15 [interquartile range (IQR) 12–17; range 3–47] years, and median weight 57 [IQR 45–71; range 15–134] kg. There were no ICE-related acute or post-procedural complications. There were 139 ICE cases matched to no ICE controls. Baseline demographics and anthropometrics were similar between cases and controls. Fluoroscopy exposure (P = 0.02), procedure duration (P = 0.01), and arrhythmia recurrence (P = 0.01) were significantly lower in ICE cases.
Intracardiac echocardiography in paediatric and CHD ablations is safe and reduces procedure duration, fluoroscopy exposure, and arrhythmia recurrence. However, not every arrhythmia substrate requires ICE use. Thoughtful selection will ensure the judicious and strategic application of ICE to enhance outcomes.
In this manuscript, we detail the largest descriptive cohort to date of intracardiac echocardiography (ICE) use in paediatric and congenital electrophysiology study and ablation. This cohort includes patients spanning a diverse range of sizes and congenital heart disease. We assess for ablation outcomes and intraprocedural and post-procedural complications attributable to ICE use. We then conducted a case–control series comparing ICE cases to matched controls to evaluate potential procedural outcome benefits associated with ICE use.
In this revised manuscript submission, we have made updates in accordance with reviewers’ comments, as detailed in the Response to Reviewers Letter.
Introduction
First described in adult electrophysiology (EP) in the mid-1990s for transseptal puncture, intracardiac echocardiography (ICE) is now commonly used in adult electrophysiology studies (EPSs). Its applications in ablation cases include, but are not limited to, guiding catheter placement, facilitating transseptal and trans-baffle access, and optimizing the ablation catheter-tissue interface. Advantages cited in adult EP literature include ablation success, complication prevention, and shorter fluoroscopy and procedural duration. Collectively, these factors are thought to result in decreased resource utilization.1–4
Though widely used in adult EPS, ICE utilization in paediatric and congenital EPS has been slower5,6 and may be limited by concerns for complications such as valvar and vascular injuries. As such, it is unknown whether ICE use in paediatric and congenital EPS can improve acute success rates, prevent complications, reduce procedural duration, fluoroscopy exposure, and decrease arrhythmia recurrence. The overall procedural advantages and risk for complications in this population remain undefined.
The purpose of this study is to describe our centre’s experience using ICE in paediatric and congenital ablations. We hypothesized that ICE would reduce fluoroscopy and procedural duration, improve acute ablation success rates, and decrease arrhythmia recurrence rates without increasing acute or post-procedural complication rates.
Methods
Patient population and study design
We performed a retrospective review of all EPS at Primary Children’s Hospital from 1 February 2013–1 February 2023. The study protocol was approved under a waiver of consent by the institutional review boards at Primary Children’s Hospital and the University of Utah.
Patients who met the following criteria were included in the descriptive ICE cohort: (1) ICE was used during the EPS, (2) age ≤ 18 years with or without congenital heart disease (CHD) at the time of EPS, and (3) age > 18 years with CHD. Patients ≥ 18 years without CHD were excluded.
For the matched cohort, ICE cases were matched 1:1 to no ICE controls by EPS operator, arrhythmia mechanism(s), arrhythmia sidedness, presence of pre-excitation, presence of CHD, and CHD categorization as follows: biventricular CHD, Ebstein anomaly, Fontan circulation, or intracardiac baffle. Matching by body surface area (BSA) ± 0.15 m2 was additionally performed in paediatric patients with BSA < 1.5 m2. Patients were excluded for any of the following: age > 18 years without CHD, history of ablation within the preceding 5 years, genetic diagnosis associated with arrhythmias, no inducible arrhythmia during EPS, or if no ablation was performed. Intracardiac echocardiography cases without an appropriate control match were excluded from comparative analyses but included in the descriptive ICE cohort described above.
Electrophysiology study and intracardiac echocardiography
All studies were performed using either the 9 F ViewFlex™ Xtra ICE Catheter system (Abbott Laboratories, Abbott Park, IL, USA) or the 8 F Biosense Webster ICE catheter (Diamond Bar, CA, USA).7 Femoral venous access for ICE was obtained utilizing an 8, 9, or 10 French introducer. All patients received general anaesthesia. Intravenous heparin (50–100 IU/kg) was administered in all cases involving transseptal or trans-baffle puncture, HD grid mapping catheter use, presence of intracardiac shunt(s), or ablations via arterial approach. Ensite Precision™ Cardiac Mapping System or EnSite™ X EP System (Abbott, St. Paul, MN) was used in all cases for three-dimensional electroanatomic mapping.
Outcomes
Intracardiac echocardiography cases were compared to no ICE controls to measure associations between ICE use and outcomes. Outcome measures included: acute ablation success, complications, arrhythmia recurrence, fluoroscopy exposure, and procedure duration. Ablation was considered acutely successful if the targeted arrhythmia substrate was eliminated and non-inducible during the 30–60 min observation period after final energy application.8 Procedural complications included: any mortality or significant morbidity including vascular and/or valvar injury, heart block, pericardial effusion, and coronary artery injury. Post-procedural electrocardiogram (ECG) was used to assess for coronary artery injury. A composite complication outcome variable was defined to comprise any of the aforementioned complications.
All patients were evaluated at 6–8 weeks after the ablation procedure, at 12 months, and thereafter as clinically indicated. Recurrence of pre-excitation was screened for with repeat ECG at clinic follow-up. Patients with a history of pre-excitation were routinely evaluated annually for at least 2 years to surveil for recurrent pre-excitation and/or symptoms suggestive of supraventricular tachycardia. Patients with CHD were followed more frequently, as indicated by their underlying lesion. Arrhythmia recurrence was evaluated only in patients with acute ablation success. Recurrences were defined as documented arrhythmias with electrophysiologic features suggestive of the previously ablated substrate or reinduction of the previously ablated substrate at the time of a follow-up EPS.
Statistical methods
Categorical variables are presented as counts with percentage in parenthesis. Continuous values are reported as median with interquartile ranges (IQRs). The overall ICE cohort was split into CHD and no CHD sub-cohorts. Comparisons of categorical outcomes were made using Pearson’s χ2 or Fisher’s exact test and continuous variables with Wilcoxon’s rank-sum test. We repeated the above approach in comparisons between matched ICE cases and no ICE controls. To determine the association between variables and outcomes, univariable logistic regression was performed on both the overall ICE cohort and the matched cohort. Variables in the univariable model with a P-value of <0.1 were included in a multivariable analysis along with selected variables from clinical assessments. The number of events limited the adjusted number of variables in multivariable analysis. Similarly, for duration outcomes, we performed gamma regression with log link function due to distribution skew. We reported odds ratio (OR) and exponential function of the Gamma regression coefficients eβ and their corresponding 95% confidence interval (CI) for the above types of regression models respectively. P-values of <0.05 were considered statistically significant. Statistical analyses were implemented using R v. 4.1.2 (R Core Team, 2022).
Results
Intracardiac echocardiography cohort
Patients and arrhythmias
Over the 10-year study period, 1262 EPS were performed. Regular ICE use began in February 2020; from introduction through the end of the study period, ICE was used in 196 of 472 EPS (Table 1). Of these 196 patients, three had an EPS only with no inducible arrhythmia and did not undergo ablation. Intracardiac echocardiography use varied by provider (Figure 1). The ICE cohort was 61.0% male and predominantly white (87%). The median age was 15 [IQR 12–17; range 3–47] years, and the median weight 57 [IQR 45–71; range 15–134] kg (Figure 2A–C). Of these, 59 (30.1%) patients weighed <50 kg. A total of 100 (51.0%) ICE cases were completed with <1 min of, or entirely without, fluoroscopy.
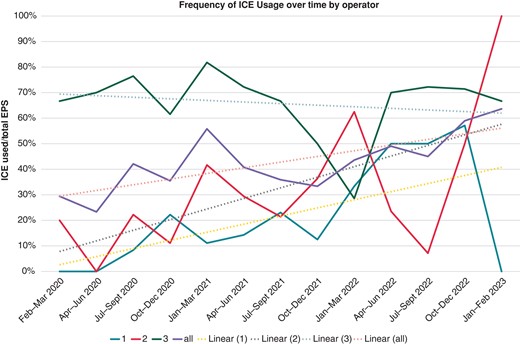
Intracardiac echocardiography usage update frequency by quarter, stratified by operator. EPS, electrophysiology study; ICE, intracardiac echocardiogram.
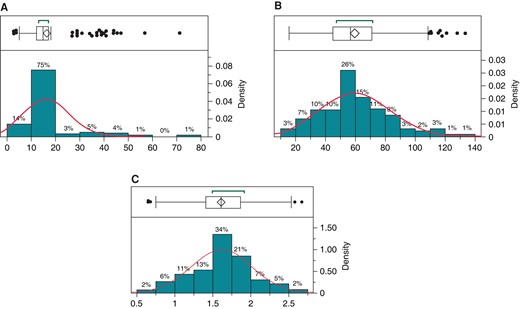
(A–C) Normal distribution and outlier plots of all ICE cases by A: age, B: weight, and C: BSA. BSA, body surface area; ICE, intracardiac echocardiogram.
| Variable . | All ICE . | CHD . | No CHD . | P-value . |
|---|---|---|---|---|
| (n = 196) . | (n = 31) . | (n = 165) . | ||
| Age (years) | 15 [12–17] | 29 [15–39] | 15 [11.5–16.5] | <0.01 |
| Female | 76 (39.0) | 10 (33.0) | 66 (40.0) | 0.42 |
| Non-white | 26 (13.3) | 4 (12.9) | 22 (13.3) | 1.0 |
| Weight (kg) | 56.8 [45.4–71.3] | 57.5 [48.8–90.9] | 56.7 [45.4–70.8] | 0.26 |
| BSA (m2) | 1.6 [1.4–1.9] | 1.6 [1.4–2.1] | 1.6 [1.4–1.8] | 0.39 |
| Pre-excitation | 62 (31.6) | 4 (12.9) | 58 (35.2) | 0.02 |
| Arrhythmia mechanisma | <0.01 | |||
| AVRT | 110 (56.0) | 4 (13.0) | 106 (64.0) | |
| AVNRT | 45 (23.0) | 2 (6.5) | 43 (26.0) | |
| IART | 26 (13.0) | 23 (74.0) | 3 (1.8) | |
| EAT | 11 (5.6) | 2 (6.5) | 9 (5.5) | |
| PVCs/VT | 9 (4.6) | 0 (0) | 9 (5.5) | |
| Ablation site | <0.01 | |||
| Right | 83 (42.4) | 11 (35.5) | 72 (43.6) | |
| Left | 88 (44.9) | 3 (9.7) | 85 (51.5) | |
| >1 ablation site | 13 (6.6) | 8 (25.8) | 5 (3.0) | |
| Trans-baffleb | 9 (4.6) | 9 (29.0) | 0 (0) | |
| No ablation | 3 (1.5) | 0 (0) | 3 (1.8) | |
| Complicationsc | 2b (1.0) | 1 (3.2) | 1 (0.6) | 0.29 |
| Fluoroscopy exposure (min) | 0.9 [0.1–3.8] | 3.8 [0.2, 9.9] | 0.7 [0.1–3.2] | <0.01 |
| Procedural time (min) | 147 [123–196] | 196 [151–255] | 144 [119.5–187] | <0.01 |
| Arrhythmia recurrence | 16 (8.2) | 6 (20.0) | 10 (6.1) | 0.01 |
| Variable . | All ICE . | CHD . | No CHD . | P-value . |
|---|---|---|---|---|
| (n = 196) . | (n = 31) . | (n = 165) . | ||
| Age (years) | 15 [12–17] | 29 [15–39] | 15 [11.5–16.5] | <0.01 |
| Female | 76 (39.0) | 10 (33.0) | 66 (40.0) | 0.42 |
| Non-white | 26 (13.3) | 4 (12.9) | 22 (13.3) | 1.0 |
| Weight (kg) | 56.8 [45.4–71.3] | 57.5 [48.8–90.9] | 56.7 [45.4–70.8] | 0.26 |
| BSA (m2) | 1.6 [1.4–1.9] | 1.6 [1.4–2.1] | 1.6 [1.4–1.8] | 0.39 |
| Pre-excitation | 62 (31.6) | 4 (12.9) | 58 (35.2) | 0.02 |
| Arrhythmia mechanisma | <0.01 | |||
| AVRT | 110 (56.0) | 4 (13.0) | 106 (64.0) | |
| AVNRT | 45 (23.0) | 2 (6.5) | 43 (26.0) | |
| IART | 26 (13.0) | 23 (74.0) | 3 (1.8) | |
| EAT | 11 (5.6) | 2 (6.5) | 9 (5.5) | |
| PVCs/VT | 9 (4.6) | 0 (0) | 9 (5.5) | |
| Ablation site | <0.01 | |||
| Right | 83 (42.4) | 11 (35.5) | 72 (43.6) | |
| Left | 88 (44.9) | 3 (9.7) | 85 (51.5) | |
| >1 ablation site | 13 (6.6) | 8 (25.8) | 5 (3.0) | |
| Trans-baffleb | 9 (4.6) | 9 (29.0) | 0 (0) | |
| No ablation | 3 (1.5) | 0 (0) | 3 (1.8) | |
| Complicationsc | 2b (1.0) | 1 (3.2) | 1 (0.6) | 0.29 |
| Fluoroscopy exposure (min) | 0.9 [0.1–3.8] | 3.8 [0.2, 9.9] | 0.7 [0.1–3.2] | <0.01 |
| Procedural time (min) | 147 [123–196] | 196 [151–255] | 144 [119.5–187] | <0.01 |
| Arrhythmia recurrence | 16 (8.2) | 6 (20.0) | 10 (6.1) | 0.01 |
Values are presented as median [interquartile range] or n (%).
AVNRT, atrioventricular nodal re-entrant tachycardia; AVRT, atrioventricular re-entrant tachycardia; BSA, body surface area; CHD, congenital heart disease; EAT, ectopic atrial tachycardia; IART, intra-atrial re-entrant tachycardia; ICE, intracardiac echocardiography; PVCs/VT, premature ventricular complexes/ventricular tachycardia.
a≥1 arrhythmia mechanisms were present in five cases.
bTrans-baffle approach included Fontan and intracardiac baffles in biventricular palliations.
cTransseptal needle entry into pericardial space during first transseptal attempt (without ICE). One case with atrial flutter that degenerated into ventricular fibrillation arrest, requiring compressions; not related to ICE usage.
| Variable . | All ICE . | CHD . | No CHD . | P-value . |
|---|---|---|---|---|
| (n = 196) . | (n = 31) . | (n = 165) . | ||
| Age (years) | 15 [12–17] | 29 [15–39] | 15 [11.5–16.5] | <0.01 |
| Female | 76 (39.0) | 10 (33.0) | 66 (40.0) | 0.42 |
| Non-white | 26 (13.3) | 4 (12.9) | 22 (13.3) | 1.0 |
| Weight (kg) | 56.8 [45.4–71.3] | 57.5 [48.8–90.9] | 56.7 [45.4–70.8] | 0.26 |
| BSA (m2) | 1.6 [1.4–1.9] | 1.6 [1.4–2.1] | 1.6 [1.4–1.8] | 0.39 |
| Pre-excitation | 62 (31.6) | 4 (12.9) | 58 (35.2) | 0.02 |
| Arrhythmia mechanisma | <0.01 | |||
| AVRT | 110 (56.0) | 4 (13.0) | 106 (64.0) | |
| AVNRT | 45 (23.0) | 2 (6.5) | 43 (26.0) | |
| IART | 26 (13.0) | 23 (74.0) | 3 (1.8) | |
| EAT | 11 (5.6) | 2 (6.5) | 9 (5.5) | |
| PVCs/VT | 9 (4.6) | 0 (0) | 9 (5.5) | |
| Ablation site | <0.01 | |||
| Right | 83 (42.4) | 11 (35.5) | 72 (43.6) | |
| Left | 88 (44.9) | 3 (9.7) | 85 (51.5) | |
| >1 ablation site | 13 (6.6) | 8 (25.8) | 5 (3.0) | |
| Trans-baffleb | 9 (4.6) | 9 (29.0) | 0 (0) | |
| No ablation | 3 (1.5) | 0 (0) | 3 (1.8) | |
| Complicationsc | 2b (1.0) | 1 (3.2) | 1 (0.6) | 0.29 |
| Fluoroscopy exposure (min) | 0.9 [0.1–3.8] | 3.8 [0.2, 9.9] | 0.7 [0.1–3.2] | <0.01 |
| Procedural time (min) | 147 [123–196] | 196 [151–255] | 144 [119.5–187] | <0.01 |
| Arrhythmia recurrence | 16 (8.2) | 6 (20.0) | 10 (6.1) | 0.01 |
| Variable . | All ICE . | CHD . | No CHD . | P-value . |
|---|---|---|---|---|
| (n = 196) . | (n = 31) . | (n = 165) . | ||
| Age (years) | 15 [12–17] | 29 [15–39] | 15 [11.5–16.5] | <0.01 |
| Female | 76 (39.0) | 10 (33.0) | 66 (40.0) | 0.42 |
| Non-white | 26 (13.3) | 4 (12.9) | 22 (13.3) | 1.0 |
| Weight (kg) | 56.8 [45.4–71.3] | 57.5 [48.8–90.9] | 56.7 [45.4–70.8] | 0.26 |
| BSA (m2) | 1.6 [1.4–1.9] | 1.6 [1.4–2.1] | 1.6 [1.4–1.8] | 0.39 |
| Pre-excitation | 62 (31.6) | 4 (12.9) | 58 (35.2) | 0.02 |
| Arrhythmia mechanisma | <0.01 | |||
| AVRT | 110 (56.0) | 4 (13.0) | 106 (64.0) | |
| AVNRT | 45 (23.0) | 2 (6.5) | 43 (26.0) | |
| IART | 26 (13.0) | 23 (74.0) | 3 (1.8) | |
| EAT | 11 (5.6) | 2 (6.5) | 9 (5.5) | |
| PVCs/VT | 9 (4.6) | 0 (0) | 9 (5.5) | |
| Ablation site | <0.01 | |||
| Right | 83 (42.4) | 11 (35.5) | 72 (43.6) | |
| Left | 88 (44.9) | 3 (9.7) | 85 (51.5) | |
| >1 ablation site | 13 (6.6) | 8 (25.8) | 5 (3.0) | |
| Trans-baffleb | 9 (4.6) | 9 (29.0) | 0 (0) | |
| No ablation | 3 (1.5) | 0 (0) | 3 (1.8) | |
| Complicationsc | 2b (1.0) | 1 (3.2) | 1 (0.6) | 0.29 |
| Fluoroscopy exposure (min) | 0.9 [0.1–3.8] | 3.8 [0.2, 9.9] | 0.7 [0.1–3.2] | <0.01 |
| Procedural time (min) | 147 [123–196] | 196 [151–255] | 144 [119.5–187] | <0.01 |
| Arrhythmia recurrence | 16 (8.2) | 6 (20.0) | 10 (6.1) | 0.01 |
Values are presented as median [interquartile range] or n (%).
AVNRT, atrioventricular nodal re-entrant tachycardia; AVRT, atrioventricular re-entrant tachycardia; BSA, body surface area; CHD, congenital heart disease; EAT, ectopic atrial tachycardia; IART, intra-atrial re-entrant tachycardia; ICE, intracardiac echocardiography; PVCs/VT, premature ventricular complexes/ventricular tachycardia.
a≥1 arrhythmia mechanisms were present in five cases.
bTrans-baffle approach included Fontan and intracardiac baffles in biventricular palliations.
cTransseptal needle entry into pericardial space during first transseptal attempt (without ICE). One case with atrial flutter that degenerated into ventricular fibrillation arrest, requiring compressions; not related to ICE usage.
Congenital heart disease and arrhythmia types are displayed in Table 1. Congenital heart disease was present in 15.8% of patients, comprising: repaired biventricular CHD (n = 13, 41.9%), Fontan circulation (n = 8, 25.8%), intracardiac baffle (n = 6, 19.4%), and Ebstein anomaly (n = 4, 12.9%). The most common arrhythmia was atrioventricular re-entrant tachycardia (AVRT), followed by atrioventricular nodal re-entry tachycardia (AVNRT), intra-atrial re-entrant tachycardia (IART), ectopic atrial tachycardia, and premature ventricular complexes/ventricular tachycardia.
Arrhythmia mechanisms differed significantly by CHD presence (P < 0.01, Table 1). In CHD patients, IART was the most common arrhythmia, occurring in 74.0% of cases. In patients with structurally normal hearts, AVRT (64.0%) followed by AVNRT (26.0%) was the most common arrhythmias. Patients without CHD were significantly more likely to have pre-excitation (P = 0.02).
Fluoroscopy and procedure duration
Congenital heart disease patients experienced significantly longer median procedure duration and fluoroscopy exposure compared to those without CHD (Table 1). On univariable analysis, CHD presence was associated with both fluoroscopy exposure and procedure duration (P = 0.03 and <0.01, respectively); male sex and age also resulted in extended fluoroscopy exposure. After adjusting for covariates in the multivariable model, CHD (OR 3.90; 95% CI 2.30–6.61; P < 0.01) and male sex (OR 1.70; 95% CI 1.15–2.53; P = 0.01) were the only variables associated with increased fluoroscopy exposure (see Supplementary material online, Table S1A). Similarly, only CHD was associated with increased procedure duration (OR 1.25; 95% CI 1.02–1.53; P = 0.04) in the multivariable models (see Supplementary material online, Table S1A and B).
Acute success and recurrence
Arrhythmias were successfully ablated in all 193 ICE cases who underwent ablation. There was an 8.2% recurrence during follow-up. Results of univariable logistic regression on 1-year arrhythmia recurrence are displayed in Table 2. Clinical variables associated with arrhythmia recurrence included: older age, higher weight, presence of CHD, and >1 ablation target. After adjusting for covariates, >1 ablation target was the only factor that neared significance (OR 4.51; 95% CI 0.99–18.96; P = 0.05).
Descriptive ICE cohort univariable and multivariable analyses: clinical factors associated with arrhythmia recurrence
| . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Variable . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Age (years) | 1.07 (1.01, 1.12) | 0.01 | — | — |
| White | 2.42 (0.46, 44.7) | 0.40 | — | — |
| Weight | 1.02 (1.00, 1.05) | 0.02 | — | — |
| BSA (m2) | 3.44 (0.92, 13.91) | 0.07 | 2.93 (0.80, 11.49) | 0.11 |
| CHD | 3.72 (1.18, 10.96) | 0.02 | 2.19 (0.59, 7.15) | 0.23 |
| >1 ablation site | 6.33 (1.54, 22.80) | <0.01 | 4.51 (0.99, 18.96) | 0.05 |
| . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Variable . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Age (years) | 1.07 (1.01, 1.12) | 0.01 | — | — |
| White | 2.42 (0.46, 44.7) | 0.40 | — | — |
| Weight | 1.02 (1.00, 1.05) | 0.02 | — | — |
| BSA (m2) | 3.44 (0.92, 13.91) | 0.07 | 2.93 (0.80, 11.49) | 0.11 |
| CHD | 3.72 (1.18, 10.96) | 0.02 | 2.19 (0.59, 7.15) | 0.23 |
| >1 ablation site | 6.33 (1.54, 22.80) | <0.01 | 4.51 (0.99, 18.96) | 0.05 |
BSA, body surface area; CHD, congenital heart disease; ICE, intracardiac echocardiogram; OR, odds ratio.
Descriptive ICE cohort univariable and multivariable analyses: clinical factors associated with arrhythmia recurrence
| . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Variable . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Age (years) | 1.07 (1.01, 1.12) | 0.01 | — | — |
| White | 2.42 (0.46, 44.7) | 0.40 | — | — |
| Weight | 1.02 (1.00, 1.05) | 0.02 | — | — |
| BSA (m2) | 3.44 (0.92, 13.91) | 0.07 | 2.93 (0.80, 11.49) | 0.11 |
| CHD | 3.72 (1.18, 10.96) | 0.02 | 2.19 (0.59, 7.15) | 0.23 |
| >1 ablation site | 6.33 (1.54, 22.80) | <0.01 | 4.51 (0.99, 18.96) | 0.05 |
| . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Variable . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Age (years) | 1.07 (1.01, 1.12) | 0.01 | — | — |
| White | 2.42 (0.46, 44.7) | 0.40 | — | — |
| Weight | 1.02 (1.00, 1.05) | 0.02 | — | — |
| BSA (m2) | 3.44 (0.92, 13.91) | 0.07 | 2.93 (0.80, 11.49) | 0.11 |
| CHD | 3.72 (1.18, 10.96) | 0.02 | 2.19 (0.59, 7.15) | 0.23 |
| >1 ablation site | 6.33 (1.54, 22.80) | <0.01 | 4.51 (0.99, 18.96) | 0.05 |
BSA, body surface area; CHD, congenital heart disease; ICE, intracardiac echocardiogram; OR, odds ratio.
Complications
There were two complications in the ICE cohort, both occurring intraprocedurally, and neither attributable to ICE use. In the first case, during fluoroscopically guided transseptal puncture without ICE, the transseptal needle entered the pericardial space. Intracardiac echocardiography was subsequently used to evaluate for an effusion. With no effusion evident, ICE was then used to guide repeat transseptal access and confirm successful entry into the left atrium. The second complication arose in an adult patient with CHD who had IART and 1:1 atrioventricular conduction that degenerated into ventricular fibrillation. After brief compressions and defibrillation, sinus rhythm was restored, allowing the procedure to continue. The IART was successfully ablated, and the patient underwent ICD implantation the following day.
Matched cohort: intracardiac echocardiography cases vs. no intracardiac echocardiography controls
Patients and arrhythmias
There were 139 (72.0%) ICE cases who were matched 1:1 to no ICE controls (total n = 278 cases) by EPS operator, arrhythmia mechanism, arrhythmia sidedness, presence of pre-excitation, presence of CHD, and CHD categorization (Table 2). There were no significant differences in age, weight, BSA, gender, or race between cases and controls. Congenital heart disease, pre-excitation, and need for transseptal puncture were equally represented between cases and controls.
Fluoroscopy exposure and procedure duration
Intracardiac echocardiography cases had significantly shortened median fluoroscopy exposure and procedural duration (Table 3, Figure 3). Subanalysis by CHD presence was conducted to identify factors associated with procedure duration and fluoroscopy exposure in patients with and without CHD (Table 4A and B). In the univariable fitted model for CHD patients, trans-baffle puncture increased fluoroscopy exposure but not procedure duration. While not reaching significance, ICE use tended to decrease overall procedure duration in patients with CHD (192 vs. 221 min; P = 0.08; OR 0.82; 95% CI 0.67–1.01; P = 0.07). Of note, during IART ablations (which represented 94.7% of CHD cases), the median procedure duration was significantly shorter when ICE was used (196.0 [IQR 120.0–235.0] vs. 236.0 [IQR 185.0–292.0] min; P = 0.02).
| Variable . | Matched ICE cases . | Matched no ICE controls . | P-valuea . |
|---|---|---|---|
| (n = 139) . | (n = 139) . | ||
| Age (years) | 15 [12–17] | 15.1 [13.1–16.8] | 0.66 |
| Female | 53 (38.1) | 69 (49.6) | 0.05 |
| Non-white | 19 (13.7) | 13 (9.4) | 0.26 |
| Weight (kg) | 56.8 [48.2–71.3] | 57.0 [46.3–71.4] | 0.60 |
| BSA (m2) | 1.6 [1.4–1.8] | 1.6 [1.4–1.8] | 0.61 |
| CHD | 19 (13.7) | 19 (13.7) | — |
| Biventricular CHD Ebstein | 7 (5.0) | 7 (5.0) | |
| Anomaly | 2 (1.4) | 2 (1.4) | |
| Fontan circulation | 5 (3.6) | 5 (3.6) | |
| Intracardiac baffle | 5 (3.6) | 5 (3.6) | |
| Arrhythmia mechanismb | — | ||
| AVRT | 81 (58.3) | 81 (58.3) | |
| AVNRT | 34 (24.5) | 34 (24.5) | |
| IART | 19 (13.7) | 19 (13.7) | |
| EAT | 3 (2.2) | 3 (2.2) | |
| PVCs/VT | 3 (2.2) | 3 (2.2) | |
| Pre-excitation | 43 (30.1) | 43 (30.1) | — |
| Arrhythmia site | — | ||
| Right | 64 (46.0) | 64 (46.0) | |
| Left | 62 (44.6) | 62 (44.6) | |
| >1 ablation site | 5 (3.6) | 5 (3.6) | |
| Trans-bafflec | 8 (5.8) | 8 (5.8) | |
| Acute procedural success | 139 (100) | 135 (97.1) | 0.12 |
| Complications | 2d (1.4) | 0 (0) | 1.0 |
| Fluoroscopy exposure (min) | 0.3 [0.1–3.5] | 2.1 [0.2–4.1] | 0.02 |
| Procedural time (min) | 141 [118.5–182] | 173 [124–219.5] | 0.01 |
| Arrhythmia recurrencee | 9 (6.5) | 23 (16.5) | 0.01 |
| Variable . | Matched ICE cases . | Matched no ICE controls . | P-valuea . |
|---|---|---|---|
| (n = 139) . | (n = 139) . | ||
| Age (years) | 15 [12–17] | 15.1 [13.1–16.8] | 0.66 |
| Female | 53 (38.1) | 69 (49.6) | 0.05 |
| Non-white | 19 (13.7) | 13 (9.4) | 0.26 |
| Weight (kg) | 56.8 [48.2–71.3] | 57.0 [46.3–71.4] | 0.60 |
| BSA (m2) | 1.6 [1.4–1.8] | 1.6 [1.4–1.8] | 0.61 |
| CHD | 19 (13.7) | 19 (13.7) | — |
| Biventricular CHD Ebstein | 7 (5.0) | 7 (5.0) | |
| Anomaly | 2 (1.4) | 2 (1.4) | |
| Fontan circulation | 5 (3.6) | 5 (3.6) | |
| Intracardiac baffle | 5 (3.6) | 5 (3.6) | |
| Arrhythmia mechanismb | — | ||
| AVRT | 81 (58.3) | 81 (58.3) | |
| AVNRT | 34 (24.5) | 34 (24.5) | |
| IART | 19 (13.7) | 19 (13.7) | |
| EAT | 3 (2.2) | 3 (2.2) | |
| PVCs/VT | 3 (2.2) | 3 (2.2) | |
| Pre-excitation | 43 (30.1) | 43 (30.1) | — |
| Arrhythmia site | — | ||
| Right | 64 (46.0) | 64 (46.0) | |
| Left | 62 (44.6) | 62 (44.6) | |
| >1 ablation site | 5 (3.6) | 5 (3.6) | |
| Trans-bafflec | 8 (5.8) | 8 (5.8) | |
| Acute procedural success | 139 (100) | 135 (97.1) | 0.12 |
| Complications | 2d (1.4) | 0 (0) | 1.0 |
| Fluoroscopy exposure (min) | 0.3 [0.1–3.5] | 2.1 [0.2–4.1] | 0.02 |
| Procedural time (min) | 141 [118.5–182] | 173 [124–219.5] | 0.01 |
| Arrhythmia recurrencee | 9 (6.5) | 23 (16.5) | 0.01 |
Values are presented as median [interquartile range] or n (%).
AVNRT, atrioventricular nodal re-entrant tachycardia; AVRT, atrioventricular re-entrant tachycardia; BSA, body surface area; EAT, ectopic atrial tachycardia; IART, intra-atrial re-entrant tachycardia; ICE, intracardiac echocardiography; IQR, interquartile range; PVCs/VT, premature ventricular complexes/ventricular tachycardia.
aMatched variable P-values are 1.0 and not elsewhere reported.
bSome patients had multiple arrhythmia mechanisms.
cTrans-baffle approach included Fontan and intracardiac baffles in biventricular palliations.
dTransseptal needle entry into pericardial space during first transseptal attempt (without ICE). One case with atrial flutter that degenerated into ventricular fibrillation arrest, requiring compressions; not related to ICE usage.
eFor the purposes of arrhythmia recurrence, only patients undergoing successful ablation were considered.
| Variable . | Matched ICE cases . | Matched no ICE controls . | P-valuea . |
|---|---|---|---|
| (n = 139) . | (n = 139) . | ||
| Age (years) | 15 [12–17] | 15.1 [13.1–16.8] | 0.66 |
| Female | 53 (38.1) | 69 (49.6) | 0.05 |
| Non-white | 19 (13.7) | 13 (9.4) | 0.26 |
| Weight (kg) | 56.8 [48.2–71.3] | 57.0 [46.3–71.4] | 0.60 |
| BSA (m2) | 1.6 [1.4–1.8] | 1.6 [1.4–1.8] | 0.61 |
| CHD | 19 (13.7) | 19 (13.7) | — |
| Biventricular CHD Ebstein | 7 (5.0) | 7 (5.0) | |
| Anomaly | 2 (1.4) | 2 (1.4) | |
| Fontan circulation | 5 (3.6) | 5 (3.6) | |
| Intracardiac baffle | 5 (3.6) | 5 (3.6) | |
| Arrhythmia mechanismb | — | ||
| AVRT | 81 (58.3) | 81 (58.3) | |
| AVNRT | 34 (24.5) | 34 (24.5) | |
| IART | 19 (13.7) | 19 (13.7) | |
| EAT | 3 (2.2) | 3 (2.2) | |
| PVCs/VT | 3 (2.2) | 3 (2.2) | |
| Pre-excitation | 43 (30.1) | 43 (30.1) | — |
| Arrhythmia site | — | ||
| Right | 64 (46.0) | 64 (46.0) | |
| Left | 62 (44.6) | 62 (44.6) | |
| >1 ablation site | 5 (3.6) | 5 (3.6) | |
| Trans-bafflec | 8 (5.8) | 8 (5.8) | |
| Acute procedural success | 139 (100) | 135 (97.1) | 0.12 |
| Complications | 2d (1.4) | 0 (0) | 1.0 |
| Fluoroscopy exposure (min) | 0.3 [0.1–3.5] | 2.1 [0.2–4.1] | 0.02 |
| Procedural time (min) | 141 [118.5–182] | 173 [124–219.5] | 0.01 |
| Arrhythmia recurrencee | 9 (6.5) | 23 (16.5) | 0.01 |
| Variable . | Matched ICE cases . | Matched no ICE controls . | P-valuea . |
|---|---|---|---|
| (n = 139) . | (n = 139) . | ||
| Age (years) | 15 [12–17] | 15.1 [13.1–16.8] | 0.66 |
| Female | 53 (38.1) | 69 (49.6) | 0.05 |
| Non-white | 19 (13.7) | 13 (9.4) | 0.26 |
| Weight (kg) | 56.8 [48.2–71.3] | 57.0 [46.3–71.4] | 0.60 |
| BSA (m2) | 1.6 [1.4–1.8] | 1.6 [1.4–1.8] | 0.61 |
| CHD | 19 (13.7) | 19 (13.7) | — |
| Biventricular CHD Ebstein | 7 (5.0) | 7 (5.0) | |
| Anomaly | 2 (1.4) | 2 (1.4) | |
| Fontan circulation | 5 (3.6) | 5 (3.6) | |
| Intracardiac baffle | 5 (3.6) | 5 (3.6) | |
| Arrhythmia mechanismb | — | ||
| AVRT | 81 (58.3) | 81 (58.3) | |
| AVNRT | 34 (24.5) | 34 (24.5) | |
| IART | 19 (13.7) | 19 (13.7) | |
| EAT | 3 (2.2) | 3 (2.2) | |
| PVCs/VT | 3 (2.2) | 3 (2.2) | |
| Pre-excitation | 43 (30.1) | 43 (30.1) | — |
| Arrhythmia site | — | ||
| Right | 64 (46.0) | 64 (46.0) | |
| Left | 62 (44.6) | 62 (44.6) | |
| >1 ablation site | 5 (3.6) | 5 (3.6) | |
| Trans-bafflec | 8 (5.8) | 8 (5.8) | |
| Acute procedural success | 139 (100) | 135 (97.1) | 0.12 |
| Complications | 2d (1.4) | 0 (0) | 1.0 |
| Fluoroscopy exposure (min) | 0.3 [0.1–3.5] | 2.1 [0.2–4.1] | 0.02 |
| Procedural time (min) | 141 [118.5–182] | 173 [124–219.5] | 0.01 |
| Arrhythmia recurrencee | 9 (6.5) | 23 (16.5) | 0.01 |
Values are presented as median [interquartile range] or n (%).
AVNRT, atrioventricular nodal re-entrant tachycardia; AVRT, atrioventricular re-entrant tachycardia; BSA, body surface area; EAT, ectopic atrial tachycardia; IART, intra-atrial re-entrant tachycardia; ICE, intracardiac echocardiography; IQR, interquartile range; PVCs/VT, premature ventricular complexes/ventricular tachycardia.
aMatched variable P-values are 1.0 and not elsewhere reported.
bSome patients had multiple arrhythmia mechanisms.
cTrans-baffle approach included Fontan and intracardiac baffles in biventricular palliations.
dTransseptal needle entry into pericardial space during first transseptal attempt (without ICE). One case with atrial flutter that degenerated into ventricular fibrillation arrest, requiring compressions; not related to ICE usage.
eFor the purposes of arrhythmia recurrence, only patients undergoing successful ablation were considered.
Matched cohort univariable analysis: clinical factors associated with fluoroscopy exposure and procedural duration in patients with (A) congenital heart disease and (B) structurally normal hearts
| A. Congenital heart disease (n = 38) . | |||
|---|---|---|---|
| Outcome . | Variable . | OR (95% CI) . | P-value . |
| Fluoroscopy exposure | Trans-baffle | 4.90 (1.73, 13.86) | <0.01 |
| ICE used | 0.99 (0.43, 2.30) | 0.99 | |
| Procedure duration | Trans-baffle | 0.96 (0.78, 1.18) | 0.68 |
| ICE used | 0.82 (0.67, 1.01) | 0.07 | |
| B. Structurally normal hearts (n = 240) | |||
| Outcome | Variable | OR (95% CI) | P-value |
| Fluoroscopy exposure | Ablation site | ||
| Right | Reference | — | |
| Left | 9.57 (5.62, 16.30) | <0.01 | |
| >1 ablation site | 7.33 (0.39, 136.86) | 0.18 | |
| Transseptal puncture | 11.10 (6.50, 18.96) | <0.01 | |
| ICE used | 0.63 (0.42, 0.95) | 0.03 | |
| Procedure duration | Ablation site | ||
| Right | Reference | — | |
| Left | 0.87 (0.78, 0.97) | 0.01 | |
| >1 ablation site | 1.02 (0.56, 1.86) | 0.95 | |
| Transseptal puncture | 0.88 (0.79, 0.98) | 0.03 | |
| ICE used | 0.91 (0.82, 1.02) | 0.10 | |
| A. Congenital heart disease (n = 38) . | |||
|---|---|---|---|
| Outcome . | Variable . | OR (95% CI) . | P-value . |
| Fluoroscopy exposure | Trans-baffle | 4.90 (1.73, 13.86) | <0.01 |
| ICE used | 0.99 (0.43, 2.30) | 0.99 | |
| Procedure duration | Trans-baffle | 0.96 (0.78, 1.18) | 0.68 |
| ICE used | 0.82 (0.67, 1.01) | 0.07 | |
| B. Structurally normal hearts (n = 240) | |||
| Outcome | Variable | OR (95% CI) | P-value |
| Fluoroscopy exposure | Ablation site | ||
| Right | Reference | — | |
| Left | 9.57 (5.62, 16.30) | <0.01 | |
| >1 ablation site | 7.33 (0.39, 136.86) | 0.18 | |
| Transseptal puncture | 11.10 (6.50, 18.96) | <0.01 | |
| ICE used | 0.63 (0.42, 0.95) | 0.03 | |
| Procedure duration | Ablation site | ||
| Right | Reference | — | |
| Left | 0.87 (0.78, 0.97) | 0.01 | |
| >1 ablation site | 1.02 (0.56, 1.86) | 0.95 | |
| Transseptal puncture | 0.88 (0.79, 0.98) | 0.03 | |
| ICE used | 0.91 (0.82, 1.02) | 0.10 | |
CHD, congenital heart disease; CI, confidence interval; ICE, intracardiac echocardiography; OR, odds ratio.
Matched cohort univariable analysis: clinical factors associated with fluoroscopy exposure and procedural duration in patients with (A) congenital heart disease and (B) structurally normal hearts
| A. Congenital heart disease (n = 38) . | |||
|---|---|---|---|
| Outcome . | Variable . | OR (95% CI) . | P-value . |
| Fluoroscopy exposure | Trans-baffle | 4.90 (1.73, 13.86) | <0.01 |
| ICE used | 0.99 (0.43, 2.30) | 0.99 | |
| Procedure duration | Trans-baffle | 0.96 (0.78, 1.18) | 0.68 |
| ICE used | 0.82 (0.67, 1.01) | 0.07 | |
| B. Structurally normal hearts (n = 240) | |||
| Outcome | Variable | OR (95% CI) | P-value |
| Fluoroscopy exposure | Ablation site | ||
| Right | Reference | — | |
| Left | 9.57 (5.62, 16.30) | <0.01 | |
| >1 ablation site | 7.33 (0.39, 136.86) | 0.18 | |
| Transseptal puncture | 11.10 (6.50, 18.96) | <0.01 | |
| ICE used | 0.63 (0.42, 0.95) | 0.03 | |
| Procedure duration | Ablation site | ||
| Right | Reference | — | |
| Left | 0.87 (0.78, 0.97) | 0.01 | |
| >1 ablation site | 1.02 (0.56, 1.86) | 0.95 | |
| Transseptal puncture | 0.88 (0.79, 0.98) | 0.03 | |
| ICE used | 0.91 (0.82, 1.02) | 0.10 | |
| A. Congenital heart disease (n = 38) . | |||
|---|---|---|---|
| Outcome . | Variable . | OR (95% CI) . | P-value . |
| Fluoroscopy exposure | Trans-baffle | 4.90 (1.73, 13.86) | <0.01 |
| ICE used | 0.99 (0.43, 2.30) | 0.99 | |
| Procedure duration | Trans-baffle | 0.96 (0.78, 1.18) | 0.68 |
| ICE used | 0.82 (0.67, 1.01) | 0.07 | |
| B. Structurally normal hearts (n = 240) | |||
| Outcome | Variable | OR (95% CI) | P-value |
| Fluoroscopy exposure | Ablation site | ||
| Right | Reference | — | |
| Left | 9.57 (5.62, 16.30) | <0.01 | |
| >1 ablation site | 7.33 (0.39, 136.86) | 0.18 | |
| Transseptal puncture | 11.10 (6.50, 18.96) | <0.01 | |
| ICE used | 0.63 (0.42, 0.95) | 0.03 | |
| Procedure duration | Ablation site | ||
| Right | Reference | — | |
| Left | 0.87 (0.78, 0.97) | 0.01 | |
| >1 ablation site | 1.02 (0.56, 1.86) | 0.95 | |
| Transseptal puncture | 0.88 (0.79, 0.98) | 0.03 | |
| ICE used | 0.91 (0.82, 1.02) | 0.10 | |
CHD, congenital heart disease; CI, confidence interval; ICE, intracardiac echocardiography; OR, odds ratio.
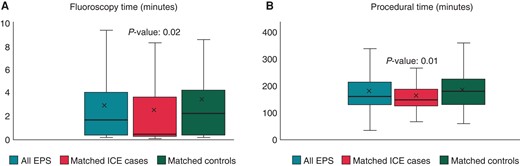
(A and B) Box-whisker plots of cohort and matched cohort EPS distributed by use or no use of ICE as relates to A: fluoroscopy time and B: procedural time. ICE, intracardiac echocardiogram.
In patients with structurally normal hearts, the median procedure duration was also significantly shortened with ICE use (137 vs. 157 min, P = 0.03). These ICE-associated reductions in procedure duration were further pronounced with a subset analysis of patients without transseptal punctures (140.5 [IQR 119.3–185.8] min with ICE vs. 195 [IQR 147–234] min without; P < 0.01). This was also true in a subset analysis of procedure duration in only AVNRT cases (139 [IQR 121.5–175.5] min with ICE vs. 193 [IQR 157–221.5] min without; P < 0.01).
In the univariable model fitted by presence or absence of CHD, ICE use was not associated with a significant reduction in procedure duration (OR 0.91; 95% CI 0.82–1.02; P = 0.1; Table 4); however, ICE did significantly decrease fluoroscopy exposure (OR 0.63; 95% CI 0.42–0.95; P = 0.03). Notably, in patients with structurally normal hearts, left-sided ablation targets and transseptal punctures were associated with increased fluoroscopy exposure (left vs. right P < 0.01; transseptal P = 0.03) and shortened procedure duration (left vs. right P = 0.01; transseptal P = 0.01).
Acute success and recurrence
The overall acute success rate in the matched ICE cases was 100%, compared with 97% in the control group (P = 0.06) (Table 2). The incidence of arrhythmia recurrence was lower in the ICE group (6.5% vs. 14.8%; P = 0.03). In the univariable analysis of the matched cohort, clinical factors associated with arrhythmia recurrence included: presence of CHD and absence of ICE use (Table 5). After accounting for covariates, the only factor associated with an increased risk of arrhythmia recurrence was the absence of ICE use (OR 0.37; 95% CI 0.15–0.84; P = 0.02).
Matched cohort univariable and multivariable analyses: clinical factors associated with arrhythmia recurrence
| . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Variable . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Age (years) | 1.03 (0.99, 1.07) | 0.12 | — | — |
| White | 0.57 (0.21, 1.80) | 0.29 | — | — |
| Weight (kg) | 1.01 (1.00, 1.03) | 0.08 | — | — |
| BSA (m2) | 2.24 (0.79, 6.58) | 0.13 | — | — |
| CHD | 2.57 (0.94, 6.34) | 0.05 | 2.48 (0.82, 6.68) | 0.08 |
| >1 ablation site | 2.95 (0.42, 13.56) | 0.20 | 1.94 (0.24, 10.86) | 0.48 |
| ICE used | 0.4 (0.17, 0.89) | 0.03 | 0.37 (0.15, 0.84) | 0.02 |
| . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Variable . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Age (years) | 1.03 (0.99, 1.07) | 0.12 | — | — |
| White | 0.57 (0.21, 1.80) | 0.29 | — | — |
| Weight (kg) | 1.01 (1.00, 1.03) | 0.08 | — | — |
| BSA (m2) | 2.24 (0.79, 6.58) | 0.13 | — | — |
| CHD | 2.57 (0.94, 6.34) | 0.05 | 2.48 (0.82, 6.68) | 0.08 |
| >1 ablation site | 2.95 (0.42, 13.56) | 0.20 | 1.94 (0.24, 10.86) | 0.48 |
| ICE used | 0.4 (0.17, 0.89) | 0.03 | 0.37 (0.15, 0.84) | 0.02 |
BSA, body surface area; CHD, congenital heart disease; ICE, intracardiac echocardiogram; OR, odds ratio.
Matched cohort univariable and multivariable analyses: clinical factors associated with arrhythmia recurrence
| . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Variable . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Age (years) | 1.03 (0.99, 1.07) | 0.12 | — | — |
| White | 0.57 (0.21, 1.80) | 0.29 | — | — |
| Weight (kg) | 1.01 (1.00, 1.03) | 0.08 | — | — |
| BSA (m2) | 2.24 (0.79, 6.58) | 0.13 | — | — |
| CHD | 2.57 (0.94, 6.34) | 0.05 | 2.48 (0.82, 6.68) | 0.08 |
| >1 ablation site | 2.95 (0.42, 13.56) | 0.20 | 1.94 (0.24, 10.86) | 0.48 |
| ICE used | 0.4 (0.17, 0.89) | 0.03 | 0.37 (0.15, 0.84) | 0.02 |
| . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Variable . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Age (years) | 1.03 (0.99, 1.07) | 0.12 | — | — |
| White | 0.57 (0.21, 1.80) | 0.29 | — | — |
| Weight (kg) | 1.01 (1.00, 1.03) | 0.08 | — | — |
| BSA (m2) | 2.24 (0.79, 6.58) | 0.13 | — | — |
| CHD | 2.57 (0.94, 6.34) | 0.05 | 2.48 (0.82, 6.68) | 0.08 |
| >1 ablation site | 2.95 (0.42, 13.56) | 0.20 | 1.94 (0.24, 10.86) | 0.48 |
| ICE used | 0.4 (0.17, 0.89) | 0.03 | 0.37 (0.15, 0.84) | 0.02 |
BSA, body surface area; CHD, congenital heart disease; ICE, intracardiac echocardiogram; OR, odds ratio.
Discussion
Catheter ablation has become to the standard of care for paediatric and CHD patients with arrhythmias.9 With ablation success rates as high as 96% and complication and recurrence rates as low as 4% and 5% in paediatric EPS, respectively,10–13 opportunities to further improve on the margins become increasingly challenging. Our study indicates that integrating ICE into the ablation procedure represents one such opportunity, and it should be considered a standard tool in paediatric and congenital EPS.
This single-centre retrospective study represents the largest cohort of paediatric and CHD patients undergoing EPS with ICE guidance. In our review of 196 patients, we found no ICE-related procedural complications. Rather, ICE was found to have a favourable effect on patient and procedural outcomes by decreasing fluoroscopic exposure, shortening procedural duration, and reducing arrhythmia recurrence rates.
The overall complication rate in our study was 1%. One of these cases was needle entry into the pericardial space during fluoroscopically guided transseptal puncture. Intracardiac echocardiography was used during the procedure solely to evaluate the sequelae of the complication and facilitate a reattempt. This case illustrates how the direct visualization provided by ICE can be valuable for troubleshooting complications and enhancing the safety of the procedure.
It is noteworthy that ICE was not associated with any vascular complications across a broad range of ages and patient sizes—the youngest patient was 3 years, and the lowest weight was ∼15 kg. This is particularly significant because the necessity for a relatively large introducer venous sheath (8–10 F) has often deterred the use of ICE in paediatric EPS.9 One approach we have used to reduce the risk of vascular injury in smaller patients (<30 kg) is to exchange a reference catheter for the ICE catheter, with the ability to replace the reference later should it be needed. Larger patients (>50 kg) may tolerate an additional venous access site dedicated for ICE use.
In addition to reducing procedural complications, incorporating ICE into the ablation procedure led to shortened procedure duration, as evidenced in the matched cohort. Significant reductions in procedural times were particularly notable within specific patient subpopulations such as CHD patients with IART and non-CHD patients with AVNRT where the median differences between ICE and no ICE controls amounted to 40 and 54 min, respectively. Because this study was conducted during the learning phase of ICE integration into our workflow, we anticipate that our findings may underestimate the full potential benefits of ICE in safely reducing procedure duration.
While ICE resulted in decreased fluoroscopy exposure, the reductions attributed to ICE were less profound compared to the reductions observed in procedure duration. This might be explained in part by the fact that fluoroscopy use in contemporary EPS is already limited and any differences in fluoroscopy exposure were more strongly associated with specific aspects of the procedure, such as the necessity for transseptal or trans-baffle puncture and sidedness of the ablation target. This notwithstanding, these reductions ought not to be discounted. Intracardiac echocardiography use allows for incremental reductions in exposure, enabling a larger number of patients to avoid fluoroscopy exposure (when compared to controls). This potentiates the possibility of achieving completely ‘fluoro-less’ cases, which are now commonplace in our lab. This aligns with the pursuit of the as low as reasonable achievable (ALARA) principle in cardiac catheterization and EPS14–16 that is based on the hypothesis that there is no threshold below which ionizing radiation is free from harmful effects to the operator and patient.17,18 This importance is only further pronounced in paediatric patients due to their extended years of potential risk and exposure, especially in CHD patients who frequently require multiple procedures involving ionizing radiation.19,20 Lastly, we should not underestimate the long-term musculoskeletal and health benefits to the operator and staff as a result of reduced lead apron usage.21
Beyond its capacity to minimize radiation exposure, ICE played a pivotal role in reducing arrhythmia recurrence and improving patient outcomes. At 1-year follow-up, patients, both with structurally normal hearts and CHD, who underwent ICE-guided ablations experienced a significantly lower arrhythmia recurrence rate of 6.5%, compared to 14.8% recurrence rate observed in the control group. We suspect that this benefit may be more pronounced among CHD patients. In the descriptive ICE cohort, CHD patients exhibited significantly higher arrhythmia recurrence rates when compared to non-CHD patients (20.0% vs. 6.1%). Moreover, in the univariable analysis, CHD patients had 2.6-fold increased odds of recurrence when compared to those without CHD (P < 0.05). When controlling for ICE use in the multivariable model however, the significance of CHD diminished (P = 0.08). In a cohort with a larger number of CHD patients, the significance of CHD may become more pronounced. Nonetheless, in the multivariable analysis, the only factor associated with a reduction in arrhythmia recurrence was the utilization of ICE. This likely speaks to the advantage that ICE confers in being able to directly visualize catheter contact, leading to a more effective and ultimately enduring intervention.
One instance illustrating the advantages conferred by ICE in performing a complex ablation procedure involved a patient with d-transposition of the great arteries and Mustard palliation. The patient had typical atrial flutter and bilateral femoral vein occlusion. In this case, ICE was inserted through the sheath accessing the right internal jugular vein into the inferior limb of the Mustard baffle. The ablation catheter was placed using a retrograde aortic approach. Intracardiac echocardiography imaging substantially enhanced the precision of catheter manipulation, enabling the successful creation of an ablation line despite the complex anatomy and catheter approach.
Incorporating ICE into procedures must consider the potential costs. The ViewFlex™ Xtra, the preferred ICE catheter at our institution, carries a list price of 2890 USD—the actual price will vary based on negotiated contracts with healthcare institutions. While one must consider such additional procedural expenses, the reductions in arrhythmia recurrence associated with ICE utilization are expected to mitigate the need for repeat EP studies and the associated resource utilization; these encompass various factors, such as laboratory time and usage, staff compensation, exposure to anaesthesia, the electrophysiologist’s involvement, and the utilization of standard catheters, among others. Considering the lower incidences of recurrence demonstrated here, these points deserve emphasis. However, not all arrhythmic substrates are equal, and a higher level of technology is not always required to achieve success. Careful selection of cases for ICE use may lead to cost savings. Further cost–benefit analyses are needed to accurately predict the extent of these benefits.
Limitations
Limitations of this study include those inherent to a retrospective single-centre study design. Despite our detailed matching algorithm, it was difficult to account for nuanced differences in arrhythmia location and mechanism in our patient population. Furthermore, since ICE cases were matched to controls that pre-dated ICE incorporation, there are likely variations in operator skills and experience between the two groups. Though no acute vascular injuries were observed, late femoral or iliac vein occlusion may have been undetected, as these may only become apparent upon attempting vascular access during repeat procedures. Lastly, due to the lack of comprehensive cost data, a thorough cost–benefit analysis of ICE utilization was not possible; thus, these benefits are not currently quantifiable and can only be inferred.
Conclusions
Intracardiac echocardiography use in paediatric and CHD ablations is safe and is not associated with higher complication rates. Additionally, it reduces procedure duration, fluoroscopy exposure, and arrhythmia recurrence. Intracardiac echocardiography has the potential for improving workflow efficiency and ablation success in both cases with CHD and with structurally normal hearts. However, not all arrhythmic substrates are equal and the application of higher levels of technology should be judicious. Further nuanced investigation is needed to better elaborate the cost–benefit analysis of increased resource utilization vs. gains in procedural efficiency and success rates.
Supplementary material
Supplementary material is available at Europace online.
Funding
This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UM1TR004409 (formerly 5UL1TR001067-05, 8UL1TR000105 and UL1RR025764).
Conflict of interest: T.P. receives compensation for speaking and teaching from Abbott Medical. The authors have no other conflicts of interest to disclose.
Data availability
All relevant data are within the manuscript and its supporting information files. Additional data may be requested from the corresponding author.
References
Author notes
T.P. and M.N. contributed equally.



