-
PDF
- Split View
-
Views
-
Cite
Cite
Silvia Liliana Ruiz Roa, Edson Zangiacomi Martinez, Clarissa Silva Martins, Sonir Rauber Antonini, Margaret de Castro, Ayrton Custódio Moreira, Postnatal Ontogeny of the Circadian Expression of the Adrenal Clock Genes and Corticosterone Rhythm in Male Rats, Endocrinology, Volume 158, Issue 5, 1 May 2017, Pages 1339–1346, https://doi.org/10.1210/en.2016-1782
Close - Share Icon Share
Abstract
The postnatal synchronization of the circadian variation of the adrenal clock genes in mammals remains unknown. We evaluated the postnatal ontogeny of daily variation of clock genes (Clock/Bmal1/Per1/Per2/Per3/Cry1/Cry2/Rorα/Rev-Erbα) and steroidogenesis-related genes (Star and Mc2r) in rat adrenals and its relationship with the emergence of plasma corticosterone rhythm using cosinor analysis. Plasma corticosterone circadian rhythm was detected from postnatal day (P)1, with morning acrophase, between zeitgeber time (ZT)0 and ZT2. From P14, there was a nocturnal acrophase of corticosterone at ZT20, which was associated with pups’ eye opening. From P3 there was a circadian variation of the mRNA expression of Bmal1, Per2, Per3, and Cry1 genes with morning acrophase, whereas Rev-Erbα had nocturnal acrophase. From P14, Bmal1, Per2, Per3, and Cry1 acrophases advanced by approximately 10 hours, as compared with early neonatal days, becoming vespertine-nocturnal. In all postnatal ages, Per2 and Cry1 circadian profiles were synchronized in phase with the circadian rhythm of plasma corticosterone, whereas Bmal1 was in antiphase. An adult-like Star circadian rhythm profile was observed only from P21. In conclusion, our original data demonstrated a progressive postnatal maturation of the circadian variation of the adrenal clock genes in synchrony with the development of the corticosterone circadian rhythm in rats.
The endogenous circadian rhythm of the hypothalamic-pituitary-adrenal axis is part of the predictive homeostasis, which allows organisms to adapt to environmental changes. Periodic factors, such as light/dark, sleep/wake, food ingestion, rest/activity cycles, and social cues, synchronize the circadian rhythms (1–3). In mammals, the adult circadian oscillators are synchronized by the suprachiasmatic nucleus (SCN), known as the “master” clock, which receives direct photic input from the retina by the light/dark cycle.
The mechanism of cell-autonomous circadian clocks depends on two core clock proteins, CLOCK and BMAL1, which act as transcriptional activators of an autoregulatory negative feedback loop (4). These proteins heterodimerize in cytoplasm forming a complex that, in the nucleus, activates their target genes, Periods (Per1, Per2, and Per3) and Cryptochromes (Cry1 and Cry2), which form a repressor complex that interacts with CLOCK-BMAL1, resulting in inhibition of their own transcription (5). Bmal1 (also known as Arntl) and other first-loop Clock genes increase the mRNA levels of Rev-Erbα (Nr1d1) and Rorα, which in turn repress or activate the expression of Bmal1, respectively (6).
During the fetal stage, the rodent SCN exhibits no rhythms or only low-amplitude rhythms in clock gene expression (7–9). The amplitude of the rhythms of the gene expression in SCN gradually increases during postnatal development and achieves adult-like levels from P10 (10).
The development of the hypothalamic-pituitary-adrenal axis circadian rhythm was previously described in neonatal rats with predominant vespertine corticosterone plasma acrophase around postnatal day (P)16 (11–13). This pattern follows the gradual maturation of the SCN (14)
Circadian rhythm has been detected in several peripheral fetal organs in rats even when the fetal SCN has not been completely functional (15, 16). Among these, the rat fetal adrenal glands demonstrate corticosterone and clock gene circadian activities from 18 days of gestation (17).
The postnatal ontogeny of the daily variation of adrenal clock genes in rats remains unknown. Therefore, the aim of the current study was to evaluate the postnatal ontogeny of daily variation of Clock, Bmal1, Per1, Per2, Per3, Cry1, Cry2, Rorα, and Rev-Erbα clock genes and the steroidogenesis-related genes Star and Mc2r, in adrenal neonatal rats and its relationship with the emergence of plasma corticosterone rhythm using cosinor analysis.
Materials and Methods
Experimental animals
Adult timed-pregnant female Wistar rats were maintained individually in cages at 21 ± 2°C with a 12-hour light/dark cycle (lights on at 0700 hours) with free access to food and water. The day of delivery was designated P0. At P1 the litters were separated and limited to eight pups per mother. Only male pups were studied at P1, P3, P6, P12, P14, P16, P21, and P24. All experiments were approved by the Animal Ethics Committee of the Ribeirao Preto Medical School of University of Sao Paulo (Protocol no. 149/2012).
Experimental protocol
The pups stayed with their mothers throughout the experiment until weaning was completed at P21. The pups sampled at P24 were fed laboratory chow diet independently of their mothers. During the experiment, the mothers and pups had free access to food and water. On each sampling day, when the pups were studied during the darkness period, sampling was performed under red light. The time of lights-on was zeitgeber time (ZT)0, and sampling was performed from ZT0 until ZT20. At postnatal ages P1, P3, P6, P12, P14, P16, P21, and P24, pups were decapitated at 4-hour intervals over a 24-hour period, with a mean of eight pups at each time point. Trunk blood was collected for corticosterone measurement as previously described (18). Rabbit anti-corticosterone antibody was used (Table 1) (catalog number: C8784, RRID: AB_259131; Sigma-Aldrich, Saint Louis, MO). The assay sensitivity was 0.15 μg/dL, and the inter- and intra-assay variations were 4.8% and 6.7%, respectively.
| Peptide/Protein Target . | Name of Antibody . | Manufacturer, Catalog #, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . | Research Resource Identifier . |
|---|---|---|---|---|---|
| Corticosterone | Rabbit anti-corticosterone antibody, unconjugated | Sigma C8784 | Rabbit; polyclonal | 01:20 | AB_259131 |
| Peptide/Protein Target . | Name of Antibody . | Manufacturer, Catalog #, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . | Research Resource Identifier . |
|---|---|---|---|---|---|
| Corticosterone | Rabbit anti-corticosterone antibody, unconjugated | Sigma C8784 | Rabbit; polyclonal | 01:20 | AB_259131 |
| Peptide/Protein Target . | Name of Antibody . | Manufacturer, Catalog #, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . | Research Resource Identifier . |
|---|---|---|---|---|---|
| Corticosterone | Rabbit anti-corticosterone antibody, unconjugated | Sigma C8784 | Rabbit; polyclonal | 01:20 | AB_259131 |
| Peptide/Protein Target . | Name of Antibody . | Manufacturer, Catalog #, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . | Research Resource Identifier . |
|---|---|---|---|---|---|
| Corticosterone | Rabbit anti-corticosterone antibody, unconjugated | Sigma C8784 | Rabbit; polyclonal | 01:20 | AB_259131 |
For molecular studies, the adrenals were dissected at P3, P6, P14, P16, P21, and P24, flash-frozen in a dry ice–isopentene bath at −30°C, and transferred to freezer at −80°C until processing.
RNA isolation and real-time quantitative reverse transcription polymerase chain reaction
Total RNA was isolated from each right adrenal tissue sample using TRIzol reagent (Thermo Fisher Scientific Inc., Life Technologies Corporation, Carlsbad, CA) according to the manufacturer’s instructions. RNA concentrations were determined by spectrophotometry assays at 260 nm, with an acceptable range of 1.6 to 2.0 ng/µL. The RNA quality was assessed by electrophoresis using 1.2% agarose gel. cDNA synthesis was performed with the High Capacity cDNA Reverse Transcription kit and MultiScribe enzyme (Thermo Fisher Scientific Inc., Applied Biosystems, Foster City, CA). Quantitative real-time polymerase chain reaction (qRT-PCR) was carried on Applied Biosystems 7500 real-time PCR system.
The mRNA expression levels of components of adrenal “Clock” and steroidogenesis-related systems, including Clock (Rn00573120_m1), Bmal1l (Rn00577590_m1), Per1 (Rn01496757_m1), Per2 (Rn01427704_m1), Per3 (Rn00709499_m1), Cry1 (Rn01503063_m1), Cry2 (Rn01485701_m1), Rora (Rn01173769_m1), Rev-Erbα (Rn01460662_m1), Mc2r (Rn01491505_m1), and Star (Rn00580695_m1) genes, were analyzed using the TaqMan gene expression assay method (Thermo Fisher Scientific Inc.) for qRT-PCR. Each qRT-PCR reaction was performed in duplicate. Water (instead of cDNA) was used as a negative control. Housekeeping genes GAPDH (4352338E) and ACTB (4352340E) were run for each cDNA sample. For each sample, the threshold cycle (Ct) was determined and normalized to the average of the two housekeeping genes (ΔCt = Ct Unknown – Ct Housekeeping genes). The determination of gene transcript levels in each sample was obtained by the 2−ΔΔCt method (19). The median value obtained from adrenal tissues from pups studied to each different postnatal ages was compared with the median value obtained from rats decapitated at P24-ZT0.
Statistical analysis
Plasma corticosterone concentrations and adrenal gene expression profiles were evaluated by cosinor analysis (20). For cosinor analysis, data were fitted with two alternative regression models to differentiate between rhythmic and nonrhythmic expression: either a horizontal straight line (null hypothesis) or a single cosine curve (alternative hypothesis), as defined by the equation Y = mesor + {amplitude·cos[2·π(X − acrophase)/wavelength]}, with a constant wavelength of 24 hours. The amplitude was defined as the difference between the peak or trough and the mean value of a cosine curve, and acrophase was defined as the phase angle of the peak of a cosine curve. The regression methods implemented in SAS 9.4 software (SAS institute, Cary, NC, USA) and R 3.2.2 Software (The R foundation, Vienna, Austria) for graphics were applied.
The circadian rhythm in plasma corticosterone concentrations and in adrenal gene expression profiles was considered when the cosinor analysis of the data fitted a cosine curve and the mesor was out of limits of the 95% confidence interval of acrophase. For better visualization, in Figures 1 and 2, the value at ZT0 was replotted as ZT24.
Categorical variables were compared using Fisher’s exact test. Significance level was fixed at P < 0.05.
Results
Plasma corticosterone circadian rhythm
Figure 1 shows the plasma corticosterone concentrations from P1 to P24. The cosinor analysis identified a circadian oscillatory pattern on all postnatal days except P6. At P1, the corticosterone acrophase was 0909 ± 1.85 hours and was also maintained at morning on P3 (0835 ± 1.85 hours) and P12 (0719 ± 1.65 hours). Conversely, the acrophase was nocturnal from P14 (2223 ± 1.25 hours), P16 (2241 ± 0.98 hours), P21 (2051 ± 1.08 hours), and P24 (1958 ± 0.92 hours) (Fig. 1; Table 2). There was an association between higher values of nocturnal plasma corticosterone levels with the numbers of pups with eyes open at P14 (Fisher’s exact test, P < 0.01).
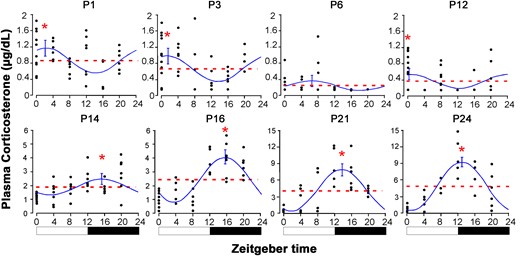
Circadian plasma corticosterone concentrations (μg/dL) at ZT0, 4, 8, 12, 16, and 20 in rats during postnatal ontogenesis at P1, P3, P6, P12, P14, P16, P21, and P24. Dotted line: mesor; solid line: cosine curve and 95% confidence interval of acrophase. *Differences between peak and mesor concentrations. Black bars represent dark phase.
Data on Cosinor Analysis of the Expression of Clock Genes and Steroidogenic Related Genes in Adrenal and Plasma Corticosterone Levels at Different Postnatal Days
| Postnatal Day . | Cosinor Data . | Clock . | Bmal1 . | Per1 . | Per2 . | Per3 . | Cry1 . | Cry2 . | Rora . | Rev-Erbα . | Mc2r . | Star . | Cort . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | Acro ± SD, h | 1151 ± 1.22 | 1728 ± 0.88 | . | 1132 ± 1.27 | 0643 ± 0.32 | 1419 ± 0.98 | 1205 ± 2.42 | . | 0226 ± 1.02 | 1709 ± 1.85 | 0907 ± 2.65 | 0835 ± 1.85 |
| Amp ± SE, 2− ΔΔCt | 0.28 ± 0.05 | 0.36 ± 0.04 | 0.42 ± 0.20 | 2.04 ± 0.36 | 2.39 ± 0.31 | 0.53 ± 0.08 | 0.35 ± 0.12 | 0.1 ± 0.05 | 0.44 ± 0.07 | 0.2 ± 0.05 | 0.25 ± 0.11 | 0.32 ± 0.08 | |
| Mesor ± SE | 1.09 ± 0.03 | 1.05 ± 0.03 | 2.71 ± 0.14 | 4.79 ± 0.25 | 6.54 ± 0.23 | 1.29 ± 0.05 | 2.34 ± 0.08 | 1.15 ± 0.04 | 0.92 ± 0.05 | 1.06 ± 0.03 | 1.33 ± 0.08 | 0.66 ± 0.06 | |
| 95% CI | 1.26–1.49a | 1.31–1.51a | 2.68–3.59 | 6.01–7.66a | 8.20–9.64a | 1.63–2.00a | 2.41–2.98a | 1.13–1.38 | 0.31–0.65a | 1.14–1.38a | 1.34–1.82a | 0.78–1.17a | |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | 0.05 | <0.01 | <0.01 | <0.01 | 0.01 | |
| 6 | Acro ± SD, h | . | 1947 ± 1.98 | . | 1148 ± 1.60 | 0945 ± 1.20 | 1219 ± 1.32 | 1159 ± 1.90 | . | 0116 ± 1.30 | 0048 ± 2.07 | . | . |
| Amp ± SE, 2− ΔΔCt | 0.02 ± 0.03 | 0.15 ± 0.03 | 0.5 ± 0.25 | 0.98 ± 0.23 | 1.41 ± 0.22 | 0.29 ± 0.06 | 0.43 ± 0.13 | 0.005 ± 0.06 | 0.37 ± 0.07 | 0.11 ± 0.04 | 0.13 ± 0.11 | 0.12 ± 0.06 | |
| Mesor ± SE | 0.97 ± 0.02 | 0.55 ± 0.03 | 2.74 ± 0.17 | 2.97 ± 0.16 | 3.27 ± 0.16 | 1.17 ± 0.04 | 1.85 ± 0.08 | 0.94 ± 0.04 | 0.74 ± 0.05 | 0.55 ± 0.02 | 1.37 ± 0.08 | 0.24 ± 0.04 | |
| 95% CI | 0.90–1.08 | 0.33–0.47a | 2.69–3.79 | 3.42–4.48a | 4.21–5.16a | 1.32–1.60a | 1.98–2.57a | 0.80–1.08 | 0.20–0.54a | 0.36–0.53a | 1.26–1.73 | 0.23–0.49 | |
| P value | 0.4 | <0.01 | 0.08 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | |
| 14 | Acro ± SD, h | 0308 ± 2.87 | 0325 ± 1.45 | . | 2107 ± 1.90 | 1609 ± 1.85 | 2231 ± 2.53 | . | 0056 ± 1.32 | 1331 ± 1.30 | . | . | 2223 ± 1.25 |
| Amp ± SE, 2− ΔΔCt | 0.1 ± 0.04 | 0.24 ± 0.04 | 0.21 ± 0.12 | 1.36 ± 0.33 | 0.67 ± 0.16 | 0.38 ± 0.13 | 0.15 ± 0.09 | 0.26 ± 0.06 | 0.55 ± 0.08 | 0.06 ± 0.04 | 0.18 ± 0.09 | 0.57 ± 0.17 | |
| Mesor ± SE | 1.08 ± 0.03 | 0.74 ± 0.03 | 1.93 ± 0.08 | 4.91 ± 0.24 | 3.03 ± 0.12 | 1.2 ± 0.09 | 0.94 ± 0.06 | 1.31 ± 0.04 | 1.13 ± 0.05 | 0.71 ± 0.03 | 1.39 ± 0.06 | 1.91 ± 0.12 | |
| 95% CI | 0.89–1.08 | 0.39–0.61a | 1.45–2.00 | 2.79–4.31a | 3.32–4.10a | 0.50–1.13a | 0.89–1.30 | 0.91–1.18a | 1.49–1.86a | 0.67–0.88 | 1.01–1.40 | 0.93–1.73a | |
| P value | 0.03 | 0.01 | 0.03 | 0.01 | <0.01 | 0.26 | 0.09 | <0.01 | <0.01 | 0.18 | 0.05 | <0.01 | |
| 16 | Acro ± SD, h | 0715 ± 1.63 | 0434 ± 1.15 | . | 2051 ± 1.35 | 1514 ± 1.63 | 2350 ± 0.98 | . | . | 1345 ± 0.80 | 1025 ± 1.70 | . | 2241 ± 0.98 |
| Amp ± SE, 2− ΔΔCt | 0.09 ± 0.03 | 0.15 ± 0.02 | 0.23 ± 0.30 | 1.92 ± 0.34 | 0.96 ± 0.21 | 0.43 ± 0.06 | 0.18 ± 0.14 | 0.11 ± 0.11 | 0.42 ± 0.04 | 0.15 ± 0.03 | 0.22 ± 0.15 | 1.65 ± 0.21 | |
| Mesor ± SE | 1.05 ± 0.02 | 0.56 ± 0.02 | 3.04 ± 0.21 | 5.37 ± 0.24 | 3.44 ± 0.15 | 1.35 ± 0.04 | 1.85 ± 0.10 | 1.41 ± 0.08 | 0.84 ± 0.03 | 0.67 ± 0.02 | 2.0 ± 0.11 | 2.42 ± 0.15 | |
| 95% CI | 1.06–1.21a | 0.36–0.47a | 2.54–4.00 | 2.67–4.23a | 3.86–4.93a | 0.79–1.05a | 1.67–2.37 | 1.25–1.79 | 1.15–1.36a | 0.74–0.90a | 1.85–2.59 | 0.25–1.28a | |
| P value | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| 21 | Acro ± SD, h | 0331 ± 1.68 | 0518 ± 0.97 | 2226 ± 1.93 | 2315 ± 0.87 | 1836 ± 1.65 | 0041 ± 0.72 | . | . | 1315 ± 0.47 | . | 1810 ± 1.65 | 2051 ± 1.08 |
| Amp ± SE, 2− ΔΔCt | 0.22 ± 0.06 | 0.59 ± 0.08 | 1.05 ± 0.28 | 5.04 ± 0.63 | 1.14 ± 0.23 | 0.93 ± 0.09 | 0.17 ± 0.04 | 0.06 ± 0.09 | 1.46 ± 0.11 | 0.03 ± 0.07 | 0.55 ± 0.11 | 3.76 ± 0.50 | |
| Mesor ± SE | 1.5 ± 0.04 | 1.17 ± 0.06 | 3.2 ± 0.19 | 7.76 ± 0.43 | 3.9 ± 0.18 | 1.42 ± 0.06 | 1.94 ± 0.06 | 1.8 ± 0.07 | 1.98 ± 0.07 | 1.36 ± 0.05 | 2.58 ± 0.08 | 4.09 ± 0.37 | |
| 95% CI | 1.14–1.42a | 1.57–1.95a | 1.53–2.78a | 1.33–4.11a | 4.43–5.66a | 0.26–0.70a | 1.90–2.32 | 1.64–2.07 | 3.18–3.69a | 1.22–1.55 | 2.85–3.43a | 0.77–1.44a | |
| P value | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | 0.01 | 0.52 | <0.01 | 0.14 | <0.01 | <0.01 | |
| 24 | Acro ± SD, h | . | 0628 ± 0.95 | . | 2251 ± 0.92 | 1858 ± 0.82 | 0013 ± 0.72 | 1955 ± 1.90 | 0400 ± 2.28 | 1354 ± 0.58 | 1715 ± 1.20 | 1916 ± 1.05 | 1958 ± 0.92 |
| Amp ± SE, 2− ΔΔCt | 0.07 ± 0.09 | 0.35 ± 0.04 | 0.17 ± 0.18 | 1.87 ± 0.24 | 0.93 ± 0.09 | 0.94 ± 0.11 | 0.25 ± 0.09 | 0.28 ± 0.09 | 1.06 ± 0.09 | 0.5 ± 0.07 | 0.69 ± 0.08 | 4.36 ± 0.46 | |
| Mesor ± SE | 1.01 ± 0.03 | 0.55 ± 0.03 | 1.64 ± 0.13 | 2.65 ± 0.17 | 1.84 ± 0.07 | 1.33 ± 0.07 | 1.44 ± 0.07 | 1.11 ± 0.06 | 1.27 ± 0.06 | 1.31 ± 0.05 | 1.86 ± 0.06 | 4.85 ± 0.35 | |
| 95% CI | 0.85–1.05 | 0.81–1.00a | 1.08–1.87 | 0.24–1.32a | 2.51–3.03a | 0.14–0.62a | 1.00–1.39a | 0.69–1.06a | 2.11–2.57a | 1.63–1.99a | 2.34–2.76a | 0.46–1.42a | |
| P value | 0.38 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Postnatal Day . | Cosinor Data . | Clock . | Bmal1 . | Per1 . | Per2 . | Per3 . | Cry1 . | Cry2 . | Rora . | Rev-Erbα . | Mc2r . | Star . | Cort . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | Acro ± SD, h | 1151 ± 1.22 | 1728 ± 0.88 | . | 1132 ± 1.27 | 0643 ± 0.32 | 1419 ± 0.98 | 1205 ± 2.42 | . | 0226 ± 1.02 | 1709 ± 1.85 | 0907 ± 2.65 | 0835 ± 1.85 |
| Amp ± SE, 2− ΔΔCt | 0.28 ± 0.05 | 0.36 ± 0.04 | 0.42 ± 0.20 | 2.04 ± 0.36 | 2.39 ± 0.31 | 0.53 ± 0.08 | 0.35 ± 0.12 | 0.1 ± 0.05 | 0.44 ± 0.07 | 0.2 ± 0.05 | 0.25 ± 0.11 | 0.32 ± 0.08 | |
| Mesor ± SE | 1.09 ± 0.03 | 1.05 ± 0.03 | 2.71 ± 0.14 | 4.79 ± 0.25 | 6.54 ± 0.23 | 1.29 ± 0.05 | 2.34 ± 0.08 | 1.15 ± 0.04 | 0.92 ± 0.05 | 1.06 ± 0.03 | 1.33 ± 0.08 | 0.66 ± 0.06 | |
| 95% CI | 1.26–1.49a | 1.31–1.51a | 2.68–3.59 | 6.01–7.66a | 8.20–9.64a | 1.63–2.00a | 2.41–2.98a | 1.13–1.38 | 0.31–0.65a | 1.14–1.38a | 1.34–1.82a | 0.78–1.17a | |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | 0.05 | <0.01 | <0.01 | <0.01 | 0.01 | |
| 6 | Acro ± SD, h | . | 1947 ± 1.98 | . | 1148 ± 1.60 | 0945 ± 1.20 | 1219 ± 1.32 | 1159 ± 1.90 | . | 0116 ± 1.30 | 0048 ± 2.07 | . | . |
| Amp ± SE, 2− ΔΔCt | 0.02 ± 0.03 | 0.15 ± 0.03 | 0.5 ± 0.25 | 0.98 ± 0.23 | 1.41 ± 0.22 | 0.29 ± 0.06 | 0.43 ± 0.13 | 0.005 ± 0.06 | 0.37 ± 0.07 | 0.11 ± 0.04 | 0.13 ± 0.11 | 0.12 ± 0.06 | |
| Mesor ± SE | 0.97 ± 0.02 | 0.55 ± 0.03 | 2.74 ± 0.17 | 2.97 ± 0.16 | 3.27 ± 0.16 | 1.17 ± 0.04 | 1.85 ± 0.08 | 0.94 ± 0.04 | 0.74 ± 0.05 | 0.55 ± 0.02 | 1.37 ± 0.08 | 0.24 ± 0.04 | |
| 95% CI | 0.90–1.08 | 0.33–0.47a | 2.69–3.79 | 3.42–4.48a | 4.21–5.16a | 1.32–1.60a | 1.98–2.57a | 0.80–1.08 | 0.20–0.54a | 0.36–0.53a | 1.26–1.73 | 0.23–0.49 | |
| P value | 0.4 | <0.01 | 0.08 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | |
| 14 | Acro ± SD, h | 0308 ± 2.87 | 0325 ± 1.45 | . | 2107 ± 1.90 | 1609 ± 1.85 | 2231 ± 2.53 | . | 0056 ± 1.32 | 1331 ± 1.30 | . | . | 2223 ± 1.25 |
| Amp ± SE, 2− ΔΔCt | 0.1 ± 0.04 | 0.24 ± 0.04 | 0.21 ± 0.12 | 1.36 ± 0.33 | 0.67 ± 0.16 | 0.38 ± 0.13 | 0.15 ± 0.09 | 0.26 ± 0.06 | 0.55 ± 0.08 | 0.06 ± 0.04 | 0.18 ± 0.09 | 0.57 ± 0.17 | |
| Mesor ± SE | 1.08 ± 0.03 | 0.74 ± 0.03 | 1.93 ± 0.08 | 4.91 ± 0.24 | 3.03 ± 0.12 | 1.2 ± 0.09 | 0.94 ± 0.06 | 1.31 ± 0.04 | 1.13 ± 0.05 | 0.71 ± 0.03 | 1.39 ± 0.06 | 1.91 ± 0.12 | |
| 95% CI | 0.89–1.08 | 0.39–0.61a | 1.45–2.00 | 2.79–4.31a | 3.32–4.10a | 0.50–1.13a | 0.89–1.30 | 0.91–1.18a | 1.49–1.86a | 0.67–0.88 | 1.01–1.40 | 0.93–1.73a | |
| P value | 0.03 | 0.01 | 0.03 | 0.01 | <0.01 | 0.26 | 0.09 | <0.01 | <0.01 | 0.18 | 0.05 | <0.01 | |
| 16 | Acro ± SD, h | 0715 ± 1.63 | 0434 ± 1.15 | . | 2051 ± 1.35 | 1514 ± 1.63 | 2350 ± 0.98 | . | . | 1345 ± 0.80 | 1025 ± 1.70 | . | 2241 ± 0.98 |
| Amp ± SE, 2− ΔΔCt | 0.09 ± 0.03 | 0.15 ± 0.02 | 0.23 ± 0.30 | 1.92 ± 0.34 | 0.96 ± 0.21 | 0.43 ± 0.06 | 0.18 ± 0.14 | 0.11 ± 0.11 | 0.42 ± 0.04 | 0.15 ± 0.03 | 0.22 ± 0.15 | 1.65 ± 0.21 | |
| Mesor ± SE | 1.05 ± 0.02 | 0.56 ± 0.02 | 3.04 ± 0.21 | 5.37 ± 0.24 | 3.44 ± 0.15 | 1.35 ± 0.04 | 1.85 ± 0.10 | 1.41 ± 0.08 | 0.84 ± 0.03 | 0.67 ± 0.02 | 2.0 ± 0.11 | 2.42 ± 0.15 | |
| 95% CI | 1.06–1.21a | 0.36–0.47a | 2.54–4.00 | 2.67–4.23a | 3.86–4.93a | 0.79–1.05a | 1.67–2.37 | 1.25–1.79 | 1.15–1.36a | 0.74–0.90a | 1.85–2.59 | 0.25–1.28a | |
| P value | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| 21 | Acro ± SD, h | 0331 ± 1.68 | 0518 ± 0.97 | 2226 ± 1.93 | 2315 ± 0.87 | 1836 ± 1.65 | 0041 ± 0.72 | . | . | 1315 ± 0.47 | . | 1810 ± 1.65 | 2051 ± 1.08 |
| Amp ± SE, 2− ΔΔCt | 0.22 ± 0.06 | 0.59 ± 0.08 | 1.05 ± 0.28 | 5.04 ± 0.63 | 1.14 ± 0.23 | 0.93 ± 0.09 | 0.17 ± 0.04 | 0.06 ± 0.09 | 1.46 ± 0.11 | 0.03 ± 0.07 | 0.55 ± 0.11 | 3.76 ± 0.50 | |
| Mesor ± SE | 1.5 ± 0.04 | 1.17 ± 0.06 | 3.2 ± 0.19 | 7.76 ± 0.43 | 3.9 ± 0.18 | 1.42 ± 0.06 | 1.94 ± 0.06 | 1.8 ± 0.07 | 1.98 ± 0.07 | 1.36 ± 0.05 | 2.58 ± 0.08 | 4.09 ± 0.37 | |
| 95% CI | 1.14–1.42a | 1.57–1.95a | 1.53–2.78a | 1.33–4.11a | 4.43–5.66a | 0.26–0.70a | 1.90–2.32 | 1.64–2.07 | 3.18–3.69a | 1.22–1.55 | 2.85–3.43a | 0.77–1.44a | |
| P value | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | 0.01 | 0.52 | <0.01 | 0.14 | <0.01 | <0.01 | |
| 24 | Acro ± SD, h | . | 0628 ± 0.95 | . | 2251 ± 0.92 | 1858 ± 0.82 | 0013 ± 0.72 | 1955 ± 1.90 | 0400 ± 2.28 | 1354 ± 0.58 | 1715 ± 1.20 | 1916 ± 1.05 | 1958 ± 0.92 |
| Amp ± SE, 2− ΔΔCt | 0.07 ± 0.09 | 0.35 ± 0.04 | 0.17 ± 0.18 | 1.87 ± 0.24 | 0.93 ± 0.09 | 0.94 ± 0.11 | 0.25 ± 0.09 | 0.28 ± 0.09 | 1.06 ± 0.09 | 0.5 ± 0.07 | 0.69 ± 0.08 | 4.36 ± 0.46 | |
| Mesor ± SE | 1.01 ± 0.03 | 0.55 ± 0.03 | 1.64 ± 0.13 | 2.65 ± 0.17 | 1.84 ± 0.07 | 1.33 ± 0.07 | 1.44 ± 0.07 | 1.11 ± 0.06 | 1.27 ± 0.06 | 1.31 ± 0.05 | 1.86 ± 0.06 | 4.85 ± 0.35 | |
| 95% CI | 0.85–1.05 | 0.81–1.00a | 1.08–1.87 | 0.24–1.32a | 2.51–3.03a | 0.14–0.62a | 1.00–1.39a | 0.69–1.06a | 2.11–2.57a | 1.63–1.99a | 2.34–2.76a | 0.46–1.42a | |
| P value | 0.38 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Abbreviations: 95% CI, 95% confidence interval of acrophase; Acro, acrophase; Amp, amplitude; Cort, plasma corticosterone concentrations in pups at P3, P6, P14, P16, P21, and P24; SD, standard deviation; SE, standard error.
The P values refer to a hypothesis test with null hypothesis that the amplitude is equal to zero.
Presence of circadian rhythm was considered when the mesor was out of limits of the 95% CI of acrophase.
Data on Cosinor Analysis of the Expression of Clock Genes and Steroidogenic Related Genes in Adrenal and Plasma Corticosterone Levels at Different Postnatal Days
| Postnatal Day . | Cosinor Data . | Clock . | Bmal1 . | Per1 . | Per2 . | Per3 . | Cry1 . | Cry2 . | Rora . | Rev-Erbα . | Mc2r . | Star . | Cort . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | Acro ± SD, h | 1151 ± 1.22 | 1728 ± 0.88 | . | 1132 ± 1.27 | 0643 ± 0.32 | 1419 ± 0.98 | 1205 ± 2.42 | . | 0226 ± 1.02 | 1709 ± 1.85 | 0907 ± 2.65 | 0835 ± 1.85 |
| Amp ± SE, 2− ΔΔCt | 0.28 ± 0.05 | 0.36 ± 0.04 | 0.42 ± 0.20 | 2.04 ± 0.36 | 2.39 ± 0.31 | 0.53 ± 0.08 | 0.35 ± 0.12 | 0.1 ± 0.05 | 0.44 ± 0.07 | 0.2 ± 0.05 | 0.25 ± 0.11 | 0.32 ± 0.08 | |
| Mesor ± SE | 1.09 ± 0.03 | 1.05 ± 0.03 | 2.71 ± 0.14 | 4.79 ± 0.25 | 6.54 ± 0.23 | 1.29 ± 0.05 | 2.34 ± 0.08 | 1.15 ± 0.04 | 0.92 ± 0.05 | 1.06 ± 0.03 | 1.33 ± 0.08 | 0.66 ± 0.06 | |
| 95% CI | 1.26–1.49a | 1.31–1.51a | 2.68–3.59 | 6.01–7.66a | 8.20–9.64a | 1.63–2.00a | 2.41–2.98a | 1.13–1.38 | 0.31–0.65a | 1.14–1.38a | 1.34–1.82a | 0.78–1.17a | |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | 0.05 | <0.01 | <0.01 | <0.01 | 0.01 | |
| 6 | Acro ± SD, h | . | 1947 ± 1.98 | . | 1148 ± 1.60 | 0945 ± 1.20 | 1219 ± 1.32 | 1159 ± 1.90 | . | 0116 ± 1.30 | 0048 ± 2.07 | . | . |
| Amp ± SE, 2− ΔΔCt | 0.02 ± 0.03 | 0.15 ± 0.03 | 0.5 ± 0.25 | 0.98 ± 0.23 | 1.41 ± 0.22 | 0.29 ± 0.06 | 0.43 ± 0.13 | 0.005 ± 0.06 | 0.37 ± 0.07 | 0.11 ± 0.04 | 0.13 ± 0.11 | 0.12 ± 0.06 | |
| Mesor ± SE | 0.97 ± 0.02 | 0.55 ± 0.03 | 2.74 ± 0.17 | 2.97 ± 0.16 | 3.27 ± 0.16 | 1.17 ± 0.04 | 1.85 ± 0.08 | 0.94 ± 0.04 | 0.74 ± 0.05 | 0.55 ± 0.02 | 1.37 ± 0.08 | 0.24 ± 0.04 | |
| 95% CI | 0.90–1.08 | 0.33–0.47a | 2.69–3.79 | 3.42–4.48a | 4.21–5.16a | 1.32–1.60a | 1.98–2.57a | 0.80–1.08 | 0.20–0.54a | 0.36–0.53a | 1.26–1.73 | 0.23–0.49 | |
| P value | 0.4 | <0.01 | 0.08 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | |
| 14 | Acro ± SD, h | 0308 ± 2.87 | 0325 ± 1.45 | . | 2107 ± 1.90 | 1609 ± 1.85 | 2231 ± 2.53 | . | 0056 ± 1.32 | 1331 ± 1.30 | . | . | 2223 ± 1.25 |
| Amp ± SE, 2− ΔΔCt | 0.1 ± 0.04 | 0.24 ± 0.04 | 0.21 ± 0.12 | 1.36 ± 0.33 | 0.67 ± 0.16 | 0.38 ± 0.13 | 0.15 ± 0.09 | 0.26 ± 0.06 | 0.55 ± 0.08 | 0.06 ± 0.04 | 0.18 ± 0.09 | 0.57 ± 0.17 | |
| Mesor ± SE | 1.08 ± 0.03 | 0.74 ± 0.03 | 1.93 ± 0.08 | 4.91 ± 0.24 | 3.03 ± 0.12 | 1.2 ± 0.09 | 0.94 ± 0.06 | 1.31 ± 0.04 | 1.13 ± 0.05 | 0.71 ± 0.03 | 1.39 ± 0.06 | 1.91 ± 0.12 | |
| 95% CI | 0.89–1.08 | 0.39–0.61a | 1.45–2.00 | 2.79–4.31a | 3.32–4.10a | 0.50–1.13a | 0.89–1.30 | 0.91–1.18a | 1.49–1.86a | 0.67–0.88 | 1.01–1.40 | 0.93–1.73a | |
| P value | 0.03 | 0.01 | 0.03 | 0.01 | <0.01 | 0.26 | 0.09 | <0.01 | <0.01 | 0.18 | 0.05 | <0.01 | |
| 16 | Acro ± SD, h | 0715 ± 1.63 | 0434 ± 1.15 | . | 2051 ± 1.35 | 1514 ± 1.63 | 2350 ± 0.98 | . | . | 1345 ± 0.80 | 1025 ± 1.70 | . | 2241 ± 0.98 |
| Amp ± SE, 2− ΔΔCt | 0.09 ± 0.03 | 0.15 ± 0.02 | 0.23 ± 0.30 | 1.92 ± 0.34 | 0.96 ± 0.21 | 0.43 ± 0.06 | 0.18 ± 0.14 | 0.11 ± 0.11 | 0.42 ± 0.04 | 0.15 ± 0.03 | 0.22 ± 0.15 | 1.65 ± 0.21 | |
| Mesor ± SE | 1.05 ± 0.02 | 0.56 ± 0.02 | 3.04 ± 0.21 | 5.37 ± 0.24 | 3.44 ± 0.15 | 1.35 ± 0.04 | 1.85 ± 0.10 | 1.41 ± 0.08 | 0.84 ± 0.03 | 0.67 ± 0.02 | 2.0 ± 0.11 | 2.42 ± 0.15 | |
| 95% CI | 1.06–1.21a | 0.36–0.47a | 2.54–4.00 | 2.67–4.23a | 3.86–4.93a | 0.79–1.05a | 1.67–2.37 | 1.25–1.79 | 1.15–1.36a | 0.74–0.90a | 1.85–2.59 | 0.25–1.28a | |
| P value | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| 21 | Acro ± SD, h | 0331 ± 1.68 | 0518 ± 0.97 | 2226 ± 1.93 | 2315 ± 0.87 | 1836 ± 1.65 | 0041 ± 0.72 | . | . | 1315 ± 0.47 | . | 1810 ± 1.65 | 2051 ± 1.08 |
| Amp ± SE, 2− ΔΔCt | 0.22 ± 0.06 | 0.59 ± 0.08 | 1.05 ± 0.28 | 5.04 ± 0.63 | 1.14 ± 0.23 | 0.93 ± 0.09 | 0.17 ± 0.04 | 0.06 ± 0.09 | 1.46 ± 0.11 | 0.03 ± 0.07 | 0.55 ± 0.11 | 3.76 ± 0.50 | |
| Mesor ± SE | 1.5 ± 0.04 | 1.17 ± 0.06 | 3.2 ± 0.19 | 7.76 ± 0.43 | 3.9 ± 0.18 | 1.42 ± 0.06 | 1.94 ± 0.06 | 1.8 ± 0.07 | 1.98 ± 0.07 | 1.36 ± 0.05 | 2.58 ± 0.08 | 4.09 ± 0.37 | |
| 95% CI | 1.14–1.42a | 1.57–1.95a | 1.53–2.78a | 1.33–4.11a | 4.43–5.66a | 0.26–0.70a | 1.90–2.32 | 1.64–2.07 | 3.18–3.69a | 1.22–1.55 | 2.85–3.43a | 0.77–1.44a | |
| P value | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | 0.01 | 0.52 | <0.01 | 0.14 | <0.01 | <0.01 | |
| 24 | Acro ± SD, h | . | 0628 ± 0.95 | . | 2251 ± 0.92 | 1858 ± 0.82 | 0013 ± 0.72 | 1955 ± 1.90 | 0400 ± 2.28 | 1354 ± 0.58 | 1715 ± 1.20 | 1916 ± 1.05 | 1958 ± 0.92 |
| Amp ± SE, 2− ΔΔCt | 0.07 ± 0.09 | 0.35 ± 0.04 | 0.17 ± 0.18 | 1.87 ± 0.24 | 0.93 ± 0.09 | 0.94 ± 0.11 | 0.25 ± 0.09 | 0.28 ± 0.09 | 1.06 ± 0.09 | 0.5 ± 0.07 | 0.69 ± 0.08 | 4.36 ± 0.46 | |
| Mesor ± SE | 1.01 ± 0.03 | 0.55 ± 0.03 | 1.64 ± 0.13 | 2.65 ± 0.17 | 1.84 ± 0.07 | 1.33 ± 0.07 | 1.44 ± 0.07 | 1.11 ± 0.06 | 1.27 ± 0.06 | 1.31 ± 0.05 | 1.86 ± 0.06 | 4.85 ± 0.35 | |
| 95% CI | 0.85–1.05 | 0.81–1.00a | 1.08–1.87 | 0.24–1.32a | 2.51–3.03a | 0.14–0.62a | 1.00–1.39a | 0.69–1.06a | 2.11–2.57a | 1.63–1.99a | 2.34–2.76a | 0.46–1.42a | |
| P value | 0.38 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Postnatal Day . | Cosinor Data . | Clock . | Bmal1 . | Per1 . | Per2 . | Per3 . | Cry1 . | Cry2 . | Rora . | Rev-Erbα . | Mc2r . | Star . | Cort . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | Acro ± SD, h | 1151 ± 1.22 | 1728 ± 0.88 | . | 1132 ± 1.27 | 0643 ± 0.32 | 1419 ± 0.98 | 1205 ± 2.42 | . | 0226 ± 1.02 | 1709 ± 1.85 | 0907 ± 2.65 | 0835 ± 1.85 |
| Amp ± SE, 2− ΔΔCt | 0.28 ± 0.05 | 0.36 ± 0.04 | 0.42 ± 0.20 | 2.04 ± 0.36 | 2.39 ± 0.31 | 0.53 ± 0.08 | 0.35 ± 0.12 | 0.1 ± 0.05 | 0.44 ± 0.07 | 0.2 ± 0.05 | 0.25 ± 0.11 | 0.32 ± 0.08 | |
| Mesor ± SE | 1.09 ± 0.03 | 1.05 ± 0.03 | 2.71 ± 0.14 | 4.79 ± 0.25 | 6.54 ± 0.23 | 1.29 ± 0.05 | 2.34 ± 0.08 | 1.15 ± 0.04 | 0.92 ± 0.05 | 1.06 ± 0.03 | 1.33 ± 0.08 | 0.66 ± 0.06 | |
| 95% CI | 1.26–1.49a | 1.31–1.51a | 2.68–3.59 | 6.01–7.66a | 8.20–9.64a | 1.63–2.00a | 2.41–2.98a | 1.13–1.38 | 0.31–0.65a | 1.14–1.38a | 1.34–1.82a | 0.78–1.17a | |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | 0.05 | <0.01 | <0.01 | <0.01 | 0.01 | |
| 6 | Acro ± SD, h | . | 1947 ± 1.98 | . | 1148 ± 1.60 | 0945 ± 1.20 | 1219 ± 1.32 | 1159 ± 1.90 | . | 0116 ± 1.30 | 0048 ± 2.07 | . | . |
| Amp ± SE, 2− ΔΔCt | 0.02 ± 0.03 | 0.15 ± 0.03 | 0.5 ± 0.25 | 0.98 ± 0.23 | 1.41 ± 0.22 | 0.29 ± 0.06 | 0.43 ± 0.13 | 0.005 ± 0.06 | 0.37 ± 0.07 | 0.11 ± 0.04 | 0.13 ± 0.11 | 0.12 ± 0.06 | |
| Mesor ± SE | 0.97 ± 0.02 | 0.55 ± 0.03 | 2.74 ± 0.17 | 2.97 ± 0.16 | 3.27 ± 0.16 | 1.17 ± 0.04 | 1.85 ± 0.08 | 0.94 ± 0.04 | 0.74 ± 0.05 | 0.55 ± 0.02 | 1.37 ± 0.08 | 0.24 ± 0.04 | |
| 95% CI | 0.90–1.08 | 0.33–0.47a | 2.69–3.79 | 3.42–4.48a | 4.21–5.16a | 1.32–1.60a | 1.98–2.57a | 0.80–1.08 | 0.20–0.54a | 0.36–0.53a | 1.26–1.73 | 0.23–0.49 | |
| P value | 0.4 | <0.01 | 0.08 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | |
| 14 | Acro ± SD, h | 0308 ± 2.87 | 0325 ± 1.45 | . | 2107 ± 1.90 | 1609 ± 1.85 | 2231 ± 2.53 | . | 0056 ± 1.32 | 1331 ± 1.30 | . | . | 2223 ± 1.25 |
| Amp ± SE, 2− ΔΔCt | 0.1 ± 0.04 | 0.24 ± 0.04 | 0.21 ± 0.12 | 1.36 ± 0.33 | 0.67 ± 0.16 | 0.38 ± 0.13 | 0.15 ± 0.09 | 0.26 ± 0.06 | 0.55 ± 0.08 | 0.06 ± 0.04 | 0.18 ± 0.09 | 0.57 ± 0.17 | |
| Mesor ± SE | 1.08 ± 0.03 | 0.74 ± 0.03 | 1.93 ± 0.08 | 4.91 ± 0.24 | 3.03 ± 0.12 | 1.2 ± 0.09 | 0.94 ± 0.06 | 1.31 ± 0.04 | 1.13 ± 0.05 | 0.71 ± 0.03 | 1.39 ± 0.06 | 1.91 ± 0.12 | |
| 95% CI | 0.89–1.08 | 0.39–0.61a | 1.45–2.00 | 2.79–4.31a | 3.32–4.10a | 0.50–1.13a | 0.89–1.30 | 0.91–1.18a | 1.49–1.86a | 0.67–0.88 | 1.01–1.40 | 0.93–1.73a | |
| P value | 0.03 | 0.01 | 0.03 | 0.01 | <0.01 | 0.26 | 0.09 | <0.01 | <0.01 | 0.18 | 0.05 | <0.01 | |
| 16 | Acro ± SD, h | 0715 ± 1.63 | 0434 ± 1.15 | . | 2051 ± 1.35 | 1514 ± 1.63 | 2350 ± 0.98 | . | . | 1345 ± 0.80 | 1025 ± 1.70 | . | 2241 ± 0.98 |
| Amp ± SE, 2− ΔΔCt | 0.09 ± 0.03 | 0.15 ± 0.02 | 0.23 ± 0.30 | 1.92 ± 0.34 | 0.96 ± 0.21 | 0.43 ± 0.06 | 0.18 ± 0.14 | 0.11 ± 0.11 | 0.42 ± 0.04 | 0.15 ± 0.03 | 0.22 ± 0.15 | 1.65 ± 0.21 | |
| Mesor ± SE | 1.05 ± 0.02 | 0.56 ± 0.02 | 3.04 ± 0.21 | 5.37 ± 0.24 | 3.44 ± 0.15 | 1.35 ± 0.04 | 1.85 ± 0.10 | 1.41 ± 0.08 | 0.84 ± 0.03 | 0.67 ± 0.02 | 2.0 ± 0.11 | 2.42 ± 0.15 | |
| 95% CI | 1.06–1.21a | 0.36–0.47a | 2.54–4.00 | 2.67–4.23a | 3.86–4.93a | 0.79–1.05a | 1.67–2.37 | 1.25–1.79 | 1.15–1.36a | 0.74–0.90a | 1.85–2.59 | 0.25–1.28a | |
| P value | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| 21 | Acro ± SD, h | 0331 ± 1.68 | 0518 ± 0.97 | 2226 ± 1.93 | 2315 ± 0.87 | 1836 ± 1.65 | 0041 ± 0.72 | . | . | 1315 ± 0.47 | . | 1810 ± 1.65 | 2051 ± 1.08 |
| Amp ± SE, 2− ΔΔCt | 0.22 ± 0.06 | 0.59 ± 0.08 | 1.05 ± 0.28 | 5.04 ± 0.63 | 1.14 ± 0.23 | 0.93 ± 0.09 | 0.17 ± 0.04 | 0.06 ± 0.09 | 1.46 ± 0.11 | 0.03 ± 0.07 | 0.55 ± 0.11 | 3.76 ± 0.50 | |
| Mesor ± SE | 1.5 ± 0.04 | 1.17 ± 0.06 | 3.2 ± 0.19 | 7.76 ± 0.43 | 3.9 ± 0.18 | 1.42 ± 0.06 | 1.94 ± 0.06 | 1.8 ± 0.07 | 1.98 ± 0.07 | 1.36 ± 0.05 | 2.58 ± 0.08 | 4.09 ± 0.37 | |
| 95% CI | 1.14–1.42a | 1.57–1.95a | 1.53–2.78a | 1.33–4.11a | 4.43–5.66a | 0.26–0.70a | 1.90–2.32 | 1.64–2.07 | 3.18–3.69a | 1.22–1.55 | 2.85–3.43a | 0.77–1.44a | |
| P value | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | 0.01 | 0.52 | <0.01 | 0.14 | <0.01 | <0.01 | |
| 24 | Acro ± SD, h | . | 0628 ± 0.95 | . | 2251 ± 0.92 | 1858 ± 0.82 | 0013 ± 0.72 | 1955 ± 1.90 | 0400 ± 2.28 | 1354 ± 0.58 | 1715 ± 1.20 | 1916 ± 1.05 | 1958 ± 0.92 |
| Amp ± SE, 2− ΔΔCt | 0.07 ± 0.09 | 0.35 ± 0.04 | 0.17 ± 0.18 | 1.87 ± 0.24 | 0.93 ± 0.09 | 0.94 ± 0.11 | 0.25 ± 0.09 | 0.28 ± 0.09 | 1.06 ± 0.09 | 0.5 ± 0.07 | 0.69 ± 0.08 | 4.36 ± 0.46 | |
| Mesor ± SE | 1.01 ± 0.03 | 0.55 ± 0.03 | 1.64 ± 0.13 | 2.65 ± 0.17 | 1.84 ± 0.07 | 1.33 ± 0.07 | 1.44 ± 0.07 | 1.11 ± 0.06 | 1.27 ± 0.06 | 1.31 ± 0.05 | 1.86 ± 0.06 | 4.85 ± 0.35 | |
| 95% CI | 0.85–1.05 | 0.81–1.00a | 1.08–1.87 | 0.24–1.32a | 2.51–3.03a | 0.14–0.62a | 1.00–1.39a | 0.69–1.06a | 2.11–2.57a | 1.63–1.99a | 2.34–2.76a | 0.46–1.42a | |
| P value | 0.38 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Abbreviations: 95% CI, 95% confidence interval of acrophase; Acro, acrophase; Amp, amplitude; Cort, plasma corticosterone concentrations in pups at P3, P6, P14, P16, P21, and P24; SD, standard deviation; SE, standard error.
The P values refer to a hypothesis test with null hypothesis that the amplitude is equal to zero.
Presence of circadian rhythm was considered when the mesor was out of limits of the 95% CI of acrophase.
Ontogeny of the rhythmicity of the clock system genes and steroidogenesis-related genes in adrenal tissue
Table 2 and Fig. 2 summarize the data of the cosinor analysis and present the acrophase, amplitude, and mesor of the mRNA expression of clock genes and steroidogenic-related genes in adrenal of rats at different postnatal days as well as these parameters regarding plasma corticosterone levels. There was a circadian variation of the mRNA expression of Bmal1, Per2, Per3, Cry1, and Rev-Erbα genes from P3 (P < 0.05) and a reversal of their acrophases from P14, when they attained the adult-like patterns. Clock circadian rhythmicity was observed only at P3, P16, and P21. No circadian rhythm of Per1, Cry2, and Rorα genes was observed.
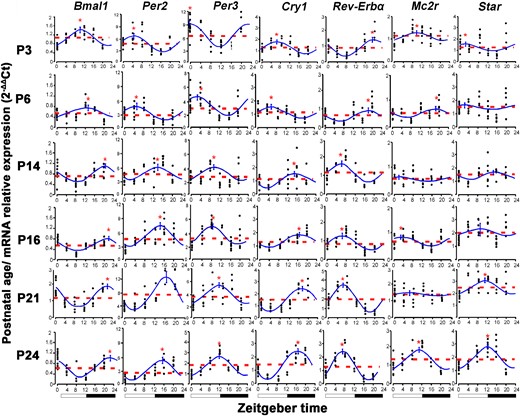
Circadian profiles of adrenal clock and steroidogenesis-related genes expression (2−ΔΔCt) at ZT0, 4, 8, 12, 16, and 20 in rats during postnatal ontogenesis at P3, P6, P14, P16, P21 and P24. Dotted line: mesor; solid line: cosine curve. *Differences between peak and mesor gene expression. Black bars represent dark phase.
The expression of Per2, Per3, and Cry1 genes in adrenal glands showed similar profiles. The acrophases of these genes were observed at light phase in the early neonatal period. However, at P14 they advanced by approximately 10 hours and reached the adult circadian profile at P21 with nocturnal acrophase (Table 2; Fig. 2).
The expression of the Bmal1 gene in adrenal showed the vespertine acrophase at P3 and P6, whereas from P14 and beyond it was observed at the end of the dark phase. In contrast, the Rev-Erbα gene showed acrophase in the middle of the dark period at P3 and P6. Then, from P14, it presented a peak in the middle of light phase, attaining the adult circadian pattern. Thus, Bmal1 and Rev-Erbα gene expression was always in antiphase during ontogeny.
The steroidogenesis-related Star and Mc2r genes presented variable expression during the early neonatal period. The adult-like Star profile was attained at P21 with acrophase at 1810 ± 1.65 hours, whereas Mc2r reached it at P24 with acrophase at 1715 ± 1.20 hours (Table 2; Fig. 2).
Relationship of the acrophases of plasma corticosterone and clock genes in adrenal tissue during postnatal development
Table 3 and Fig. 3 show the pattern of plasma corticosterone and each clock gene acrophases during the postnatal development. The acrophases of Per2 and Cry1 genes and plasma corticosterone were in synchrony in all postnatal stages. In contrast, Bmal1 acrophase occurred by a mean of 7.8 hours after plasma corticosterone acrophase at P3, P14, P16, P21, and P24. In addition, the acrophases of Rev-Erbα and Per3 genes in adrenal occurred by 7.4 hours and 3.6 hours, respectively, before plasma circadian corticosterone acrophase in all postnatal stages.
Comparison Between the Acrophases of Plasma Corticosterone and Clock Gene Expression During Postnatal Development in Rats
| . | Acrophase (h) . | P Value . | ||||
|---|---|---|---|---|---|---|
| P3 . | P14 . | P16 . | P21 . | P24 . | ||
| Corticosterone | 0835 ± 1.85 | 2223 ± 1.25 | 2241 ± 0.98 | 2051 ± 1.08 | 1958 ± 0.92 | |
| Per2 | 1132 ± 1.27 | 2107 ± 1.90 | 2051 ± 1.35 | 2315 ± 0.87 | 2251 ± 0.92 | |
| Delay, h | −3 | ∼1 | ∼2 | −2 | −3 | 0.37 |
| Per3 | 0643 ± 0.32 | 1609 ± 1.85 | 1514 ± 1.63 | 1836 ± 1.65 | 1858 ± 0.82 | 0.04 |
| Delay, h | ∼2 | ∼6 | ∼7 | ∼2 | ∼1 | |
| Cry1 | 1419 ± 0.98 | 2231 ± 2.53 | 2350 ± 0.98 | 0041 ± 0.72 | 0013 ± 0.72 | 0.05 |
| Delay, h | ∼6 | ≈ | −1 | −4 | −4 | |
| Bmal1 | 1728 ± 0.88 | 0325 ± 1.45 | 0434 ± 1.15 | 0518 ± 0.97 | 0628 ± 0.95 | 0.001 |
| Delay, h | −9 | −5 | −6 | −9 | −10 | |
| Rev-Erbα | 0226 ± 1.02 | 1331 ± 1.30 | 1345 ± 0.80 | 1315 ± 0.47 | 1354 ± 0.58 | 0.001 |
| Delay, h | ∼6 | ∼9 | ∼9 | ∼7 | ∼6 | |
| Mc2r | 1709 ± 1.85 | — | 1025 ± 1.70 | — | 1715 ± 1.20 | 0.78 |
| Delay, h | −8 | — | −12 | — | ∼2 | |
| Star | 0907 ± 2.65 | — | — | 1810 ± 1.65 | 1916 ± 1.05 | 0.52 |
| Delay, h | −1 | — | — | ∼2 | ≈ | |
| . | Acrophase (h) . | P Value . | ||||
|---|---|---|---|---|---|---|
| P3 . | P14 . | P16 . | P21 . | P24 . | ||
| Corticosterone | 0835 ± 1.85 | 2223 ± 1.25 | 2241 ± 0.98 | 2051 ± 1.08 | 1958 ± 0.92 | |
| Per2 | 1132 ± 1.27 | 2107 ± 1.90 | 2051 ± 1.35 | 2315 ± 0.87 | 2251 ± 0.92 | |
| Delay, h | −3 | ∼1 | ∼2 | −2 | −3 | 0.37 |
| Per3 | 0643 ± 0.32 | 1609 ± 1.85 | 1514 ± 1.63 | 1836 ± 1.65 | 1858 ± 0.82 | 0.04 |
| Delay, h | ∼2 | ∼6 | ∼7 | ∼2 | ∼1 | |
| Cry1 | 1419 ± 0.98 | 2231 ± 2.53 | 2350 ± 0.98 | 0041 ± 0.72 | 0013 ± 0.72 | 0.05 |
| Delay, h | ∼6 | ≈ | −1 | −4 | −4 | |
| Bmal1 | 1728 ± 0.88 | 0325 ± 1.45 | 0434 ± 1.15 | 0518 ± 0.97 | 0628 ± 0.95 | 0.001 |
| Delay, h | −9 | −5 | −6 | −9 | −10 | |
| Rev-Erbα | 0226 ± 1.02 | 1331 ± 1.30 | 1345 ± 0.80 | 1315 ± 0.47 | 1354 ± 0.58 | 0.001 |
| Delay, h | ∼6 | ∼9 | ∼9 | ∼7 | ∼6 | |
| Mc2r | 1709 ± 1.85 | — | 1025 ± 1.70 | — | 1715 ± 1.20 | 0.78 |
| Delay, h | −8 | — | −12 | — | ∼2 | |
| Star | 0907 ± 2.65 | — | — | 1810 ± 1.65 | 1916 ± 1.05 | 0.52 |
| Delay, h | −1 | — | — | ∼2 | ≈ | |
Data are acrophases ± 95% confidence interval. Symbols: ∼, advance; −, anticipatory; ≈, equal; —, absence of circadian rhythm.
Comparison Between the Acrophases of Plasma Corticosterone and Clock Gene Expression During Postnatal Development in Rats
| . | Acrophase (h) . | P Value . | ||||
|---|---|---|---|---|---|---|
| P3 . | P14 . | P16 . | P21 . | P24 . | ||
| Corticosterone | 0835 ± 1.85 | 2223 ± 1.25 | 2241 ± 0.98 | 2051 ± 1.08 | 1958 ± 0.92 | |
| Per2 | 1132 ± 1.27 | 2107 ± 1.90 | 2051 ± 1.35 | 2315 ± 0.87 | 2251 ± 0.92 | |
| Delay, h | −3 | ∼1 | ∼2 | −2 | −3 | 0.37 |
| Per3 | 0643 ± 0.32 | 1609 ± 1.85 | 1514 ± 1.63 | 1836 ± 1.65 | 1858 ± 0.82 | 0.04 |
| Delay, h | ∼2 | ∼6 | ∼7 | ∼2 | ∼1 | |
| Cry1 | 1419 ± 0.98 | 2231 ± 2.53 | 2350 ± 0.98 | 0041 ± 0.72 | 0013 ± 0.72 | 0.05 |
| Delay, h | ∼6 | ≈ | −1 | −4 | −4 | |
| Bmal1 | 1728 ± 0.88 | 0325 ± 1.45 | 0434 ± 1.15 | 0518 ± 0.97 | 0628 ± 0.95 | 0.001 |
| Delay, h | −9 | −5 | −6 | −9 | −10 | |
| Rev-Erbα | 0226 ± 1.02 | 1331 ± 1.30 | 1345 ± 0.80 | 1315 ± 0.47 | 1354 ± 0.58 | 0.001 |
| Delay, h | ∼6 | ∼9 | ∼9 | ∼7 | ∼6 | |
| Mc2r | 1709 ± 1.85 | — | 1025 ± 1.70 | — | 1715 ± 1.20 | 0.78 |
| Delay, h | −8 | — | −12 | — | ∼2 | |
| Star | 0907 ± 2.65 | — | — | 1810 ± 1.65 | 1916 ± 1.05 | 0.52 |
| Delay, h | −1 | — | — | ∼2 | ≈ | |
| . | Acrophase (h) . | P Value . | ||||
|---|---|---|---|---|---|---|
| P3 . | P14 . | P16 . | P21 . | P24 . | ||
| Corticosterone | 0835 ± 1.85 | 2223 ± 1.25 | 2241 ± 0.98 | 2051 ± 1.08 | 1958 ± 0.92 | |
| Per2 | 1132 ± 1.27 | 2107 ± 1.90 | 2051 ± 1.35 | 2315 ± 0.87 | 2251 ± 0.92 | |
| Delay, h | −3 | ∼1 | ∼2 | −2 | −3 | 0.37 |
| Per3 | 0643 ± 0.32 | 1609 ± 1.85 | 1514 ± 1.63 | 1836 ± 1.65 | 1858 ± 0.82 | 0.04 |
| Delay, h | ∼2 | ∼6 | ∼7 | ∼2 | ∼1 | |
| Cry1 | 1419 ± 0.98 | 2231 ± 2.53 | 2350 ± 0.98 | 0041 ± 0.72 | 0013 ± 0.72 | 0.05 |
| Delay, h | ∼6 | ≈ | −1 | −4 | −4 | |
| Bmal1 | 1728 ± 0.88 | 0325 ± 1.45 | 0434 ± 1.15 | 0518 ± 0.97 | 0628 ± 0.95 | 0.001 |
| Delay, h | −9 | −5 | −6 | −9 | −10 | |
| Rev-Erbα | 0226 ± 1.02 | 1331 ± 1.30 | 1345 ± 0.80 | 1315 ± 0.47 | 1354 ± 0.58 | 0.001 |
| Delay, h | ∼6 | ∼9 | ∼9 | ∼7 | ∼6 | |
| Mc2r | 1709 ± 1.85 | — | 1025 ± 1.70 | — | 1715 ± 1.20 | 0.78 |
| Delay, h | −8 | — | −12 | — | ∼2 | |
| Star | 0907 ± 2.65 | — | — | 1810 ± 1.65 | 1916 ± 1.05 | 0.52 |
| Delay, h | −1 | — | — | ∼2 | ≈ | |
Data are acrophases ± 95% confidence interval. Symbols: ∼, advance; −, anticipatory; ≈, equal; —, absence of circadian rhythm.
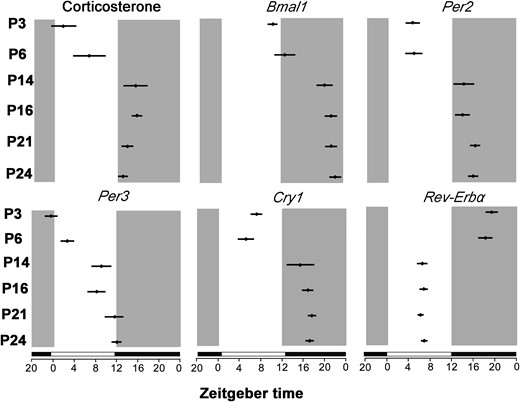
Acrophase changes (hours) in adrenal clock genes expression (Bmal1, Per2, Per3, Cry1, and Rev-Erbα) and plasma corticosterone concentrations at ZT0, 4, 8, 12, 16, and 20 in rats at P3, P6, P14, P16, P21, and P24. Circles indicate acrophase ± 95% confidence interval. Black bars represent the dark phase.
The pattern of acrophases of Mc2r and Star, steroidogenesis-related genes, and plasma corticosterone circadian rhythm during postnatal development seems to be in phase in P24 animals at 1715 ± 1.20 hours and 1916 ± 1.05 hours, respectively (Table 3; Fig. 3).
Discussion
Our data on postnatal ontogeny of daily variation expression of the clock genes in the adrenal of rats demonstrated the presence of circadian rhythm since the early postnatal period, which attained the adult circadian profile from P14. Furthermore, our data show that, depending on the adrenal clock genes their acrophases are in phase, antiphase, or in anticipatory phase with corticosterone circadian rhythm during different stages of postnatal development.
In the current study, the cosinor analysis identified plasma corticosterone circadian rhythm from P1 with acrophase at morning (between ZT0 and ZT2). Two important issues in this original finding deserve further discussion. First, plasma corticosterone rhythmicity was observed at very early postnatal age, which has been never described. Second, the acrophase at early postnatal age was detected in the morning, which can be ascribed to the time of breastfeeding. As already well established, in the light phase, the mother approaches the litter to feed the pups, although mother activity is predominantly nocturnal. In addition, mother body temperature at the time of breastfeeding and milk melatonin circadian rhythm may contribute as synchronizer agents (21–23). Although it is well established that circadian rhythms are self-sustained intrinsic molecular mechanisms observed during mammalian prenatal and postnatal stages, one issue not completely resolved is the role of maternal rhythmicity in the entrainment of fetal and neonatal rhythms (21–23). Thus, we hypothesized that in the early postnatal stage, external rhythmic cues derived from the maternal environment might entrain a circadian rhythm in corticosterone secretion on the pups in spite of the absence of synchronization in SCN cells. From P14, as previously described (11–13), there was a nocturnal increase of corticosterone concentrations. The well-known adult rat circadian rhythm acrophase was observed at ZT12, and nadir was observed at ZT0.
From P10, SCN neurogenesis and intercellular network are completed, allowing the full rhythmicity in the clock gene expression in the SCN cells (9, 10). Of note, we found an association between higher values of nocturnal plasma corticosterone levels with the numbers of pups with eyes open at P14. The light entrainment was traditionally explained by the concept of a hierarchical system, in which light synchronizes the SCN clock genes that subsequently aligns the peripheral clocks. More recently, experiments with SCN clock-deficient mice indicated that light information might sustain clock gene rhythms directly in peripheral tissues. The light information is conveyed via retinal input to nonclock neurons in the SCN to the adrenal gland through a sympathetic route (24–26).
Few studies have examined the postnatal development of the circadian rhythms of clock genes in peripheral tissues; most of them were performed in liver, colon, and heart (27–30). The diurnal expression of clock-related genes in the adrenal gland has been studied in fetal (17) and in adult rats (15). However, to our knowledge, no research has performed a longitudinal study to evaluate the ontogeny of adrenal clock genes (Clock, Bmal1, Per1, Per2, Per3, Cry1, Cry2, Rorα, and Rev-Erbα) and steroidogenesis-related genes (Star and Mc2r) in rats from the early postnatal period until adulthood. Moreover, cosinor analysis allowed us to better characterize the circadian rhythm of clock genes and their relationship with plasma corticosterone rhythm ontogeny.
We demonstrated a circadian variation of the mRNA expression of Bmal1, Per2, Per3, and Cry1 genes with morning acrophase, whereas Rev-Erbα presented nocturnal acrophase from P3. From P14, Bmal1, Per2, Per3, and Cry1 acrophases advance by approximately 10 hours compared with early neonatal periods and become vespertine-nocturnal, compatible with the adult circadian profile, as previously described (15). Our findings suggest that from the early postnatal ages, Bmal1 acrophase occurs later than Per2, Per3, and Cry1 acrophases. These findings are in accordance with the temporal relationship in adult rats between the protein products of CLOCK:BMAL1 heterodimer, which regulate positively the Per and Cry genes that ultimately feed back and inhibit Clock/Bmal1 transcriptional activity that allows the molecular circadian cycle to start over (31, 32)
The REV-ERB protein competes for and inhibits the retinoic acid-related orphan receptor response element binding sites within the promoter of Bmal1 (33). Indeed, our study showed that the Bmal1 and Rev-Erbα gene expressions were in antiphase during all postnatal ontogeny. The adult profile of Bmal1 and Rev-Erbα acrophases was achieved after P14. Bmal1/Rev-Erbα molecular loop has been demonstrated to be necessary for fine-tuning regulation of the circadian rhythmicity, providing the necessary delay to the cycle at near 24 h (34). In addition, it has been described that glucocorticoids modulate the expression of circadian rhythm of the clock genes in peripheral tissues but not in SCN (35, 36). Our data indicate that the adrenal circadian molecular interactions, which are well described in adult rats, are present since early postnatal ages. In addition, the synergy of the two interlocking molecular feedback loops supports the robustness of circadian rhythm since very early age. Per2 and Cry1 circadian profiles were synchronized in phase with the circadian rhythm of plasma corticosterone in all postnatal ages, whereas Bmal1 was in antiphase.
Previous studies have documented that the expression of the circadian genes Per1 and Per2 are rhythmically but differentially expressed in the adrenal cortex and medulla in adult mice or primates (37, 38). However, in the current study we did not dissect the adrenal cortex from the medulla in neonatal rats. Therefore, in neonatal rats, the role of the medulla circadian clock genes and their relationship with the adrenal cortex circadian clock genes and plasma corticosterone have not been explored
Using cosinor analysis, our study also demonstrated circadian variation of Star during the very early neonatal period (P3); however, at P6, P14, and P16, Star expression was flattened with no statistical evidence of circadian rhythm, and at P21 and P24 Star expression achieved an adult circadian rhythm profile. The Star rhythmic expression and corticosterone circadian rhythm had been described in a pool of 15 fetal adrenal glands at E18 (17). In that study and in the current study, the acrophases were coincident with that of the corticosterone rhythm. These data are supported by the role of StAR protein as one of the pacemakers of adrenal steroid biosynthesis. CLOCK:BMAL1 heterodimer acts on the Star upstream cis-elements of the murine promoter, indicating a direct link between the adrenocortical clocks and the regulation of glucocorticoid production (39). It has been shown that, in addition to StAR, several of its transcriptional regulators are also rhythmically expressed in the adrenal gland, such as the nuclear receptors steroidogenic factor 1, nerve growth factor IB, and dose-sensitive sex reversal. Whether transcriptional activation of StAR's regulators is under control of the clock gene machinery is also not currently known, including at neonatal ages (40).
In summary, our data demonstrate the synchronization of the adrenal clock gene circadian variation and, consequently, the corticosterone circadian rhythm during postnatal ontogeny. This synchronization during the early phase of the postnatal ontogeny seems to depend on the external rhythmic synchronizers derived mainly from maternal environment cues. In the later phase, the entrainment of these adrenal circadian variations may shift from maternal and nonphotic to photic cues. At this phase, in addition to light, daily pattern of food ingestion and animal motor activity can be synchronizer agents responsible for circadian changes after weaning. Taken together, our data demonstrate a progressive maturation of adrenal molecular circadian variations during rat ontogeny and its synchrony with the corticosterone circadian rhythm.
Abbreviations:
- P
postnatal day
- qRT-PCR
quantitative real-time polymerase chain reaction
- SCN
suprachiasmatic nucleus
- ZT
zeitgeber time.
Acknowledgments
The authors thank Wendy Turatti, Rogerio Zuliani, and José Roberto Silva from the Laboratory of Endocrinology in our Institution for technical support.
This work was supported by S.L.R.R fellowship 2012/22164-2 and by Grants 2007/58365-3 and 2013/09799-1 from the Sao Paulo Research Foundation and by A.C.M Grant 314279/2009 from the National Council of Technological and Scientific Development.
Author contributions: S.L.R.R. was responsible for the experimental design, execution, data analysis, data interpretation, and manuscript preparation. E.Z.M performed statistical analyses. C.S.M contributed to the data interpretation, manuscript preparation, and some experimental design. S.R.A and M.C. were equally responsible for data interpretation and manuscript preparation. A.C.M was responsible for supervision of research, data interpretation, and manuscript preparation.
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
Address all correspondence and requests for reprints to: Ayrton C. Moreira, MD, PhD, Department of Internal Medicine, Ribeirao Preto Medical School, University of Sao Paulo, Av Bandeirantes, 3900, Ribeirao Preto, SP 14049-900, Brazil. E-mail: [email protected].



