-
PDF
- Split View
-
Views
-
Cite
Cite
Jung Jin Lim, Chae Young Han, Dong Ryul Lee, Benjamin K. Tsang, Ring Finger Protein 6 Mediates Androgen-Induced Granulosa Cell Proliferation and Follicle Growth via Modulation of Androgen Receptor Signaling, Endocrinology, Volume 158, Issue 4, 1 April 2017, Pages 993–1004, https://doi.org/10.1210/en.2016-1866
Close - Share Icon Share
Abstract
The destiny of the ovarian follicle (growth or atresia) is tightly regulated by the actions and interactions of endocrine, paracrine, and autocrine factors. Although androgens are known to be important in the regulation of folliculogenesis, whether they facilitate or suppress follicular growth has been controversial, and the mechanisms involved are not fully understood. Moreover, the role and regulation of androgen receptor (AR) in mediating androgen signaling during follicular development is not clear. Here, we report that the active androgen dihydrotestosterone upregulates the expression of AR and its E3 ligase ring finger protein 6 (RNF6), increasing site-specific AR polyubiquitination and AR transcriptional activity for soluble Kit ligand (sKit-L) expression in preantral follicle growth. RNF6 silencing suppressed dihydrotestosterone-induced AR ubiquitination (lysine residue 63) and proliferation and suppressed apoptosis in preantral granulosa cells, with these responses being overcome by the presence of exogenous sKit-L. Taken together, our findings support the notion that RNF6 plays an important role in androgen-induced, follicle-stage–dependent follicle growth and that it acts by facilitating AR-mediated granulosa cell sKit-L expression and proliferation. Our findings offer insights into the regulatory mechanism of androgen action in ovarian follicular growth.
Ovarian folliculogenesis involves development of the follicle from primordial, primary, preantral, and antral stages to the preovulatory follicle stage (1). During this coordinated series of transitions, follicular cell proliferation, differentiation, and apoptosis are tightly regulated by a complex network of paracrine, autocrine, and endocrine signals, including androgens (2, 3). It is well established that the cellular actions of androgens are mediated via the binding to and activation of androgen receptor (AR) (4). AR, a transcription factor, regulates gene transcription in androgen-dependent cell growth and proliferation (5) and plays important regulatory roles in follicular development (6). However, depending on the stage of follicular development, androgens can promote or suppress ovarian follicle growth (2), although the reasons for these apparently conflicting findings are unclear.
Protein ubiquitination plays an important role in the regulation of protein stability, trafficking, and function (7) and involves three sequential steps: E1 for ubiquitin activation, E2 for ubiquitin conjugation, and E3 for ubiquitin ligation (8). The intracellular levels of AR are regulated by ubiquitination and proteasomal degradation (9), although whether and how AR ubiquitination influences its biological activities is less clear. Small nuclear RING (really interesting new gene) finger proteins are E3 ligases and are nuclear receptor coregulators. Ring finger protein 6 (RNF6), a member of this family, induces AR ubiquitination (10, 11) and is believed to promote AR transcriptional activity in prostate cancer cells (12). However, the relative expression of RNF6 and its role in the regulation of ovarian folliculogenesis is largely unknown. Moreover, if and how it interacts with AR in the regulation of ovarian follicular growth and function is not clear.
Kit ligand (Kit-L; also known as stem cell factor, steel factor, and mast cell growth factor), produced in granulosa cells (GCs), signals through its receptor Kit in the oocyte (13) and stimulates oocyte growth and folliculogenesis (14). Although androgen is known to upregulate GC expression of Kit-L and its action in the ovarian follicle (15), if and how AR and RNF6 are involved in the regulation of Kit-L gene expression and ovarian preantral follicular growth in response by androgen is not known.
The overall objective for the present studies was to examine the expression and role of RNF6 in cell fate determination during ovarian follicular development and its regulation by androgen. In particular, we investigated how RNF6 regulates AR signaling and its down-stream target gene Kit-L. Our overall hypothesis was that androgenic regulation of ovarian follicular development is follicular stage dependent, a phenomenon dependent on the GC level and intracellular localization of RNF6. RNF6 regulates GC fate and follicular growth via modulation of AR content and their transcriptional activity on Kit-L expression. We have shown that RNF6 plays an important role in androgen-induced preantral follicle growth by modulation of AR signaling.
Materials and Methods
Reagents
Pregnant mare's serum gonadotropin, diethylstilbestrol, flutamide, and Duolink In Situ PLA detection reagents were purchased from Sigma (St. Louis, MO), and 5α-dihydrotestosterone was purchased from Steraloids (Newport, RI). Adeno-associated virus system for scramble and RNF6 were from Applied Biological Materials Inc. (Richmond, BC, Canada). Recombinant rat Kit-L was from R&D Systems (Minneapolis, MN). The Kit-L ELISA kit was purchased from Abcam (Cambridge, MA). All sets of polymerase chain reaction (PCR) primers were purchased from Integrated DNA Technologies (Redwood, CA).
Animal preparation
Female Sprague Dawley rats (13 to 14 and 21 days old) were obtained from Charles River Canada (Montreal, QC, Canada), and all animal procedures were carried out in accordance with the Canadian Council on Animal Care guidelines and approved by the University of Ottawa Animal Care Committee.
Follicle isolation and culture
Preantral follicles (which include early-stage antral follicles; 110 to 150 µm) and antral follicles” (150 to 200 µm) from 13- to 14-day-old rats were mechanically isolated and individually cultured (16).
Primary culture of rat GCs and small interfering RNA transfection
GCs from preantral follicles (diethylstilbestrol-primed immature rats; day 21, 1 mg/d, subcutaneously, for 3 consecutive days) and antral follicles (pregnant mare's serum gonadotropin–injected immature rats; day 21, 10 IU, subcutaneously, for 1 day) were isolated by follicular puncture (17). GC cultures were transfected with RNF6 small interfering RNA (siRNA) (RSS314876; Thermo Fisher Scientific, Mississauga, ON, Canada) (AGA GAA CGC AGA GGC ACC GAU UAU A) or scramble siRNA (AM4611; Thermo Fisher Scientific) using Lipofectamine 2000 (Thermo Fisher Scientific) as described previously with modifications (17).
Cell cycle analysis
Cell cycle was analyzed by flow cytometry as described previously (18).
Apoptosis assay
Apoptosis in 5-µm ovarian sections and GC cultures were determined by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay using an In Situ Cell Death Detection Kit (Roche Applied Science, Mannheim, Germany) (19).
Immunoprecipitation and Western blot analysis
GC cultures were harvested by trypsin treatment and lysed in a buffer [0.25 M sucrose, 10 mM Tris (pH 7.5), 1 mM EDTA]. Cell lysates were precleared for 1 hour with protein A/G PLUS-agarose, and the supernatant fractions were incubated with RNF6 or AR antibodies (4°C, overnight) and treated with protein A/G PLUS-agarose (4°C, 4 hours). The immunoprecipitates were then washed three times with Tris-buffered saline. After heating (95°C, 5 minutes), the coimmunoprecipitates were subjected to immunoblotting as described previously (17) using antibodies described in Supplemental Table 1. Immunoreactive bands were densitometrically scanned and normalized with glyceraldehyde 3-phosphate dehydrogenase.
Real-time quantitative PCR analysis
Extraction of GC total RNAs and real-time quantitative PCR analysis were performed as previously described (16). The primers are shown in Supplemental Table 2. Data were analyzed by the 2-∆∆CT method.
Immunofluorescence microscopy
Immunofluorescence (IF) experiments were performed as described (19) with antibodies and conditions as indicated in Supplemental Table 1. At least 300 cells were counted per experimental group.
Proximity ligation assay
AR-RNF6 interaction was assessed as described previously (20) using the proximity ligation assay (PLA) kit. PLA allows specific AR-RNF6 interaction be examined in situ.
ELISA for soluble kit-ligand
Concentration of soluble kit-ligand was assessed by Mouse SCF ELISA Kit according to the manufacturer's protocol.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation was performed as described previously (21). Purified DNA was analyzed by traditional PCR methods or quantitative PCR (Supplemental Table 2).
Intrafollicular injection of RNF6 siRNA
Adeno-associated virus GFP-tagged RNF6 siRNA was microinjected into preantral follicles as described previously (16). The RNF6 adeno-associated virus siRNA sequence was designed based on its rat cDNA sequence. At the end of the 4-day culture period, follicles were fixed and embedded, as described previously (19) for TUNEL (apoptosis) or IF (Ki67).
Statistical analysis
One-, two-, or three-way analysis of variance (ANOVA) was used to assess the effects of and interactions between variables, followed by multiple comparison by the Bonferroni post hoc test using Prism v.5 (GraphPad, San Diego, CA) and Sigma plot v.12 (Systat Software, San Jose, CA).
Results
The regulation of GC proliferation and follicular growth by androgen is follicular stage dependent
Although androgen has been shown to increase proliferation of preantral GCs and prostate cancer cells (22–24), whether these responses are dependent on cellular differentiated status is not known. To examine the follicular stage–dependent regulation of ovarian follicular growth by androgen, rat preantral follicles (110 to 149 µm diameter) and antral follicles (150 to 210 µm diameter) were cultured for 4 days with dihydrotestosterone (DHT) (0 and 1 μm). Preantral and antral follicles exhibited minimal growth in the absence of DHT (P > 0.05) [Fig. 1(a)]. Addition of DHT to the culture media significantly increased preantral follicular growth starting on day 2 [P< 0.05 versus control (CTL)], and preantral follicular growth increased markedly over the duration of the culture period. In contrast, DHT elicited a slight and nonsignificant increase in antral follicular growth (P > 0.05 versus CTL) [Fig. 1(a)]. The DHT-induced preantral follicle growth was associated with changes in GC fate, as evident by increased cell proliferation (Ki67-positive; P < 0.001) and decreased apoptosis (TUNEL-positive; P < 0.001) in GCs isolated from preantral follicles and cultured with DHT, whereas the opposite responses (P < 0.01 and P < 0.05, respectively) were observed in GCs from antral follicles [Fig. 1(b); Supplemental Fig. 1A).
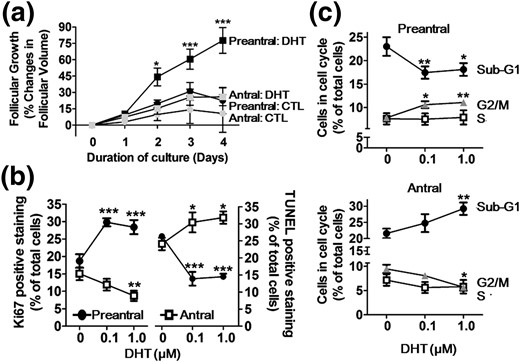
DHT-induced growth of preantral follicles but not antral follicles is associated with increased proliferation and decreased apoptosis in GCs in vitro. (a) Preantral follicles (110 to 150 μm) and antral follicle (151 to 200 μm) isolated from 13- to 14-day-old rats were cultured with or without DHT (1 μm; 4 days). Results are expressed as change in follicular volume and as means ± standard error of the mean of 80 follicles (n = 3 replicates for each experiments with 30/30/20 follicles per group). Data were analyzed by three-way ANOVA and Bonferroni post hoc test. In preantral follicles: *P < 0.05; ***P < 0.001 versus CTL. In antral follicles: P > 0.05 versus CTL. (b) GCs, isolated from preantral and antral follicles of 21-day-old rats injected with diethylstilbestrol (1 mg/d, subcutaneously, 3 days) and pregnant mare's serum gonadotropin (10 IU, intraperitoneally; 48 hours), respectively, were cultured with DHT (0 to 1 µm, 24 hours). Proliferating and apoptotic cells were determined by Ki67-IF and TUNEL assay, respectively, and expressed as percentage of total cells. Data were analyzed by two-way ANOVA and Bonferroni post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001 versus CTL. (c) Flow cytometric analysis of cell cycle progression with propidium iodide. Data were analyzed by one-way ANOVA and Bonferroni post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001 versus CTL.
Flow cytometric studies indicated DHT increased the percentage of preantral GCs in G2/M phase (P < 0.01) and decreased preantral GCs in sub-G1 (P < 0.01) [Fig. 1(c)]. DHT treatment of GCs from antral follicles resulted in a decreased percentage of cells in G2/M phase and an increased percentage of cells in sub-G1 phase [Fig. 1(c); Supplemental Fig. 1B). Taken together, our findings demonstrate that the influence of androgen on follicular growth is dependent on follicle stage. Whereas androgen promotes GC proliferation, suppresses apoptosis, and stimulates preantral follicle growth, the opposite is true at the antral stage of development.
AR and RNF6 are predominantly expressed in GC
We compared the relative expression of AR in the theca cells, GCs, and oocytes at different stages of follicular development by IF. AR fluorescence intensity in the GCs was significantly higher than in the oocytes and theca cells (P < 0.001), and its levels increased with follicular development (Supplemental Fig. 2A). As in AR expression, RNF6 was abundantly expressed in all cell types examined, with significantly higher level in GCs (P < 0.001). However, in contrast to the increased GC AR levels with follicular growth, RNF6 levels were decreased as the preantral follicles developed to the antral stage (Supplemental Fig. 2B). Our findings indicate that GC RNF6 expression is positively associated with androgen-induced follicular development and that its content is tightly regulated to ensure optimal androgen-mediated preantral follicle growth.
Androgen stimulates GC RNF6 and AR expression and nuclear uptake in preantral but not antral follicular stages of development in vitro
DHT significantly increased GC RNF6 and AR contents, both responses being dependent on follicular stage of development (two-way ANOVA: DHT treatment, P < 0.01; follicular stage, P < 0.01). RNF6 contents in the GCs from preantral follicles (P < 0.01 versus CTL) but not antral follicles (P > 0.05 versus CTL) were increased in the presence of DHT [Fig. 2(a)]. Similarly, whereas AR content in GCs from preantral follicles was significantly upregulated by DHT in vitro (P < 0.01 versus respective CTL), as has previously been demonstrated (25), that from the antral follicles was unaffected by DHT (P > 0.05 versus respective CTL) [Fig. 2(b)]. Immunofluorescence localization in the GCs isolated from preantral follicles indicates a greater abundance of RNF6 in the cytoplasmic area when compared with just nucleus or nucleus and cytoplasm (N+C), but this finding was not significantly affected by DHT in vitro. In contrast, the number of cells with RNF6 localized in both N+C was markedly increased by DHT (P < 0.001) in a concentration-dependent manner (P < 0.01, 0.1 µm DHT versus CTL; P < 0.001, 1 µm DHT versus CTL). However, intracellular localization of RNF6 in GCs from antral follicle was not influenced by androgen treatment [Fig. 2(c)].
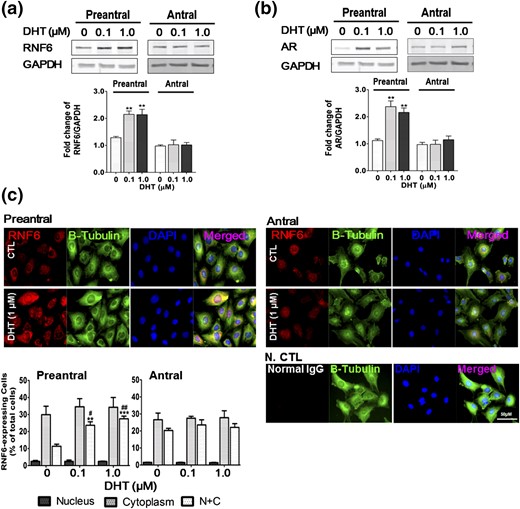
DHT increases RNF6 and AR contents and nuclear RNF6 localization in GCs from preantral and antral follicles in vitro. GCs isolated from preantral and antral follicles were cultured with DHT (0 to 1 µm, 24 hours), and changes in (a) RNF6 and (b) AR contents were assessed by Western blot. Results are expressed as means ± standard error of the mean (n = 4) and analyzed by two-way ANOVA and Bonferroni post hoc test. **P < 0.01 versus CTL. (c) Subcellular localization of RNF6 was analyzed by IF and is shown as percentage of total cells. Data were analyzed by two-way ANOVA and Bonferroni post hoc test. N+C RNF6 localization: **P < 0.01; ***P < 0.001 versus CTL. #P < 0.05; ##P < 0.01 (DHT 1.0 µm versus DHT 0.1 µm). Normal rabbit IgG was used for negative control (N CTL). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
GCs from preantral follicles exhibited a greater abundance of AR in the nucleus when compared with cytoplasm and N+C (P < 0.001), and the percentage of the cells with nuclear AR localization was increased with DHT in a concentration-dependent manner (P < 0.01, 0.1 µm DHT versus CTL; P < 0.001, 1 µm DHT versus CTL). In contrast, although DHT increased the percentage of AR expressing GCs from antral follicles (P < 0.05, 1 µm DHT versus CTL), this increase was relatively modest compared with cells from preantral follicles (0.5-fold versus 7-fold) (Supplemental Fig. 3). Taken together, these results suggest that RNF6 and AR contents and their signaling, as regulated by androgen, play a more important role in promoting follicle growth at the preantral stage than at later stages of development.
RNF6 and AR accumulate and interact in the nucleus in response to androgen
The intracellular colocalization [nucleus, cytoplasm, or both (N+C)] of RNF6 and AR in GCs cultured with DHT was examined by IF [Fig. 3(a)]. Colocalization of RNF6 and AR was most evident in the nucleus when compared with cytoplasm or N+C in GCs from preantral follicles, and this was significantly upregulated by DHT in vitro (P < 0.001 versus CTL). Although the percentage of GCs from antral follicles showing nuclear AR-RNF6 colocalization was also increased by DHT treatment (P < 0.001 versus CTL), the response was considerably lower (preantral versus antral: 38.9 ± 3.4 versus 12.2 ± 0.2) [Fig. 3(a)].
![Androgen induces follicular stage–specific nuclear colocalization and interaction of RNF6 and AR in GCs in vitro. (a) GCs from preantral and antral follicles were cultured with DHT (0 and 1 µm, 24 hours). Staining for AR (green) and RNF6 (red) was analyzed and quantified using ImageJ (JACoP). AR-RNF6 colocalization (red) was analyzed using DAPI (blue, nucleus) and anti–β-tubulin (gray, cytoplasm). Data were analyzed by two-way ANOVA and Bonferroni post hoc test. ***P < 0.001 versus CTL. (b) AR-RNF6 interaction in GCs was assessed by PLA using Duolink ImageTool. The number of AR-RNF6 interactions (red spot) and their cellular localizations were determined [nucleus (blue), cytoplasm (green)] and analyzed by two-way ANOVA and Bonferroni post hoc test. ***P < 0.001 versus CTL; ###P < 0.001 versus DHT. Cyt, cytoplasm; Nuc, nucleus.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/endo/158/4/10.1210_en.2016-1866/2/m_en.2016-1866f3.jpeg?Expires=1750332185&Signature=S6YPzUG4xHnPOpzvg5sJrtRDA7o9-TkwZIBgrmUcHYGCoXpccxMkBa-vHX~gcqEBy7ZX8aqhwEJbKTfdXPeSC3EroIzLC1ig7HJlmzCDobL9KDc05QO39mVgr3DZcM9oCd~DnDp0~pQYounpOorSr1ruMIn8eeUkL6eSOjB2S2lyVJGM9a6t1tyYHCIkLUFYwqorqE7kOV0iWCzeqF3Ibw0Sg8c1y8nEHEAoWZzm7YGk8-wBlN7K39gQRSp~Xly4suqv6VNmqCWzVC6tvFP4oLuN04fTCxr8YudfuPeX05-tkcI9Z1h9PFJnzUL0EX6OzqHYpMvSk8dqn8PmJBxyqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Androgen induces follicular stage–specific nuclear colocalization and interaction of RNF6 and AR in GCs in vitro. (a) GCs from preantral and antral follicles were cultured with DHT (0 and 1 µm, 24 hours). Staining for AR (green) and RNF6 (red) was analyzed and quantified using ImageJ (JACoP). AR-RNF6 colocalization (red) was analyzed using DAPI (blue, nucleus) and anti–β-tubulin (gray, cytoplasm). Data were analyzed by two-way ANOVA and Bonferroni post hoc test. ***P < 0.001 versus CTL. (b) AR-RNF6 interaction in GCs was assessed by PLA using Duolink ImageTool. The number of AR-RNF6 interactions (red spot) and their cellular localizations were determined [nucleus (blue), cytoplasm (green)] and analyzed by two-way ANOVA and Bonferroni post hoc test. ***P < 0.001 versus CTL; ###P < 0.001 versus DHT. Cyt, cytoplasm; Nuc, nucleus.
The RNF6-AR interaction was higher in the nuclear fraction than in the cytoplasmic fraction from GCs of preantral follicles and was increased by the presence of DHT. In contrast, the AR-RNF6 interaction was minimal in nuclear and cytoplasmic fractions from antral GCs and was not affected by the androgen (data not shown). To better quantify the RNF6-AR interaction and to demonstrate that the interaction was indeed direct, we assessed this response in the cultured GCs by the PLA [Fig. 3(b)]. The RNF6-AR interaction at the nucleus (i.e., the number of PLA signals per cell) was markedly increased with DHT in GCs from preantral follicles (P < 0.001, 1 µm DHT versus CTL). Moreover, a more extensive RNF6-AR interaction was evident in the nucleus of DHT-treated GCs from preantral follicles compared with antral follicles (P < 0.001; preantral versus antral: 14.7 ± 0.9 versus 4.1 ± 0.8) [Fig. 3(b)]. These results demonstrate that the RNF6-AR interaction and AR signaling are modulated by androgen in a follicular stage–dependent manner. The androgen-induced RNF6-AR interaction in the nucleus of GCs supports the notion that androgen may play an important role in the regulation of gene transcription involved in the androgenic stimulation of preantral follicle growth.
DHT promotes RNF6-mediated lysine residue 63–linked polyubiquitination of AR
To determine if DHT-induced nuclear RNF6-AR interaction in the GCs is associated with AR ubiquitination, poly- and monoubiquitination of AR at lysine residue 48 (K48) and lysine residue 63 (K63) was examined in GCs from preantral follicles cultured for different durations (0, 3, 6, 12 hours) without or with DHT by coprecipitation. At 12 h, DHT increased polyubiquitination of AR K63 (P < 0.01, DHT versus CTL: 3.6 ± 0.5 versus 1.2 ± 0.3) but not at K48 (P > 0.05) [Fig. 4(a)]. Additionally, monoubiquitination of AR at K63 and K48 was not significantly affected by DHT (Supplemental Fig. 4A).
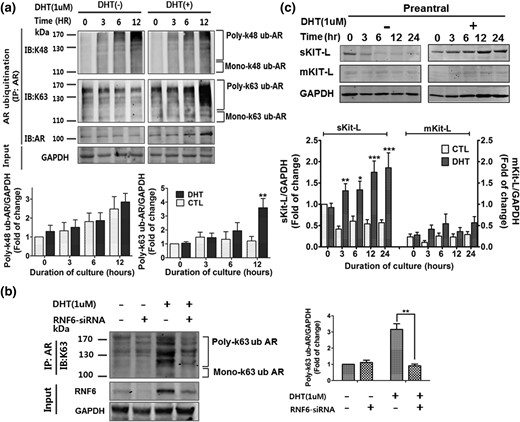
RNF6 silencing attenuates DHT-induced K63-linked polyubiquitination of AR, and DHT upregulates its down-stream target sKit-L. (a) GCs from preantral and antral follicles were cultured with DHT (0 to 1 µm, 24 hours), subjected to immunoprecipitation with anti-AR, and followed by immunoblotting with anti-K48 ubiquitin or K63 ubiquitin. Results are expressed as means ± standard error of the mean (SEM) (n = 3) and analyzed by two-way ANOVA and Bonferroni post hoc test. **P < 0.01 versus CTL. (b) GCs were transfected with RNF6 siRNA (50 nM, 12 hours; scramble siRNA as control) and then treated with DHT (1 µm, 24 hours). Cells were immunoprecipitated using anti-AR, and the precipitate was immunoblotted with anti-K63 ubiqutin antibody. Results are expressed as means ± SEM (n = 3) and analyzed by two-way ANOVA and Bonferroni post hoc test. **P < 0.01, DHT versus DHT + RNF6 siRNA. (c) Changes in contents of sKit-L or mKit-L in GC cultures were assessed by Western blotting, expressed as means ± SEM (n = 3), and analyzed by two-way ANOVA and Bonferroni post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001 versus DHT at 0 hours. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
To investigate the role of RNF6 in androgen-induced K63-linked AR polyubiquitination in GCs, the influence of RNF6 downregulation on DHT-induced K63-linked AR polyubiquitination in cultured GCs from preantral follicles was examined by IP and Western blot. DHT markedly increased K63-linked AR polyubiquitination in GCs, and this response was significantly inhibited by RNF6 silencing (P < 0.01; DHT versus DHT + RNF6 siRNA: 3.2 ± 0.3 versus 0.9 ± 0.1) [Fig. 4(b)]. The aforementioned changes showed that RNF6 plays an important regulatory role on K63-linked AR polyubiquitination in the regulation of AR transcriptional activity in GCs in preantral follicles.
Androgen increases the content of soluble Kit-L, an AR down-stream target in GCs
DHT increased the content of soluble Kit-L (sKit-L) but not membrane-bound Kit-L (mKit-L) in GCs from preantral follicles in a time-dependent manner in vitro (two-way ANOVA: DHT, P < 0.01; duration, P < 0.01; DHT × duration, P < 0.05) [Fig. 4(c)]. sKit-L contents in GCs and their spent media showed a similar response to DHT (two-way ANOVA: DHT, P < 0.01; duration, P < 0.01; DHT × duration, P < 0.01) [Supplemental Fig. 4B]. Because DHT-induced polyubiquitination of AR occurs at K63 rather than at K48, our findings raise the interesting possibility that RNF6 plays a more important regulatory role on AR transcriptional activity in the regulation of sKit-L expression than AR degradation in GCs in preantral follicles.
RNF6 is important for AR binding to the Kit-L promoter region and sKit-L expression
To investigate the role of RNF6 in androgen-induced Kit-L expression in GCs, the influence of RNF6 silencing (siRNA) and the specific AR antagonist flutamide on DHT-induced sKit-L promoter binding of AR (chromatin immunoprecipitation assay), sKit-L and mKit-L mRNA abundance (quantitative reverse transcription PCR), and sKit-L protein contents (ELISA and Western blot) in cultured GCs from preantral follicles was examined. Effective downregulation was confirmed by Western blotting (Supplemental Fig. 5). Figure 5(a) demonstrates that although DHT markedly increased AR binding onto the promoter region of the KIT-L gene in GCs, this response was significantly attenuated by RNF6 silencing (P < 0.01; 6.4 ± 0.7 versus 1.8 ± 1.3) or treatment with flutamide (P < 0.01; 6.4 ± 0.7 versus 0.9 ± 0.2). The aforementioned changes in the AR–sKit-L promoter binding were also associated with down-stream responses. Whereas the sKit-L mRNA abundance and protein contents in GCs were significantly upregulated by DHT in vitro, they were significantly decreased in the presence of RNF6 siRNA (sKit-L mRNA: 7.5 ± 1.5 versus 0.8 ± 0.4, P < 0.001; sKit-L content: 3.3 ± 0.8 versus 0.9 ± 0.1, P < 0.001) or flutamide (sKit-L mRNA: 7.5 ± 1.5 versus 1.4 ± 0.9, P < 0.01; sKit-L content: 3.3 ± 0.8 versus 1.1 ± 0.2, P < 0.01) [Fig. 5(b) and 5(c)]. However, the abundance of mKit-L mRNA was not different in all groups, irrespective of the presence of DHT (P > 0.05) [Fig. 5(b)]. As observed in previous results, sKit-L contents in the GCs and spent media were likewise modulated by DHT, RNF6 silencing, and blockade of AR action (P < 0.001) [Fig. 5(d)]. Taken together, these results suggest that, in addition to AR, RNF6 plays an important role in androgen-induced sKit-L gene expression in GCs during preantral follicle development.
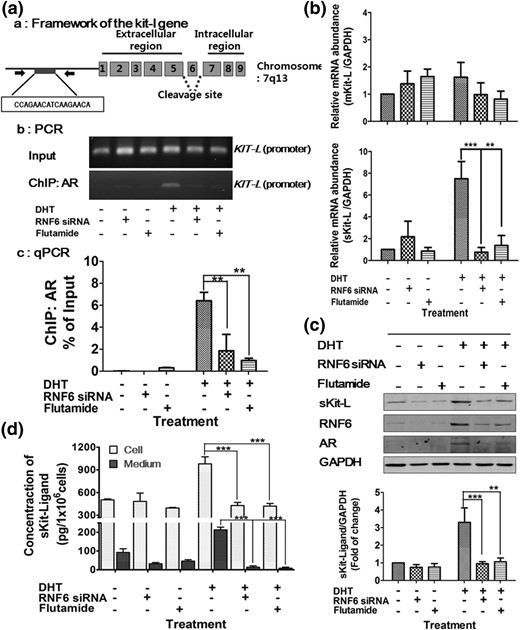
DHT increases GC AR–Kit-L promoter binding and sKit-L expression, with the latter response being sensitive to downregulation of AR or RNF6. (a) GCs were treated with flutamide or transfected with RNF6 siRNA (scramble siRNA as control as described in Fig. 4) and then treated with DHT (1 µm, 24 hours). (a) AR binding to the KIT-L promoter of the rat KIT-L gene; the predicted AR sequence was located at the promoter region from −1 to −3000 upstream, and the amplicon targeting AR bound Kit-ligand was designed in region of −179 and −36 bp where ARE consensus binding (–CCA GAA CAT CAA GAA CA–) is located. (b) Chromatin immunoprecipitation (ChIP) assay: GCs were cross-linked, sonicated, and subjected to ChIP with anti-AR antibody. Precipitated DNA was visualized on agarose gel and (c) analyzed by quantitative PCR (qPCR). The data are presented as the percentage change in the immunoprecipitated PCR products normalized based on the input PCR (as 100%). Results are expressed as means ± standard error of the mean (SEM) (n = 3) and analyzed by two-way ANOVA and Bonferroni post hoc test. **P < 0.01***; P < 0.001 versus DHT. (b) mRNA abundance of sKit-L and mKit-L in GCs was analyzed by quantitative PCR and normalized to glyceraldehyde 3-phosphate dehydrogenase. Results are expressed as means ± SEM (n = 3) and analyzed by two-way ANOVA and Bonferroni post hoc test. **P < 0.01; ***P < 0.001 versus DHT. (c) sKit-L contents of GCs were analyzed by Western blot, expressed as means ± SEM (n = 3), and analyzed by two-way ANOVA and Bonferroni post hoc test. **P < 0.01; ***P < 0.001 versus DHT. (d) Concentration of sKit-L in GCs and spent media were analyzed by ELISA, presented as means ± SEM (n = 3) of sKit-L in pg/106 cells, and analyzed by two-way ANOVA and Bonferroni post hoc test. **P < 0.01; ***P < 0.001 versus DHT. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
To investigate the role of Kit-L in androgen-induced, RNF6-mediated preantral follicle growth, rat RNF6 adeno-associated virus siRNA (RNF6 siRNA) and its scramble control (scramble siRNA) were injected into the GC layers of preantral follicles, and DHT-induced GC proliferation, apoptosis, and follicular growth were assessed over a 4-day culture period. DHT significantly increased the growth of scramble siRNA-injected preantral follicles [% change of follicle growth of scramble siRNA/DHT(+)/sKit-L(−) follicles versus Scramble siRNA/DHT(−)/sKit-L(−) follicle at day 3: 55.9 ± 5.4 versus 22.9 ± 5.4, P < 0.001; at day 4: 89.0 ± 9.5 versus 27.8 ± 4.4, P < 0.001]. However, the RNF6 siRNA–injected preantral follicles exhibited a significant decrease in DHT-induced preantral follicular growth [RNF6 siRNA/DHT(+)/sKit-L(−) follicles versus Scramble siRNA/DHT(+)/sKit-L(−) follicles; day 3: 16.5 ± 3.4 versus 55.9 ± 5.4, P < 0.001; day 4: 21.4 ± 3.8 versus 89.0 ± 9.5, P < 0.001]. These latter responses were overcome by the presence of exogenous sKit-L [RNF6 siRNA/DHT(+)/sKit-L(+) follicles versus RNF6 siRNA/DHT(+)/Kit-L(−) follicles; day 3: 59.4 ± 5.6 versus 16.5 ± 3.4, P < 0.001; day 4: 93.8 ± 9.5 versus 21.4 ± 3.8, P < 0.001] [Fig. 6(a)]. Although RNF6 silencing attenuated DHT-induced proliferation [Fig. 6(b)] and suppressed apoptosis [Fig. 6(c)] in preantral GCs, these responses were also overcome by the addition of sKit-L in the culture medium. Taken together, our findings support the notion that RNF6 plays an important role in androgen-induced preantral follicle growth and that it acts by facilitating AR-mediated GC sKit-L expression and proliferation.
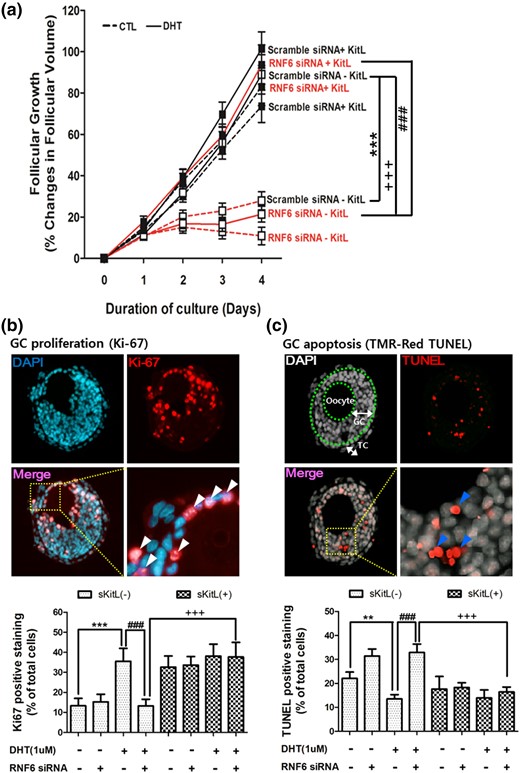
Exogenous sKit-L restores preantral follicular growth downregulated by RNF6 silencing in vitro. (a) RNF6 siRNA was microinjected into the GC layer of preantral follicle (110 to 150 μm) from 14-day-old rats and cultured for 4 days with DHT (0 and 1 µm) in the absence or presence of sKit-L (100 ng/mL). Changes in follicular volume (assessed daily) were expressed as means ± standard error of the mean (SEM) of 25 follicles (n = 3 replicates for each experiment with 10/10/5 follicles per group). ***P < 0.01 versus CTL; ###P < 0.001 versus DHT alone; +++P < 0.001 versus DHT and RNF6 siRNA. After the 4-day culture period, proliferating or apoptotic cells in the cultured follicles were analyzed by Ki67 (b) or TUNEL (c) and expressed as means ± SEM of 12 follicles (n = 3 replicates for each experiment with four follicles per group) and as a percentage of total cell number. (b) ***P < 0.01 versus CTL; ###P < 0.001 versus DHT alone; +++P < 0.001 versus DHT and RNF6 siRNA. (c **P < 0.01 versus CTL; ###P < 0.001 versus DHT alone; +++P < 0.001 versus DHT and RNF6 siRNA. TC, theca cell layer. White (Ki67) or blue (TUNEL) arrow heads indicate positive signal.
Discussion
Evidence indicates that androgen plays an important role in the regulation of ovarian follicular development, although the nature of this regulation is unclear. In the current study, we have investigated the role of RNF6 in the action of androgen in determining the destiny of the developing follicle. We have demonstrated that DHT promotes follicle growth in a stage-dependent manner via changes in GC proliferation and apoptosis. In contrast to antral follicles, which responded to DHT with minimal or suppressed follicle growth, androgenic stimulation of the preantral follicles results in increased GC expression of RNF6 and AR, their interaction, site-specific AR polyubiquitination, and sKit-L expression and in increased follicular growth. These observations suggest that androgen-induced expression and the RNF6-AR interaction, which regulate ubiquitin-mediated AR transcriptional activity and sKit-L expression, are positive regulatory events during preantral follicular growth and that these signaling mechanisms are compromised as the preantral follicle transition to the antral stage.
Androgens play an important role in ovarian follicular growth and development (2, 25). However, whether androgen promotes or suppresses follicle growth is controversial. Whereas earlier studies indicate that androgen is a negative regulator of follicular development that increases GC apoptosis, enhances follicular atresia, and reduces fertility in rodents (26, 27), others reported that animals exposed to androgens exhibited increased GC and theca cell proliferation, increased follicular recruitment and growth, and enhanced ovulation rates (2, 28). Although androgen receptor knockout mice have normal ovarian and oviductal morphology, they exhibit considerable reproductive defects, including fewer pups per litter, defective folliculogenesis, and premature ovarian failure (29, 30). In vivo and in vitro studies have suggested that androgen promotes preantral follicle growth, accelerating development into antral follicles and inhibiting follicular atresia (31).
Although it is well established that androgen and FSH interact in the regulation of ovarian follicular growth, the steroid is known to augment follicular FSH receptor expression in primate follicles (32). Treatment of primate GCs from preantral follicles with FSH and DHT leads to a dramatic increase in steroidogenesis and proliferation, whereas androgen treatment of GCs from large antral follicles leads to an inhibition of FSH activity, which leads to apoptosis (33–35). Whether this interaction also involves the regulation of RNF6-mediated, site-specific AR ubiquitination and subsequent enhanced gene expression remains to be determined. Nonetheless, our current findings not only support earlier reports that androgen promotes follicular growth (2, 27) but also demonstrate how these responses could be attenuated at different cellular process, thus providing insights into reason(s) for the apparent contradictory responses. In this context, our current studies show that DHT significantly increased androgen receptor and follicular growth in the preantral follicles but not in the antral follicles in vitro. In GC cultures, DHT significantly increased the percentage of Ki67-positive preantral GCs in G2/M phase (proliferating population) and decreased those that are TUNEL-positive in Sub-G1 phase (apoptotic population). In contrast, the androgen elicited opposite responses in GCs from antral follicles under the same conditions, suggesting that the role of androgen in the regulation of ovarian folliculogenesis is follicle stage dependent.
AR activity is regulated by a variety of post-translational modifications, including ubiquitination, phosphorylation, acetylation, methylation, and sumoylation (9). Protein ubiquitination regulates various cellular processes and may involve the attachment of ubiquitin to substrates as a single ubiquitin (monoubiquitination) or as a ubiquitin chain (polyubiquitination). Ub contains seven lysine residues (K6, K11, K27, K29, K33, K48, and K63) through which the ubiquitination chain extends (36). Whereas it is well established that polyubiquitination at K48 leads to AR degradation by the 26S proteasome (37), less is known about polyubiquitination at K63 and how it alters AR activity and localization (38). Although biochemical and mass spectrometry analysis indicates interactions between RNF6 and AR (12), there is no report demonstrating the RNF6-AR interaction and whether RNF6 directly modulates AR transcriptional activity during androgen-induced ovarian follicular growth. Our current results show that DHT increases GC RNF6 and AR content in the preantral follicles in vitro. RNF6 interacts directly with AR in the GC nucleus, and this interaction is increased by DHT. Also, DHT promotes site-specific polyubiquitination of AR (K63- but not K48-linked), raising the possibility that RNF6 plays an important role in androgen-induced gene transcription during preantral follicle growth.
Microarray data from ARKO mice (29) indicate that Kit-L, a growth factor involved in the oocyte-GC regulatory loop in the regulation of folliculogenesis (39), is an AR target gene (40). Kit-L is a pleiotropic growth factor that exerts an influence on target cells through binding its cognate tyrosine kinase receptor, c-Kit, and regulates ovarian follicle cell survival, migration, and proliferation (41). In the mouse, Kit-L is expressed in GCs, whereas c-Kit receptor is confined to the oocytes and theca interna cells (14, 42). In the rat, Kit-L expression in follicles is localized to GCs (41, 43), supporting the concept that Kit-L may be an important paracrine promoter of early follicle development. In vitro ovarian follicle studies confirm that Kit-L enhances preantral follicle growth (44). However, whether the GC RNF6-AR interaction is involved in androgen-induced KIT-L expression and its transcriptional activation is unclear. Results from our studies support the concept that androgen-induced preantral follicle growth is mediated through enhanced KIT-L expression and increased cell proliferation and suppressed apoptosis in GCs. Androgen increased the nuclear RNF6-AR interaction and KIT-L promoter binding. Whereas downregulation of AR or RNF6 decreases AR binding to KIT-L promoter region and sKit-L contents in GCs, exogenous sKit-L attenuates RNF6 siRNA-induced downregulation of preantral GC proliferation and increases apoptosis and preantral follicular growth stimulated by androgen. These findings provide strong support for an important role of RNF6 in androgen-induced, Kit-L–mediated preantal follicular growth.
In conclusion, our present study provides insight into the role of RNF6 in AR signaling in the regulation of GC fate during follicle growth. RNF6 is a novel positive mediator in androgen-induced AR ubiquitination, KIT-L expression, and GC proliferation. To facilitate future investigation into the role and regulation of RNF6 in the androgenic control of ovarian follicular development, the following hypothetical model is proposed (Fig. 7). Androgen upregulates RNF6 and AR expression, increasing site-specific AR polyubiquitination and AR transcriptional activity for sKit-L expression. sKit-L indirectly increases proliferation and suppress apoptosis of GCs in a paracrine manner, probably mediated through the expression and action of oocyte-derived GDF9, and promotes preantral follicular growth (40, 45). Although these findings are intriguing, their pathologic significance remains to be explored. It is of interest to note that individuals with polycystic ovary syndrome often exhibit hyperandrogenemia increased preantral follicle growth but suppressed antral development as well as ovulatory dysfunction (46). Whether and how these follicular stage–dependent AR responses are important in the observed differential follicular growth in polycystic ovary syndrome (PCOS) remains to be determined. Investigation of the AR/RNF6 signaling pathway in the DHT-induced PCOS rat model may uncover important clues about the pathophysiology of PCOS and may lead to novel therapeutic approaches for AR-related reproductive diseases. Our findings contribute to the current understanding of the cellular basis of androgen action in ovarian follicular growth.
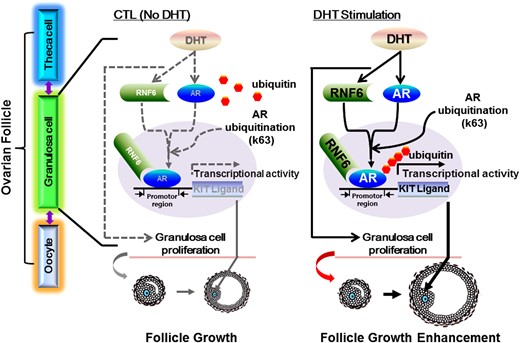
A hypothetical model for RNF6 as a mediator of androgen-induced GC proliferation and preantral follicle growth. Androgen upregulates RNF6 and AR expression, increasing RNF6-mediated, site-specific AR polyubiquitination and AR transcriptional activity for sKit-L expression. sKit-L increases proliferation and suppresses apoptosis of GCs and promotes preantral follicular development.
Abbreviations:
- ANOVA
analysis of variance
- AR
androgen receptor
- CTL
control
- DHT
dihydrotestosterone
- GC
granulosa cell
- IF
immunofluorescence
- K48
lysine residue 48
- K63
lysine residue 63
- Kit-L
Kit ligand
- mKit-L
membrane-bound Kit ligand
- PCOS
polycystic ovary syndrome
- PCR
polymerase chain reaction
- PLA
proximity ligation assay
- RNF6
ring finger protein 6
- siRNA
small interfering RNA
- sKit-L
soluble Kit ligand
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
Acknowledgments
This work was supported by grant MOP-119381 from the Canadian Institutes of Health Research (to B.K.T.); by Grants 2009-0093821 and 2015M3A9C6028961 from the National Research Foundation of Korea (D.R.L.); by postdoctoral fellowships from the IHDCYH-Training Program in Reproduction, Early Development, and the Impact on Health (to J.J.L.); and by the Basic Science Research Program through the National Research Foundation of Korea funded by Ministry of Education Grant NRF-2015R1A6A3A03020098 (to J.J.L.).
Author contributions: J.J.L. and B.K.T. designed the research plan; J.J.L. and C.Y.H. performed the experiments; B.K.T. contributed new reagents/analytic tools; J.J.L., D.R.L., and B.K.T. analyzed experimental data; J.J.L., D.R.L. and B.K.T. wrote the paper; D.R.L. and B.K.T. provided financial support for the project.
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
Address all correspondence and requests for reprints to: Benjamin K. Tsang, PhD, Ottawa Hospital Research Institute, The Ottawa Hospital–General Campus, Critical Care Wing, 3rd Floor, Room W3107, 501 Smyth Road, Ottawa, Ontario, Canada K1H 8L6 E-mail: [email protected].



