-
PDF
- Split View
-
Views
-
Cite
Cite
Joseph Okafor, Anastasia Vamvakidou, Alexander R Lyons, Rajdeep Khattar, Mitral annular fibrous band—a unique morphological variant of a rare congenital mitral valve anomaly: a case report, European Heart Journal - Case Reports, Volume 6, Issue 11, November 2022, ytac444, https://doi.org/10.1093/ehjcr/ytac444
Close - Share Icon Share
Abstract
Left atrial bands are rare and can be associated with mitral valve dysfunction, heart failure, and stroke. Most cases are identified on autopsy, and the demonstration in vivo is very uncommon. Various anatomical configurations have been reported. This description of a mitral annular fibrous band contributes to the literature as the first reported case to traverse the supravalvular mitral inflow region, without involving the left atrium.
A 59-year-old man with a history of metastatic duodenal carcinoma was admitted with a 2-week history of fever and rigors. Inflammatory markers were elevated and blood cultures positive for Enterococcus feacium. Transoesophageal echocardiography performed to investigate for infective endocarditis revealed a 2.3 cm long, thin fibrous band attached to the posterior mitral annulus and extending to the base of the middle scallop of the anterior mitral valve leaflet causing localized tethering, but no valve dysfunction. The band was felt to represent a bystander anatomic variant unrelated to the sepsis, which was most likely gastrointestinal in origin. The patient responded well to intravenous antibiotics.
The presence of an abnormal intracardiac structure in the setting of occult infection should always raise the suspicion of infective endocarditis. Using detailed 2D multiplanar and 3D transoesophageal echocardiography, we were able to identify the anomalous band and exclude any overt infective vegetations attached to the band or the leaflets. Once identified, treatment options range from conservative management to surgical resection and mitral valve surgery if concomitant valvular dysfunction is demonstrated.
Mitral annular fibrous band is a rare variant of the anomalous left atrial band (LAB), a congenital cardiac abnormality with an estimated prevalence of 2% at autopsy.
Most cases of LAB are benign but can be associated with mitral regurgitation, mitral stenosis, heart failure, cryptogenic stroke, and atrial fibrillation.
Three-dimensional transoesophageal echocardiography is the tool of choice to define LAB attachments, assess for mitral valve dysfunction, and exclude infective endocarditis.
Introduction
Autopsy findings have shown that left atrial bands (LABs) occur in approximately 2% of the population.1 However, the demonstration of LABs in vivo is very uncommon. With recent advances in cardiovascular imaging, isolated cases have been identified on transthoracic echocardiography, transoesophageal echocardiography, computed tomography, and magnetic resonance imaging. Incidental cases have also been observed during cardiac surgery or catheter ablation.2,3 LABs are composed of fibrous and muscular tissue which may vary in location, length and thickness. The vast majority of LABs originate from the inter-atrial septum in the region of the fossa ovalis and then attach to one of the other walls of the left atrium (LA).1 Such cases tend to be incidental findings unrelated to the clinical presentation. However, in some cases, LABs may extend from the inter-atrial septum and attach to a mitral valve (MV) leaflet or pulmonary vein, and this may lead to MV dysfunction, heart failure or atrial arrhythmias.4 In rare cases, LABs have also been associated with cryptogenic stroke.
Timeline
| Time . | Events . |
|---|---|
| Index presentation | The patient was admitted to hospital after presenting with fever and rigors. Blood cultures were positive for Enterococcus faecium and appropriate intravenous antibiotics were commenced. |
| Day 4 | Transthoracic echocardiogram revealed a linear structure in the region of the mitral apparatus but no mobile vegetation. |
| 1 week | Patient responded well to treatment and was discharged to continue intravenous antibiotic therapy in the community. |
| 2 weeks | Patient underwent transoesophageal echocardiography which revealed structure to be a mitral annular fibrous band. Infective endocarditis was excluded and antibiotics stopped. |
| 8 weeks | Patient remained clinically stable at the time of clinic follow-up, with normalization of inflammatory markers. Septic episode felt to be of gastrointestinal origin. |
| Time . | Events . |
|---|---|
| Index presentation | The patient was admitted to hospital after presenting with fever and rigors. Blood cultures were positive for Enterococcus faecium and appropriate intravenous antibiotics were commenced. |
| Day 4 | Transthoracic echocardiogram revealed a linear structure in the region of the mitral apparatus but no mobile vegetation. |
| 1 week | Patient responded well to treatment and was discharged to continue intravenous antibiotic therapy in the community. |
| 2 weeks | Patient underwent transoesophageal echocardiography which revealed structure to be a mitral annular fibrous band. Infective endocarditis was excluded and antibiotics stopped. |
| 8 weeks | Patient remained clinically stable at the time of clinic follow-up, with normalization of inflammatory markers. Septic episode felt to be of gastrointestinal origin. |
| Time . | Events . |
|---|---|
| Index presentation | The patient was admitted to hospital after presenting with fever and rigors. Blood cultures were positive for Enterococcus faecium and appropriate intravenous antibiotics were commenced. |
| Day 4 | Transthoracic echocardiogram revealed a linear structure in the region of the mitral apparatus but no mobile vegetation. |
| 1 week | Patient responded well to treatment and was discharged to continue intravenous antibiotic therapy in the community. |
| 2 weeks | Patient underwent transoesophageal echocardiography which revealed structure to be a mitral annular fibrous band. Infective endocarditis was excluded and antibiotics stopped. |
| 8 weeks | Patient remained clinically stable at the time of clinic follow-up, with normalization of inflammatory markers. Septic episode felt to be of gastrointestinal origin. |
| Time . | Events . |
|---|---|
| Index presentation | The patient was admitted to hospital after presenting with fever and rigors. Blood cultures were positive for Enterococcus faecium and appropriate intravenous antibiotics were commenced. |
| Day 4 | Transthoracic echocardiogram revealed a linear structure in the region of the mitral apparatus but no mobile vegetation. |
| 1 week | Patient responded well to treatment and was discharged to continue intravenous antibiotic therapy in the community. |
| 2 weeks | Patient underwent transoesophageal echocardiography which revealed structure to be a mitral annular fibrous band. Infective endocarditis was excluded and antibiotics stopped. |
| 8 weeks | Patient remained clinically stable at the time of clinic follow-up, with normalization of inflammatory markers. Septic episode felt to be of gastrointestinal origin. |
We describe a case of a mitral annular fibrous band extending from the posterior annulus to the base of the anterior mitral leaflet with no involvement of the LA, identified during the investigation of sepsis.
Case presentation
A 59-year-old man of Indian origin was admitted with a 2-week history of systemic upset, fever and rigors. His past medical history included duodenal carcinoma with liver metastases for which he had undergone a Whipple’s procedure 5 years earlier, followed by adjuvant chemotherapy and immunotherapy. In the month prior to presentation, chemotherapy had been reinstituted for disease progression. In addition, he had type II diabetes mellitus, previous immunotherapy-related colitis and was receiving therapeutic low molecular weight heparin for a recent pulmonary thrombo-embolism. Clinical examination revealed normal heart sounds with no audible murmurs or external stigmata of endocarditis. Blood pressure was 128/70 mmHg. Heart rate was 90 b.p.m.
His 12-lead electrocardiogram showed normal sinus rhythm. Laboratory data revealed elevated inflammatory markers. His blood cultures were positive for Enterococcus feacium. Transthoracic echocardiography revealed a linear structure in the region of the MV apparatus at the annular level seen best in the apical three-chamber view (Figure 1). The attachments were not clearly identified, but MV function appeared unaffected with no regurgitation or stenosis. All other valves were structurally and functionally normal. Left ventricular size was normal with an ejection fraction of 60%. Both atria were of normal size, right ventricular size and function were also normal, and there was no evidence of pulmonary hypertension. Intravenous antibiotics consisting of Ertapenem and Teicoplanin were commenced based on sensitivities to the cultured organism, with the intention to cover for suspected infective endocarditis.
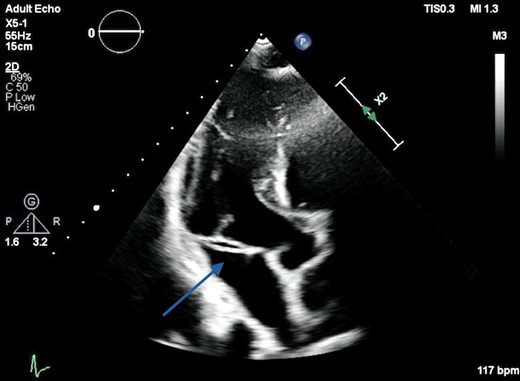
Transthoracic echocardiography apical three-chamber view demonstrating a linear echogenic structure in the region of the mitral annulus measuring 23 mm in length in the anteroposterior plane (arrow).
The sepsis responded well to antibiotics, and after 2 weeks of treatment, he underwent 2D and 3D transoesophageal echocardiography (TOE) to further assess the MV. Imaging in the mid-oesophageal plane revealed a 2.3 cm long, thin fibrous band attached to the posterior mitral annulus, and extending to the base of the middle scallop of the anterior MV leaflet causing localized tethering, but no mitral valve dysfunction (Supplementary material online, Video S1). The band appeared mildly thickened in its mid-segment but no mobile component or focal soft tissue masses were identified (Figure 2). The other valves were also structurally and functionally normal. A sessile soft tissue mass with a maximum dimension of 1.4 cm was noted at the roof of the right atrium, consistent with thrombus (Figure 3) with no obstruction to vena cava drainage. Saline microbubble contrast echocardiography showed that the inter-atrial septum was intact, with no evidence of a shunt defect.
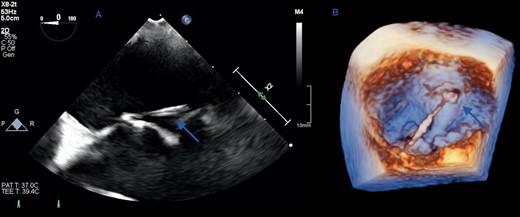
(A) Two-dimensional transoesophageal echocardiographic view of the mitral fibrous band in the horizontal mid-oesophageal plane showing an attachment at the base of the anterior mitral valve leaflet (arrow). (B) Three-dimensional reconstruction of the mitral valve in the ‘surgeon’s view’ shows the band in its entirety measuring 23 mm in length, running perpendicular to the commissural line from the posterior mitral annulus to the base of the middle scallop of the anterior leaflet (arrows).
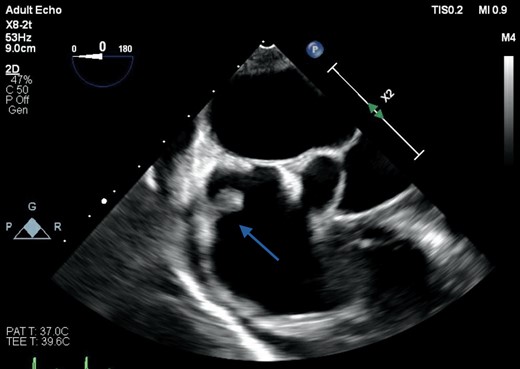
Transoesophageal echocardiography of both atria and the interatrial septum, showing a sessile soft tissue mass attached to the roof of the right atrium measuring 1 cm in maximum length and consistent with a thrombus (arrow).
The patient’s course was uncomplicated and the sepsis was thought to have originated from disease progression of his duodenal carcinoma and subsequent immunotherapy-related colitis rather than infective endocarditis. The mitral annular fibrous band was felt to represent a bystander anatomic variant causing no disruption to MV function and most likely unrelated to the sepsis; therefore no intervention was required.
Discussion
To our knowledge, this is the first case of a fibrous band traversing the supravalvular mitral inflow region, attached at one end to the mitral annulus, extending to the anterior mitral valve leaflet and not involving the LA. The absence of a valvular lesion or mobile vegetation attached to the band along with an alternative plausible cause for the sepsis allowed us to exclude infective endocarditis.
The presence of myocardial or fibromuscular bands in the ventricles is well recognized and commonly seen. However, LABs are much less common and rarely encountered in normal life. In 1972, Olsen and Valentine5 reported an incidental autopsy finding of a LAB in a patient who died of a thrombotic right coronary artery occlusion. The LAB extended from the region of the fossa ovalis to the root of the aorta near the anterior leaflet of the MV. In their paper, 18 previous reports of LABs were quoted since 1890, all originating from the region of the foramen ovale and attached to the anterior or posterior MV leaflet, or straddling the left atrial cavity. In 1993, Yamashita et al.1 reported a series of 1100 autopsy cases in which anomalous LABs were found in 22 cases (2%). In 19 cases, the anomalous band connected to 2 areas of the LA, one of which was the LA side of the fossa ovalis and the other was one of the other LA walls. The sizes ranged from 1.5 to 4 mm in width, from 0.5 to 2 mm in thickness, and from 4 to 55 mm in length. Histopathological findings revealed the anomalous bands to be composed of fibrous and muscular tissue with no Purkinje cells, consistent with normal myocardial fibres.
Since then a total of 18 cases of LABs have been described in vivo.4,6–9 In all cases, the LABs originated from the inter-atrial septum, and attached to a MV leaflet in 50% of cases, other LA walls in 39% of cases and a pulmonary vein in 11% of cases. Those attached to the left atrial walls were largely incidental findings, whereas those with MV involvement mainly caused mitral regurgitation, with the exception of one case which led to mitral stenosis. Additional reported complications include cryptogenic stroke (24%) and atrial fibrillation (18.7%).5,10 The high incidence of stroke may be related to the increased prevalence of patent foramen ovale (PFO) in this population.1 In addition, about a quarter of patients have a prominent Chiari network. Anticoagulation may be considered in the setting of atrial fibrillation especially when a PFO is present. If the MV leaflets are involved, surgical resection of the band with annuloplasty repair or replacement of the MV may be performed.1,2,8
Concurrently, a right atrial mass was identified in the context of multiple previous central venous catheter insertions to facilitate chemotherapy; the most recent catheter had been removed only weeks before the TOE. The mass almost certainly reflected thrombus formation due to a nidus of chronic interaction between catheter edge and endocardium. A saline microbubble contrast study was performed to confirm the absence of an interatrial shunt defect, excluding any potential for systemic cardio-embolism.
Our case of a mitral annular fibrous band involving the base of the anterior MV leaflet was an incidental finding associated with normal MV function and no disturbance of blood flow given the thin, discrete morphology. The presence of an abnormal intracardiac structure in the setting of occult infection should always raise the suspicion of infective endocarditis. It is important to be able to differentiate between a fibrous band and vegetation. The absence of a mobile, oscillating component on TOE imaging makes endocarditis less likely. Fibrous bands display a more linear appearance in contrast to the rounded or irregular form of vegetation. The ability to track the origin and insertion of the structure to an atrial wall or pulmonary vein is more characteristic of a fibrous band. Using detailed 2D multiplanar and 3D TOE, we were able to identify the anomalous band and exclude any overt infective vegetations attached to the band or the MV leaflets.
Lead author biography
 Dr Joseph Okafor is a National Heart & Lung Institute Clinical Research Fellow and Cardiology Registrar in NW London. He graduated from the University of Sheffield Medical School in 2013. He holds a keen interest in multi-modality structural interventional imaging, structural heart disease and advanced echocardiography.
Dr Joseph Okafor is a National Heart & Lung Institute Clinical Research Fellow and Cardiology Registrar in NW London. He graduated from the University of Sheffield Medical School in 2013. He holds a keen interest in multi-modality structural interventional imaging, structural heart disease and advanced echocardiography.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Funding: None declared.
References
Author notes
Conflict of interest: None declared.




Comments