-
PDF
- Split View
-
Views
-
Cite
Cite
Gabriele Dell’Era, Alessandro Veroli, Anna Degiovanni, Giuseppe Patti, Coronary sinus for cardiac resynchronization therapy: leave it alone and go for the branch! a case report, European Heart Journal - Case Reports, Volume 6, Issue 11, November 2022, ytac436, https://doi.org/10.1093/ehjcr/ytac436
Close - Share Icon Share
Abstract
Coronary sinus is the target of an increasing number of percutaneous interventional procedures. Thus, in some patients, conventional cardiac resynchronization therapy (CRT) may not be feasible or preferable, and ‘alternative’ CRT approaches should be applied.
We present the case of a successful CRT via direct left bundle branch permanent pacing (LBBP) in a patient with relative contraindication to conventional CRT because of previous percutaneous indirect mitral annuloplasty.
LBBP is emerging as a promising technique for physiological cardiac pacing and CRT. It may represent the technique of choice when coronary sinus is not viable for the implant of a conventional left ventricular catheter.
Conduction system pacing (CSP) is a physiological alternative to standard ventricular pacing and conventional cardiac resynchronization therapy.
Among CSP, left bundle branch pacing is especially interesting, due to easiness, reproducibility, excellent electrical measures and ability to correct intraventricular conduction delays.
CSP consents to leave the coronary sinus apart, therefore suitable for other interventional procedures in the same patient.
Introduction
Cardiac resynchronization therapy (CRT) is indicated with Class I recommendation in heart failure with reduced ejection fraction (HFrEF) patients having EF < 35%, NYHA Class II–IV despite optimal therapy and QRS duration >150 ms with left bundle branch (LBB) block morphology.1 Conventional CRT is performed delivering pacing stimuli via two ventricular catheters, one placed in the right ventricle and the other in a branch of the coronary sinus (preferably lying on the epicardium of left ventricular postero-lateral wall or on the most delayed area of mechanical activation). However, an increasing number of percutaneous interventional techniques now utilize the coronary sinus (CS) as target, such as Reducer device implantation for refractory angina,2 electrophysiology procedures, mainly for diagnostic and ablation interventions in patients with supraventricular and ventricular arrhythmias, and valve annuloplasty for severe mitral regurgitation (MR).
Timeline
| 2019 | Non-ST myocardial infarction (NSTEMI) and percutaneous coronary intervention (PCI) on left anterior descending (LAD) artery |
| 2021 | Heart failure with reduced EF (HFrEF) |
| February 2022 | Percutaneous mitral annuloplasty with implantation of the Carillon device for severe mitral regurgitation (MR) |
| May 2022 | Acute decompensation in HFrEF and development of left bundle branch (LBB) block |
| June 2022 | Cardiac resynchronization therapy (CRT) with left bundle branch pacing (LBBP) |
| 2019 | Non-ST myocardial infarction (NSTEMI) and percutaneous coronary intervention (PCI) on left anterior descending (LAD) artery |
| 2021 | Heart failure with reduced EF (HFrEF) |
| February 2022 | Percutaneous mitral annuloplasty with implantation of the Carillon device for severe mitral regurgitation (MR) |
| May 2022 | Acute decompensation in HFrEF and development of left bundle branch (LBB) block |
| June 2022 | Cardiac resynchronization therapy (CRT) with left bundle branch pacing (LBBP) |
| 2019 | Non-ST myocardial infarction (NSTEMI) and percutaneous coronary intervention (PCI) on left anterior descending (LAD) artery |
| 2021 | Heart failure with reduced EF (HFrEF) |
| February 2022 | Percutaneous mitral annuloplasty with implantation of the Carillon device for severe mitral regurgitation (MR) |
| May 2022 | Acute decompensation in HFrEF and development of left bundle branch (LBB) block |
| June 2022 | Cardiac resynchronization therapy (CRT) with left bundle branch pacing (LBBP) |
| 2019 | Non-ST myocardial infarction (NSTEMI) and percutaneous coronary intervention (PCI) on left anterior descending (LAD) artery |
| 2021 | Heart failure with reduced EF (HFrEF) |
| February 2022 | Percutaneous mitral annuloplasty with implantation of the Carillon device for severe mitral regurgitation (MR) |
| May 2022 | Acute decompensation in HFrEF and development of left bundle branch (LBB) block |
| June 2022 | Cardiac resynchronization therapy (CRT) with left bundle branch pacing (LBBP) |
We here present the case of a patient with dilated cardiomyopathy, needing CRT after percutaneous correction of MR via indirect percutaneous annuloplasty with Carillon Mitral Contour System (Cardiac Dimensions, Washington, US). This is a right heart transcatheter device composed by two hooks and a connecting shaping ribbon that, placed in the CS, uses the heart’s venous anatomy to chinch the mitral apparatus, reduce functional MR and improve MR, functional class and left ventricle geometry.3 When a device is already deployed in the CS, the placement of a permanent lead for CRT may be difficult, unfeasible or undesirable.4,5
In our patient, we therefore opted for LBB pacing (LBBP) instead of conventional CRT. Conduction system pacing (CSP) is already recommended1 as an alternative when CRT implant fails or is not possible, and leaves the CS free for future procedures.6,7 LBBP is an emerging CSP technique to overcome technical issues and weaknesses of a pure Hisian pacing, providing easiness of performance, optimal and stable electrical measures, high rate of success, and very low rate of complications.8,9,10
Case presentation
An 84-year-old woman with systemic hypertension, diabetes mellitus, hypercholesterolaemia, chronic kidney disease, and rheumatic polymyalgia was referred to our Institution. She had suffered from non-ST segment elevation myocardial infarction in 2019, treated with PCI and drug-eluting stent implantation on the left anterior descending artery, with a residual moderate left ventricular dysfunction (EF 40%) and paroxysmal atrial fibrillation episodes. During follow-up, despite optimal medical therapy (bisoprolol, sacubitril/valsartan, furosemide, statin and apixaban started at discharge; linagliptin started 3 months before), she developed symptomatic HFrEF and severe functional MR because of annulus enlargement. Taking in consideration patient’s age and the very high surgical risk, the heart team opted for percutaneous MR correction. A thorough imaging evaluation was performed to choose the best technique: transcatheter edge-to-edge valvuloplasty was excluded due to posterior leaflet hypoplasia with short P2 scallop and exaggerate annulus enlargement. Therefore, an indirect annuloplasty using the Carillon device was performed in February 2022.11,12 Coronary angiography had shown no further coronary stenosis. At 3 months after Carillon device implantation, she was again admitted for decompensated HF: left ventricular EF was 31%, residual MR was mild, with a good result of annuloplasty, and EKG showed sinus rhythm with new-onset LBB block (Figure 1A and 1B), not present before (see Supplementary material online, Figure S1). Nor chest pain, nor troponin elevation were observed at admission; thus, LBB was considered as an ‘electrical’ evolution of HF. After a course of intravenous diuretics, CRT (without defibrillator backup because of age and co-morbidities) was proposed. However, the placement of a conventional left ventricular CRT lead through the CS could have been problematic, due to presence of the in-situ Carillon device. After collecting the informed consent, we attempted a direct LBBP; following the technique first described by Huang et al13 a preformed delivery catheter (Selectra 3D 65–42, Biotronik SE & Co. KG; Berlin, Germany) was introduced in the right ventricle through the tricuspid valve and then rotated on the interventricular septum; a standard stylet-driven active fixation lead (Solia S60, Biotronik), with the helix pre-extended, was advanced in the delivery catheter and progressively screwed through the septal myocardium during continuous unipolar pacing; when LBB capture was obtained (Figure 2), electrical measures were checked and the lead was left in place. An active fixation lead (Solia S53, Biotronik) was then positioned in the right atrial appendage according to the conventional technique and a dual-chamber pacemaker (Enitra 8 DR-T, Biotronik) was implanted without acute complications (Figure 3). Procedural time was 90 min and fluoroscopy lasted 10 min. After implantation, LBB lead parameters were satisfactory (capture threshold 1,2 V × 0,5 ms, sensing 15 mV, impedance 625 Ohm). Post-procedural EKG showed complete QRS correction with a narrowing from 156 to 96 ms (Figure 4). At 24 h, ventricular lead performance was optimal (capture threshold 0,5 V × 0,5 ms, sensing 11,7 mV, impedance 332 Ohm), LBB capture was confirmed and echocardiography showed an acute recovery of bi-ventricular synchrony. To obtain a constant and complete ventricular stimulation, avoiding prolonged atrio-ventricular (AV) conduction and potential ‘fusion’ pacing, the paced AV delay was set at 150 ms, sensed AV delay at 130 ms and dynamic AV interval (shortening of AV delay with increase in heart rate) was activated. The patient was discharged 6 days after pacemaker implant and outpatient follow-up was planned. After 2 months, LBB lead parameters were excellent (capture threshold 0,5 V × 0,4 ms, impedance 312 Ohm, sensing 24,4 mV) and echocardiography showed improvement in ventricular contraction (EF 43 vs. 31%), reduction of both left ventricular end-diastolic volume (92 vs. 100 mL) and diameter (40 vs. 50 mm) (see Supplementary material online, Video S1, Supplementary material online, Video S2), as well as amelioration of bi-ventricular synchrony, as assessed by strain analysis (Figure 5).
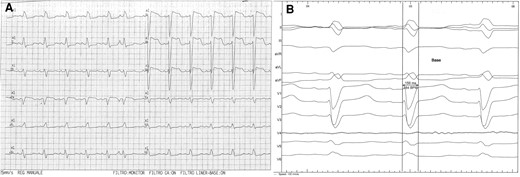
Baseline 12-lead EKG. (A) Standard recording, showing sinus rhythm and complete left bundle branch block; (B) recording in the lab at 100 mm/s, showing QRS duration and morphology.
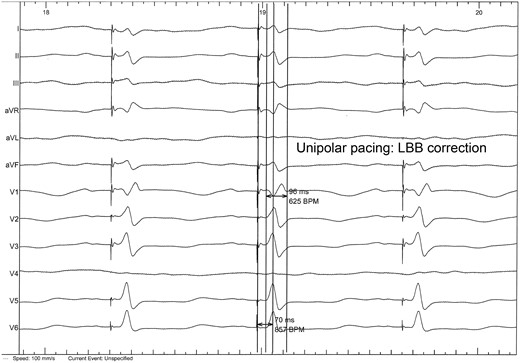
Left bundle branch capture during lead positioning: rSR’ complex in V1 lead with narrowed QRS and rapid intrinsecoid deflection in V6.
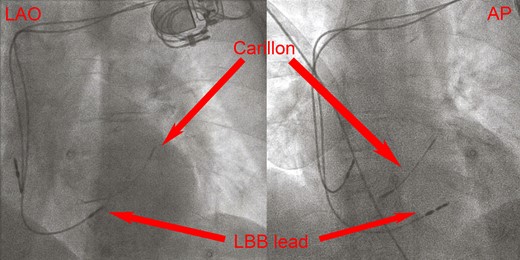
Left anterior oblique and antero–posterior fluoroscopy of the implanted lead. Note the ventricular lead (left bundle branch lead) pointing through the myocardium of the mid septum and the Carillon device placed in the coronary sinus.
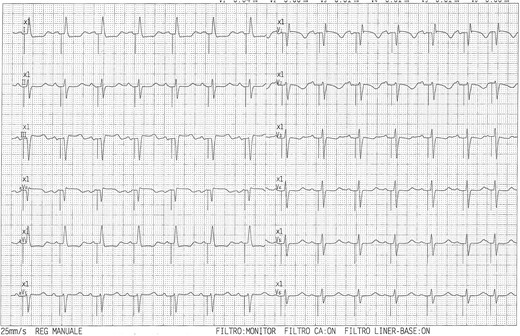
Twelve-lead EKG with left bundle branch pacing and narrow, near-normal, QRS duration.
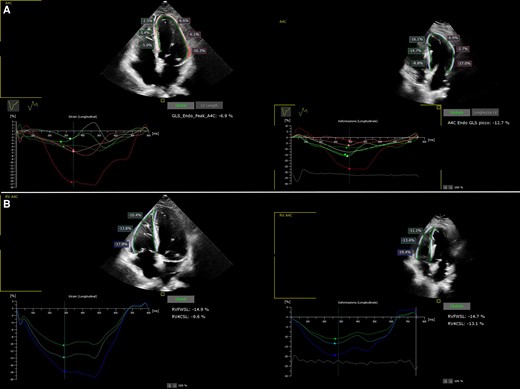
Strain curves of the left (panel A) and right (panel B) ventricle before and 2 months after LLBP, showing recovering in mechanical synchrony.
Discussion
In the case described, conventional CRT via the CS could have been feasible according to some reports.14,15,16 However, difficulties in manoeuvering the lead could be anticipated, besides a potential increase in the risk of infection of the previously implanted Carillon device. The simultaneous presence of a lead and a device in the CS makes the management of infections and electric malfunctions more difficult, with open chest surgery being required in case of complications. We therefore opted for LBBP, performing a simpler and faster procedure without the added bulk of a left ventricular lead and an effective electrical correction of the LBB. In prospective trials, LBBP showed promising results when compared with standard CRT, providing a superior proportion of responders to therapy and a better electrical and echocardiographic reverse remodelling.17,18 In the HIS-SYNC trial and sub-analysis His bundle pacing (HBP) proved effective in obtaining superior CRT compared to bi-ventricular pacing.19,20 However, a correction of intraventricular delay was not obtained via HBP in 48% of patients and pacing threshold may be significantly higher than that obtainable via direct LBBP. In this aspect, LBBP is associated with higher success rates and better electrical measures and will be probably preferred in the future.
To the best of our knowledge, this is the first case of CRT by LBBP in a patient with pre-existing device in the CS. The optimal result of this CRT procedure supports its utilization for treating at the best patients with non-viable CS or the choice to leave CS alone and free for future diagnostic or therapeutic options.
Lead author biography
 Dr. Gabriele Dell’Era was born in Novara, Italy, in 1980. He graduated in University of Eastern Piedmont in 2005 and specialized in Cardiology in the same University in 2010. He is the chief of electrophysiology lab in the Department of Thoracic and Cardiovascular Diseases at Maggiore della Carità Hospital in Novara, directed by Prof. Giuseppe Patti. Dr. Dell’Era has 14 years experience in electrophysiology, pacing, and arrhythmias management.
Dr. Gabriele Dell’Era was born in Novara, Italy, in 1980. He graduated in University of Eastern Piedmont in 2005 and specialized in Cardiology in the same University in 2010. He is the chief of electrophysiology lab in the Department of Thoracic and Cardiovascular Diseases at Maggiore della Carità Hospital in Novara, directed by Prof. Giuseppe Patti. Dr. Dell’Era has 14 years experience in electrophysiology, pacing, and arrhythmias management.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Acknowledgements
The authors thank the medical (Dr. De Vecchi Federica, Dr. Ghiglieno Chiara, Dr. Porcellini Stefano, and Dr. Santagostino Matteo) and the nursing (Bonanno Luca, De Benedetti Simone, and Tomasoni Adriano) staff for the constant support to our research.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Funding: None declared.
References
Author notes
Conflict of interest: None declared.




Comments