-
PDF
- Split View
-
Views
-
Cite
Cite
Jwan A Naser, Mohamad R Hemu, Patricia A Pellikka, Considerations in caseous calcification of the mitral annulus: a case report, European Heart Journal - Case Reports, Volume 6, Issue 11, November 2022, ytac442, https://doi.org/10.1093/ehjcr/ytac442
Close - Share Icon Share
Abstract
Caseous calcification of the mitral annulus is an extremely rare variant of mitral annulus calcification occurring in <1% of cases. The degeneration of caseous masses could act as a source of embolic strokes and a nidus for infective endocarditis (IE).
A man in his sixties presented with transient left arm weakness. His history was pertinent for bioprosthetic aortic valve replacement secondary to endocarditis and recent pneumococcal pneumonia complicated by empyema and bacteraemia. He was still on intravenous antibiotics when he presented. Evaluation including magnetic resonance imaging of the brain, transoesophageal echocardiography, and computed tomography (CT) of the chest revealed multifocal embolic strokes, degenerative bioprosthetic aortic valve dysfunction, mitral annular calcification with mobile calcific masses, and persistent empyema. 18F-fluorodeoxyglucose–positron emission tomography–CT showed indeterminate activity across a portion of the posterior mitral leaflet and no activity on the bioprosthetic aortic valve. The patient was deemed high risk for surgery and was treated with 6-week course of antibiotics both for the empyema and the possible IE of the native mitral valve. Repeat echocardiography 40 days later showed stable mitral masses. At 4 months of follow up, the patient had no evidence of recurrent clinical strokes.
Caseous calcification of the mitral annulus is a rare but an increasingly recognized predisposing factor for embolic strokes and IE. Treatment ideally involves surgical resection of the calcified masses in such cases.
Mitral annular calcification and caseous calcification of the mitral annulus are risk factors for embolic strokes.
Mitral annular calcification and caseous calcification of the mitral annulus can act as nidus for infective endocarditis.
Introduction
Mitral annular calcification (MAC) is a chronic degenerative process involving the fibrous mitral annulus, predominantly the posterior leaflet.1 It is associated with older age, female sex, advanced kidney disease, chest irradiation, and atherosclerotic risk factors.1 Caseous calcification of the mitral annulus (CCMA) is a rare variant of MAC that occurs in <1% of cases and is described as soft periannular calcification consisting of a mixture of calcium, fatty acids, and cholesterol with a toothpaste-like texture.2 Caseous calcification of the mitral annulus manifests as masses of echogenic texture on echocardiography which often appear with a more lucent centre surrounded by a more dense, calcified rim. Both MAC and CCMA have been associated with embolic strokes and increased risk of infective endocarditis (IE).3–5
Timeline
| 8 years prior to presentation | Aortic valve endocarditis Bioprosthetic aortic valve replacement and coronary artery bypass graft |
| 2 weeks prior to presentation | Streptococcus pneumoniae complicated by empyema and bacteraemia Increased bioprosthetic aortic valve gradient from baseline Mitral annular calcification with stable mitral valve function Intravenous ceftriaxone and thoracostomy tube for empyema |
| Presentation (11-day hospital stay) | Transient left arm weakness Imaging evidence of multifocal embolic strokes Stable bioprosthetic aortic valve gradient found to be due to degeneration of the bioprosthesis Multiple highly mobile masses found on the native mitral valve 18F-fluorodeoxyglucose–positron emission tomography–computed tomography (18F-FDG-PET-CT) with indeterminate activity across a portion of posterior mitral leaflet and no activity on the aortic valve Patient was deemed high risk for surgical intervention Empiric treatment for both empyema and possible native mitral valve IE was pursued |
| Follow up at 4–6 weeks | Stable mitral annular masses Completed empiric treatment for both empyema and possible native mitral valve IE Stable aortic valve gradient consistent with moderate degenerative stenosis |
| Follow up at 4 months | No recurrence of clinical stroke |
| 8 years prior to presentation | Aortic valve endocarditis Bioprosthetic aortic valve replacement and coronary artery bypass graft |
| 2 weeks prior to presentation | Streptococcus pneumoniae complicated by empyema and bacteraemia Increased bioprosthetic aortic valve gradient from baseline Mitral annular calcification with stable mitral valve function Intravenous ceftriaxone and thoracostomy tube for empyema |
| Presentation (11-day hospital stay) | Transient left arm weakness Imaging evidence of multifocal embolic strokes Stable bioprosthetic aortic valve gradient found to be due to degeneration of the bioprosthesis Multiple highly mobile masses found on the native mitral valve 18F-fluorodeoxyglucose–positron emission tomography–computed tomography (18F-FDG-PET-CT) with indeterminate activity across a portion of posterior mitral leaflet and no activity on the aortic valve Patient was deemed high risk for surgical intervention Empiric treatment for both empyema and possible native mitral valve IE was pursued |
| Follow up at 4–6 weeks | Stable mitral annular masses Completed empiric treatment for both empyema and possible native mitral valve IE Stable aortic valve gradient consistent with moderate degenerative stenosis |
| Follow up at 4 months | No recurrence of clinical stroke |
| 8 years prior to presentation | Aortic valve endocarditis Bioprosthetic aortic valve replacement and coronary artery bypass graft |
| 2 weeks prior to presentation | Streptococcus pneumoniae complicated by empyema and bacteraemia Increased bioprosthetic aortic valve gradient from baseline Mitral annular calcification with stable mitral valve function Intravenous ceftriaxone and thoracostomy tube for empyema |
| Presentation (11-day hospital stay) | Transient left arm weakness Imaging evidence of multifocal embolic strokes Stable bioprosthetic aortic valve gradient found to be due to degeneration of the bioprosthesis Multiple highly mobile masses found on the native mitral valve 18F-fluorodeoxyglucose–positron emission tomography–computed tomography (18F-FDG-PET-CT) with indeterminate activity across a portion of posterior mitral leaflet and no activity on the aortic valve Patient was deemed high risk for surgical intervention Empiric treatment for both empyema and possible native mitral valve IE was pursued |
| Follow up at 4–6 weeks | Stable mitral annular masses Completed empiric treatment for both empyema and possible native mitral valve IE Stable aortic valve gradient consistent with moderate degenerative stenosis |
| Follow up at 4 months | No recurrence of clinical stroke |
| 8 years prior to presentation | Aortic valve endocarditis Bioprosthetic aortic valve replacement and coronary artery bypass graft |
| 2 weeks prior to presentation | Streptococcus pneumoniae complicated by empyema and bacteraemia Increased bioprosthetic aortic valve gradient from baseline Mitral annular calcification with stable mitral valve function Intravenous ceftriaxone and thoracostomy tube for empyema |
| Presentation (11-day hospital stay) | Transient left arm weakness Imaging evidence of multifocal embolic strokes Stable bioprosthetic aortic valve gradient found to be due to degeneration of the bioprosthesis Multiple highly mobile masses found on the native mitral valve 18F-fluorodeoxyglucose–positron emission tomography–computed tomography (18F-FDG-PET-CT) with indeterminate activity across a portion of posterior mitral leaflet and no activity on the aortic valve Patient was deemed high risk for surgical intervention Empiric treatment for both empyema and possible native mitral valve IE was pursued |
| Follow up at 4–6 weeks | Stable mitral annular masses Completed empiric treatment for both empyema and possible native mitral valve IE Stable aortic valve gradient consistent with moderate degenerative stenosis |
| Follow up at 4 months | No recurrence of clinical stroke |
Case presentation
A non-Hispanic white man in his sixties presented to the emergency department with transient left arm numbness. He denied chest pain, dyspnoea, or left arm pain. He was found to be hypotensive with blood pressure of 71/53 mmHg, heart rate of 102 beats per minute, respiratory rate of 33 breath per minute, and temperature of 36.7°C. On physical examination, he had no focal neurological signs, Grade 3 holosystolic murmur at the left sternal border and Grade 3 ejection systolic murmur at right sternal border, and cool skin. He had a haemoglobin of 8.6 g/dL (reference 13.2–16.6 g/dL) and leucocyte count of 17 × 109/L (reference 3.4–9.6 × 109/L). His lactate was 3.1 mmol/L. Troponin was 1803 ng/L (normal ≤15 ng/L) at baseline and was increasing. An electrocardiogram demonstrated old left bundle branch block with no evidence for a new heart block. Computed tomography of the head without contrast was unremarkable. He was admitted to the hospital and treated with intravenous fluids, moderate intensity heparin, and loading doses of aspirin and clopidogrel.
Relevant medical history included bioprosthetic aortic valve replacement secondary to endocarditis 8 years before presentation, coronary artery disease (CAD) needing single-vessel coronary artery bypass graft 8 years before presentation and drug-eluting stent 2 years before presentation, heart failure with reduced ejection fraction (HFrEF), end-stage kidney disease with history of renal transplantation and chronic immunosuppression on haemodialysis, and hospitalization 2 weeks earlier due to pan-susceptible Streptococcus pneumoniae pneumonia complicated by empyema and transient bacteraemia (two sets of blood cultures <10 min apart on Day 1 were positive). During hospitalization, C-reactive protein was 365 mg/L (reference: ≤8.0 mg/L), and transthoracic echocardiography (TTE) showed stable ejection fraction of 35%, mean aortic valve gradient 33 mmHg with mild regurgitation, and MAC with mild–moderate regurgitation. In comparison, baseline TTE performed 8 years earlier, had shown transaortic mean gradient of 11 mmHg and mild mitral regurgitation. The patient’s empyema had been evacuated with a thoracostomy tube that had been removed and he had been discharged on intravenous ceftriaxone. Outpatient follow up with repeat TTE had been scheduled. His medications included low-dose aspirin, high-intensity atorvastatin, metoprolol succinate 50 mg daily, prednisone 10 mg daily, and tacrolimus 1 mg twice daily.
During hospitalization at our centre, TTE showed ejection fraction of 35%, stable moderate–severe left ventricle (LV) dilation, transaortic mean gradient of 30 mmHg, indexed aortic valve prosthetic orifice area by Doppler of 0.55 cm2/m2, dimensionless index of 0.29, acceleration time of 79 ms, stroke volume index of 36 mL/m2, mild transaortic regurgitation and fused, heavily calcified posterior cusps of the aortic bioprosthetic valve, as well as MAC with masses protruding into both the LV and left atrium (Figure 1, Supplementary material online, Video S1) with mild–moderate mitral regurgitation. Troponin continued to increase, and a coronary angiogram showed stable severe CAD without culprit lesions.
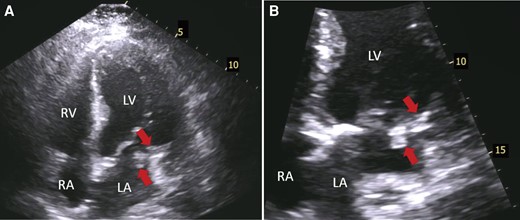
Transthoracic echocardiography showing masses over mitral annulus calcification. (A) Four-chamber apical view shows at least two echogenic masses (arrows) over calcified posterior mitral leaflet. (B) Zoomed four-chamber apical view showing more closely the aforementioned masses (arrows). LV, left ventricle; LA, left atrium; RV, right ventricle; RA, right atrium.
Due to concerns of bioprosthetic aortic valve endocarditis in the setting of recent bacteraemia and to evaluate further the masses seen on the mitral valve, a transoesophageal echocardiogram (TEE) was obtained. Transoesophageal echocardiography showed the posterior cusps of the bioprosthetic aortic valve to be echo-bright, thickened, and immobile and the anterior cusp to have some retained mobility, suggesting degeneration of the valve. There were no masses on the aortic valve. However, the mitral annulus appeared to be moderately calcified, and multiple highly mobile masses were noted predominantly on the atrial surface of the posterior leaflet, protruding into both the LV and left atrium (Figure 2, Supplementary material online, Video S2). On reviewing the CT scan of the chest obtained on admission to assess for persisting empyema, the masses on the mitral annulus appeared calcified with a mixture of high- and low-density areas (Figure 3). 18F-fluorodeoxyglucose–positron emission tomography–CT scan was subsequently obtained to evaluate for IE and showed indeterminate activity along a portion of the calcified mitral annulus [maximal standardized uptake value (SUVmax) of 3.2] indicating possible infection (Figure 4). There was no activity along the bioprosthetic aortic valve. Magnetic resonance imaging of the brain showed numerous acute and subacute infarcts involving both cerebral and cerebellar hemispheres suggesting embolic aetiology. Repeated blood cultures were persistently negative. Magnetic resonance angiography of the head and neck was negative for occlusion or high-grade stenosis in the arteries of the head and neck.
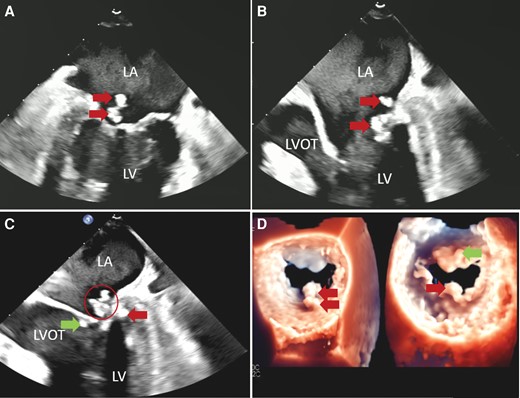
Transoesophageal echocardiography of the mitral valve masses. (A) Mid-oesophageal two-chamber view shows two echogenic masses (arrows) on the atrial side of the calcified posterior mitral leaflet. (B) Mid-oesophageal three-chamber view shows these masses (arrows) are highly mobile projecting into both the left atrium and left ventricle. (C) Mid-oesophageal three-chamber view shows there are additional masses on the ventricular side of the posterior (arrow on right side of the image) and anterior (arrow on left side of the image) leaflets. Note one of the masses (circled) has a mixture of high- and low-density areas suggesting caseous calcification of the mitral annulus. (D) 3-D images of the atrial (left) and ventricular (right) sides of the mitral valve also demonstrating the masses.
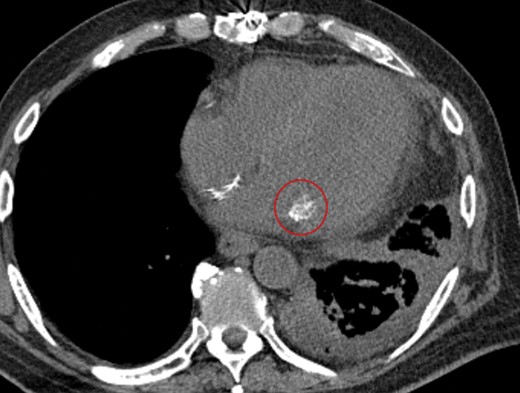
Noncontrast computed tomography scan of the chest shows caseous calcification of the mitral annulus. Axial view shows that at least one of the masses (circled) on the posterior mitral annulus appeared calcified with a mixture of high- and low-density areas suggesting caseous calcification of the mitral annulus.
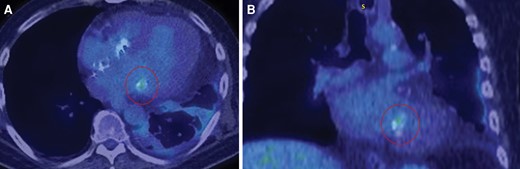
18F-fluorodeoxyglucose–positron emission tomography–computed tomography scan showing uptake along a portion of mitral annulus. Shows activity (circled) along a portion of the calcified mitral annulus suggesting possible infective endocarditis in the axial view (A) and coronal view (B).
The patient was deemed to be high risk for surgical mitral and aortic valve replacement (Society of Thoracic Surgeons risk score 36.4%). Therefore, the possible mitral valve endocarditis and empyema were treated medically with a 6-week course of intravenous ceftriaxone, and he was discharged home. While hospitalized, he was continued on haemodialysis. Goal-directed medical therapy titration for HFrEF was attempted inpatient but deferred to the outpatient setting. Repeat TTE at 1 month showed a transaortic mean gradient of 30 mmHg (stroke volume index of 52 mL/m2). Repeat TEE at 6 weeks showed stable size of the mitral masses (Figures 5 and 6, Supplementary material online, Video S3). He was clinically improving. At 4 months of follow up, he had no evidence of recurrent clinical strokes or transient ischaemic attacks.
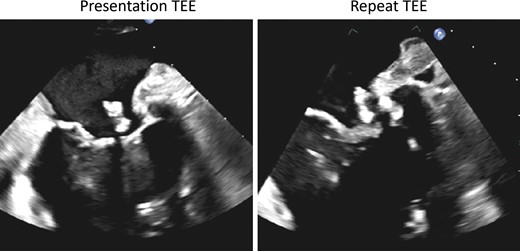
Follow-up transoesophageal echocardiography of the mitral masses. On follow-up transoesophageal echocardiography (right panel) after completion of 6-week course of antibiotics, the mitral masses were found to be of similar size compared with the baseline transoesophageal echocardiography (left panel).
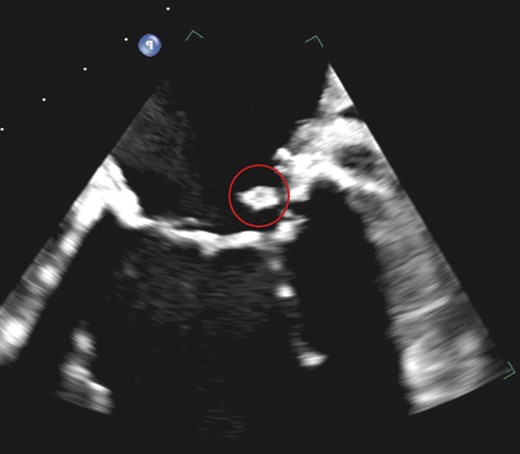
Follow-up transoesophageal echocardiography of the mitral masses suggests persisting caseous calcification of the mitral annulus. Note the previous mass which had has a mixture of high- and low-density areas suggesting caseous calcification of the mitral annulus still has heterogenicity.
Discussion
The patient presented with transient left arm numbness and was found to have multifocal embolic strokes. He met three minor modified Duke criteria (positive blood cultures not meeting major criterion, predisposing heart condition, systemic emboli) and thus had possible IE. We proceeded with 18F-FDG-PET-CT scan, which has been noted to have high sensitivity (93%) and negative predictive value (94%) for prosthetic IE and a potential role in reclassifying cases of possible native IE into definite.6 The aortic prosthesis did not show any activity on 18F-FDG-PET-CT scan, making aortic IE unlikely. A portion of the calcified mitral annulus was found to have mild indeterminate activity (SUVmax 3.2) indicating possible infection.
The finding that the masses on the mitral annulus appeared calcified with a mixture of high- and low-density areas on CT scan is typical for CCMA, and the echo-bright nature on echocardiography suggests chronicity to the masses. Although MAC and CCMA have been found to be associated with increased uptake on 18F-FDG-PET-CT scan (given that inflammation and calcification are important in their pathophysiology),7 Infective endocarditis remained an important possibility. On one hand, multiple cases describing IE of CCMA exist in the literature.4 In fact, MAC and CCMA are thought to act as nidus for infection.5 Interestingly, a previous study showed that vegetations originated from MAC itself rather than the mitral leaflet in a quarter of cases.4 Furthermore, the 18F-FDG-PET-CT scan was obtained after 2 weeks of appropriate antibiotics, which are known to result in decreased SUV activity.8 On the other hand, our patient had nonpersistent pneumococcal bacteraemia, and S. pneumoniae is a rare cause of IE.9 Given the lack of a surgical specimen, IE superimposed on CCMA with subsequent healed vegetations could not be ruled out, and the patient, who also had empyema, was treated with a 6-week course of antibiotics.
Caseous calcification of the mitral annulus itself has been described as a predisposing factor for embolic strokes with risk as high as 19.2%.3 This is higher than the risk associated with MAC not accompanied with CCMA (11.8%, P < 0.05).3 Potential mechanisms include spontaneous fistulization in CCMAs resulting in caseous material that leaks into left atrium and ventricle and embolizes, dislodgement of caseous necrotic material directly from CCMA, and embolization of calcium and cholesterol particles.3 Infective endocarditis superimposed on CCMA would be expected to have an even more substantial risk of embolic strokes.
Treatment of CCMA resulting in severe mitral dysfunction or embolic strokes is indicated and involves surgical replacement of the valve or resection of the masses, respectively. Asymptomatic patients are usually treated conservatively with frequent reported transformation to typical MAC or resolution of the masses with time.10 Elective surgical resection in asymptomatic patients with CCMA and acceptable surgical risk should be considered given the risk of embolic strokes.3 Given his high risk for surgical intervention, the patient’s preference for conservative management, and the possibility that embolic strokes were related to IE superimposed on CCMA, he was treated conservatively with no recurrence of clinical strokes at 4 months of follow up.
Lead author biography
 Dr Jwan A. Naser is a resident at the Clinician Investigator Track in Internal Medicine—Cardiology at Mayo Clinic, Rochester. She finished her medical school at Jordan University of Science and Technology. Her research interests include echocardiography, valvular heart diseases, atrial fibrillation, and artificial intelligence.
Dr Jwan A. Naser is a resident at the Clinician Investigator Track in Internal Medicine—Cardiology at Mayo Clinic, Rochester. She finished her medical school at Jordan University of Science and Technology. Her research interests include echocardiography, valvular heart diseases, atrial fibrillation, and artificial intelligence.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that verbal consent for submission of this case report has been obtained from the patient or their next of kin in accordance with COPE guidelines.
Funding: None declared.
References
Author notes
Conflict of interest: None declared.
- antibiotics
- positron-emission tomography
- heart neoplasms
- endocarditis
- echocardiography
- transesophageal echocardiography
- bacterial endocarditis
- mitral valve annular calcification
- computed tomography
- mitral valve
- cerebrovascular accident
- embolic stroke
- bacteremia
- empyema
- disease susceptibility
- follow-up
- pneumonia, pneumococcal
- surgical procedures, operative
- chest
- persistence
- brain mri
- upper extremity paresis
- antibiotic therapy, intravenous
- excision
- anulus fibrosus of mitral orifice
- calcification
- tissue degeneration
- bioprosthetic aortic valve replacement
- posterior commissural leaflet of mitral valve
- aortic valve bioprosthesis




Comments