-
PDF
- Split View
-
Views
-
Cite
Cite
Paul V Viscuse, David J Bartlett, Thomas A Foley, Hector I Michelena, Post-ischaemic exuberant left ventricular mass: thrombus vs. tumour—case report, European Heart Journal - Case Reports, Volume 2, Issue 3, September 2018, yty077, https://doi.org/10.1093/ehjcr/yty077
Close - Share Icon Share
Abstract
We present a case that illustrates the diagnostic challenge of differentiating thrombus from tumour when confronted with a large left ventricular (LV) cardiac mass.
A 43-year-old Caucasian woman polysubstance-abuser presented to a regional hospital with an ST-elevation myocardial infarction and underwent aspiration-thrombectomy and successful circumflex artery bare metal stenting. She was noted to have an exuberant LV mass on transthoracic echocardiogram the following day and transferred to our care. Transthoracic echocardiogram, transoesophageal echocardiogram, and cardiac magnetic resonance imaging were performed in an attempt to characterize the mass with conflicting findings for either thrombus or tumour. The mass was surgically excised and final pathology indicated a fibrin-rich thrombus.
The association of the mass with an infarcted area of the left ventricle supported the diagnosis of thrombus. However, due to the size and some imaging features a myxoma could not be completely ruled out. Atypical presentations of thrombus can be difficult to differentiate from cardiac tumours.
It can be diagnostically challenging to differentiate thrombus from myxoma as non-invasive imaging findings with echocardiography and magnetic resonance imaging may demonstrate potentially confusing characteristics.
Various imaging characteristics and pro-thrombotic risk factors (i.e. cocaine use) can assist with determining the diagnosis of a cardiac mass.
Introduction
An intracavitary left ventricular (LV) mass is a rare finding, which may lead to complications such as systemic embolization or LV outflow tract (LVOT) obstruction. The most common type is a thrombus, which can arise following a myocardial infarction and may present as a mobile mass though its incidence is lower in the current era of rapid reperfusion.1 Primary cardiac tumours are very rare with prevalence of 0.0017–0.28%.2–5 The most common primary cardiac neoplasm in adults is a myxoma, which presents more frequently in the left atrium and rarely in the left ventricle.2 Differentiating cardiac thrombus from a tumour, when presented with a large LV mobile mass, can be a diagnostic challenge despite the various modality options available.
Timeline
| Upon presentation to emergency room |
|
| Day 1, 0830 |
|
| Day 1, 1830 |
|
| Day 2, 1055 |
|
| Day 2, 1322 |
|
| Day 3, 1305 |
|
| Day 6, 0814 |
|
| Day 6, 1130 |
|
| Day 7, 1500 |
|
| Day 8 |
|
| Day 11 |
|
| Three months after admission |
|
| Upon presentation to emergency room |
|
| Day 1, 0830 |
|
| Day 1, 1830 |
|
| Day 2, 1055 |
|
| Day 2, 1322 |
|
| Day 3, 1305 |
|
| Day 6, 0814 |
|
| Day 6, 1130 |
|
| Day 7, 1500 |
|
| Day 8 |
|
| Day 11 |
|
| Three months after admission |
|
| Upon presentation to emergency room |
|
| Day 1, 0830 |
|
| Day 1, 1830 |
|
| Day 2, 1055 |
|
| Day 2, 1322 |
|
| Day 3, 1305 |
|
| Day 6, 0814 |
|
| Day 6, 1130 |
|
| Day 7, 1500 |
|
| Day 8 |
|
| Day 11 |
|
| Three months after admission |
|
| Upon presentation to emergency room |
|
| Day 1, 0830 |
|
| Day 1, 1830 |
|
| Day 2, 1055 |
|
| Day 2, 1322 |
|
| Day 3, 1305 |
|
| Day 6, 0814 |
|
| Day 6, 1130 |
|
| Day 7, 1500 |
|
| Day 8 |
|
| Day 11 |
|
| Three months after admission |
|
Case
A 43-year-old Caucasian woman with hypertension, obesity (body mass index 30.2 kg/m2), 13 pack-year tobacco use, marijuana use two times per-week, and prior crack-cocaine smoking for 2 years followed by current inhaled cocaine use once per week presented to her local emergency department after waking up vomiting with chest pain radiating to her shoulders and neck. She reported smoking marijuana on the day prior to the onset of symptoms. She denied any prior history of intravenous drug use. Electrocardiogram (ECG) reportedly revealed ST-elevation in leads I and aVL. Troponin-I levels were reported at 0.205 ng/mL [<0.056 ng/mL]. She had already taken 325 mg aspirin during the onset of symptoms and was given a 300 mg clopidogrel load. She was transferred to a percutaneous coronary intervention (PCI)-capable hospital where an extensive thrombus in the left circumflex artery was noted with a 99% stenosis on emergency coronary angiogram (Figure 1, Supplementary material online, Video S1). Thrombectomy was performed and a bare metal stent was deployed without use of a glycoprotein IIb/IIIa inhibitor (Figure 1, Supplementary material online, Video S2). A bare metal stent was likely chosen due to concerns for bleeding risk secondary to a prior history of bleeding erosive gastritis from non-steroidal anti-inflammatory drug use with subsequent anaemia requiring multiple transfusions. It is also possible that her polysubstance abuse history prompted outside physicians to choose the stent with the lowest dual anti-platelet therapy course possible due to potential non-compliance. The following morning, a transthoracic echocardiogram (TTE) noted a large mass within the left ventricle. She was transferred to our institution on intravenous heparin and dual antiplatelet therapy for further evaluation.
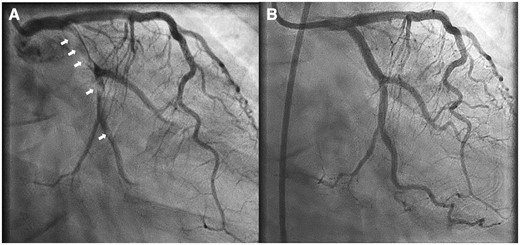
Right anterior oblique caudal coronary angiogram shows extensive thrombosis (arrows) of the circumflex artery (A) and excellent post-percutaneous coronary intervention result (B).
Upon admission, she had heart rate of 102 b.p.m., blood pressure of 121/72 mmHg, oxygen saturation of 98% on room air, respiratory rate of 16 b.p.m., and temperature of 36.9°C. Physical examination was notable for a comfortable appearing patient in no acute distress, alert and oriented, regular heart rate and rhythm with normal S1 and S2, no murmurs appreciated, no jugulovenous distention, no carotid bruits, normal pulses palpated in all extremities, no extremity oedema, lungs clear bilaterally, and a stable appearing right femoral haematoma. Initial laboratory tests demonstrated iron deficiency anaemia (haemoglobin 7.4 g/dL [12.0–15.5 g/dL], mean corpuscular volume 68.2 fL [81.6–98.3 fL], peripheral smear showing hypochromic, microcytic red blood cells), leucocytosis (leucocytes 14.4 × 109/L [3.5–10.5 × 109/L]), with normal electrolytes, and creatinine. Values within square brackets are normal reference ranges. Further cardiac risk factor workup revealed low-density lipoprotein 70 mg/dL [<100 mg/dL], high-density lipoprotein 53 mg/dL [>50 mg/dL], total cholesterol 165 mg/dL [<200 mg/dL], and triglycerides 210 mg/dL [<150 mg/dL]. Chest X-ray was normal. Repeat ECG showed a left posterior fascicular block and sinus tachycardia.
A TTE confirmed a medium echogenicity, large mobile LV mass measuring 6.2 × 2.7 cm, which traversed the LV cavity from the lateral wall into the LVOT making contact with mitral and aortic valve leaflets, with apparent attachment to the mid-lateral wall (Figure 2, Supplementary material online, Videos S3 and S4). Left ventricular ejection fraction was measured at 55% with normal chamber size and mid-lateral hypokinesis was noted. Trivial mitral valve regurgitation was noted but otherwise no valvular dysfunction was appreciated. A differential diagnosis of thrombus vs. tumour was suggested. A cardiac magnetic resonance imaging (MRI) was done the following day and showed subendocardial myocardial late enhancement of the lateral and inferolateral LV walls extending from near the cardiac base almost to the apex, consistent with myocardial infarction of the left circumflex artery territory. On MRI, the mass was of 6.0 × 2.0 × 1.7 cm size, extending from the lateral wall infarction into the LVOT, with attachment site mostly at the posteromedial papillary muscle. The association of the mass with a known infarction favoured the diagnosis of thrombus. Although first pass perfusion images demonstrated no obvious enhancement (see Supplementary material online, Video S5), delayed post-contrast images demonstrated mildly increased central signal within the mass, which had been felt to be possibly consistent with a myxoma (Figure 3). However, an short inversion time (TI) (205 ms) was used for nulling the myocardium in order to look for an infarct (Figure 4) which led to the confusion. A long TI (400–600 ms) was not used to evaluate specifically for thrombus. Due to the proximity of the mass to the aortic valve in the LVOT, it was felt that the patient was at particularly high risk for embolization and warranted timely surgical intervention.
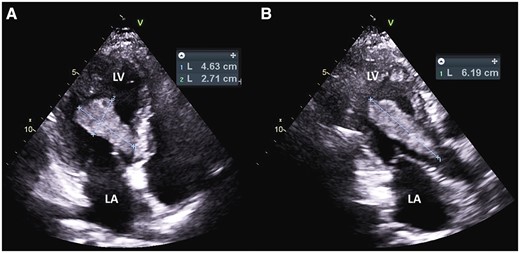
Transthoracic apical four-chamber view of the mass (A) and apical three-chamber view (B). LA, left atrium; LV, left ventricle.
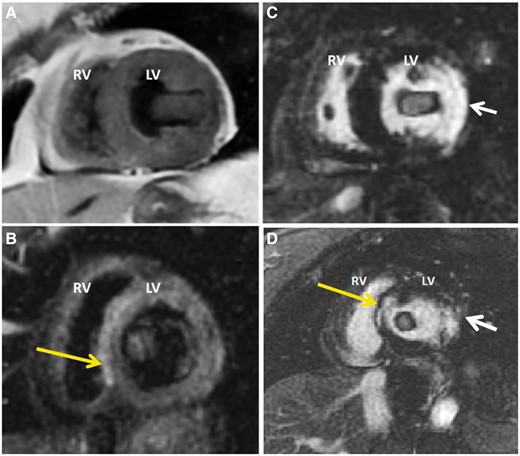
Short-axis double inversion recovery (A) and triple inversion recovery (B) images demonstrate an ovoid mass that is approximately isointense to myocardium. The triple inversion recovery images emphasize T2 (fluid) signal and employ a Short TI Inversion Recovery (STIR) pulse as part of the three inversion sequences to suppress fat. Post-contrast short axis (C) and three-chamber (D) myocardial late enhancement images at short TI shows ‘enhancement’ of mass likely due to short TI that led to confusion of potential myxoma. Late enhancement images also demonstrate a full thickness lateral wall myocardial infarction (white arrows), which are more consistent with adherent thrombus. High signal intensity in the septum on T2 is likely small septal infarct that is also demonstrated on delayed enhancement (yellow arrows). LV, left ventricle; RV, right ventricle.
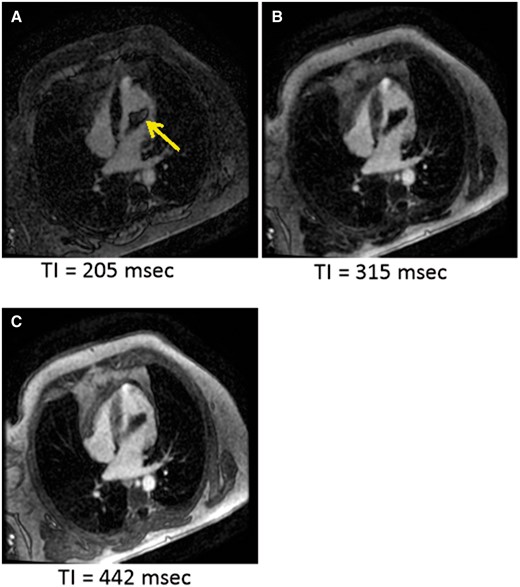
Images from TI scout sequence. TI 205 ms (A) shows central bright area in mass (yellow arrow), which was also seen on the delayed enhancement image and is likely due to short TI (thrombus not nulled). Note: bright area is not see at other time points (B) and (C).
Three days later, the patient underwent successful surgical resection of the mass. The pre-cardiopulmonary bypass transoesophageal echocardiogram noted severe hypokinesis of the lateral wall with a mobile mass attached to an akinetic portion by a stalk (Figure 5, Supplementary material online, Videos S6 and S7), and mass protrusion into the LVOT in systole, without significant obstruction (Figure 5, Supplementary material online, Videos S8 and S9). Normal Doppler configuration by pulsed-wave was noted with LVOT Vmax 1.2 m/s and normal Doppler configuration by continuous-wave was noted with Vmax 1.6 m/s. Attached to the just-inserted haemodynamic-monitoring catheter in the right atrium was a fresh linear thrombus, near the junction of the superior vena cava and right atrium (see Supplementary material online, Video S10). Post-cardiopulmonary bypass imaging confirmed adequate removal of the mass. Initial frozen specimens were labelled as possible myxoma mixed with clot but final pathology revealed a fibrin-rich thrombus consistent with an acute thrombus with some early organization indicating a small area of likely pre-existing mural thrombus indicating a possible acute over chronic thrombus (Figure 6).
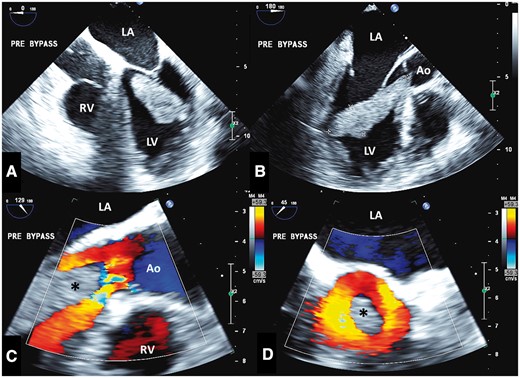
(A) Pre-bypass IOTEE shows mass in four-chamber format (A) and long-axis format (B). The zoomed long-axis (C) and short-axis (D) views of the left ventricular outflow tract show the mass (asterisk) protruding with preserved mostly laminar systolic flow (colour Doppler) around it. Ao, aorta; IOTEE: intraoperative transoesophageal echocardiography; LA, left atrium; LV, left ventricle; RV, right ventricle.
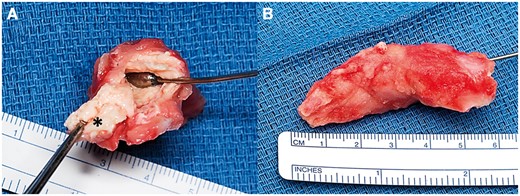
Intraoperative photographs of the excised left ventricular mass reveal a tan-red, somewhat friable mass (A and B) with a firm attachment to the subjacent endocardium (asterisk, A).
Due to her highly pro-thrombotic state as evidenced by multiple thrombotic complications, she underwent a thrombophilia workup. Hypercoagulable workup related to arterial thrombosis was negative including lupus anticoagulant, phospholipid antibodies (IgM 10.0 MPL [<15.0 MPL negative], IgG <9.4 GPL [<15.0 GPL negative]), beta-2 glycoprotein 1 antibodies (IgM <9.4 U/mL [<15.0 U/mL], IgG <9.4 U/mL [<15.0 U/mL]), and JAK2 V617F mutation (negative). Values within square brackets are normal reference ranges. She was dismissed from the hospital on clopidogrel 75 mg daily and warfarin with a goal international normalized ratio 2.0–3.0. Warfarin was felt to be essential for the extensive thrombosis of the circumflex coronary artery and left ventricle. Triple therapy with aspirin was deferred due to the higher conferred bleeding risk specifically in the setting of her history of significant GI bleed. She was scheduled to follow-up in our Thrombophilia Clinic for further evaluation consisting of cancer screening and additional thrombophilia laboratory work up but did not show for her appointment and was lost to follow-up.
Discussion
This case was unique in that a very large thrombus developed along the lateral LV wall in a very short time despite timely coronary intervention and a preserved LV ejection fraction >50%. An LV thrombus is typically seen within 2 weeks after myocardial infarction and may be related to abnormal flow profiles.6,7 Its incidence has declined to approximately 4–15% in the era of PCI or fibrinolysis for ST-elevation myocardial infarction (STEMI).6 Furthermore, LV thrombus is more likely to occur following an anterior myocardial infarction and when the ejection fraction is ≤40%.7 Septal wall infarcts make up approximately 11% of LV thrombus formation and 3% are inferoposterior wall infarcts.7 Another unique feature was the exuberant size of the thrombus. A cardiac myxoma can range from a few millimeters up to 15 cm and usually arise from the septum of the left atrium (∼90%) and rarely from the left ventricle.8
Also of interest was the massive amount of intracoronary thrombus at the time of STEMI and the sudden formation of thrombi on the haemodynamic-catheter inserted during the surgery, all suggesting a heightened prothrombotic state. Our patient was a long-term cocaine user; cocaine abuse has been cited as a risk factor for vascular disease and a prothrombotic state. A similar case has been reported that describes a STEMI in a 29 year old male regular cocaine user and smoker who was treated with thrombolytic therapy. He was acutely found to have a 3.0 × 4.5 cm intraventricular apical mural thrombus on echo for which he was treated with warfarin.9 The effects of cocaine on vascular function and blood coagulation are secondary to the loss of the protective functions of the endothelium. Cocaine causes the release of endothelin-1, which is a potent vasoconstrictor and is associated with a decrease in production of nitric oxide, a potent vasodilator.10,11 Additionally, cocaine promotes the influx of calcium and/or release of intracellular calcium leading to vasoconstriction.10,12 The increased vasoconstriction can lead to endothelial damage and subsequent increase in fibrinogen and von Willebrand Factor causing platelet aggregation and eventually thrombosis.10,13,14 Additionally, cocaine has been implicated in increasing the activity of plasma plasminogen activator inhibitor, which reduces plasmin activity and fibrinolysis.15 Our patient was also a smoker and tobacco use has been associated with increased risk of acute coronary events.16
Aside from noting the location an extension of thrombus, it becomes important therapeutically to determine the age of thrombus. Histopathological findings were consistent with both acute and chronic features of thrombus. Thrombus T1- and T2-signal characteristics are dependent on the age-related pathological changes. An acute thrombus has a higher concentration of oxyhaemoglobin, which will generate intermediate signal intensity on both T1–T2 weighted images. As the thrombus matures, the haemoglobin becomes deoxygenated and will be further oxidized to methaemoglobin, a paramagnetic molecule, which lowers the T1-weighted signal. The T2-weighted signal intensity in subacute thrombus is increased from higher water content caused by red blood cell lysis. Further aging of the thrombus results in dehydration and the deposition of fibrin lowering the signal intensity on T1- and T2-weighted images.17 Evidence of calcification can also indicate chronicity of a thrombus though magnetic resonance (MR) is less sensitive than computed tomography for detection.18 Gadolinium study in cardiovascular MR is ideal for thrombus identification. Early gadolinium enhancement imaging is applied during the first 2 min after administration of contrast creating intermediate signal intensity for the blood pool and myocardium, which extenuates the presence of a thrombus since it has low signal intensity from lack of contrast uptake. Long gadolinium enhancement imaging occurs about 7–10 min after contrast administration, and results in low signal intensity for T1-weight images due to the lack of contrast uptake. However, there are circumstances when a chronic thrombus has an organized architecture and becomes vascularized which can result in enhancement on LGE imaging.17,19–21 In our case, the use of an short TI to null the myocardium to look for an infarct likely led to the ‘enhancement’ of the thrombus. Ideally, a longer TI of around 400–600 ms would have allowed for more specific evaluation of thrombus.22 At 442 ms, no enhancement is seen but the edges do get brighter due to partial volume effects as the thrombus moves out of the plain of the image (Figure 4). This could be viewed as enhancement if not viewed in context with the thrombus moving. Phase-sensitive inversion recovery images were not available at the time of evaluation and would have also been helpful to confirm or exclude enhancement of the mass. The absence of true enhancement in this case could then indicate a potentially fresh thrombus. Fibrin-specific MRI contrast has been reported to further detect arterial and venous thrombi.23 In mice, fibrin-specific contrast was found to be potentially helpful in identifying thrombi in vivo that are amenable to thrombolysis.24
Based on the organizing histology of the thrombus, it is possible that our patient had ‘stuttering’ obstruction of the circumflex artery prior to her presentation, which might have created the regional wall motion abnormalities that favoured thrombus formation, even before she presented with the florid STEMI and would potentially favour an acute on chronic thrombus. Unfortunately, an LV gram was not obtained at the regional hospital during catheterization to assess if the mass was present at the time of the STEMI presentation. Infective thrombus could also be considered though she did not exhibit any fevers, chills, sweats, or malaise and did not have a history of intravenous drug use. We submit the hypothesis that our patient simply developed a very early LV thrombus acutely secondary to the lateral STEMI in the setting of a pro-thrombotic state related to cocaine and tobacco use. The combination of an akinetic region and her hypercoagulable state could have allowed for rapid and extensive thrombus formation despite timely stent placement. This is supported by the absence of ischaemic symptoms prior to presentation (as to suggest a stuttering coronary lesion), the extensive circumflex artery thrombosis, finding of LV thrombus, and the formation of thrombi on the monitoring catheter during surgery. Nonetheless, this remains speculative.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.




Comments