-
PDF
- Split View
-
Views
-
Cite
Cite
James V. Briskie, Frequency of Egg Rejection by Potential Hosts of the New Zealand Cuckoos, The Condor: Ornithological Applications, Volume 105, Issue 4, 1 November 2003, Pages 719–727, https://doi.org/10.1093/condor/105.4.719
Close - Share Icon Share
Abstract
Host-specific brood parasites use a limited diversity of host species to raise their young. The two species of parasitic cuckoo that occur in New Zealand, Shining Cuckoo (Chrysococcyx lucidus) and Long-tailed Cuckoo (Eudynamys taitensis), are both host specific despite the availability of other apparently suitable species to act as hosts. To determine if host specificity has been shaped by the widespread occurrence of egg rejection among potential hosts, I tested the response of nine native passerine species to brood parasitism by the experimental addition of artificial cuckoo eggs to their nests. Artificial Shining Cuckoo eggs were rejected at least some of the time in eight of nine species tested, but levels of rejection were generally low. The majority of hosts accepted artificial Long-tailed Cuckoo eggs, and only the Brown Creeper (Mohoua novaeseelandiae) rejected eggs of both cuckoos. The occurrence of egg rejection in species currently not used as hosts (e.g., New Zealand Robin [Petroica australis]) suggests that they may have been parasitized by cuckoos in the past. Although egg rejection appears to limit the number of species currently suitable as hosts, it is not clear why acceptor species such as Fantails (Rhipidura fuliginosa) and Bellbirds (Anthornis melanura) are rarely parasitized, nor why egg mimicry as a counteradaptation to rejector species such as Brown Creepers and the New Zealand Pipit (Anthus novaeseelandiae) is poorly developed. On islands with depauperate avifaunas such as New Zealand, it is possible that the evolution of antiparasite adaptations in a small number of potential hosts may restrict the ability of brood parasites to evolve generalist strategies of host use.
Frecuencia de Rechazo de Huevos por Hospederos Potenciales de los Cucos de Nueva Zelanda
Resumen. Los parásitos de cría hospedero-específico utilizan una diversidad limitada de especies hospederas para criar a sus pichones. Las dos especies de cucos parásitos que se encuentran en Nueva Zelanda, Chrysococcyx lucidus y Eudynamys taitensis, son hospedero-específicas a pesar de la disponibilidad de otras especies aparentemente apropiadas para servir como hospederas. Para determinar si la especificidad de hospedero ha sido moldeada por una alta frecuencia de rechazo de huevos por parte de los hospederos potenciales, puse a prueba la respuesta de nueve especies de paserinos nativos ante el parasitismo de cría añadiendo huevos de cucos en sus nidos experimentalmente. Los huevos artificiales de C. lucidus fueron rechazados al menos algunas veces en ocho de las nueve especies estudiadas, pero los niveles de rechazo fueron generalmente bajos. La mayoría de los hospederos aceptaron los huevos artificiales de E. taitensis, y sólo Mohoua novaeseelandiae rechazó los huevos de ambos cucos. La ocurrencia de rechazo de huevos en especies no utilizadas actualmente como hospederos (e.g., Petroica australis) sugiere que éstas podrían haber sido parasitadas por cucos en el pasado. Aunque el rechazo de huevos parece limitar el número de especies actualmente apropiadas para servir como hospederas, no es claro por qué especies que aceptan los huevos (como Rhipidura fuliginosa y Anthornis melanura) son raramente parasitadas, ni por qué está poco desarrollado el mimetismo de huevos como una contra-adaptación ante especies que rechazan los huevos (como M. novaeseelandiae y Anthus novaeseelandiae). En islas con avifaunas empobrecidas como Nueva Zelanda, es posible que la evolución de adaptaciones antiparásitos en un pequeño número de hospederos potenciales restrinja la habilidad de los parásitos de cría para evolucionar hacia estrategias generalistas de uso de hospederos.
Introduction
Obligate brood parasitism has evolved in about 100 species of birds worldwide, but the majority of studies have focused on the Brown-headed Cowbird (Molothrus ater) of North America and the European Cuckoo (Cuculus canorus) of Eurasia. These two species illustrate a striking dichotomy among brood parasites: some species are host specific, parasitizing only one or a few species, while others are generalists and use many different hosts. For example, the Brown-headed Cowbird has been recorded parasitizing 220 species across its range in the United States and Canada (Friedmann and Kiff 1985) while 90% of all parasitism records of the European Cuckoo in the United Kingdom involve only five host species (Brooke and Davies 1987). Why some species of brood parasite are specialized while others adopt a more generalist strategy is not clear (Rothstein et al. 2002).
Host specialization is extreme in the two species of parasitic cuckoo found in New Zealand and its offshore islands. The Long-tailed Cuckoo (Eudynamys taitensis) is endemic to New Zealand and parasitizes only three species, in the genus Mohoua: Whitehead (Mohoua albicilla) on the North Island, and Brown Creeper (M. novaeseelandiae) and Yellowhead (M. ochrocephala) on the South Island (McLean 1988). The Shining Cuckoo (Chrysococcyx lucidus) is found throughout Australasia and the South Pacific, but in the main islands of New Zealand it parasitizes only the endemic Grey Warbler (Gerygone igata; Gill 1983). In contrast, Shining Cuckoos in Australia are more generalist: their eggs have been recorded in the nests of 82 species, with at least 10 species fledging cuckoo chicks (Brooker and Brooker 1989a). Although the passerine avifauna of New Zealand is less diverse than that of Australia, the high specificity of cuckoos in New Zealand occurs despite the availability of at least a dozen other potentially suitable hosts. Why should cuckoos in New Zealand be so host specific?
In this study I tested whether host specificity of New Zealand cuckoos is due to the widespread evolution of egg rejection among the pool of potential hosts. Perhaps the development of antiparasite adaptations among a large proportion of the potential host community has forced cuckoos to specialize on the few hosts that lack these defenses. By the use of experimental egg additions I tested whether some potential hosts in New Zealand are not parasitized because of their ability to recognize and reject a cuckoo's egg.
Methods
The response of native passerines to experimentally introduced cuckoo eggs was tested during four austral summers (October–December) from 1998–2001. The main study area was located at Kowhai Bush (42°23′S, 173°37′E), a 240-ha block of native forest located approximately 10 km from the town of Kaikoura, on the South Island of New Zealand. Seven native passerines nest in this forest: Rifleman (Acanthisitta chloris), Bellbird (Anthornis melanura), Grey Warbler, New Zealand Robin (Petroica australis), Brown Creeper, Fantail (Rhipidura fuliginosa), and Silvereye (Zosterops lateralis). The avifauna of this area has been the subject of numerous studies (e.g., Powlesland 1981, Gill 1982, 1983), and the site has been described by Gill (1980). Two additional sites were also used to test the response of species absent from the Kaikoura area: Motuara Island (41°05′S, 174°16′E) was visited to test the responses of Saddlebacks (Philesturnus carunculatus), and Maud Island (41°01′S, 173°16′E) was used to test New Zealand Pipits (Anthus novaeseelandiae). Both islands are located in the Marlborough Sounds at the north end of the South Island. Shining Cuckoos occur commonly in all three study areas. Long-tailed Cuckoos currently occur only as migrants on the three study sites, but they likely bred in these areas before widespread forest clearance and the introduction of mammalian predators.
I located nests by systematically searching suitable habitat and by following adult birds as they returned to their nests. Nests were flagged with numbered tape and then revisited at intervals of 3 to 5 days to monitor their progress. Nests found during the laying and incubation phases were used to estimate the natural rate of parasitism by cuckoos. Eggs of Shining Cuckoos are nonmimetic and were easily distinguished from host eggs (Fig. 1). Eggs of Long-tailed Cuckoos are similar in color and spotting pattern to many native species but could be readily differentiated by size. Both species of New Zealand cuckoos are migratory, and because many native passerines initiate clutches before cuckoos arrive on the breeding grounds, these early nests are unavailable for parasitism. Thus, I only included nests monitored during the laying season of cuckoos (from early October to late December) when estimating rates of parasitism (Gill 1982). Although I tried to find as many nests as possible on the study areas, it is likely that a number were missed (particularly nests high in the canopy) and this may have biased estimates of parasitism rate.

Eggs of cuckoos and their potential hosts in New Zealand. Far left column, from top to bottom: real Shining Cuckoo (olive-green; 18.5 × 12.5 mm), real Long-tailed Cuckoo (creamy-white with brownish blotches; 23.0 × 17.0 mm). Second column from left: model eggs painted to resemble each cuckoo species, respectively. Middle column, top to bottom: real eggs of Grey Warbler (white with reddish-brown speckling; 17.0 × 12.0 mm), Rifleman (white; 16.0 × 12.5 mm), and Silvereye (pale blue; 17.5 × 13.0 mm). Second column from right, top to bottom: real eggs of Fantail (white with light brown speckling; 16.0 × 12.0 mm), New Zealand Robin (cream with purplish-brown spots; 25.0 × 18.5 mm), and Bellbird (pinkish-white with reddish-brown spots; 23.0 × 16.0 mm). Far right column, top to bottom: real eggs of New Zealand Pipit (cream with heavy brown blotches; 23.0 × 17.0 mm), Brown Creeper (white to dark pink with reddish-brown speckling; 18.5 × 14.0 mm), and Saddleback (gray or white with dark blotches; 29.0 × 22.0 mm). Color descriptions and measurements of eggs from Heather and Robertson (1996)
The response of birds to cuckoo parasitism was tested by experimentally adding artificial eggs to their nests, as done previously by other workers (Rothstein 1975, Davies and Brooke 1989). Eggs were made by molding modeling clay around lightweight styrofoam balls and painting them to mimic either those of the Shining Cuckoo or the Long-tailed Cuckoo (Fig. 1). This allowed me to produce model eggs similar in size and mass to that of real eggs (real Shining Cuckoo eggs: 1.85 ± 0.06 [SD] g, n = 4, Gill 1983, model eggs: 1.94 ± 0.10 g, n = 10; real Long-tailed Cuckoo eggs: 3.64 g, [estimated using Hoyt 1979 and egg dimensions in Heather and Robertson 1996], model eggs: 3.77 ± 0.12 g, n = 10). It was not possible to use real cuckoo eggs because of their rarity, and therefore I could not test whether the response of birds to clay eggs differed from that of real eggs. However, previous studies on other brood parasites have demonstrated that artificial eggs generally elicit similar responses to those of real eggs (Rothstein 1975, Davies and Brooke 1989).
Artificial cuckoo eggs were added either during the laying (at least one host egg present) or early incubation period of the host (days 1–5 of incubation). A single artificial cuckoo egg was added to each nest and no host eggs were removed. Both species of New Zealand cuckoo normally remove a host egg during the act of parasitism, but Davies and Brooke (1988) have shown that removal does not affect the probability of the host rejecting the parasitic egg in European hosts. As some of the species tested in this study are endangered, it was not ethical to remove host eggs. Eggs were added between 07:00 and 12:00, which coincides with the time Shining Cuckoos lay their eggs (Brooker et al. 1988); it is not known what time Long-tailed Cuckoos lay eggs. To avoid excessive disturbance, I added cuckoo eggs after the host had naturally left the nest. In nearly all instances I was able to add the cuckoo egg and leave the vicinity of the nest before the host returned (the only exceptions were at some Fantail and Silvereye nests, in which both parents incubate and nests were unattended for only short periods). I watched each nest for 10 min after adding the egg to record the initial response of the host upon its return. Nests were then checked 24 hr later to determine if the hosts rejected the model egg. If the cuckoo egg was still present after 24 hr, I rechecked the nest 4 days later. At both nest checks, I waited until the host naturally left the nest before checking its contents. If the egg was still being incubated after 5 days, I removed it and considered the cuckoo egg accepted (Rothstein 1975, Davies and Brooke 1989). If the egg was present but cold, I watched the nest for at least 30 min to see if a bird returned. In all such instances (n = 9), no adults ever returned to these nests and I classified them as deserted. This was confirmed on subsequent visits to all these nests after the 5-day period. The cuckoo egg was considered ejected if it was missing from the nest but the nest was still active. In some instances, ejections occurred within a few minutes of the host returning to the nest and I witnessed these directly. Most birds in this study were not color banded, so some individuals may have been tested twice. However, to minimize this problem, I avoided parasitizing nests within the same general area as a nest tested previously, and I tested no single nest more than once.
Nine species were tested (Table 1). Model Shining Cuckoo eggs were added to a total of 79 host nests, while 51 nests were tested with Long-tailed Cuckoo eggs (combined total = 130 nests). The number of tests per egg type per species ranged from 1 to 16 (Table 1). Sample sizes were small for some species either because of their rarity (e.g., only about 500 Saddlebacks survive on the South Island) or because nests were difficult to reach (e.g., Brown Creepers generally nest high in the canopy). Nonetheless, positive results from a few tests can indicate whether a species ever rejects parasitic eggs even if the rejection rate cannot be estimated (Rothstein 1975).
Rates of parasitism by Shining Cuckoos and artificial-egg rejection rates for nine passerine species in New Zealand. Natural rate of parasitism indicates the number of nests parasitized by cuckoos relative to number monitored. Rejection rate indicates the number (%) of model eggs rejected relative to the number of artificially parasitized nests (one model egg per parasitized nest)
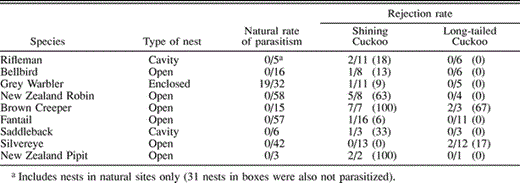
Rates of parasitism by Shining Cuckoos and artificial-egg rejection rates for nine passerine species in New Zealand. Natural rate of parasitism indicates the number of nests parasitized by cuckoos relative to number monitored. Rejection rate indicates the number (%) of model eggs rejected relative to the number of artificially parasitized nests (one model egg per parasitized nest)

Results
I followed 234 nests from early laying through incubation during the time cuckoos were present on the study sites. Only the Grey Warbler was naturally parasitized by the Shining Cuckoo (Table 1). No cuckoo eggs were observed in any of the other eight species monitored, although some of these species are rejectors (see below), and parasitism would have been missed if cuckoo eggs were laid and ejected between nest visits. This confirms the extreme host specificity of the Shining Cuckoo in New Zealand found previously (Gill 1983, 1998). No instances of parasitism by the Long-tailed Cuckoo were observed.
Eight of the nine species tested showed at least some rejection of artificial Shining Cuckoo eggs (Table 1). The Brown Creeper rejected Shining Cuckoo eggs in all seven nests tested. High levels of rejection were also found in New Zealand Robins (5 of 8 nests). Although sample size was small, rejection of Shining Cuckoo eggs also appeared to occur in the New Zealand Pipit (2 of 2 nests). Low levels of rejection (<33% of nests) were observed in five species (Rifleman, Bellbird, Fantail, Grey Warbler, and Saddleback). No rejections of Shining Cuckoo eggs by Silvereyes were observed. There was also no rejection observed in the 19 nests of the Grey Warbler that were naturally parasitized by Shining Cuckoos.
In contrast, rejection of artificial Long-tailed Cuckoo eggs was observed in only two of the nine species tested (Table 1). Only the Brown Creeper (2 of 3 nests) and Silvereye (2 of 11 nests) rejected Long-tailed Cuckoo eggs. In all other experiments, Long-tailed Cuckoo eggs were accepted and incubated by hosts for the 5-day duration of the test period (Table 1).
Cuckoo eggs were rejected either by egg ejection or nest desertion (Table 2). The type of response depended on the type of parasitic egg. All four rejections of Long-tailed Cuckoo eggs were by nest desertion. In contrast, desertion accounted for only 5 of 20 rejections of Shining Cuckoo eggs. The remainder were ejected from the nest by the hosts (Fisher exact test: P < 0.01). The Brown Creeper was the only species to regularly reject eggs of both cuckoos: Shining Cuckoo eggs were typically ejected (6 of 7 rejections), while nests parasitized with Long-tailed Cuckoo eggs were deserted (2 of 2 rejections). It is possible that this difference was an artifact of using artificial eggs: one of the Long-tailed Cuckoo eggs added to a Brown Creeper nest was covered with peck marks, suggesting this nest was deserted only after the host was unable to eject the clay egg from its nest.
Mode of rejection by nine New Zealand passerine species to experimental Shining and Long-tailed Cuckoo parasitism. Figures represent number of nests
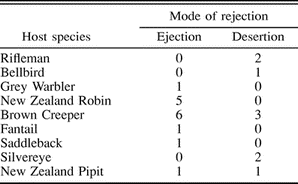
Mode of rejection by nine New Zealand passerine species to experimental Shining and Long-tailed Cuckoo parasitism. Figures represent number of nests

Despite my attempt to minimize nest disturbance, it is possible that some nest desertions were caused by my activities and were not an adaptive response to brood parasitism. The rates of desertion observed in unparasitized nests suggest that this was not the case for the Brown Creeper: the desertion rate at creeper nests experimentally parasitized with Long-tailed Cuckoo eggs (2 of 3 nests) was significantly different from control nests not parasitized but visited regularly (0 of 12 nests; Fisher exact test: P = 0.03). However, the rate of desertion of Silvereye nests parasitized with Long-tailed Cuckoo eggs (2 of 12 nests) was similar to that observed in nests visited regularly but not parasitized (2 of 21 nests; Fisher exact test: P = 0.34). Likewise, the rate of desertion at Rifleman nests experimentally parasitized with Long-tailed Cuckoo eggs (2 of 11 nests) was not significantly different from that observed in control nests (0 of 15 nests; Fisher exact test: P = 0.17). This indicates that nest desertions in these species may not be a response to brood parasitism but to my visits or some other factor.
Egg ejection occurred within 10 min of birds returning to their nest at two New Zealand Robin nests and at one Fantail nest. At both robin nests, the female robin grasped the Shining Cuckoo egg entirely in its bill and then flew out of sight while holding the egg. At the single case of ejection at a Fantail nest, the adult (sex unknown) pushed the egg out of the nest while trying to peck it. The process took a few minutes and the egg fell to the ground under the nest. The remaining 13 instances of egg ejection occurred after the 10-min observation bout and were not witnessed directly. No host eggs were damaged in any of the 15 nests in which ejection occurred. I also did not notice any damage to host eggs in nests where rejection did not occur.
Discussion
I found that some potential cuckoo hosts in New Zealand rejected parasitic eggs experimentally added to their nests. The likelihood of rejection varied among species, with the Brown Creeper and New Zealand Robin showing relatively high levels of rejection, whereas species such as the Fantail and Silvereye rejected parasitic eggs only rarely. Rejection rates of artificial cuckoo eggs were somewhat greater against eggs of the Shining Cuckoo than the Long-tailed Cuckoo, but in neither case did there seem to be a lack of suitable, alternative hosts that would restrict cuckoo parasitism. Although the evolution of egg rejection among some currently unused hosts (e.g., New Zealand Robin, Brown Creeper, and New Zealand Pipit) limits the pool of suitable hosts, these results cannot explain why New Zealand cuckoos rarely use acceptor species such as Silvereyes and Bellbirds. Cuckoos in New Zealand do not seem forced to specialize because of widespread rejection among unparasitized species.
The occurrence of egg rejection in at least some potential host species in New Zealand contrasts with the apparent lack of similar behavior among the hosts of the Shining Cuckoo in Australia (Brooker and Brooker 1989b). These authors suggested that selection for either egg mimicry or egg crypsis in Australian cuckoos is not the result of host discrimination but instead has been due to selective removal of eggs by other cuckoos. The assumption that a lack of host rejection is also characteristic of the New Zealand avifauna (Gill 1998) has added to an emerging paradigm that brood-parasite–host coevolution in Australasian birds is somehow different from the rest of the world. My results indicate that this is not the case for the New Zealand avifauna, and it suggests that further studies need to be carried out to confirm whether the lack of host rejection in Australia is indeed widespread.
The egg color of most potential hosts in my study was similar to that of the Long-tailed Cuckoo (white or cream base with a variable amount of dark markings), while the dark and solid olive-green color of the Shining Cuckoo egg differed markedly (Fig. 1). However, this does not necessarily mean that Shining Cuckoo eggs would be more readily distinguished than Long-tailed Cuckoo eggs, as recognition ability may depend on light levels within the nest. Species nesting in cavities or enclosed nests (e.g., Grey Warbler, Rifleman, Saddleback) should be less likely to reject parasitic eggs simply because low light levels prevent them from detecting a dissimilar egg. In contrast, species nesting in open nests (e.g., New Zealand Robin, Brown Creeper, Silvereye) are less constrained in this way and should show higher levels of egg rejection. This appeared to be the case (Table 1), with the levels of rejection of artificial Shining Cuckoo eggs averaging only 20% (range 0–33%) in enclosed and cavity nesters but 47% (range 0–100%) in open nesters. A similar pattern was evident with Long-tailed Cuckoo eggs, with no rejection by any cavity nesters but some rejection in open nesters (Table 1).
The dark-colored egg of the Shining Cuckoo does not mimic any of its hosts, either in Australia (Brooker and Brooker 1989a) or New Zealand (Gill 1998). Instead, it has been suggested that its dark color is an adaptation to reduce its visibility in the dimly lit nests of their main hosts (Brooker and Brooker 1989b). For example, the Grey Warbler builds an enclosed nest in which the color of any egg must be difficult to distinguish, particularly when a bird enters its nest and blocks light through the entrance. In this situation, a dark egg should be less visible either by the host or by another brood parasite (Brooker and Brooker 1989b). Although such cryptic egg coloration may be advantageous to brood parasites using enclosed nests, the same color could limit their ability to simultaneously exploit open-nesting species, in which their eggs would be relatively conspicuous. This might explain why the Shining Cuckoo is limited to parasitizing the Grey Warbler, although cavity nesting also occurs in the Stitchbird (Notiomystis cincta), Rifleman, and Yellowhead, and none are hosts to Shining Cuckoos.
Egg mimicry might also be expected to evolve as an adaptation to exploit open-nesting species that reject mimetic eggs (e.g., Brown Creeper, New Zealand Pipit) but there is no evidence that this has occurred in any populations of the Shining Cuckoo. Egg mimicry appears more likely between Long-tailed Cuckoo eggs and those of their hosts, as all have similar ground color and markings. Whether this is the result of coevolution or a common phylogenetic history needs to tested further. It is interesting to note that Brown Creepers rejected some of the Long-tailed Cuckoo egg models in my experiments. This species is the main host of the Long-tailed Cuckoo on the South Island, and it is possible that the similar eggs of the two species are a result of mimicry by the cuckoo. Further studies are required using both naturally parasitized nests and nonmimetic models to determine how dissimilar eggs must be before they are rejected and whether egg mimicry of other hosts occurs in the Long-tailed Cuckoo.
Some studies of experimental host responses to foreign eggs have shown that the presence of a model cuckoo at the nest can increase the probability of host rejection (Moksnes and Røskaft 1989, but see Braa et al. 1992). Presumably this occurs because the detection of the cuckoo near the nest alerts the host to the likelihood of parasitism. In my study, I did not present a cuckoo at the nest, and I added cuckoo eggs only once the parents had left their nests naturally. This meant that hosts had no cues to the fact they had been parasitized except the sudden appearance of another egg in their nest. The results presented in this study thus provide a conservative test of whether each potential host species has a tendency to reject parasitic eggs. Nonetheless, observations of potential hosts mobbing free-living cuckoos on the study area indicates that some species recognize cuckoos as a threat and respond accordingly (JVB, unpubl. data). It is possible that some species recorded as acceptors in this study might reject eggs if they witnessed the parasitism. It also might increase the rejection rate in those species (e.g., Fantail, Saddleback) that showed a low level of rejection.
As rejection of parasitic eggs can cost hosts through ejection or damage of their own eggs, a host should weigh the decision of whether to reject according to the probability it has been parasitized (Davies et al. 1996). Because Long-tailed Cuckoos do not currently breed on the study sites, hosts may perceive the risk of parasitism by this species as negligible, leading to the low rejection rate of their eggs that I observed. Such context-dependent responses were used by Brooke et al. (1998) to explain the rapid decline in egg ejection by Reed Warblers (Acrocephalus scirpaceus) as European Cuckoos decreased over their study areas. However, there is no reason to think that potential hosts should tune their behavior to different parasitic species when more than one is present. Hosts that discriminate against parasitic eggs do so by learning the appearance of their own eggs and rejecting anything that is sufficiently different (Rothstein 1982a, 1982b). Thus, a species that rejected a Shining Cuckoo egg would also be expected to discriminate against Long-tailed Cuckoo eggs using the same behavioral mechanisms. On the other hand, lower levels of rejection of Long-tailed Cuckoo eggs may be due to their greater similarity with host eggs.
Apart from egg rejection, the widespread evolution of other antiparasite adaptations may also have forced New Zealand cuckoos to specialize. For example, some hosts in North America attack Brown-headed Cowbirds and it has been suggested that this may minimize their risk of parasitism (Robertson and Norman 1976, Gill et al. 1997). It is possible that similar aggressive behavior may make some New Zealand hosts difficult for cuckoos to parasitize. For example, Bellbirds are particularly aggressive to other species in the forest community at Kaikoura, including cuckoos, and so they may prevent parasitism through interspecific territory defense. Whether such aggressive defense is sufficient to deter cuckoo parasitism is unknown. The responses of various hosts to cuckoos near their nests needs to be quantified before this hypothesis can be tested further.
The observation that some species reject cuckoo eggs but are seldom observed today as hosts suggests that they may have been hosts in the past (Davies and Brooke 1989). Species such as the New Zealand Robin may have once been used as hosts by Shining Cuckoos but then evolved egg ejection. This would have forced cuckoos to switch to less discriminating hosts and could lead to the specific nature of parasitism currently observed. It is interesting to note that brood parasites with extreme levels of generalism, such as Brown-headed Cowbirds, exploit host communities in which rejection is rare compared to that encountered by the more specialized European Cuckoo (Davies 2000). If the ancestors of New Zealand cuckoos originally pursued a generalist strategy like their counterparts in Australia, they may have switched to a more specialized strategy once a critical proportion of the host community evolved antiparasite defenses.
An alternative possibility is that egg recognition and rejection among species such as New Zealand Robins and Brown Creepers evolved as an adaptation to conspecific brood parasitism and that this protected them from cuckoo parasitism as an incidental byproduct. The extent of conspecific brood parasitism is not known for any of the hosts studied here, so this possibility cannot be ruled out. However, the similarity of conspecific eggs relative to those of heterospecifics suggests egg recognition would be highly developed if it evolved in response to conspecific brood parasitism. Because several host species tested in this study were capable of rejecting Shining Cuckoo eggs but then did not reject Long-tailed Cuckoo eggs (which were bigger and differed in spotting pattern from those of the host), egg discrimination is generally not as finely tuned as would be expected if parasitism by conspecifics was responsible for the evolution of egg rejection. Thus, it is more likely that egg rejection evolved when such hosts were previously subject to interspecific brood parasitism (Rothstein and Robinson 1998).
Finally, host specificity in New Zealand cuckoos might also be a direct consequence of recent changes in the avifauna. Since the arrival of humans in New Zealand, about 40% of the avifauna have become extinct (Holdaway et al. 2001). Maori settlement led to the extinction of at least two passerines; another four passerine species have disappeared since the start of European settlement, and several more have greatly reduced ranges. With a more diverse avifauna, it is possible that New Zealand cuckoos were more generalist in the past, but have been forced into specialization by the recent decline and extinction of their hosts. Unfortunately, there is no way to test this hypothesis, except to note that all of the extinct species were potentially suitable as hosts based on their size and insectivorous diet. On the other hand, the specificity of cuckoos today, despite the availability of apparently suitable but unused hosts such as Bellbirds, Fantails, and Silvereyes, suggests that the pattern of host selection in the past may not have been very different from that observed today.
Host specificity of cuckoos is not unique to New Zealand. The South Pacific region is home to a number of cuckoo species, and the island groups of Fiji, New Caledonia, and Vanuatu each have 1 or 2 resident species of cuckoos. Although the patterns of host use are poorly known in these areas, all seem to be relatively host specific. For example, the Fan-tailed Cuckoo (Cacomantis flabelliformes) in Fiji is known to parasitize only the Fiji Bush-warbler (Cettia ruficapilla) while Australian populations of this parasite use 17 species as biological hosts (Watling 1982, Brooker and Brooker 1989a). Likewise, the Shining Cuckoo in New Caledonia appears to use only the Fan-tailed Warbler (G. flavolateralis; Barré and Dutson 2000). Previous studies of brood parasitism have been centered primarily in Europe and America, where brood parasites typically have a greater diversity of available hosts. In contrast, few studies have examined coevolution between hosts and parasites on islands with depauperate avifaunas. Because of the diversity of host-specific and host-generalist cuckoos in Australasia and the South Pacific, this region provides an ideal area in which to test ideas about how host availability and host defenses have influenced the evolution of host specificity by parasitic birds.
Acknowledgments
I thank Craig Barnett, Jane Cross, Rodney Garrard, Chris Imesch, Ole Jacobsen, Rachel Johnson, Emily King, Myles Mackintosh, Craig Morley, Andrew Ward, Belinda Whyte, and Kerryn Wratt for help in finding and monitoring nests. The Canterbury Regional Council allowed me to work at Kowhai Bush, while the Department of Conservation provided access to Motuara and Maud Islands. I thank Jack van Berkel for his support and the use of facilities at the Edward Percival Field Station. Peter Gaze, Bill Cash, and Steve Ward provided logistical support for work in the Marlborough Sounds. Funding was provided by a grant from the Brian Mason Scientific Trust and the University of Canterbury. I thank James Rivers, Steve Rothstein, and an anonymous reviewer for commenting on an earlier version of this manuscript. This study was approved by the Animal Ethics Committee of the University of Canterbury and the New Zealand Department of Conservation.
Literature Cited



