-
PDF
- Split View
-
Views
-
Cite
Cite
Paula Sackmann, Juan Carlos Reboreda, A Comparative Study of Shiny Cowbird Parasitism of Two Large Hosts, the Chalk-Browed Mockingbird and the Rufous-Bellied Thrush, The Condor: Ornithological Applications, Volume 105, Issue 4, 1 November 2003, Pages 728–736, https://doi.org/10.1093/condor/105.4.728
Close - Share Icon Share
Abstract
It is usually accepted that generalist brood parasites should avoid using hosts larger than themselves because host chicks may outcompete parasite chicks for food. We studied the interactions between the Shiny Cowbird (Molothrus bonariensis) and two common hosts larger than the parasite, the Chalk-browed Mockingbird (Mimus saturninus) and the Rufous-bellied Thrush (Turdus rufiventris). For each host we determined (1) frequency and intensity of parasitism during the breeding season, (2) nesting success, egg survival, hatching success, and chick survival in unparasitized and parasitized nests, and (3) antiparasitic defenses. We also determined Shiny Cowbird egg survival, hatching success, and chick survival in both hosts. Parasitism reached 50% in mockingbirds and 66% in thrushes. In both species the main cost of parasitism was egg destruction through punctures. Hatching success, survival of host chicks, and nest survival did not differ between unparasitized and parasitized nests. Both hosts rejected parasitic white-morph eggs but accepted spotted-morph ones, even though they were significantly smaller than host eggs. The proportion of cowbirds fledged per egg laid in successful mockingbird and thrush nests was 0.4 and 0.6, respectively. Considering nest survival, reproductive success of Shiny Cowbirds was 0.15 in mockingbird nests and 0.17 in thrush nests. These values are similar to or higher than cowbird success with smaller hosts. Our results indicate that host quality is not only determined by host-parasite differences in body size, and that other factors, such as host defenses and nest survivorship, should be considered.
Un Estudio Comparado del Parasitismo de Molothrus bonariensis en dos Hospedadores de Gran Tamaño, Mimus saturninus y Turdus rufiventris
Resumen. Es aceptado generalmente que los parásitos de cría generalistas deberían evitar utilizar hospedadores de mayor tamaño corporal porque los pichones del hospedador podrían desplazar a sus pichones en la competencia por alimento. Se estudiaron las interacciones entre Molothrus bonariensis y dos hospedadores frecuentes de mayor tamaño que el parásito, Mimus saturninus y Turdus rufiventris. Para cada hospedador se determinó (1) frecuencia e intensidad de parasitismo durante la temporada reproductiva, (2) éxito de nidificación, supervivencia de huevos, éxito de eclosión y supervivencia de pichones en nidos no parasitados y parasitados, y (3) defensas antiparasitarias. También se determinó el éxito reproductivo del parásito en ambos hospedadores. El porcentaje de nidos parasitados fue 50% en Mimus saturninus y 66% en Turdus rufiventris. En ambas especies, el principal costo del parasitismo fue la destrucción de huevos por picaduras. El éxito de eclosión, la supervivencia de pichones y el éxito de nidificación fueron semejantes entre nidos no parasitados y parasitados. Ambos hospedadores rechazaron los huevos parásitos del morfo blanco pero aceptaron los del morfo manchado, si bien éstos fueron de menor tamaño que los del hospedador. La proporción de volantones de Molothrus bonariensis por huevo puesto en nidos exitosos de Mimus saturninus y Turdus rufiventris fue 0.4 y 0.6, respectivamente. Considerando la supervivencia de los nidos, el éxito reproductivo fue 0.15 en Mimus saturninus y 0.17 en Turdus rufiventris. Estos valores son similares o mayores que los reportados para hospedadores de menor tamaño que el parásito. Nuestros resultados indican que la calidad del hospedador no está sólo determinada por las diferencias en tamaño corporal entre el hospedador y el parásito y que otros factores, como defensas del hospedador y supervivencia de los nidos, deberían ser considerados.
Introduction
Brood parasites employ a reproductive strategy in which they lay eggs in nests of other birds (hosts) which incubate and provide food for the parasite's offspring. Some parasites are specialists and use only a few hosts, while others are generalists using a large number of them (Payne 1977, Rothstein 1990, Rothstein and Robinson 1998).
Among the parasitic cowbirds, Shiny Cowbirds (Molothrus bonariensis) and Brown-headed Cowbirds (M. ater) are extreme generalists (Friedmann and Kiff 1985, Ortega 1998). Generalist brood parasites face an array of potential hosts that differ in how easily they can be parasitized and in the quality of parental care they can provide. Some hosts prevent parasitism by aggressively defending their nests from parasites (Robertson and Norman 1976, Burhans et al. 2001) or having high levels of nest attentiveness (Scott 1977, Mermoz and Fernández 1999). In addition, hosts may recognize and reject parasitic eggs (Rothstein 1982, Sealy and Bazin 1995, Peer et al. 2000). If the parasite's egg is accepted, the quality of parental care becomes an important factor determining the reproductive success of the parasite. Parasite eggs that are larger or smaller than host eggs may not be properly incubated (Petit 1991, Peer and Bollinger 1997), and food delivered by the host may not be suitable for the development of parasite chicks (Middleton 1991, Kozlovic et al. 1996). In addition, larger host chicks may outcompete parasite chicks for food (Scott and Lemon 1996, Peer and Bollinger 1997). Another important factor determining the reproductive success of the parasite is nest survivorship (Mason 1986a).
Because host species vary in antiparasitic defenses, quality of parental care, and nest survivorship, it may be expected that generalist brood parasites would have developed host preferences. However, the extent to which cowbirds select the most favorable hosts within a community remains unclear. In some cases cowbirds avoid parasitizing hosts that reject their eggs (Sealy and Bazin 1995, Peer and Bollinguer 1997), but in other cases they parasitize rejector species (Rothstein 1976, Scott 1977, Mason 1986b) or species that do not feed cowbird chicks an appropriate diet (Rothstein 1976, Kozlovic 1996).
Female cowbirds can lay 40 eggs in a breeding season (Scott and Ankney 1980, Jackson and Roby 1992) leading to an alternative hypothesis that they might follow a “shotgun” strategy (Rothstein 1990, Kattan 1997). According to this view, cowbirds would parasitize hosts in relation to their abundance instead of selecting high quality hosts.
Shiny Cowbirds are obligate brood parasites of more than 200 host species (Friedmann and Kiff 1985, Ortega 1998). In the grasslands of Argentina and Uruguay, they lay either white (unspotted) or spotted eggs (Hudson 1874, Friedmann 1929). The frequency of the white morph varies from 20% (Massoni and Reboreda 1998) to 50% (Fraga 1978). Some hosts accept both egg morphs, while others accept only spotted eggs (Mason 1986a, Mermoz and Reboreda 1994, Massoni and Reboreda 1998).
It is not clear whether Shiny Cowbirds select hosts or not. Wiley (1988) found that they did not parasitize hosts in relation to their abundance and that the breeding season of cowbirds coincided with their high-quality hosts (species that fledged more than 55% of cowbird hatchlings). Food habits and egg size of these hosts were similar to those of Shiny Cowbirds, indicating that they might choose hosts partly on the basis of this combination. On the other hand, other studies have shown that Shiny Cowbirds usually parasitize hosts that are larger than themselves (Gochfeld 1979, Mason 1986b, Mermoz and Reboreda 1994) or that feed their brood with an unsuitable diet (i.e., fruits and seeds instead of animal protein; Lichtenstein 1998).
The Chalk-browed Mockingbird (Mimus saturninus; 75 g; Rabuffetti and Reboreda, unpubl. data), and the Rufous-bellied Thrush (Turdus rufiventris; 80 g; Llambias et al., unpubl. data) are considerably larger than Shiny Cowbirds (females 40–45 g, males 50–55 g, Reboreda et al. 1996). Shiny Cowbirds appear to have a low reproductive success in Chalk-browed Mockingbird nests as a result of the lower competitive ability of parasitic nestlings (Fraga 1985). Similarly, Rufous-bellied Thrushes appear to be poor hosts for Shiny Cowbirds mainly as a result of diet and size difference between parasitic and host chicks (Lichtenstein 1998). However, Shiny Cowbirds parasitize these hosts at frequencies of 50–80% (Salvador 1984, Fraga 1985, Lichtenstein 1998), which may indicate that Shiny Cowbirds do not select hosts according to their quality.
In this work we investigated the interactions of Shiny Cowbirds with Chalk-browed Mockingbirds and Rufous-bellied Thrushes. For each host we determined frequency and intensity of parasitism during the course of the breeding season, antiparasitic defenses, impact of Shiny Cowbird parasitism on their reproductive success, and reproductive success of the parasite in their nests, in terms of nest survival and survival of eggs and chicks in successful nests.
Methods
Study Site
The study was carried out at Rio Luján (34°16′S, 58°56′W), Buenos Aires Province, Argentina, during the breeding seasons (September–January) of 1995 and 1996. The study site is a farm of 230 ha forested with trees of both native (Celtis tala and Parkinsonia aculeata) and exotic (mainly Pinus sp., Cupressus sp., Crataegus sp., and Populus sp.) origin.
We followed the fates of 53 mockingbird nests (14 found during construction, 15 during laying, and 24 in early incubation), and 44 thrush nests (18 found during construction, 13 during laying, and 13 in early incubation). Most nests were found by systematic search in an area of approximately 10 ha. Mockingbirds usually nested in Celtis tala trees at heights of 1.0–4.5 m (mean ± SE: 2.2 ± 0.1 m, n = 53 nests) while thrushes usually nested in Cupressus plants at heights of 0.4–3.5 m (1.9 ± 0.1 m, n = 44 nests). In each nest we measured the eggs with calipers (length and width) to the nearest 0.1 mm and marked them with waterproof ink. Nests were numbered with flagging tape placed 5–10 m away and visited at 2–3 day intervals until either the chicks fledged or the nest failed. In each visit we recorded the number of host and parasite eggs and chicks, and the occurrence of cracks or punctures in eggs.
Data Collection
Host and parasite reproductive success
We estimated the number of eggs laid by the host (clutch size) from nests found during construction, egg laying, and early incubation. We assumed that the clutch was complete when the number of host eggs remained constant for at least two consecutive days. We considered as parasitized those clutches that had cowbird eggs or nestlings at any stage of the nesting cycle. To estimate the frequency of parasitism and its intensity (number of parasitic eggs per parasitized nest) we only considered nests found in construction, laying, or early incubation and for which the host completed laying.
We calculated egg survival rate as the proportion of eggs laid by the host or the parasite that were present in the nest at the time of hatching. Similarly, we calculated hatching success as the number of hatchlings divided by the number of eggs present in the nest at the time of hatching, and chick survival as the number of fledglings divided by the number of hatchlings.
For each host we estimated nest mortality rate using Mayfield's exposure method (Mayfield 1975). Daily nest mortality rate was estimated as the number of nests lost divided by the total number of days those nests were under observation. The variance of daily mortality rate (V) was estimated as: V = [(nest days − nest losses) × nest losses]/nest days3 (Johnson 1979). When we did not know the exact day of the nest loss, we assumed that it occurred in the middle of the interval between our visits (Mayfield 1975). Daily mortality rate was calculated for two nest stages: eggs (laying and incubation) and nestlings. We compared stage-specific daily mortality rates within and between hosts using the program CONTRAST (Hines and Sauer 1989). Nest survival at each nest stage was calculated as (1 − DMR)t, where DMR is the daily mortality rate and t is the length in days of the nest stage. Nesting success was calculated as the product of nest survival during the egg and nestling stages.
Host defensive behavior
To evaluate nest attentiveness we visited nests of both hosts at morning (from 07:00 to 12:00) and afternoon (from 12:01 to 19:00) from the day the host started laying until the third day after incubation started (5–6 days). We selected these days because the majority of events of Shiny Cowbird parasitism occur during this period (Massoni and Reboreda 1998, Mermoz and Reboreda 1999). At each visit we recorded the presence at the nest of members of the pair. We measured an attentiveness index (Scott 1977) as the number of visits at which at least one member of the pair was in the nest or <2 m from it divided by the total number of visits.
We tested whether the presence of a parasite close to the nest triggered any defensive behavior (either agonistic responses toward the parasite or sitting in the nest). We used models of a female Shiny Cowbird and a Firewood-gatherer (Anumbius annumbi) as a control species. The latter species is quite common at the study site, is similar in size and color to the female Shiny Cowbird, but has a different head and body shape and does not parasitize nests. Following Hobson and Sealy (1989), each trial consisted of a sequential presentation of the Shiny Cowbird and control models. The model was mounted 1.5 m high on a pole, pointing toward the nest approximately 1–1.5 m away. We did nine trials in mockingbird nests (one trial per nest). In order to control for an order effect, in four trials the cowbird model was presented first, while the control species was first in the other five. Similarly, we did 11 trials in thrush nests (in six the cowbird model was presented first, while the control species was first in the other five). All trials were conducted during laying or the first three days of incubation. We recorded the behavior of the host during the first 5 min after any member of the pair returned to the nest. We classified host behavior as nest protection when the host stayed within 2 m of the nest, or aggression, when the host showed any agonistic display toward the model.
Egg rejection
To evaluate whether the hosts rejected Shiny Cowbird eggs of the white or spotted morphs we carried out artificial parasitism experiments. We used either spotted Shiny Cowbird eggs (mean ± SE, length: 22.6 ± 0.3 mm, width: 17.8 ± 0.1 mm, n = 34) or Picui Ground-Dove (Columbina picui) white eggs (length: 22.7 ± 0.2 mm, width: 17.6 ± 0.2 mm, n = 25). We used Picui Ground-Dove white eggs instead of Shiny cowbird white eggs because we did not have enough eggs of this morph for conducting the experiments. Picui Ground-Dove eggs used in these experiments were collected from deserted nests and did not differ significantly from Shiny Cowbird eggs of the white morph found in our study site. In each experiment the egg was placed inside the nest during egg laying. Following Rothstein (1975), we considered that the parasitic egg had been accepted if it remained in the nest for at least 5 days after the experimental introduction. We did 30 artificial parasitism experiments in mockingbird nests, 22 of which provided complete data, suitable for analysis (the nest was not depredated during the following 5 days after artificial parasitism). Similarly, we did 31 experiments in thrush nests, 20 of which we could analyze.
Statistical Analysis
We used nonparametric statistics due to small sample sizes and lack of normality of the data. Statistical tests were performed using StatView 5.0 (SAS Institute Inc. 1998) with P < 0.05. Values reported are means ± SE.
Results
Nest Availability and Incidence of Parasitism
First nesting attempts occurred in early September for mockingbirds and in late September for thrushes. For both species nesting attempts peaked during late October (Fig. 1). The frequency of parasitism in mockingbirds was 50% (25 of 50 nests); in thrushes it was 66% (27 of 41 nests, χ21 = 1.6, P > 0.2). Considering only the months during which both species nested (October, November, and December), the frequency of parasitism was similar (65% for mockingbirds and 68% for thrushes). To test for a seasonal effect on frequency of parasitism we performed a logistic regression between occurrence of parasitism in a nest (dependent variable) and time of the breeding season at which the first egg was laid (independent variable). For this analysis we divided the breeding season into 15-day periods. In both species there was a significant increase in the frequency of parasitism with time of breeding (mockingbirds: χ21 = 3.9, P < 0.05; thrushes: χ21 = 4.3, P < 0.05; Fig. 1).
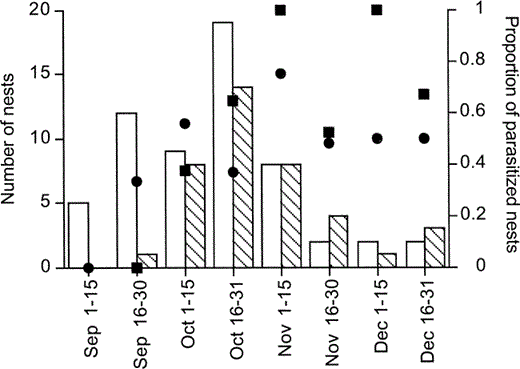
Seasonal patterns of nest availability and Shiny Cowbird parasitism in the Chalk-browed Mockingbird (open bars) and the Rufous-bellied Thrush (striped bars) in Buenos Aires Province, Argentina. Bars show the number of available nests through the breeding season. Black circles and squares show respectively the proportion of mockingbird and thrush nests that were parasitized in each period
The intensity of Shiny Cowbird parasitism in mockingbird nests was 2.0 ± 0.3 eggs (n = 25); in thrush nests it was 1.7 ± 0.2 eggs (n = 25, Mann-Whitney U-test, Z = −0.7, P > 0.4). In both species approximately 50% of the parasitized nests received more than one parasite egg (13 of 25, range 2–5 eggs for mockingbirds and 11 of 25, range 2–4 eggs for thrushes). We did not detect a seasonal effect on intensity of parasitism in either mockingbirds (Kruskal-Wallis test, H2 = 3.0, P > 0.2) or thrushes (Kruskal-Wallis test H3 = 3.9, P > 0.2).
Nest Survival and Nesting Success
For both hosts, daily nest mortality rates were higher during incubation than during the nestling stage (mockingbirds: 0.054 ± 0.010 vs. 0.011 ± 0.006, χ21 = 12.1, P < 0.001, thrushes: 0.065 ± 0.013 vs. 0.022 ± 0.011, χ21 = 6.6, P < 0.01), but stage-specific mortality rates did not differ between hosts (egg stage: χ21 = 0.4, P > 0.5, nestling stage: χ21 = 0.7, P > 0.5). Nest survival during egg and nestling stages was 0.43 and 0.87 for mockingbirds and 0.37 and 0.77 for thrushes. Overall nesting success was 0.38 and 0.28 for mockingbirds and thrushes respectively.
Impact of Shiny Cowbird Parasitism on Host Reproductive Success
There were no differences in daily nest mortality rates during the egg stage between unparasitized and parasitized nests (mockingbirds: 0.052 ± 0.016 vs. 0.056 ± 0.014, χ21 = 0.04, P > 0.8; thrushes: 0.074 ± 0.024 vs. 0.060 ± 0.016, χ21 = 0.24, P > 0.6). Nest survival during the egg stage in unparasitized and parasitized nests was 0.45 and 0.42 for mockingbirds and 0.32 and 0.39 for thrushes.
For both hosts, the main cost of parasitism was egg punctures inflicted by Shiny Cowbirds when they visited the nests. The percentage of mockingbird nests with egg punctures was 29% (14 of 48) while for thrushes it was 46% (17 of 37). Egg punctures were more frequent in parasitized than in unparasitized mockingbird nests (11 of 23 vs. 3 of 25, respectively, Fisher's exact test P < 0.01), but there was no difference between groups in thrushes (13 of 24 vs. 4 of 13, respectively, Fisher's exact test P > 0.3).
Clutch size of mockingbirds did not differ between unparasitized and parasitized nests (3.2 ± 0.1 eggs, n = 25 vs. 3.0 ± 0.1 eggs, n = 24, Mann-Whitney U-test, Z = −1.3, P > 0.1) but as a result of egg punctures, the number of eggs at hatching was lower in parasitized than in unparasitized nests (2.2 ± 0.3 eggs, n = 24 vs. 2.8 ± 0.2 eggs, n = 25, Z = −2.5, P < 0.01). Although egg survival was lower in parasitized nests (Z = −2.5, P < 0.01), we did not detect differences between groups either in hatching success (Z = −1.2, P > 0.2) or chick survival (Z = −0.4, P > 0.7, Fig. 2 A).
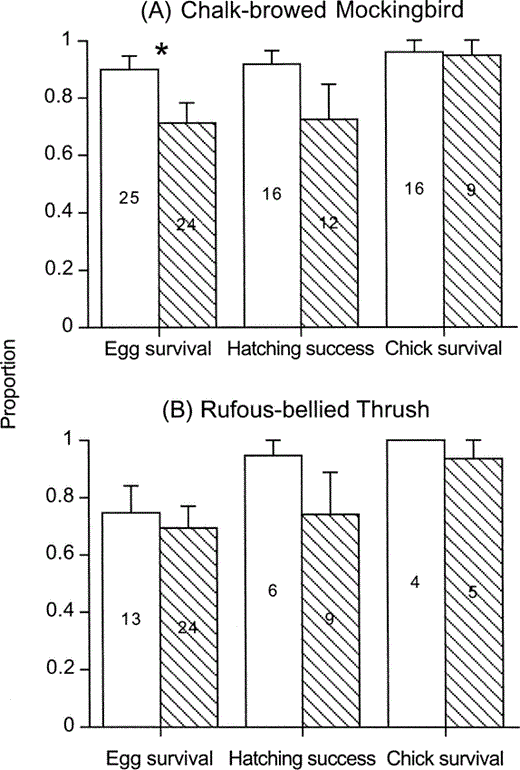
Proportions (mean ± SE) of host eggs that survived until hatching, host chicks that hatched, and host chicks that fledged in unparasitized (white bars) and parasitized (striped bars) nests of (A) the Chalk-browed Mockingbird and (B) the Rufous-bellied Thrush. Sample size (nests) is indicated at bar center
Clutch size in thrushes did not differ between unparasitized and parasitized nests (2.8 ± 0.1 eggs, n = 13 vs. 2.7 ± 0.1 eggs, n = 24, Z = −0.9, P > 0.3). Similarly, because egg punctures affected both unparasitized and parasitized nests we did not detect differences between these groups in the number of eggs at hatching (unparasitized: 2.2 ± 0.3 eggs, n = 13; parasitized: 1.9 ± 0.2 eggs, n = 24, Z = −0.9, P > 0.3). In this species, egg survival, hatching success, and chick survival did not differ between unparasitized and parasitized nests (Z = −0.3, P > 0.7, Z = 0.8, P > 0.4, Z = 0.9, P > 0.3, respectively, Fig. 2B).
Egg survival in parasitized nests was similar in mockingbirds and thrushes (Z = −0.01, P > 0.9), but egg survival in unparasitized nests tended to be lower in thrush nests (Z = −1.7, P = 0.08, Fig. 2).
Host Defenses
Nest attentiveness
The attentiveness index was 0.67 (n = 60) for mockingbirds and 0.68 (n = 50) for thrushes. There were no differences in the attention index between morning and afternoon for either mockingbirds (0.69 vs. 0.62, χ2 = 0.08, P > 0.7) or thrushes (0.61 vs. 0.67, χ2 = 0.05, P > 0.4). In several instances we found a thrush sitting in the nest before laying had started, but we never observed this behavior in mockingbirds.
Nest defense
Mockingbirds responded in three of nine trials with high levels of aggression toward the cowbird model (in these three cases the model was presented first). This aggressive behavior consisted of hovering over the model and attacking it with pecks and leg-strikes while emitting alarm calls. In the other six trials one or both birds stayed close to the nest watching the cowbird model. The same response was observed toward the control species in all the trials. For thrushes, in all cases one or both birds remained close to the nest and emitted alarm calls when the cowbird or the control model was presented (independent of the order of model presentation). The thrushes never sat on the nest as a response to the cowbird or the control model. We observed several instances of mockingbirds, but not thrushes, attacking live cowbirds. The only agonistic interactions of thrushes that we observed were intraspecific.
Egg rejection
Mockingbirds rejected white eggs in all 12 cases and spotted eggs in 0 of 10 cases (Fisher's exact test, P < 0.001). White eggs were rejected within the first 24 hr after we parasitized the nest. On two occasions we observed the mockingbird rejecting the egg during its first visit to the nest. In these cases the bird grasped the egg, flew approximately 10 m away, and dropped it. Thrushes rejected white eggs in 9 of 12 cases and spotted eggs in 0 of 8 cases (Fisher's exact test, P < 0.001). In this species egg rejections occurred up to 4 days after we parasitized the nest.
We found Shiny Cowbird white eggs in 9% of mockingbird nests (4 of 45). We found two of these eggs in nests where laying had not started, one in a nest during laying, and the other in a nest in incubation. The two nests parasitized before the host had started laying were deserted. We did not find white eggs in thrush nests.
Shiny Cowbird spotted eggs in naturally parasitized nests were significantly smaller than host eggs. For this analysis we compared the parasite egg (or the largest parasite egg in nests with multiple parasitism) with the smallest host egg. Shiny Cowbird eggs in mockingbird nests were 23.6 ± 0.4 mm long and 18.2 ± 0.2 mm wide. Host eggs were 27.6 ± 0.2 mm long and 20.5 ± 0.2 mm wide (n =19 nests, Wilcoxon signed-ranks tests, both Z = −3.7, P < 0.001). Similarly, Shiny Cowbird eggs in thrush nests were 22.9 ± 0.4 mm long and 17.9 ± 0.2 mm wide while host eggs were 29.2 ± 0.2 mm long and 21.3 ± 0.2 mm wide (n = 22 nests, Wilcoxon signed-ranks tests, both Z = −4.0, P < 0.001).
Reproductive Success of the Parasite
In both hosts survival of parasite eggs during incubation was high (0.88–0.95), but hatching success and chick survival were lower (Fig. 3). We did not detect significant differences between hosts in survival of parasite eggs (Mann-Whitney U-test, Z = −1.1, P > 0.2), hatching success (Z = −0.4, P > 0.7), or survival of parasite chicks (Z = −0.7, P > 0.5, Fig. 3). Shiny Cowbird chicks that fledged had on average two mockingbird nestmates (range 0–4) and 1.3 thrush nestmates (range 0–2). The success of Shiny Cowbird eggs estimated as the product of egg survival, hatching success, and chick survival was 0.4 in mockingbird nests and 0.6 in thrush nests. Taking into account host nesting success (estimated from daily nest mortality rates), the proportion of Shiny Cowbird chicks fledged per egg laid in mockingbird nests was 0.15, while in thrush nests it was 0.17.
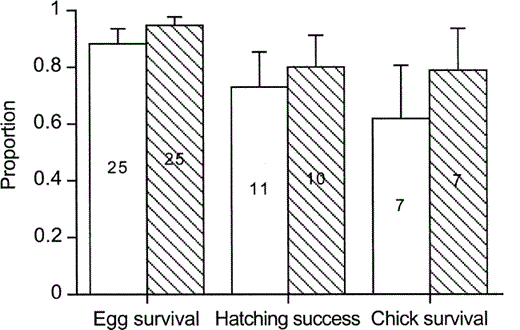
Proportions (mean ± SE) of parasite eggs that survived until hatching, parasite chicks that hatched, and parasite chicks that fledged in nests of the Chalk-browed Mockingbird (white bars) and the Rufous-bellied Thrush (striped bars). Sample size (nests) is indicated at bar center
Discussion
Shiny Cowbirds parasitized mockingbirds and thrushes with a similar frequency and intensity. The small difference between hosts may be accounted by early mockingbird nesting attempts avoiding parasitism. The frequency of parasitism we observed in mockingbird nests (50%) was lower than that reported by Salvador (1984) in a study conducted in the province of Cordoba, Argentina (86%), and by Fraga (1985) in a study conducted in the province of Buenos Aires, Argentina (73%). The frequency of thrush parasitism we observed (66%) was larger than that reported by Lichtenstein (1998) also in Buenos Aires Province (49%). Our results confirm that, although these species are considerably larger than Shiny Cowbirds, they are common hosts. The frequencies of parasitism we measured may underestimate actual values because some nests recorded as unparasitized could have been parasitized (with white eggs) that were rejected before we detected them.
For both hosts, the main cost of parasitism was the destruction of eggs. Parasitized mockingbirds and thrushes did not suffer losses from hatching success or nestling competition. This result as well as those from studies of smaller (Massoni and Reboreda 1998, 2002) and larger (Mermoz and Reboreda 1994) hosts, indicates that the main impact of Shiny Cowbirds on host reproductive success is the destruction of eggs in both parasitized and unparasitized nests.
Another potential cost of brood parasitism is the decrease in nesting success as a result of the desertion of parasitized nests (Petit 1991, Clotfelter and Yasukawa 1999, Massoni and Reboreda 1998). We did not detect differences in daily mortality rates between unparasitized and parasitized nests of either species. However, mockingbirds deserted nests that were parasitized before laying had started, which can be considered a cost of parasitism.
Considering that the main impact of parasitism for these hosts was the puncture of eggs, one would expect them to have developed defenses that minimize this cost (i.e., nest attentiveness and defense). However, neither nest attentiveness nor the agonistic displays towards the parasite were important. Both species spent 65–70% of their time attending the nest, less time than spent by the Scarlet-headed Blackbird (Amblyramphus holosericeus). In this species, at least one of the parents remains close to the nest during 95–98% of the laying and incubation time, and this high level of nest attentiveness is associated with a very low frequency of parasitism (Mermoz and Fernández 1999). In addition, nest attentiveness in mockingbirds and thrushes was not higher during the morning, when most parasitism occurs (Hoy and Ottow 1964). In terms of nest defense behavior, only the mockingbird showed differential responses toward parasite and control models, and only in some cases.
The most clear antiparasitic defense in these hosts was the rejection of parasite eggs of the white morph. Egg ejection can only eliminate part of the costs associated with lower hatchability of host eggs in parasitized nests, or competition between parasite and host chicks, which were not significant for these hosts. Both hosts accepted parasite eggs of the spotted morph even though they were significantly smaller than host eggs. Thus, difference in size did not cue egg discrimination in these hosts, as happens in the Rufous Hornero (Furnarius rufus, Mason and Rothstein 1986). It is likely that the similarity between host eggs and spotted parasite eggs made it difficult for hosts to discriminate between them. In addition, at the time the parasite egg is laid the host has already paid the main cost of parasitism (egg punctures). Therefore, a low selection pressure favoring rejection of spotted eggs may be expected.
The frequency of white eggs in mockingbird nests (9%) was lower than that reported in hosts that do not reject white eggs such as the Rufous-collared Sparrow (Zonotrichia capensis; 50% white eggs in nests, Fraga 1978) and the Yellow-winged Blackbird (Agelaius thilius; 20%, Massoni and Reboreda 1998). This difference is consistent with the mockingbird tendency to reject white eggs, which we observed in our experiments. It is also possible that Shiny Cowbird females that lay white eggs avoided parasitizing these hosts because they reject white eggs. Although we cannot reject this possibility, this was not the case for Shiny Cowbirds parasitizing the Brown-and-yellow Marshbird (Pseudoleistes virescens), another host that rejects white eggs and accepts spotted ones (Mermoz and Reboreda 1999).
The main factors that affected Shiny Cowbird success in nests that fledged chicks were hatchability and chick survival. Although there was a high percentage of multiply parasitized nests, we observed few cases of parasitic eggs with punctures (1 of 25 in each host). Survival of Shiny Cowbird chicks in mockingbird nests (0.62) was similar to Fraga's (1985) survival rate of 0.60, but the value we observed in thrushes (0.79) was considerably higher than that reported by Lichtenstein (1998). In Lichtenstein's (1998) study, cowbird chicks died of starvation in 69% of experimentally created broods containing one host and one cowbird chick. Lichtenstein (2001) also reported that parasitic chicks were fed significantly less than host young and that the poor success of Shiny Cowbird chicks was not simply due to competition with their larger nestmates, but may also have involved parental discrimination. The effects of competition between chicks and parental discrimination were not very important in our study, as successful Shiny Cowbird chicks had on average only 1.3 thrush nestmates.
Taking into account the survival of host nests, the reproductive success of Shiny Cowbirds was approximately 15% in mockingbird and 17% in thrush nests. These values are probably an overestimate of the actual reproductive success of Shiny Cowbirds, because both hosts reject parasite eggs of the white morph. The frequency of white eggs in Buenos Aires province varies between 20% and 50% (Fraga 1978, Massoni and Reboreda 1998). However, even considering a frequency of white eggs of 50% (and therefore an overestimation of 100% in Shiny Cowbird reproductive success), the host quality of mockingbirds and thrushes is similar or better than that observed in hosts smaller than the parasite that do not reject white eggs, like Rufous-collared Sparrows (7%, Fraga 1978) or Yellow-winged Blackbirds (3%, Massoni and Reboreda 1998). Thus, the high frequency of parasitism we observed in these hosts is not contradictory with the relatively low survival rate of parasite chicks in their nests. The quality of mockingbirds and thrushes as hosts is not only determined by Shiny Cowbird chicks' success at competing for food with their nestmates. Other factors, such as host antiparasitic defenses and nest survivorship, should be considered.
Acknowledgments
We thank Juan C. Corley, Gustavo J. Fernández, Myriam E. Mermoz, and Fabián Rabuffetti for useful discussion. David Dobkin and two anonymous reviewers suggested additional improvements to a previous version of this manuscript. PS was supported by a fellowship from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). JCR is a Research Fellow of CONICET. This work was supported by CONICET (grant PID 0798/98), University of Buenos Aires (grant TW88), and Agencia Nacional de Promoción Científica y Tecnológica (grant 01-09237).
Literature Cited
Author notes
Corresponding author: [email protected]



