-
PDF
- Split View
-
Views
-
Cite
Cite
Julien Coussement, Christopher H Heath, Matthew B Roberts, Rebekah J Lane, Tim Spelman, Olivia C Smibert, Anthony Longhitano, Orla Morrissey, Blake Nield, Monica Tripathy, Joshua S Davis, Karina J Kennedy, Sarah A Lynar, Lucy C Crawford, Simeon J Crawford, Benjamin J Smith, Andrew P Gador-Whyte, Rose Haywood, Andrew A Mahony, Julia C Howard, Genevieve B Walls, Gabrielle M O’Kane, Matthew T Broom, Caitlin L Keighley, Olivia Bupha-Intr, Louise Cooley, Jennifer A O’Hern, Justin D Jackson, Arthur J Morris, Caroline Bartolo, Adrian R Tramontana, Katherine C Grimwade, Victor Au Yeung, Roy Chean, Emily Woolnough, Benjamin W Teh, Sharon C A Chen, Monica A Slavin, the Australian and New Zealand Study Group for Cryptococcosis in Patients Without HIV Infection , Current Epidemiology and Clinical Features of Cryptococcus Infection in Patients Without Human Immunodeficiency Virus: A Multicenter Study in 46 Hospitals in Australia and New Zealand, Clinical Infectious Diseases, Volume 77, Issue 7, 1 October 2023, Pages 976–986, https://doi.org/10.1093/cid/ciad321
Close - Share Icon Share
Abstract
Patients without human immunodeficiency virus (HIV) are increasingly recognized as being at risk for cryptococcosis. Knowledge of characteristics of cryptococcosis in these patients remains incomplete.
We conducted a retrospective study of cryptococcosis in 46 Australian and New Zealand hospitals to compare its frequency in patients with and without HIV and describe its characteristics in patients without HIV. Patients with cryptococcosis between January 2015 and December 2019 were included.
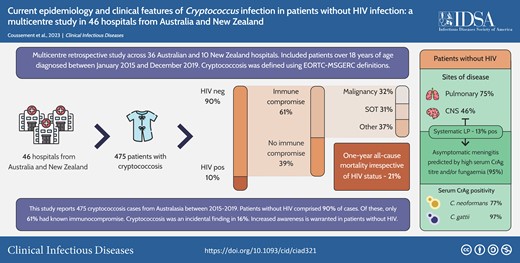
Of 475 patients with cryptococcosis, 90% were without HIV (426 of 475) with marked predominance in both Cryptococcus neoformans (88.7%) and Cryptococcus gattii cases (94.3%). Most patients without HIV (60.8%) had a known immunocompromising condition: cancer (n = 91), organ transplantation (n = 81), or other immunocompromising condition (n = 97). Cryptococcosis presented as incidental imaging findings in 16.4% of patients (70 of 426). The serum cryptococcal antigen test was positive in 85.1% of tested patients (319 of 375); high titers independently predicted risk of central nervous system involvement. Lumbar puncture was performed in 167 patients to screen for asymptomatic meningitis, with a positivity rate of 13.2% where meningitis could have been predicted by a high serum cryptococcal antigen titer and/or fungemia in 95% of evaluable cases. One-year all-cause mortality was 20.9% in patients without HIV and 21.7% in patients with HIV (P = .89).
Ninety percent of cryptococcosis cases occurred in patients without HIV (89% and 94% for C. neoformans and C. gattii, respectively). Emerging patient risk groups were evident. A high level of awareness is warranted to diagnose cryptococcosis in patients without HIV.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/currentepidemiology-and-clinical-features-of-cryptococcus-infection-in-patients-without-hiv-infection-a-multicentre-study-in-46-hospitalsfrom-australia-and-new-zealand
Cryptococcus infection is a major concern in persons living with human immunodeficiency virus (HIV), with a particularly high incidence and mortality in sub-Saharan Africa. A 2020 estimate suggested that annually, around 152 000 cases of cryptococcal meningitis occur globally, with 112 000 cryptococcal-related deaths [1]. In many developed countries, however, HIV-associated cryptococcosis has become uncommon due to the availability of highly effective antiretroviral therapy [1]. In contrast, Cryptococcus infections in patients without HIV are increasingly appreciated, including in solid organ transplant recipients, other patients receiving immunosuppressive therapy, and patients who are otherwise considered immunocompetent [2–11].
Despite increasing awareness, knowledge of the epidemiology and clinical features of cryptococcosis in patients without HIV remains limited [8, 9, 12]. Previous studies have suggested a lower rate of central nervous system (CNS) involvement or a decreased sensitivity of serum Cryptococcus antigen (CrAg) testing in patients without HIV compared with patients with HIV [13]. However, these studies were limited by the relatively small number of HIV-negative patients with cryptococcosis (often <150–200 per study) [6, 7, 9, 12, 14, 15] and/or by lack of detailed clinical information [8, 15, 16]. Furthermore, many of the cases were diagnosed before 2010 [6–8, 12, 16]. Patients without HIV now represent a rapidly changing and heterogeneous group of patients with either new forms of immunosuppression that favor the emergence of new subgroups of immunocompromised patients or novel risks in otherwise healthy patients [17]. Finally, most studies were reported from the United States [6–9, 14]; whether their findings can be applied elsewhere is unclear. This is particularly important in Australia and New Zealand where the epidemiology of Cryptococcus infections not only varies according to patient host groups but also by cryptococcal species, given our relatively high incidence of Cryptococcus gattii infections [16, 18].
In the only population-based study in Australia and New Zealand during 1994–1997, having underlying HIV accounted for 43% of all cases of cryptococcosis; 26% of cases occurred in other immunocompromised hosts, and 31% occurred in apparently immunocompetent patients [16]. To update these data in an era of changing host risks, we conducted a multicenter retrospective study to compare the current frequency of cryptococcosis in patients without HIV with that of HIV-associated cryptococcosis and to describe the epidemiology and features of cryptococcosis in patients without HIV.
METHODS
Study Design
We performed a multicenter retrospective study across 36 Australian and 10 New Zealand hospitals (Supplementary Figure 1). Hospitals were recruited through the Australia and New Zealand Mycoses Interest Group of the Australasian Society for Infectious Diseases. Both metropolitan and regional hospitals participated; all had at least 1 infectious diseases physician on staff. Cases were identified by screening microbiology, histopathology, and the hospital medical record information systems. This study was approved by ethics committees at all sites with a waiver for informed consent.
Inclusion Criteria, Definitions, and Microbiological Methods Used
Adults aged ≥18 years with cryptococcosis diagnosed between 1 January 2015 and 31 December 2019 were included. This study period was selected because Australasian guidelines on cryptococcosis were published in 2014 [19]. Cryptococcosis was defined using the European Organization for Research and Treatment of Cancer–Mycoses Study Group Education and Research Consortium definitions [20] and categorized as proven or probable (Supplementary Materials). Possible cases (ie, without mycological evidence of cryptococcosis) and patients with asymptomatic colonization of the airways (without clinical/radiological signs of cryptococcosis) were excluded. The date of diagnosis was defined as the day when the clinical sample that first led to the diagnosis of cryptococcosis was collected. Dissemination was defined as infection of at least 2 noncontiguous sites. CrAg testing was performed using either the Immuno-Mycologics Inc (IMMY, Norman, OK) CrAg lateral flow assay (41 of 46 sites) or the IMMY Latex-Cryptococcus antigen test (5 of 46 sites). Based on prior evidence [12, 21, 22] and distribution of titers obtained in the present study (data not shown), we defined a high serum CrAg titer as ≥1:320 where the lateral flow assay (IMMY) was used and ≥1:256 where the latex agglutination test (IMMY) was used. Cryptococcus species were assigned as Cryptococcus neoformans complex or C. gattii complex using standard mycological methods [23]. Patients were categorized into rural vs metropolitan on the basis of their residential postcodes. Additional definitions are provided in the Supplementary Material.
Data Collected
Data were collected using a standardized electronic data capture system (REDCap). We collected information on patient demographics and presence of at least 1 of the 4 following underlying immunocompromising conditions: diagnosed with HIV, solid organ transplantation, cancer, or other immunocompromising disease (ie, systemic inflammatory disease, primary immunodeficiency, or other immunocompromising condition/receipt of immunosuppressive medications). Additionally, the presence of medical conditions such as diabetes, chronic kidney disease, or liver disease was recorded using the Charlson comorbidity index. While we captured extensive data for patients without HIV (see case report form in the Supplementary Material), only minimal data were collected for patients with HIV (ie, date of diagnosis, causative Cryptococcus species, 1-year survival status).
Statistical Analyses
Summary statistics were undertaken to describe our cohort. Categorical data were summarized as counts and percentages. Continuous variables were summarized as means and standard deviations for normally distributed variables, and median and interquartile ranges (IQRs) were used for nonnormally distributed continuous variables. To compare the characteristics of infection between the subgroups of patients without HIV, categorical variables were compared using the Pearson χ2 test or Fischer exact test (as appropriate), and continuous variables were compared using 1-way analysis of variance, a Kruskal–Wallis test, a t test, or a rank sum test (as appropriate). Associations between a priori selected patient and infection factors and a high serum CrAg titer were analyzed using univariable and multivariable logistic regression. Associations between selected patient factors with C. neoformans infection (compared with C. gattii) were also analyzed. A Hosmer–Lemeshow test was used to assess goodness of fit. One-year survival was assessed using Kaplan–Meier curves and compared between groups using log-rank tests. A 2-sided P value of < .05 was considered to be statistically significant. All analyses were performed in Stata version 16.1 (StataCorp, College Station, TX).
RESULTS
Study Population and HIV+ to HIV– Ratio
A total of 475 patients from 46 hospitals were identified. Approximately 90% (426 of 475) of these cases were without HIV. This marked predominance of cases without HIV was observed for both culture-confirmed C. neoformans cases (88.7%, 228 of 257) and C. gattii cases (94.3%, 82 of 87), as well as cases diagnosed based on serum CrAg and/or histopathology results only without culture (88.4%, 114 of 129; Supplementary Table 1).
While the yearly number of cases with HIV remained stable during the study period, the number of cases without HIV doubled between 2015 and 2019 (Supplementary Figure 2).
Description of HIV-Negative Patients With Cryptococcosis
Characteristics of the 426 HIV-negative patients with cryptococcosis are shown in Table 1. Overall, there was a male predominance with a male-to-female ratio of 2:1 (284 males, 142 females). There was also an association between residing in a rural area and having C. gattii infection compared with C. neoformans infection (crude odds ratio [OR], 2.61; 95% confidence interval [CI], 1.56 to 4.38; P < .001). Cryptococcosis gattii cases occurred almost exclusively in Australia (81 of 82 patients, 98.8%).
Characteristics of the 426 Patients Without Human Immunodeficiency Virus With Cryptococcosis
| . | Patients With At Least One Known Underlying Immunocompromising Condition(s) Before Cryptococcosis (n = 259)a . | . | . | ||
|---|---|---|---|---|---|
| Characteristic . | Cancer (n = 82)a . | Solid Organ Transplant (n = 81)a . | Other Immunocompromising condition (n = 96)a . | Patients Without Known Underlying Immunocompromising Condition (n = 167) . | P Value b . |
| Male | 62 (75.6) | 57 (70.4) | 51 (53.1) | 114 (68.3) | .009 |
| Age at diagnosis, y mean ± SD | 67 ± 13 | 54.9 ± 12.2 | 61.2 ± 15 | 54 ± 15.3 | < .001 |
| Rural residence (vs metropolitan) | 35 (42.7) | 29 (35.8) | 25 (26.0) | 70 (41.9) | .05 |
| Charlson comorbidity index at diagnosis, mean ± SD | 3.5 ± 2 | 3.2 ± 1.6 | 1.8 ± 1.5 | 0.9 ± 1.5 | < .001 |
| Smoking status, n = 351 | |||||
| Current | 6 (9.1) | 3 (4.9) | 11 (13.4) | 32 (22.5) | .02 |
| Former | 27 (40.9) | 20 (32.8) | 30 (36.6) | 42 (25.6) | |
| Never | 33 (50) | 38 (62.3) | 41 (50) | 68 (47.9) | |
| Cryptococcus species | |||||
| C. neoformans | 50 (61) | 51 (63) | 65 (67.7) | 62 (37.1) | < .001 |
| C. gattii | 9 (11) | 6 (7.4) | 7 (7.3) | 60 (35.9) | |
| Unknown (no positive culture) | 23 (28) | 24 (29.6) | 23 (24.0) | 44 (26.4) | |
| Other | 0 (0) | 0 (0) | 1 (1) | 1 (0.6) | |
| Proven cryptococcosis per European Organization for Research and Treatment of Cancer-Mycoses Study Group Education and Research Consortium criteria (vs probable) | 59 (72) | 58 (71.6) | 72 (75.0) | 128 (76.7) | .79 |
| Site(s) of infection | |||||
| Lung | 62 (75.6) | 60 (74.1) | 68 (70.8) | 130 (77.8) | .65 |
| Lung (isolated) | 38 (46.3) | 30 (37.0) | 43 (44.8) | 65 (38.9) | .51 |
| CNS | 30 (36.6) | 36 (44.4) | 42 (43.8) | 87 (52.1) | .13 |
| Skin | 7 (8.5) | 14 (17.3) | 9 (9.4) | 2 (1.2) | < .001 |
| Blood | 18 (22.0) | 15 (18.5) | 16 (16.7) | 13 (7.8) | .01 |
| Urine | 1 (1.2) | 3 (3.7) | 1 (1) | 2 (1.2) | .55 |
| Other | 6 (7.3) | 11 (13.6) | 3 (3.1) | 15 (9) | .08 |
| Disseminated (≥2 sites) | 29 (35.6) | 37 (45.7) | 30 (31.3) | 75 (44.9) | .09 |
| Presenting signs/symptoms | |||||
| No symptoms | 19 (23.2) | 16 (19.8) | 13 (13.5) | 22 (13.2) | .16. |
| Fever ≥38°C | 26 (31.7) | 19 (23.5) | 32 (33.3) | 28 (16.8) | .008 |
| Respiratory symptoms (cough, sputum production, and/or dyspnea) | 30 (36.6) | 27 (33.3) | 41 (42.7) | 65 (38.9) | .62 |
| Neurological features | 30 (36.6) | 36 (44.4) | 41 (42.7) | 82 (49.1) | .30 |
| Headaches (isolated) | 6 (7.3) | 11 (13.6) | 9 (9.4) | 21 (12.6) | .50 |
| Meningism | 8 (9.8) | 11 (13.6) | 13 (13.5) | 34 (20.4) | .13 |
| Altered mental status (Glasgow score ≤13) | 6 (7.3) | 7 (8.6) | 15 (15.6) | 18 (10.8) | .29 |
| Seizures | 3 (3.7) | 0 (0) | 3 (3.1) | 10 (6) | .11 |
| Other neurological symptoms/sign | 24 (29.3) | 20 (24.7) | 25 (26.0) | 54 (32.3) | .56 |
| Skin lesion(s) | 7 (8.5) | 14 (17.3) | 9 (9.4) | 2 (1.2) | < .001 |
| If symptomatic: time from onset of symptoms to diagnosis, d, median (IQR), n = 334 | 21 (8–35) | 13 (5–30) | 23.5 (13–60) | 35 (11–60) | .002 |
| Blood test results at presentation | |||||
| C-reactive protein level, mg/L, median (IQR), n = 342 | 34 (8.8–83) | 11.3 (3–44.5) | 20 (4.2–64) | 7.1 (3–25) | < .001 |
| Positive blood cultures, n = 287 | 18 (34) | 15 (23.1) | 16 (23.2) | 13 (13) | .025 |
| Positive serum CrAg, n = 375 | 61 (83.6) | 64 (87.7) | 71 (87.7) | 123 (83.1) | .71 |
| Neutropenia <500 mm3 | 2 (2.4) | 2 (2.5) | 1 (1) | 0 (0) | .07 |
| Biopsy with suggestive histopathology, n = 424 | 28 (34.6) | 24 (30) | 29 (30.2) | 66 (39.5) | .35 |
| CSF findings (for patients with CNS cryptococcosis) | |||||
| White blood cells, per mm3, median (IQR), n = 169 | 157 (34–350) | 65 (13–393) | 33 (10–73) | 113 (28–277) | < .001 |
| Lymphocytes, per mm3, median (IQR), n = 175 | 85 (18–209) | 47 (10–121) | 20 (6–62) | 99 (24–214) | < .001 |
| Neutrophils, per mm3, median (IQR), n = 174 | 16 (5–71) | 4 (1–16) | 4 (0–29) | 9 (1–36) | .08 |
| Protein, mg/dL, median (IQR), n = 183 | 134 (93–180) | 90 (45–147) | 103 (49–212) | 90 (58–150) | .16 |
| Glucose, mg/dL median (IQR), n = 182 | 36 (24–51) | 49 (29–68) | 34 (14–54) | 43 (27–69) | 0,16 |
| Opening pressure ≥25 cmH2O, n = 127 | 2 (11.8) | 8 (34.8) | 8 (29.6) | 28 (46.7) | .047 |
| Positive microscopy, n = 156 | 12 (52.2) | 19 (63.3) | 16 (42.1) | 36 (55.3) | .36 |
| Positive culture, n = 186 | 23 (79.3) | 28 (80) | 29 (70.7) | 58 (71.6) | < .001 |
| Positive CSF CrAg, n = 185 | 29 (100) | 35 (100) | 41 (100) | 75 (93.4) | .14 |
| Brain imaging findings (for patients with CNS cryptococcosis), n = 176 | |||||
| Cryptococcoma(s) | 6 (23.1) | 3 (9.4) | 5 (13.5) | 31 (38.3) | .003 |
| Signs of meningitis | 7 (26.9) | 6 (18.8) | 13 (35.1) | 27 (33.3) | .40 |
| Hydrocephalus | 0 (0) | 2 (6.3) | 3 (8.1) | 8 (9.9) | .45 |
| Dilated perivascular spaces | 2 (7.7) | 1 (3.1) | 1 (2.7) | 3 (3.7) | .80 |
| No related lesions reported | 13 (50) | 21 (65.6) | 20 (54.1) | 23 (28.4) | .001 |
| Chest imaging findings (for patients with lung cryptococcosis) | |||||
| Nodule(s) or mass(es) | 51 (82.3) | 43 (71.7) | 50 (73.5) | 103 (79.2) | .43 |
| Consolidation(s) | 16 (25.8) | 14 (23.3) | 26 (38.2) | 31 (23.9) | .14 |
| Interstitial pattern | 7 (11.3) | 6 (10) | 14 (20.6) | 5 (3.9) | .003 |
| Pleural effusion | 10 (16.1) | 10 (16.7) | 5 (7.4) | 11 (8.5) | .15 |
| No related lesions reported | 14 (22.6) | 17 (28.3) | 20 (29.4) | 34 (26.2) | .33 |
| . | Patients With At Least One Known Underlying Immunocompromising Condition(s) Before Cryptococcosis (n = 259)a . | . | . | ||
|---|---|---|---|---|---|
| Characteristic . | Cancer (n = 82)a . | Solid Organ Transplant (n = 81)a . | Other Immunocompromising condition (n = 96)a . | Patients Without Known Underlying Immunocompromising Condition (n = 167) . | P Value b . |
| Male | 62 (75.6) | 57 (70.4) | 51 (53.1) | 114 (68.3) | .009 |
| Age at diagnosis, y mean ± SD | 67 ± 13 | 54.9 ± 12.2 | 61.2 ± 15 | 54 ± 15.3 | < .001 |
| Rural residence (vs metropolitan) | 35 (42.7) | 29 (35.8) | 25 (26.0) | 70 (41.9) | .05 |
| Charlson comorbidity index at diagnosis, mean ± SD | 3.5 ± 2 | 3.2 ± 1.6 | 1.8 ± 1.5 | 0.9 ± 1.5 | < .001 |
| Smoking status, n = 351 | |||||
| Current | 6 (9.1) | 3 (4.9) | 11 (13.4) | 32 (22.5) | .02 |
| Former | 27 (40.9) | 20 (32.8) | 30 (36.6) | 42 (25.6) | |
| Never | 33 (50) | 38 (62.3) | 41 (50) | 68 (47.9) | |
| Cryptococcus species | |||||
| C. neoformans | 50 (61) | 51 (63) | 65 (67.7) | 62 (37.1) | < .001 |
| C. gattii | 9 (11) | 6 (7.4) | 7 (7.3) | 60 (35.9) | |
| Unknown (no positive culture) | 23 (28) | 24 (29.6) | 23 (24.0) | 44 (26.4) | |
| Other | 0 (0) | 0 (0) | 1 (1) | 1 (0.6) | |
| Proven cryptococcosis per European Organization for Research and Treatment of Cancer-Mycoses Study Group Education and Research Consortium criteria (vs probable) | 59 (72) | 58 (71.6) | 72 (75.0) | 128 (76.7) | .79 |
| Site(s) of infection | |||||
| Lung | 62 (75.6) | 60 (74.1) | 68 (70.8) | 130 (77.8) | .65 |
| Lung (isolated) | 38 (46.3) | 30 (37.0) | 43 (44.8) | 65 (38.9) | .51 |
| CNS | 30 (36.6) | 36 (44.4) | 42 (43.8) | 87 (52.1) | .13 |
| Skin | 7 (8.5) | 14 (17.3) | 9 (9.4) | 2 (1.2) | < .001 |
| Blood | 18 (22.0) | 15 (18.5) | 16 (16.7) | 13 (7.8) | .01 |
| Urine | 1 (1.2) | 3 (3.7) | 1 (1) | 2 (1.2) | .55 |
| Other | 6 (7.3) | 11 (13.6) | 3 (3.1) | 15 (9) | .08 |
| Disseminated (≥2 sites) | 29 (35.6) | 37 (45.7) | 30 (31.3) | 75 (44.9) | .09 |
| Presenting signs/symptoms | |||||
| No symptoms | 19 (23.2) | 16 (19.8) | 13 (13.5) | 22 (13.2) | .16. |
| Fever ≥38°C | 26 (31.7) | 19 (23.5) | 32 (33.3) | 28 (16.8) | .008 |
| Respiratory symptoms (cough, sputum production, and/or dyspnea) | 30 (36.6) | 27 (33.3) | 41 (42.7) | 65 (38.9) | .62 |
| Neurological features | 30 (36.6) | 36 (44.4) | 41 (42.7) | 82 (49.1) | .30 |
| Headaches (isolated) | 6 (7.3) | 11 (13.6) | 9 (9.4) | 21 (12.6) | .50 |
| Meningism | 8 (9.8) | 11 (13.6) | 13 (13.5) | 34 (20.4) | .13 |
| Altered mental status (Glasgow score ≤13) | 6 (7.3) | 7 (8.6) | 15 (15.6) | 18 (10.8) | .29 |
| Seizures | 3 (3.7) | 0 (0) | 3 (3.1) | 10 (6) | .11 |
| Other neurological symptoms/sign | 24 (29.3) | 20 (24.7) | 25 (26.0) | 54 (32.3) | .56 |
| Skin lesion(s) | 7 (8.5) | 14 (17.3) | 9 (9.4) | 2 (1.2) | < .001 |
| If symptomatic: time from onset of symptoms to diagnosis, d, median (IQR), n = 334 | 21 (8–35) | 13 (5–30) | 23.5 (13–60) | 35 (11–60) | .002 |
| Blood test results at presentation | |||||
| C-reactive protein level, mg/L, median (IQR), n = 342 | 34 (8.8–83) | 11.3 (3–44.5) | 20 (4.2–64) | 7.1 (3–25) | < .001 |
| Positive blood cultures, n = 287 | 18 (34) | 15 (23.1) | 16 (23.2) | 13 (13) | .025 |
| Positive serum CrAg, n = 375 | 61 (83.6) | 64 (87.7) | 71 (87.7) | 123 (83.1) | .71 |
| Neutropenia <500 mm3 | 2 (2.4) | 2 (2.5) | 1 (1) | 0 (0) | .07 |
| Biopsy with suggestive histopathology, n = 424 | 28 (34.6) | 24 (30) | 29 (30.2) | 66 (39.5) | .35 |
| CSF findings (for patients with CNS cryptococcosis) | |||||
| White blood cells, per mm3, median (IQR), n = 169 | 157 (34–350) | 65 (13–393) | 33 (10–73) | 113 (28–277) | < .001 |
| Lymphocytes, per mm3, median (IQR), n = 175 | 85 (18–209) | 47 (10–121) | 20 (6–62) | 99 (24–214) | < .001 |
| Neutrophils, per mm3, median (IQR), n = 174 | 16 (5–71) | 4 (1–16) | 4 (0–29) | 9 (1–36) | .08 |
| Protein, mg/dL, median (IQR), n = 183 | 134 (93–180) | 90 (45–147) | 103 (49–212) | 90 (58–150) | .16 |
| Glucose, mg/dL median (IQR), n = 182 | 36 (24–51) | 49 (29–68) | 34 (14–54) | 43 (27–69) | 0,16 |
| Opening pressure ≥25 cmH2O, n = 127 | 2 (11.8) | 8 (34.8) | 8 (29.6) | 28 (46.7) | .047 |
| Positive microscopy, n = 156 | 12 (52.2) | 19 (63.3) | 16 (42.1) | 36 (55.3) | .36 |
| Positive culture, n = 186 | 23 (79.3) | 28 (80) | 29 (70.7) | 58 (71.6) | < .001 |
| Positive CSF CrAg, n = 185 | 29 (100) | 35 (100) | 41 (100) | 75 (93.4) | .14 |
| Brain imaging findings (for patients with CNS cryptococcosis), n = 176 | |||||
| Cryptococcoma(s) | 6 (23.1) | 3 (9.4) | 5 (13.5) | 31 (38.3) | .003 |
| Signs of meningitis | 7 (26.9) | 6 (18.8) | 13 (35.1) | 27 (33.3) | .40 |
| Hydrocephalus | 0 (0) | 2 (6.3) | 3 (8.1) | 8 (9.9) | .45 |
| Dilated perivascular spaces | 2 (7.7) | 1 (3.1) | 1 (2.7) | 3 (3.7) | .80 |
| No related lesions reported | 13 (50) | 21 (65.6) | 20 (54.1) | 23 (28.4) | .001 |
| Chest imaging findings (for patients with lung cryptococcosis) | |||||
| Nodule(s) or mass(es) | 51 (82.3) | 43 (71.7) | 50 (73.5) | 103 (79.2) | .43 |
| Consolidation(s) | 16 (25.8) | 14 (23.3) | 26 (38.2) | 31 (23.9) | .14 |
| Interstitial pattern | 7 (11.3) | 6 (10) | 14 (20.6) | 5 (3.9) | .003 |
| Pleural effusion | 10 (16.1) | 10 (16.7) | 5 (7.4) | 11 (8.5) | .15 |
| No related lesions reported | 14 (22.6) | 17 (28.3) | 20 (29.4) | 34 (26.2) | .33 |
Data are n (%) unless otherwise indicated.
Abbreviations: CNS, central nervous system; CrAg, Cryptococcus antigen; CSF, cerebrospinal fluid; IQR, interquartile range; n, number of data analyzed (when at least 1 missing data); SD, standard deviation.
Ten patients had 2 immunocompromising conditions and, to avoid duplicates in data analysis, were included in only 1 subgroup after medical history review (details: 1 kidney transplant recipient with a history of vasculitis was analyzed as being an organ transplant recipient; 1 kidney transplant recipient with a history of cancer was analyzed as being an organ transplant recipient; 8 patients with a history of both cancer and another immunocompromising condition were analyzed as having another immunocompromising condition).
P values are for the comparison of the 4 subgroups of patients without human immunodeficiency virus.
P values below 0.05 are in bold, indicating statistical significance.
Characteristics of the 426 Patients Without Human Immunodeficiency Virus With Cryptococcosis
| . | Patients With At Least One Known Underlying Immunocompromising Condition(s) Before Cryptococcosis (n = 259)a . | . | . | ||
|---|---|---|---|---|---|
| Characteristic . | Cancer (n = 82)a . | Solid Organ Transplant (n = 81)a . | Other Immunocompromising condition (n = 96)a . | Patients Without Known Underlying Immunocompromising Condition (n = 167) . | P Value b . |
| Male | 62 (75.6) | 57 (70.4) | 51 (53.1) | 114 (68.3) | .009 |
| Age at diagnosis, y mean ± SD | 67 ± 13 | 54.9 ± 12.2 | 61.2 ± 15 | 54 ± 15.3 | < .001 |
| Rural residence (vs metropolitan) | 35 (42.7) | 29 (35.8) | 25 (26.0) | 70 (41.9) | .05 |
| Charlson comorbidity index at diagnosis, mean ± SD | 3.5 ± 2 | 3.2 ± 1.6 | 1.8 ± 1.5 | 0.9 ± 1.5 | < .001 |
| Smoking status, n = 351 | |||||
| Current | 6 (9.1) | 3 (4.9) | 11 (13.4) | 32 (22.5) | .02 |
| Former | 27 (40.9) | 20 (32.8) | 30 (36.6) | 42 (25.6) | |
| Never | 33 (50) | 38 (62.3) | 41 (50) | 68 (47.9) | |
| Cryptococcus species | |||||
| C. neoformans | 50 (61) | 51 (63) | 65 (67.7) | 62 (37.1) | < .001 |
| C. gattii | 9 (11) | 6 (7.4) | 7 (7.3) | 60 (35.9) | |
| Unknown (no positive culture) | 23 (28) | 24 (29.6) | 23 (24.0) | 44 (26.4) | |
| Other | 0 (0) | 0 (0) | 1 (1) | 1 (0.6) | |
| Proven cryptococcosis per European Organization for Research and Treatment of Cancer-Mycoses Study Group Education and Research Consortium criteria (vs probable) | 59 (72) | 58 (71.6) | 72 (75.0) | 128 (76.7) | .79 |
| Site(s) of infection | |||||
| Lung | 62 (75.6) | 60 (74.1) | 68 (70.8) | 130 (77.8) | .65 |
| Lung (isolated) | 38 (46.3) | 30 (37.0) | 43 (44.8) | 65 (38.9) | .51 |
| CNS | 30 (36.6) | 36 (44.4) | 42 (43.8) | 87 (52.1) | .13 |
| Skin | 7 (8.5) | 14 (17.3) | 9 (9.4) | 2 (1.2) | < .001 |
| Blood | 18 (22.0) | 15 (18.5) | 16 (16.7) | 13 (7.8) | .01 |
| Urine | 1 (1.2) | 3 (3.7) | 1 (1) | 2 (1.2) | .55 |
| Other | 6 (7.3) | 11 (13.6) | 3 (3.1) | 15 (9) | .08 |
| Disseminated (≥2 sites) | 29 (35.6) | 37 (45.7) | 30 (31.3) | 75 (44.9) | .09 |
| Presenting signs/symptoms | |||||
| No symptoms | 19 (23.2) | 16 (19.8) | 13 (13.5) | 22 (13.2) | .16. |
| Fever ≥38°C | 26 (31.7) | 19 (23.5) | 32 (33.3) | 28 (16.8) | .008 |
| Respiratory symptoms (cough, sputum production, and/or dyspnea) | 30 (36.6) | 27 (33.3) | 41 (42.7) | 65 (38.9) | .62 |
| Neurological features | 30 (36.6) | 36 (44.4) | 41 (42.7) | 82 (49.1) | .30 |
| Headaches (isolated) | 6 (7.3) | 11 (13.6) | 9 (9.4) | 21 (12.6) | .50 |
| Meningism | 8 (9.8) | 11 (13.6) | 13 (13.5) | 34 (20.4) | .13 |
| Altered mental status (Glasgow score ≤13) | 6 (7.3) | 7 (8.6) | 15 (15.6) | 18 (10.8) | .29 |
| Seizures | 3 (3.7) | 0 (0) | 3 (3.1) | 10 (6) | .11 |
| Other neurological symptoms/sign | 24 (29.3) | 20 (24.7) | 25 (26.0) | 54 (32.3) | .56 |
| Skin lesion(s) | 7 (8.5) | 14 (17.3) | 9 (9.4) | 2 (1.2) | < .001 |
| If symptomatic: time from onset of symptoms to diagnosis, d, median (IQR), n = 334 | 21 (8–35) | 13 (5–30) | 23.5 (13–60) | 35 (11–60) | .002 |
| Blood test results at presentation | |||||
| C-reactive protein level, mg/L, median (IQR), n = 342 | 34 (8.8–83) | 11.3 (3–44.5) | 20 (4.2–64) | 7.1 (3–25) | < .001 |
| Positive blood cultures, n = 287 | 18 (34) | 15 (23.1) | 16 (23.2) | 13 (13) | .025 |
| Positive serum CrAg, n = 375 | 61 (83.6) | 64 (87.7) | 71 (87.7) | 123 (83.1) | .71 |
| Neutropenia <500 mm3 | 2 (2.4) | 2 (2.5) | 1 (1) | 0 (0) | .07 |
| Biopsy with suggestive histopathology, n = 424 | 28 (34.6) | 24 (30) | 29 (30.2) | 66 (39.5) | .35 |
| CSF findings (for patients with CNS cryptococcosis) | |||||
| White blood cells, per mm3, median (IQR), n = 169 | 157 (34–350) | 65 (13–393) | 33 (10–73) | 113 (28–277) | < .001 |
| Lymphocytes, per mm3, median (IQR), n = 175 | 85 (18–209) | 47 (10–121) | 20 (6–62) | 99 (24–214) | < .001 |
| Neutrophils, per mm3, median (IQR), n = 174 | 16 (5–71) | 4 (1–16) | 4 (0–29) | 9 (1–36) | .08 |
| Protein, mg/dL, median (IQR), n = 183 | 134 (93–180) | 90 (45–147) | 103 (49–212) | 90 (58–150) | .16 |
| Glucose, mg/dL median (IQR), n = 182 | 36 (24–51) | 49 (29–68) | 34 (14–54) | 43 (27–69) | 0,16 |
| Opening pressure ≥25 cmH2O, n = 127 | 2 (11.8) | 8 (34.8) | 8 (29.6) | 28 (46.7) | .047 |
| Positive microscopy, n = 156 | 12 (52.2) | 19 (63.3) | 16 (42.1) | 36 (55.3) | .36 |
| Positive culture, n = 186 | 23 (79.3) | 28 (80) | 29 (70.7) | 58 (71.6) | < .001 |
| Positive CSF CrAg, n = 185 | 29 (100) | 35 (100) | 41 (100) | 75 (93.4) | .14 |
| Brain imaging findings (for patients with CNS cryptococcosis), n = 176 | |||||
| Cryptococcoma(s) | 6 (23.1) | 3 (9.4) | 5 (13.5) | 31 (38.3) | .003 |
| Signs of meningitis | 7 (26.9) | 6 (18.8) | 13 (35.1) | 27 (33.3) | .40 |
| Hydrocephalus | 0 (0) | 2 (6.3) | 3 (8.1) | 8 (9.9) | .45 |
| Dilated perivascular spaces | 2 (7.7) | 1 (3.1) | 1 (2.7) | 3 (3.7) | .80 |
| No related lesions reported | 13 (50) | 21 (65.6) | 20 (54.1) | 23 (28.4) | .001 |
| Chest imaging findings (for patients with lung cryptococcosis) | |||||
| Nodule(s) or mass(es) | 51 (82.3) | 43 (71.7) | 50 (73.5) | 103 (79.2) | .43 |
| Consolidation(s) | 16 (25.8) | 14 (23.3) | 26 (38.2) | 31 (23.9) | .14 |
| Interstitial pattern | 7 (11.3) | 6 (10) | 14 (20.6) | 5 (3.9) | .003 |
| Pleural effusion | 10 (16.1) | 10 (16.7) | 5 (7.4) | 11 (8.5) | .15 |
| No related lesions reported | 14 (22.6) | 17 (28.3) | 20 (29.4) | 34 (26.2) | .33 |
| . | Patients With At Least One Known Underlying Immunocompromising Condition(s) Before Cryptococcosis (n = 259)a . | . | . | ||
|---|---|---|---|---|---|
| Characteristic . | Cancer (n = 82)a . | Solid Organ Transplant (n = 81)a . | Other Immunocompromising condition (n = 96)a . | Patients Without Known Underlying Immunocompromising Condition (n = 167) . | P Value b . |
| Male | 62 (75.6) | 57 (70.4) | 51 (53.1) | 114 (68.3) | .009 |
| Age at diagnosis, y mean ± SD | 67 ± 13 | 54.9 ± 12.2 | 61.2 ± 15 | 54 ± 15.3 | < .001 |
| Rural residence (vs metropolitan) | 35 (42.7) | 29 (35.8) | 25 (26.0) | 70 (41.9) | .05 |
| Charlson comorbidity index at diagnosis, mean ± SD | 3.5 ± 2 | 3.2 ± 1.6 | 1.8 ± 1.5 | 0.9 ± 1.5 | < .001 |
| Smoking status, n = 351 | |||||
| Current | 6 (9.1) | 3 (4.9) | 11 (13.4) | 32 (22.5) | .02 |
| Former | 27 (40.9) | 20 (32.8) | 30 (36.6) | 42 (25.6) | |
| Never | 33 (50) | 38 (62.3) | 41 (50) | 68 (47.9) | |
| Cryptococcus species | |||||
| C. neoformans | 50 (61) | 51 (63) | 65 (67.7) | 62 (37.1) | < .001 |
| C. gattii | 9 (11) | 6 (7.4) | 7 (7.3) | 60 (35.9) | |
| Unknown (no positive culture) | 23 (28) | 24 (29.6) | 23 (24.0) | 44 (26.4) | |
| Other | 0 (0) | 0 (0) | 1 (1) | 1 (0.6) | |
| Proven cryptococcosis per European Organization for Research and Treatment of Cancer-Mycoses Study Group Education and Research Consortium criteria (vs probable) | 59 (72) | 58 (71.6) | 72 (75.0) | 128 (76.7) | .79 |
| Site(s) of infection | |||||
| Lung | 62 (75.6) | 60 (74.1) | 68 (70.8) | 130 (77.8) | .65 |
| Lung (isolated) | 38 (46.3) | 30 (37.0) | 43 (44.8) | 65 (38.9) | .51 |
| CNS | 30 (36.6) | 36 (44.4) | 42 (43.8) | 87 (52.1) | .13 |
| Skin | 7 (8.5) | 14 (17.3) | 9 (9.4) | 2 (1.2) | < .001 |
| Blood | 18 (22.0) | 15 (18.5) | 16 (16.7) | 13 (7.8) | .01 |
| Urine | 1 (1.2) | 3 (3.7) | 1 (1) | 2 (1.2) | .55 |
| Other | 6 (7.3) | 11 (13.6) | 3 (3.1) | 15 (9) | .08 |
| Disseminated (≥2 sites) | 29 (35.6) | 37 (45.7) | 30 (31.3) | 75 (44.9) | .09 |
| Presenting signs/symptoms | |||||
| No symptoms | 19 (23.2) | 16 (19.8) | 13 (13.5) | 22 (13.2) | .16. |
| Fever ≥38°C | 26 (31.7) | 19 (23.5) | 32 (33.3) | 28 (16.8) | .008 |
| Respiratory symptoms (cough, sputum production, and/or dyspnea) | 30 (36.6) | 27 (33.3) | 41 (42.7) | 65 (38.9) | .62 |
| Neurological features | 30 (36.6) | 36 (44.4) | 41 (42.7) | 82 (49.1) | .30 |
| Headaches (isolated) | 6 (7.3) | 11 (13.6) | 9 (9.4) | 21 (12.6) | .50 |
| Meningism | 8 (9.8) | 11 (13.6) | 13 (13.5) | 34 (20.4) | .13 |
| Altered mental status (Glasgow score ≤13) | 6 (7.3) | 7 (8.6) | 15 (15.6) | 18 (10.8) | .29 |
| Seizures | 3 (3.7) | 0 (0) | 3 (3.1) | 10 (6) | .11 |
| Other neurological symptoms/sign | 24 (29.3) | 20 (24.7) | 25 (26.0) | 54 (32.3) | .56 |
| Skin lesion(s) | 7 (8.5) | 14 (17.3) | 9 (9.4) | 2 (1.2) | < .001 |
| If symptomatic: time from onset of symptoms to diagnosis, d, median (IQR), n = 334 | 21 (8–35) | 13 (5–30) | 23.5 (13–60) | 35 (11–60) | .002 |
| Blood test results at presentation | |||||
| C-reactive protein level, mg/L, median (IQR), n = 342 | 34 (8.8–83) | 11.3 (3–44.5) | 20 (4.2–64) | 7.1 (3–25) | < .001 |
| Positive blood cultures, n = 287 | 18 (34) | 15 (23.1) | 16 (23.2) | 13 (13) | .025 |
| Positive serum CrAg, n = 375 | 61 (83.6) | 64 (87.7) | 71 (87.7) | 123 (83.1) | .71 |
| Neutropenia <500 mm3 | 2 (2.4) | 2 (2.5) | 1 (1) | 0 (0) | .07 |
| Biopsy with suggestive histopathology, n = 424 | 28 (34.6) | 24 (30) | 29 (30.2) | 66 (39.5) | .35 |
| CSF findings (for patients with CNS cryptococcosis) | |||||
| White blood cells, per mm3, median (IQR), n = 169 | 157 (34–350) | 65 (13–393) | 33 (10–73) | 113 (28–277) | < .001 |
| Lymphocytes, per mm3, median (IQR), n = 175 | 85 (18–209) | 47 (10–121) | 20 (6–62) | 99 (24–214) | < .001 |
| Neutrophils, per mm3, median (IQR), n = 174 | 16 (5–71) | 4 (1–16) | 4 (0–29) | 9 (1–36) | .08 |
| Protein, mg/dL, median (IQR), n = 183 | 134 (93–180) | 90 (45–147) | 103 (49–212) | 90 (58–150) | .16 |
| Glucose, mg/dL median (IQR), n = 182 | 36 (24–51) | 49 (29–68) | 34 (14–54) | 43 (27–69) | 0,16 |
| Opening pressure ≥25 cmH2O, n = 127 | 2 (11.8) | 8 (34.8) | 8 (29.6) | 28 (46.7) | .047 |
| Positive microscopy, n = 156 | 12 (52.2) | 19 (63.3) | 16 (42.1) | 36 (55.3) | .36 |
| Positive culture, n = 186 | 23 (79.3) | 28 (80) | 29 (70.7) | 58 (71.6) | < .001 |
| Positive CSF CrAg, n = 185 | 29 (100) | 35 (100) | 41 (100) | 75 (93.4) | .14 |
| Brain imaging findings (for patients with CNS cryptococcosis), n = 176 | |||||
| Cryptococcoma(s) | 6 (23.1) | 3 (9.4) | 5 (13.5) | 31 (38.3) | .003 |
| Signs of meningitis | 7 (26.9) | 6 (18.8) | 13 (35.1) | 27 (33.3) | .40 |
| Hydrocephalus | 0 (0) | 2 (6.3) | 3 (8.1) | 8 (9.9) | .45 |
| Dilated perivascular spaces | 2 (7.7) | 1 (3.1) | 1 (2.7) | 3 (3.7) | .80 |
| No related lesions reported | 13 (50) | 21 (65.6) | 20 (54.1) | 23 (28.4) | .001 |
| Chest imaging findings (for patients with lung cryptococcosis) | |||||
| Nodule(s) or mass(es) | 51 (82.3) | 43 (71.7) | 50 (73.5) | 103 (79.2) | .43 |
| Consolidation(s) | 16 (25.8) | 14 (23.3) | 26 (38.2) | 31 (23.9) | .14 |
| Interstitial pattern | 7 (11.3) | 6 (10) | 14 (20.6) | 5 (3.9) | .003 |
| Pleural effusion | 10 (16.1) | 10 (16.7) | 5 (7.4) | 11 (8.5) | .15 |
| No related lesions reported | 14 (22.6) | 17 (28.3) | 20 (29.4) | 34 (26.2) | .33 |
Data are n (%) unless otherwise indicated.
Abbreviations: CNS, central nervous system; CrAg, Cryptococcus antigen; CSF, cerebrospinal fluid; IQR, interquartile range; n, number of data analyzed (when at least 1 missing data); SD, standard deviation.
Ten patients had 2 immunocompromising conditions and, to avoid duplicates in data analysis, were included in only 1 subgroup after medical history review (details: 1 kidney transplant recipient with a history of vasculitis was analyzed as being an organ transplant recipient; 1 kidney transplant recipient with a history of cancer was analyzed as being an organ transplant recipient; 8 patients with a history of both cancer and another immunocompromising condition were analyzed as having another immunocompromising condition).
P values are for the comparison of the 4 subgroups of patients without human immunodeficiency virus.
P values below 0.05 are in bold, indicating statistical significance.
At least 1 immunocompromising condition was present in 259 of 426 (60.8%) HIV-negative patients with cryptococcosis: 91 had cancer, 81 were organ transplant recipients, and 97 had another immunocompromising condition (Table 1).
Of cancer patients, most had a hematological malignancy (62.6%, 57 of 91), with 19 patients each having chronic lymphocytic leukemia and non-Hodgkin lymphoma (Supplementary Table 2). Twelve cases were associated with receipt of Bruton's tyrosine kinase inhibitors. No cancer-related cases occurred in patients taking antifungal prophylaxis (Supplementary Table 2).
Regarding solid organ transplant recipients, the most commonly transplanted organs were kidney (68%, 55 of 81), lung (17%, 14 of 81), and liver (15%, 12 of 81 patients; Supplementary Table 3). The median time between transplantation and cryptococcosis was 35 months (IQR, 13 to 76), and only 11 patients (14%) had a history of acute graft rejection in the 6 months before cryptococcosis (Supplementary Table 3).
In those with an immunocompromising condition other than cancer or solid organ transplantation, the most common condition was rheumatoid arthritis (23%, 22 of 97 patients; Supplementary Table 4). There were 9 cases of cryptococcosis in patients with multiple sclerosis receiving fingolimod (see Supplementary Table 4 for additional details).
Of 167 patients who had no identifiable immunocompromising condition, only 37.1% had C. neoformans infection (62 of 167). In contrast, the presence of an immunocompromising condition was significantly associated with C. neoformans compared with C. gattii infection (OR, 7.3; 95% CI, 4.1 to 12.9; P < .001; Table 2). Using Charlson comorbidity index data, we observed that most patients (56.5%, 35 of 62) who developed C. neoformans infection despite no identifiable immunocompromising condition had at least 1 medical disorder that may have predisposed them to cryptococcosis (ie, chronic lung disease, liver disease, kidney disease, or diabetes). Additional patient details are shown in Supplementary Table 5.
Comparison Between Patients Without Human Immunodeficiency Virus (HIV) Who Had a Culture-Positive Cryptococcus neoformans Infection (n = 228) and Patients Without HIV Who Had a Culture-Positive Cryptococcus gattii Infection (n = 82)
| Characteristic . | Patients Without HIV With Cryptococcus neoformans Infection (n = 228) . | Patients Without HIV With C. gattii Infection (n = 82) . | P Value . |
|---|---|---|---|
| Male | 114 (61.4) | 56 (68.3) | .27 |
| Age at diagnosis, y mean ± standard deviation | 60.7 ± 15.5 | 52.7 ± 14.1 | < .001 |
| Australia (vs New Zealand) | 197 (86.4) | 81 (98.8) | .002 |
| Rural residence (vs metropolitan) | 70 (30.7) | 44 (53.7) | < .001 |
| Known underlying immunocompromising conditiona Cancer | 166 (72.8) 56 (24.6) | 22 (26.8) 9 (11.0) | < .001 .01 |
| Solid organ transplantation | 51 (22.4) | 6 (7.3) | .003 |
| Othera | 65 (28.5) | 7 (8.5) | < .001 |
| Charlson comorbidity index at diagnosis, median (IQR) | 2 (1–4) | 0 (0–2) | < .001 |
| Smoking status, n = 247 | |||
| Current | 17 (9.7) | 14 (19.7) | .10 |
| Former | 62 (35.2) | 23 (32.4) | |
| Never | 97 (55.1) | 34 (4) | |
| Proven cryptococcosis per European Organization for Research and Treatment of Cancer-Mycoses Study Group Education and Research Consortium criteria (vs probable) | 161 (70.6) | 71 (86.6) | .004 |
| Site(s) of infection | |||
| Lung | 150 (65.8) | 71 (86.6) | < .001 |
| Lung (isolated) | 23 (10.1) | 4 (4.9) | .18 |
| CNS | 107 (46.9) | 64 (78) | < .001 |
| Skin | 22 (9.7) | 2 (2.4) | .05 |
| Blood | 60 (26.3) | 1 (1.2) | < .001 |
| Urine | 7 (3.1) | 0 (0) | .20 |
| Other | 22 (9.7) | 5 (6.1) | .33 |
| Disseminated (≥2 sites) | 97 (42.5) | 54 (65.9) | < .001 |
| Signs/symptoms at time of presentation | |||
| No symptoms | 25 (11.0) | 6 (7.3) | .35 |
| Fever ≥38°C | 68 (29.8) | 26 (31.7) | .75 |
| Respiratory symptoms (cough, sputum production, or dyspnea) | 87 (38.2) | 31 (37.8) | .96 |
| Neurological features | 104 (45.6) | 59 (72.0) | < .001 |
| Headaches (isolated) | 26 (11.4) | 17 (20.7) | .04 |
| Meningism | 33 (14.5) | 25 (30.5) | .001 |
| Altered mental status (Glasgow score ≤13) | 30 (13.2) | 10 (12.2) | .82 |
| Seizures | 8 (3.5) | 6 (7.3) | .15 |
| Other | 78 (34.2) | 21 (25.6) | .15 |
| Skin lesion | 22 (9.7) | 2 (2.4) | .05 |
| Time from onset of symptoms to diagnosis, d, median (IQR), n = 264 | 21 (7–56) | 21 (10–60) | .34 |
| Blood test results at time of presentation C-reactive protein level, mg/L, median (IQR), n = 258 | 19.7 (4.2–58.6) | 8.4 (3.9–43) | .06 |
| Positive blood cultures, n = 225 | 60 (36.1) | 1 (1.7) | < .001 |
| Positive serum CrAg, n = 265 | 148 (77.1) | 71 (97.3) | < .001 |
| Neutropenia <500 mm3, n = 302 | 3 (1.3) | 1 (1.3) | 1 |
| Biopsy with suggestive histopathology results, n = 308 | 44 (19.5) | 32 (39) | < .001 |
| CSF findings (for patients with CNS cryptococcosis) | |||
| White blood cells, per mm3, median (IQR), n = 148 | 60 (12–146) | 113 (41–262) | .01 |
| Lymphocytes, per mm3, median (IQR), n = 155 | 52 (9–122) | 87 (42–210) | .01 |
| Neutrophils, per mm3, median (IQR), n = 154 | 6 (1–27) | 16 (3–49) | .01 |
| Protein, mg/dL, median (IQR), n = 161 | 110 (63.5–176.6) | 82 (50–113.1) | .02 |
| Glucose, mg/dL, median (IQR), n = 160 | 38.7 (18–59.2) | 45 (30.6–70.1) | .046 |
| Opening pressure ≥25 cmH2O, n = 111 | 26 (38.8) | 19 (43.2) | .62 |
| Positive microscopy, n = 138 | 56 (62.9) | 27 (55.1) | .33 |
| Positive culture, n = 164 | 89 (86.4) | 49 (80.3) | < .001 |
| Positive CSF CrAg, n = 163 | 103 (100) | 57 (95) | .02 |
| Brain imaging findings (for patients with CNS cryptococcosis), n = 154 | |||
| Cryptococcoma(s) | 11 (12) | 28 (45.2) | < .001 |
| Signs of meningitis | 31 (33.7) | 15 (24.2) | .21 |
| Hydrocephalus | 7 (7.7) | 4 (6.5) | 1 |
| Dilated perivascular spaces | 2 (2.2) | 5 (8.1) | .12 |
| No related lesions | 51 (55.4) | 19 (30.6) | .002 |
| Chest imaging findings (for patients with lung cryptococcosis) | |||
| Nodule(s) or mass(es) | 89 (59.3) | 54 (76.1) | .02 |
| Consolidation(s) | 50 (33.3) | 18 (25.4) | .23 |
| Interstitial pattern | 19 (12.7) | 4 (5.6) | .16 |
| Pleural effusion | 23 (15.3) | 6 (8.5) | .16 |
| No related lesions | 4 (2.7) | 1 (1.4) | 1 |
| Characteristic . | Patients Without HIV With Cryptococcus neoformans Infection (n = 228) . | Patients Without HIV With C. gattii Infection (n = 82) . | P Value . |
|---|---|---|---|
| Male | 114 (61.4) | 56 (68.3) | .27 |
| Age at diagnosis, y mean ± standard deviation | 60.7 ± 15.5 | 52.7 ± 14.1 | < .001 |
| Australia (vs New Zealand) | 197 (86.4) | 81 (98.8) | .002 |
| Rural residence (vs metropolitan) | 70 (30.7) | 44 (53.7) | < .001 |
| Known underlying immunocompromising conditiona Cancer | 166 (72.8) 56 (24.6) | 22 (26.8) 9 (11.0) | < .001 .01 |
| Solid organ transplantation | 51 (22.4) | 6 (7.3) | .003 |
| Othera | 65 (28.5) | 7 (8.5) | < .001 |
| Charlson comorbidity index at diagnosis, median (IQR) | 2 (1–4) | 0 (0–2) | < .001 |
| Smoking status, n = 247 | |||
| Current | 17 (9.7) | 14 (19.7) | .10 |
| Former | 62 (35.2) | 23 (32.4) | |
| Never | 97 (55.1) | 34 (4) | |
| Proven cryptococcosis per European Organization for Research and Treatment of Cancer-Mycoses Study Group Education and Research Consortium criteria (vs probable) | 161 (70.6) | 71 (86.6) | .004 |
| Site(s) of infection | |||
| Lung | 150 (65.8) | 71 (86.6) | < .001 |
| Lung (isolated) | 23 (10.1) | 4 (4.9) | .18 |
| CNS | 107 (46.9) | 64 (78) | < .001 |
| Skin | 22 (9.7) | 2 (2.4) | .05 |
| Blood | 60 (26.3) | 1 (1.2) | < .001 |
| Urine | 7 (3.1) | 0 (0) | .20 |
| Other | 22 (9.7) | 5 (6.1) | .33 |
| Disseminated (≥2 sites) | 97 (42.5) | 54 (65.9) | < .001 |
| Signs/symptoms at time of presentation | |||
| No symptoms | 25 (11.0) | 6 (7.3) | .35 |
| Fever ≥38°C | 68 (29.8) | 26 (31.7) | .75 |
| Respiratory symptoms (cough, sputum production, or dyspnea) | 87 (38.2) | 31 (37.8) | .96 |
| Neurological features | 104 (45.6) | 59 (72.0) | < .001 |
| Headaches (isolated) | 26 (11.4) | 17 (20.7) | .04 |
| Meningism | 33 (14.5) | 25 (30.5) | .001 |
| Altered mental status (Glasgow score ≤13) | 30 (13.2) | 10 (12.2) | .82 |
| Seizures | 8 (3.5) | 6 (7.3) | .15 |
| Other | 78 (34.2) | 21 (25.6) | .15 |
| Skin lesion | 22 (9.7) | 2 (2.4) | .05 |
| Time from onset of symptoms to diagnosis, d, median (IQR), n = 264 | 21 (7–56) | 21 (10–60) | .34 |
| Blood test results at time of presentation C-reactive protein level, mg/L, median (IQR), n = 258 | 19.7 (4.2–58.6) | 8.4 (3.9–43) | .06 |
| Positive blood cultures, n = 225 | 60 (36.1) | 1 (1.7) | < .001 |
| Positive serum CrAg, n = 265 | 148 (77.1) | 71 (97.3) | < .001 |
| Neutropenia <500 mm3, n = 302 | 3 (1.3) | 1 (1.3) | 1 |
| Biopsy with suggestive histopathology results, n = 308 | 44 (19.5) | 32 (39) | < .001 |
| CSF findings (for patients with CNS cryptococcosis) | |||
| White blood cells, per mm3, median (IQR), n = 148 | 60 (12–146) | 113 (41–262) | .01 |
| Lymphocytes, per mm3, median (IQR), n = 155 | 52 (9–122) | 87 (42–210) | .01 |
| Neutrophils, per mm3, median (IQR), n = 154 | 6 (1–27) | 16 (3–49) | .01 |
| Protein, mg/dL, median (IQR), n = 161 | 110 (63.5–176.6) | 82 (50–113.1) | .02 |
| Glucose, mg/dL, median (IQR), n = 160 | 38.7 (18–59.2) | 45 (30.6–70.1) | .046 |
| Opening pressure ≥25 cmH2O, n = 111 | 26 (38.8) | 19 (43.2) | .62 |
| Positive microscopy, n = 138 | 56 (62.9) | 27 (55.1) | .33 |
| Positive culture, n = 164 | 89 (86.4) | 49 (80.3) | < .001 |
| Positive CSF CrAg, n = 163 | 103 (100) | 57 (95) | .02 |
| Brain imaging findings (for patients with CNS cryptococcosis), n = 154 | |||
| Cryptococcoma(s) | 11 (12) | 28 (45.2) | < .001 |
| Signs of meningitis | 31 (33.7) | 15 (24.2) | .21 |
| Hydrocephalus | 7 (7.7) | 4 (6.5) | 1 |
| Dilated perivascular spaces | 2 (2.2) | 5 (8.1) | .12 |
| No related lesions | 51 (55.4) | 19 (30.6) | .002 |
| Chest imaging findings (for patients with lung cryptococcosis) | |||
| Nodule(s) or mass(es) | 89 (59.3) | 54 (76.1) | .02 |
| Consolidation(s) | 50 (33.3) | 18 (25.4) | .23 |
| Interstitial pattern | 19 (12.7) | 4 (5.6) | .16 |
| Pleural effusion | 23 (15.3) | 6 (8.5) | .16 |
| No related lesions | 4 (2.7) | 1 (1.4) | 1 |
Data are n (%) unless otherwise indicated. Only culture-positive cases were used for these comparisons (ie, 228 infections due to Cryptococcus neoformans vs 82 infections due to C. gattii).
Abbreviations: CNS, central nervous system; CrAg, Cryptococcus antigen; CSF, cerebrospinal fluid; IQR, interquartile range; n, number of data analyzed (when at least 1 missing data).
Defined as solid organ transplantation, cancer, and/or other immunocompromising condition (Supplementary Table 4). For the purpose of this study, other medical conditions that may be associated with varying degrees of immune deficit (eg, diabetes, chronic kidney disease, or liver disease) were not considered by themselves as “underlying immunocompromising conditions.”
P values below 0.05 are in bold, indicating statistical significance.
Comparison Between Patients Without Human Immunodeficiency Virus (HIV) Who Had a Culture-Positive Cryptococcus neoformans Infection (n = 228) and Patients Without HIV Who Had a Culture-Positive Cryptococcus gattii Infection (n = 82)
| Characteristic . | Patients Without HIV With Cryptococcus neoformans Infection (n = 228) . | Patients Without HIV With C. gattii Infection (n = 82) . | P Value . |
|---|---|---|---|
| Male | 114 (61.4) | 56 (68.3) | .27 |
| Age at diagnosis, y mean ± standard deviation | 60.7 ± 15.5 | 52.7 ± 14.1 | < .001 |
| Australia (vs New Zealand) | 197 (86.4) | 81 (98.8) | .002 |
| Rural residence (vs metropolitan) | 70 (30.7) | 44 (53.7) | < .001 |
| Known underlying immunocompromising conditiona Cancer | 166 (72.8) 56 (24.6) | 22 (26.8) 9 (11.0) | < .001 .01 |
| Solid organ transplantation | 51 (22.4) | 6 (7.3) | .003 |
| Othera | 65 (28.5) | 7 (8.5) | < .001 |
| Charlson comorbidity index at diagnosis, median (IQR) | 2 (1–4) | 0 (0–2) | < .001 |
| Smoking status, n = 247 | |||
| Current | 17 (9.7) | 14 (19.7) | .10 |
| Former | 62 (35.2) | 23 (32.4) | |
| Never | 97 (55.1) | 34 (4) | |
| Proven cryptococcosis per European Organization for Research and Treatment of Cancer-Mycoses Study Group Education and Research Consortium criteria (vs probable) | 161 (70.6) | 71 (86.6) | .004 |
| Site(s) of infection | |||
| Lung | 150 (65.8) | 71 (86.6) | < .001 |
| Lung (isolated) | 23 (10.1) | 4 (4.9) | .18 |
| CNS | 107 (46.9) | 64 (78) | < .001 |
| Skin | 22 (9.7) | 2 (2.4) | .05 |
| Blood | 60 (26.3) | 1 (1.2) | < .001 |
| Urine | 7 (3.1) | 0 (0) | .20 |
| Other | 22 (9.7) | 5 (6.1) | .33 |
| Disseminated (≥2 sites) | 97 (42.5) | 54 (65.9) | < .001 |
| Signs/symptoms at time of presentation | |||
| No symptoms | 25 (11.0) | 6 (7.3) | .35 |
| Fever ≥38°C | 68 (29.8) | 26 (31.7) | .75 |
| Respiratory symptoms (cough, sputum production, or dyspnea) | 87 (38.2) | 31 (37.8) | .96 |
| Neurological features | 104 (45.6) | 59 (72.0) | < .001 |
| Headaches (isolated) | 26 (11.4) | 17 (20.7) | .04 |
| Meningism | 33 (14.5) | 25 (30.5) | .001 |
| Altered mental status (Glasgow score ≤13) | 30 (13.2) | 10 (12.2) | .82 |
| Seizures | 8 (3.5) | 6 (7.3) | .15 |
| Other | 78 (34.2) | 21 (25.6) | .15 |
| Skin lesion | 22 (9.7) | 2 (2.4) | .05 |
| Time from onset of symptoms to diagnosis, d, median (IQR), n = 264 | 21 (7–56) | 21 (10–60) | .34 |
| Blood test results at time of presentation C-reactive protein level, mg/L, median (IQR), n = 258 | 19.7 (4.2–58.6) | 8.4 (3.9–43) | .06 |
| Positive blood cultures, n = 225 | 60 (36.1) | 1 (1.7) | < .001 |
| Positive serum CrAg, n = 265 | 148 (77.1) | 71 (97.3) | < .001 |
| Neutropenia <500 mm3, n = 302 | 3 (1.3) | 1 (1.3) | 1 |
| Biopsy with suggestive histopathology results, n = 308 | 44 (19.5) | 32 (39) | < .001 |
| CSF findings (for patients with CNS cryptococcosis) | |||
| White blood cells, per mm3, median (IQR), n = 148 | 60 (12–146) | 113 (41–262) | .01 |
| Lymphocytes, per mm3, median (IQR), n = 155 | 52 (9–122) | 87 (42–210) | .01 |
| Neutrophils, per mm3, median (IQR), n = 154 | 6 (1–27) | 16 (3–49) | .01 |
| Protein, mg/dL, median (IQR), n = 161 | 110 (63.5–176.6) | 82 (50–113.1) | .02 |
| Glucose, mg/dL, median (IQR), n = 160 | 38.7 (18–59.2) | 45 (30.6–70.1) | .046 |
| Opening pressure ≥25 cmH2O, n = 111 | 26 (38.8) | 19 (43.2) | .62 |
| Positive microscopy, n = 138 | 56 (62.9) | 27 (55.1) | .33 |
| Positive culture, n = 164 | 89 (86.4) | 49 (80.3) | < .001 |
| Positive CSF CrAg, n = 163 | 103 (100) | 57 (95) | .02 |
| Brain imaging findings (for patients with CNS cryptococcosis), n = 154 | |||
| Cryptococcoma(s) | 11 (12) | 28 (45.2) | < .001 |
| Signs of meningitis | 31 (33.7) | 15 (24.2) | .21 |
| Hydrocephalus | 7 (7.7) | 4 (6.5) | 1 |
| Dilated perivascular spaces | 2 (2.2) | 5 (8.1) | .12 |
| No related lesions | 51 (55.4) | 19 (30.6) | .002 |
| Chest imaging findings (for patients with lung cryptococcosis) | |||
| Nodule(s) or mass(es) | 89 (59.3) | 54 (76.1) | .02 |
| Consolidation(s) | 50 (33.3) | 18 (25.4) | .23 |
| Interstitial pattern | 19 (12.7) | 4 (5.6) | .16 |
| Pleural effusion | 23 (15.3) | 6 (8.5) | .16 |
| No related lesions | 4 (2.7) | 1 (1.4) | 1 |
| Characteristic . | Patients Without HIV With Cryptococcus neoformans Infection (n = 228) . | Patients Without HIV With C. gattii Infection (n = 82) . | P Value . |
|---|---|---|---|
| Male | 114 (61.4) | 56 (68.3) | .27 |
| Age at diagnosis, y mean ± standard deviation | 60.7 ± 15.5 | 52.7 ± 14.1 | < .001 |
| Australia (vs New Zealand) | 197 (86.4) | 81 (98.8) | .002 |
| Rural residence (vs metropolitan) | 70 (30.7) | 44 (53.7) | < .001 |
| Known underlying immunocompromising conditiona Cancer | 166 (72.8) 56 (24.6) | 22 (26.8) 9 (11.0) | < .001 .01 |
| Solid organ transplantation | 51 (22.4) | 6 (7.3) | .003 |
| Othera | 65 (28.5) | 7 (8.5) | < .001 |
| Charlson comorbidity index at diagnosis, median (IQR) | 2 (1–4) | 0 (0–2) | < .001 |
| Smoking status, n = 247 | |||
| Current | 17 (9.7) | 14 (19.7) | .10 |
| Former | 62 (35.2) | 23 (32.4) | |
| Never | 97 (55.1) | 34 (4) | |
| Proven cryptococcosis per European Organization for Research and Treatment of Cancer-Mycoses Study Group Education and Research Consortium criteria (vs probable) | 161 (70.6) | 71 (86.6) | .004 |
| Site(s) of infection | |||
| Lung | 150 (65.8) | 71 (86.6) | < .001 |
| Lung (isolated) | 23 (10.1) | 4 (4.9) | .18 |
| CNS | 107 (46.9) | 64 (78) | < .001 |
| Skin | 22 (9.7) | 2 (2.4) | .05 |
| Blood | 60 (26.3) | 1 (1.2) | < .001 |
| Urine | 7 (3.1) | 0 (0) | .20 |
| Other | 22 (9.7) | 5 (6.1) | .33 |
| Disseminated (≥2 sites) | 97 (42.5) | 54 (65.9) | < .001 |
| Signs/symptoms at time of presentation | |||
| No symptoms | 25 (11.0) | 6 (7.3) | .35 |
| Fever ≥38°C | 68 (29.8) | 26 (31.7) | .75 |
| Respiratory symptoms (cough, sputum production, or dyspnea) | 87 (38.2) | 31 (37.8) | .96 |
| Neurological features | 104 (45.6) | 59 (72.0) | < .001 |
| Headaches (isolated) | 26 (11.4) | 17 (20.7) | .04 |
| Meningism | 33 (14.5) | 25 (30.5) | .001 |
| Altered mental status (Glasgow score ≤13) | 30 (13.2) | 10 (12.2) | .82 |
| Seizures | 8 (3.5) | 6 (7.3) | .15 |
| Other | 78 (34.2) | 21 (25.6) | .15 |
| Skin lesion | 22 (9.7) | 2 (2.4) | .05 |
| Time from onset of symptoms to diagnosis, d, median (IQR), n = 264 | 21 (7–56) | 21 (10–60) | .34 |
| Blood test results at time of presentation C-reactive protein level, mg/L, median (IQR), n = 258 | 19.7 (4.2–58.6) | 8.4 (3.9–43) | .06 |
| Positive blood cultures, n = 225 | 60 (36.1) | 1 (1.7) | < .001 |
| Positive serum CrAg, n = 265 | 148 (77.1) | 71 (97.3) | < .001 |
| Neutropenia <500 mm3, n = 302 | 3 (1.3) | 1 (1.3) | 1 |
| Biopsy with suggestive histopathology results, n = 308 | 44 (19.5) | 32 (39) | < .001 |
| CSF findings (for patients with CNS cryptococcosis) | |||
| White blood cells, per mm3, median (IQR), n = 148 | 60 (12–146) | 113 (41–262) | .01 |
| Lymphocytes, per mm3, median (IQR), n = 155 | 52 (9–122) | 87 (42–210) | .01 |
| Neutrophils, per mm3, median (IQR), n = 154 | 6 (1–27) | 16 (3–49) | .01 |
| Protein, mg/dL, median (IQR), n = 161 | 110 (63.5–176.6) | 82 (50–113.1) | .02 |
| Glucose, mg/dL, median (IQR), n = 160 | 38.7 (18–59.2) | 45 (30.6–70.1) | .046 |
| Opening pressure ≥25 cmH2O, n = 111 | 26 (38.8) | 19 (43.2) | .62 |
| Positive microscopy, n = 138 | 56 (62.9) | 27 (55.1) | .33 |
| Positive culture, n = 164 | 89 (86.4) | 49 (80.3) | < .001 |
| Positive CSF CrAg, n = 163 | 103 (100) | 57 (95) | .02 |
| Brain imaging findings (for patients with CNS cryptococcosis), n = 154 | |||
| Cryptococcoma(s) | 11 (12) | 28 (45.2) | < .001 |
| Signs of meningitis | 31 (33.7) | 15 (24.2) | .21 |
| Hydrocephalus | 7 (7.7) | 4 (6.5) | 1 |
| Dilated perivascular spaces | 2 (2.2) | 5 (8.1) | .12 |
| No related lesions | 51 (55.4) | 19 (30.6) | .002 |
| Chest imaging findings (for patients with lung cryptococcosis) | |||
| Nodule(s) or mass(es) | 89 (59.3) | 54 (76.1) | .02 |
| Consolidation(s) | 50 (33.3) | 18 (25.4) | .23 |
| Interstitial pattern | 19 (12.7) | 4 (5.6) | .16 |
| Pleural effusion | 23 (15.3) | 6 (8.5) | .16 |
| No related lesions | 4 (2.7) | 1 (1.4) | 1 |
Data are n (%) unless otherwise indicated. Only culture-positive cases were used for these comparisons (ie, 228 infections due to Cryptococcus neoformans vs 82 infections due to C. gattii).
Abbreviations: CNS, central nervous system; CrAg, Cryptococcus antigen; CSF, cerebrospinal fluid; IQR, interquartile range; n, number of data analyzed (when at least 1 missing data).
Defined as solid organ transplantation, cancer, and/or other immunocompromising condition (Supplementary Table 4). For the purpose of this study, other medical conditions that may be associated with varying degrees of immune deficit (eg, diabetes, chronic kidney disease, or liver disease) were not considered by themselves as “underlying immunocompromising conditions.”
P values below 0.05 are in bold, indicating statistical significance.
Clinical and Radiological Presentation of Cryptococcosis in Patients Without HIV
The clinical and radiological features of cryptococcosis are shown in Table 1. Common sites of infection were the lungs (75.1%, 320 of 426) and CNS (45.8%, 195 of 426); 40.1% of patients (171 of 426) had disseminated cryptococcosis. Of note, 16.4% of cryptococcosis episodes presented as an incidental finding on imaging of asymptomatic patients (70 of 426). In symptomatic patients, the median time from symptom onset to diagnosis was 22 days (IQR, 8 to 60), and only 24.6% (105 of 426) of patients presented with fever ≥ 38°C (Table 1).
Compared with patients with C. neoformans infection, those with C. gattii infection significantly more often had CNS involvement (P < .001), evidence of brain cryptococcoma(s) (P < .001), or lung nodule(s)/mass(es) (P = .02; see Table 2 for details and additional differences between C. neoformans and C. gattii infections).
Results of Blood Tests in HIV-Negative Patients With Cryptococcosis
Serum C-reactive protein (CRP) was measured in 80.3% (342 of 426) of patients; it was normal (<10 mg/L) in almost half of those tested (155 of 342, 45.3%). The proportion of patients who had an increased blood CRP level (≥10 mg/L) was low in asymptomatic patients incidentally found to have isolated lung cryptococcosis (44%, 19 of 43 tested patients) as well as in those with symptomatic CNS cryptococcosis (56%, 94 of 169 tested patients).
Blood cultures were taken in more than two-thirds of patients and grew Cryptococcus in 21.6% (62 of 287) of tested patients, almost all with C. neoformans (60 of 62).
Serum CrAg testing was performed in 88.2% of patients at presentation (375 of 426) and was positive in 85.1% of tested patients (319 of 375). As shown in Table 2, serum CrAg was positive in 77.1% of patients with C. neoformans infection compared with 97.3% of those with C. gattii infection (P < .001). Patients with negative serum CrAg results often had isolated lung cryptococcosis (representing 75% [42 of 56] of cases with a negative serum CrAg). After adjustment for potential confounders in a multivariable model (Supplementary Table 6), serum CrAg titer positively correlated with risk of CNS involvement (adjusted OR, 6.50; 95% CI, 2.92 to 14.45; P < .001) and C. gattii infection (adjusted OR, 3.01; 95% CI, 1.29 to 7.04; P = .01).
Usefulness of Systematic Lumbar Puncture in Patients Without HIV Who Have Cryptococcosis But No Clinical Neurological Abnormalities at Presentation
A lumbar puncture (LP) was performed in 80.8% (344 of 426) of our cohort without HIV. Around half were “clinically indicated” (ie, in the presence of clinical neurological abnormalities at time of presentation), and the remainder were “systematic LPs” performed to screen for asymptomatic meningitis in patients without clinical neurological abnormalities (51.5%, 177 of 344 and 48.5%, 167 of 344, respectively).
Of the 167 patients who had systematic LPs, 22 were found to have meningitis (positivity rate, 13.2%; see Figure 1). Patients with such asymptomatic meningitis typically had cerebrospinal fluid (CSF) features that suggested low fungal burden (see Supplementary Table 7 for CSF culture results and CrAg titers).
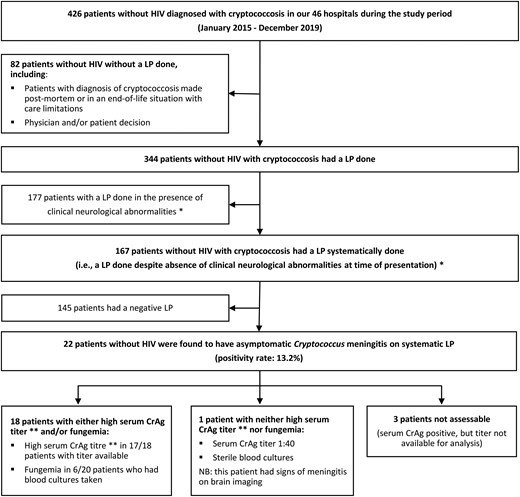
Usefulness of systematic LP in patients without HIV who have cryptococcosis but no clinical neurological abnormalities at the time of presentation. *The presence of suggestive clinical neurological abnormalities was defined as the presence of at least 1 of the following signs/symptoms: headache, meningeal symptoms, altered mental status, confusion, lethargy, seizures, syncope/falls, focal neurological signs. **A high serum CrAg titer was defined as ≥1:320 for study sites that used the Immuno-Mycologics Inc CrAg lateral flow assay and ≥1:256 for study sites that used latex agglutination. Abbreviations: CrAg: Cryptococcus antigen; HIV, human immunodeficiency virus; LP, lumbar puncture; NB, nota bene.
Next, we determined if these 22 cases of asymptomatic meningitis would have been predicted by blood tests. After exclusion of 3 of 22 patients who did not have a serum CrAg titer available for analysis, we observed that 18 of 19 patients (95%) with asymptomatic meningitis had either a high serum CrAg titer and/or fungemia (details in Figure 1 and Supplementary Table 7). The remaining patient truly had asymptomatic meningitis despite negative blood cultures and a low serum CrAg titer (1:40).
CSF Features in HIV-Negative Patients With Cryptococcosis
CSF features are presented in Table 1 for patients with an LP that showed CNS cryptococcosis. CSF CrAg was positive in nearly all patients without HIV with CNS cryptococcosis (97.3%, 180 of 185 tested patients). The CSF white cell count and CSF culture were positive in 85.2% (count >5/mm3 in 144 of 169 tested patients) and 74.2% (138 of 186 tested patients), respectively. The yield of CSF culture was low among patients with asymptomatic meningitis (positive in 31.8%, 7 of 22 tested patients).
Last, 7 patients without HIV who presented with CNS cryptococcosis had a BioFire FilmArray meningitis/encephalitis panel performed for diagnosis, and that panel was negative in 3 (Supplementary Table 8).
Mortality
One-year survival status was available for 98.5% of all study patients (468 of 475). One-year all-cause mortality did not significantly differ between patients with and without HIV (21.7%, 10 of 46 vs 20.9%, 88 of 422, respectively; P = .89).
Kaplan–Meier survival curves are shown for patients without HIV in Supplementary Figures 3–5. In these patients, most deaths occurred early after diagnosis (10-week all-cause mortality was 12.2%, 52 of 426). Subgroup analyses revealed that among patients without HIV, 1-year mortality was highest among cancer patients (34.1%, 31 of 91; Supplementary Figure 3) and lowest among patients with C. gattii infection (11.1%, 9 of 81; Supplementary Figure 5).
DISCUSSION
Our findings confirm the changing epidemiology of cryptococcosis in medically developed countries. In our study of 475 cases that occurred between 2015 and 2019 in Australia and New Zealand, 90% of the cryptococcosis episodes occurred in patients without HIV. This proportion, also observed when focusing on C. neoformans infections alone, is much higher than the proportions of 23%–44% observed in previous large multisite studies performed in the United States and France [8, 12]. It is also higher than the proportion of 57% observed in a previous population-based study conducted in Australia and New Zealand 20 years ago [16]. This may be explained by factors such as the relatively low prevalence of uncontrolled HIV in Australia and New Zealand due to widespread availability of highly effective antiretroviral therapy and the rising number of other immunocompromised patients.
While previous studies of HIV-negative patients with cryptococcosis often included cases diagnosed before 2010, our study was limited to recent years and therefore identified new groups of patients at risk of cryptococcosis, such as multiple sclerosis patients receiving fingolimod and cancer patients receiving Bruton's tyrosine kinase inhibitors. Our findings confirm previous information from case reports and small case series [24, 25] and show the importance of these emerging risk groups. A high level of awareness is warranted when these patients develop features compatible with cryptococcosis.
The rate of apparently immunocompetent patients was also higher in our cohort (almost 40%) compared with past studies on cryptococcosis. This may be attributable to the relative importance of C. gattii in our region and its well-known ability to infect previously healthy individuals [18, 26]. However, we also identified 62 patients who had C. neoformans infection despite being apparently immunocompetent. While most had at least 1 medical condition that may have fostered cryptococcosis (ie, chronic lung disease, liver disease, kidney disease, or diabetes), it is possible that some of these patients had an unrecognized immunocompromising condition. Recent studies have suggested that some C. gattii infections may be due to relatively subtle deficits, such as the presence of anti-granulocyte-macrophage-colony-stimulating factor antibodies [27, 28].
Our study confirms that CNS involvement, which represents more than 70%–90% of HIV-related cryptococcal cases [28], occurs in fewer than half of cases without HIV [3, 5, 13]. Since we collected detailed information at presentation, we were able to identify several elements that illustrate the complexity of diagnosing cryptococcosis in patients without HIV. First, one-sixth of the patients without HIV who had cryptococcosis in our study were asymptomatic at the time of diagnosis; in these patients, cryptococcosis was typically an incidental finding on chest imaging (eg, in cancer patients having follow-up imaging or in trauma patients). Second, classic indicators of infection such as fever and increased CRP level were absent in 75% and 45% of the cases, respectively. The unremarkable levels of CRP are in contrast with other invasive bacterial and fungal diseases as well as with patients who have HIV and cryptococcosis [29]. Finally, serum CrAg (which is positive in >99% of the patients who have HIV and cryptococcal meningitis [30]) was positive in only 85% of the patients without HIV in our study. Consequently, neither the absence of symptoms nor a normal CRP level nor a negative serum CrAg could be used to rule out cryptococcosis in patients without HIV with compatible lesions. Another pitfall recently described [31, 32], and observed in our study, is falsely negative results with the BioFire FilmArray meningitis/encephalitis panel in some patients with cryptococcal meningitis.
Our findings may be used to inform the debate regarding the recommendation to routinely perform an LP in HIV-negative patients with cryptococcosis, even in the absence of neurological symptoms [33]. Although we acknowledge the importance of early identification of asymptomatic meningitis, we observed that more than 85% of CSF specimens in our patients without neurological symptoms were normal. Further, we found that the combination of 2 simple blood tests (high serum CrAg titer and/or fungemia) may predict the risk of asymptomatic cryptococcal meningitis in our patients without HIV. Specifically, at least 1 of these 2 biomarkers was present in 95% of the evaluable patients found to have asymptomatic meningitis. Previous studies have evaluated the potential usefulness of serum CrAg titer and blood cultures to reduce the number of systematic LPs. However, these studies typically recruited very few patients with asymptomatic CNS involvement or included only limited information on baseline neurological status [34, 35]. Further research is needed to determine whether serum CrAg and blood cultures can be used to safely confine the use of LPs to asymptomatic patients who are the most likely to benefit.
Last, we confirm the distinctive epidemiology of C. gattii and C. neoformans in our region. In particular, our study corroborates that C. gattii cryptococcosis affects the CNS in almost 80% of the cases in our region compared with <40% of outbreak cases in North America [26]. Other clinical findings of importance include the high rate of positivity of serum CrAg in patients with C. gattii infection (around 97% vs 77% in patients without HIV with C. neoformans infection) and the very low yield from blood cultures (<2% in C. gattii cases) [36].
Our study has several limitations. We did not collect information on conditions such as excess alcohol consumption or pregnancy, which are potential predisposing factors for cryptococcosis. Our study was also conducted before the coronavirus disease 2019 pandemic, so any association with severe acute respiratory syndrome coronavirus 2 infection was not evaluable [37]. Due to the retrospective study design, not all patients had each diagnostic test performed at baseline, making it difficult to precisely estimate their sensitivity and specificity.
In conclusion, 90% of the cases of cryptococcosis recently seen in Australia and New Zealand occurred in patients without HIV. This proportion, also observed when focusing on C. neoformans infections alone, is much higher than that seen in prior population-based studies. The high number of enrolled patients and detailed clinical data contribute to a greater understanding of this difficult-to-diagnose infection, which commonly presents with nonspecific or even no symptoms.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Members of the Australian and New Zealand Study Group for Cryptococcosis in Patients Without HIV Infection (in alphabetical order). Kylie Alcorn (Southport, Queensland, Australia); Justin Beardsley (Sydney, New South Wales, Australia); Aaron Bloch (Ballarat, Victoria, Australia); Amy Crowe (Melbourne, Victoria, Australia); Wendy Doyle (Newcastle, New South Wales, Australia); Michelle England (Perth, Western Australia, Australia); David Griffin (Melbourne, Victoria, Australia); Kate Hamilton (Sydney, New South Wales, Australia); Tony M. Korman (Melbourne, Victoria, Australia); Victoria Madigan (Melbourne, Victoria, Australia); Hugh McGann (Hamilton, New Zealand); William Pratt (Wollongong, New South Wales, Australia); Sebastiaan Van Hal (Sydney, New South Wales, Australia); Prue Waters (Heidelberg, Victoria, Australia); and Eloise Williams (Melbourne, Victoria, Australia).
References
Author notes
S. C.-A. C. and M. A. S. contributed equally to this work.
Members of the Australian and New Zealand study group for cryptococcosis in patients without HIV infection:
Individual collaborators who are members of the Australian and New Zealand study group for cryptococcosis in patients without HIV infection and are not listed in the main list of authors are listed before the references.
Potential conflicts of interest. T. S. has received consulting fees for serving on advisory boards and steering committees from Biogen. O. M. has received grants from Gilead Sciences and Merck, Sharp and Dohme Australia and honoraria from Gilead Sciences; support for attending meetings from F2G; and participated on data and safety monitoring boards (DSMB) or advisory boards for Gilead Sciences and Merck, Sharp and Dohme Australia. K. J. K. has received payment for expert testimony at the 46th Society of Hospital Pharmacists of Australia National Conference. A. R. T. has received honoraria from the Medical Journal of Australia, paid to institution, and reports grants or contracts from CTRA with the University of Melbourne (reimbursement of costs paid to institution). B. W. T. is supported by a Medical Research Future Fund Investigator Fellowship; has received grants from MSD and Seqirus; has received honoraria from Pfizer, Alexion, and Janssen; and participated on DSMBs or advisory boards for CSLBehring, Takeda, and Moderna. S. C. A. C. has received educational grants from F2G and MSD Australia; reports untied educational grants from MSD Australia and F2G Pty Ltd; and reports a role as editor-in-chief for Medical Mycology (journal of ISHAM). M. A. S. has received grants from Gilead Sciences, Merck, and F2G; has received honoraria from F2G; and participated on DSMBs or advisory boards for Pfizer, Cidara, and Roche. R. J. L. reports paid participation on a GSK advisory board. S. A. L. reports grants or contracts as principal investigator on 3 projects funded through a Hot North fund grant and a UK Government Fleming Fund Grant (as part of broader funding for the Menzies School of Health Research; no salary, project costs remunerated only); unpaid participation on a DSMB or advisory board for the Australian Academy of Science and the Australian Academy of Health and Medical Sciences roundtable of experts for the House of Representatives Committee on Health and Ageing; and an unpaid role as a National Tuberculosis Advisory Committee member. M. T. B. reports an unpaid role as an Advanced Training Committee member for General Medicine for the Royal Australasian College of Physicians and an unpaid member of the Vocational Training Committee for Medical Registrars in the Auckland region for the Northern Region Alliance. C. L. K. reports an unpaid role on the Australian Society of Infectious Diseases Board of Directors. E. W. reports a role as a committee member of the Australasian Society for Infectious Diseases Equity and Diversity Committee (unpaid). K. C. G. reports a role as a member of the New Zealand Committee of the Australasian Society for Infectious Diseases. All other authors report no potential conflicts.
All authors have submitted the International Committee of Medical Journal Editors Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




