-
PDF
- Split View
-
Views
-
Cite
Cite
Po Hsien Kuo, Un In Wu, Yi Hua Pan, Jann Tay Wang, Yu Chen Wang, Hsin Yun Sun, Wang Huei Sheng, Yee Chun Chen, Shan Chwen Chang, Neutralizing Anti–Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies in Patients With Central Nervous System and Localized Cryptococcosis: Longitudinal Follow-up and Literature Review, Clinical Infectious Diseases, Volume 75, Issue 2, 15 July 2022, Pages 278–287, https://doi.org/10.1093/cid/ciab920
Close - Share Icon Share
Abstract
Neutralizing anti–granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibodies (AAbs) have been increasingly recognized to predispose healthy individuals to disseminated cryptococcosis. However, studies have only considered patients with central nervous system (CNS) infection. No longitudinal study has captured the disease spectrum and clinical course.
We prospectively enrolled adults without human immunodeficiency virus infection who had disseminated or unusual cryptococcosis. We compared the demographics, clinical features, kinetics of serum cryptococcal antigen (CrAg) titers, anti–GM-CSF AAb concentrations, and treatment outcomes between patients with (case patients) and without (control patients) anti–GM-CSF AAbs. Additional reports from the literature were also reviewed.
Twenty-three patients were enrolled, of whom 6 tested positive for anti–GM-CSF AAbs. All case patients with positive fungal cultures (5/5 [100%]) were infected with Cryptococcus gattii VGII. Among them, 3 had exclusively pulmonary involvement, and 1 had only musculoskeletal lesions. Patients with CNS cryptococcosis exhibited a higher serum concentration of anti–GM-CSF AAbs than those with extraneural cryptococcosis. Case patients had higher initial and peak levels of serum CrAg and longer duration of antigenemia compared with the control patients. All case patients who had completed antifungal therapy had favorable outcomes without recurrence.
Testing for anti–GM-CSF AAbs should be considered for not only previously healthy patients with disseminated cryptococcosis but also those with unexplained, localized cryptococcosis. Recurrence after completion of antifungal therapy was rare despite the persistence of anti–GM-CSF AAbs.
Anticytokine autoantibodies (AAbs) are a major emerging cause of secondary immunodeficiencies and susceptibilities to diverse opportunistic infections [1]. Anti–granulocyte-macrophage colony-stimulating factor (GM-CSF) AAbs, first recognized as an etiology in most cases of pulmonary alveolar proteinosis (PAP) due to defective GM-CSF–dependent surfactant clearance by pulmonary macrophages, have also gained increasing recognition as mediators of infections in otherwise healthy individuals [2]. Studies have reported that these AAbs can predispose healthy individuals to central nervous system (CNS) cryptococcosis, with a predominance of Cryptococcus gattii infection [3–12]. However, given that only case reports and few case series are available [3–12], the natural history and outcomes of anti–GM-CSF AAbs in cryptococcosis are less clear-cut compared with those of anti–interferon gamma (IFN-γ) AAbs in mycobacterial infections.
Several critical questions remain unanswered, including the spectrum of clinical manifestations, dynamic changes of serum cryptococcal antigen (CrAg) titers, treatment outcomes, and necessity of long-term prophylaxis for patients with anti–GM-CSF AAbs. Furthermore, studies on anti–GM-CSF AAbs and cryptococcosis thus far have only considered patients with disseminated or CNS infection. The association between anti–GM-CSF AAbs and cryptococcosis outside the CNS is largely unknown.
Hence, we conducted a comprehensive, longitudinal study to characterize the spectrum and natural course of cryptococcosis in human immunodeficiency virus (HIV)–negative, previously healthy patients with anti–GM-CSF AAbs, including the evolution of the AAb concentrations and neutralizing abilities and their association with disease outcomes. A secondary objective was to compare our cases with those published to date.
MATERIALS AND METHODS
Subjects and Settings
We conducted a prospective study at National Taiwan University Hospital, a tertiary medical center, between 1 January 2015 and 31 May 2021 to enroll adult patients presented with localized or disseminated cryptococcosis (Figure 1). Cryptococcal disease was defined as “proven” with compatible radiographic abnormalities and culture positivity from sterile fluid or biopsy confirmation; cryptococcosis was defined as “probable” with antigen positivity in blood or cerebrospinal fluid [13]. Disseminated cryptococcosis is defined by (i) at least 2 nonadjacent sites with documented cryptococcosis or (ii) a positive blood culture [14]. Enrolled cases with localized presentations in this study were restricted to extrapulmonary cryptococcosis, or pulmonary cryptococcosis with antigen positivity, severe disease (eg, empyema, respiratory failure), or unsatisfactory treatment response defined by persistent radiographic abnormalities or symptoms not responding to antifungal therapy. Patients with overt immunocompromised status including HIV infection, solid organ or hematopoietic stem cell transplantation, or systemic immunosuppressive therapy were excluded. All participants provided written consent for the study protocols approved by the Research Ethics Committee of the National Taiwan University Hospital (number 201412163RIND) and conducted according to the Declaration of Helsinki.
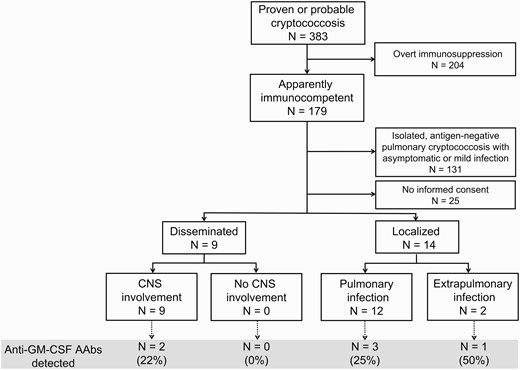
Flowchart illustrating patients enrolled and analyzed in the study. Abbreviations: AAbs, autoantibodies; CNS, central nervous system; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Data Collection and Follow-up
Patients’ demographics, medical comorbidities, and clinical presentations at disease onset were recorded. Initial and follow-up evaluations included determination of the neutralizing anti–GM-CSF AAb and anti–IFN-γ AAb levels, serum and cerebrospinal fluid CrAg, routine laboratory tests, HIV antibody screening, analysis of lymphocyte population, measurement of immunoglobulin levels, identification of autoimmune profile, interpretation of the microbiological results (cultures, antigens/antibodies, and microscopic examination), histopathological reports, and radiographic examinations. Detection for neutralizing anti–IFN-γ AAb in each participant’s serum was also performed as previously described [15].
Patients who tested positive for neutralizing anti–GM-CSF AAb were followed up every 3 months in the first year after enrollment and every 6 months in the subsequent years. Additional blood tests were performed if clinically indicated. The method for detection of CrAg had shifted from latex agglutination (IMMY, Norman, Oklahoma) to lateral flow assay (IMMY) in January 2019 at our clinical laboratory.
Detection of Neutralizing Anti–GM-CSF AAbs
Whole blood was collected from each patient and a healthy volunteer on the same day for each experiment for anti–GM-CSF AAb enzyme-linked immunosorbent assay (ELISA). Plasma was collected from fresh heparinized blood and stored at –80°C until analysis (Supplementary Materials). A participant was considered to have biologically active anti–GM-CSF AAbs if an elevated concentration of anti–GM-CSF AAbs was detected in plasma, as measured through ELISA, which could inhibit GM-CSF–induced STAT5 phosphorylation in monocytes (Supplementary Figure 1). Data were collected using FACSCalibur (BD Biosciences), analyzed using FlowJo software (Tree Star, Ashland, Oregon), and graphed with Prism 6 software (GraphPad Software, La Jolla, California). The ratio of the geometric mean fluorescence of phosphorylated STAT5 (pSTAT5) staining for unstimulated to stimulated conditions was referred to the inhibition index.
Characterization of Cryptococcus Clinical Isolates
All Cryptococcus clinical isolates were identified using matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI Biotyper, Bruker Daltonics, Bremen, Germany) and obtained for further genotyping by URA5 gene restriction fragment length polymorphism analysis as previously described [16].
Literature Review
We searched PubMed and Medline for published articles with the keywords “cryptococcosis,” “Cryptococcus species,” “Cryptococcus gattii,” “anti-granulocyte-macrophage colony-stimulating factor,” “anti-GM-CSF,” and “anticytokine autoantibodies” written in English. Case reports not retrieved by the above-mentioned keywords but mentioned in different articles were also examined.
Statistical Analysis
All statistical analyses were performed using Stata software, version 14. Categorical variables were analyzed using χ2 or Fisher exact test, as appropriate. Continuous variables were compared using the paired t test. Linear regression was used for evaluation of the correlation between the titers of anti–GM-CSF AAb and the ratio of pSTAT5 inhibition. A P value < .05 was considered to be statistically significant.
RESULTS
Clinical Features of Patients Positive for Neutralizing Anti–GM-CSF AAbs
In total, 23 patients with disseminated or unusual cryptococcosis were enrolled (Figure 1). Among these, 6 patients tested positive for neutralizing anti–GM-CSF AAbs (Figure 2), and none had neutralizing anti–IFN-γ AAbs in their sera. The median age of the positive patients was 50.5 years (interquartile range, 46–61 years), with a male predominance (83%). Five patients were infected with C. gattii, with all isolates belonging to the VGII genotype (Table 1). Histopathological examination revealed pulmonary cryptococcosis in 1 patient, although no Cryptococcus species were isolated from sputum or lung biopsy specimens. All 4 positive patients that were enrolled before 2019 had peak serum CrAg higher than 1:512 (by latex agglutination). One of the 2 patients enrolled after 2019 had a peak CrAg titer of >1:1280, and the other had 1:320, as indicated by lateral flow assay.
Characteristics of Disseminated or Unusual Cryptococcosis in Patients With and Those Without Anti–Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies
| Demographic and Clinical Variables . | AAb Positive (n = 6) . | AAb Negative (n = 17) . | P Value . |
|---|---|---|---|
| Demographics | |||
| Sex, male | 5 (83.3) | 14 (82.3) | 1.00 |
| Age, y, median (IQR) | 50.5 (46–61) | 50.0 (36–61.5) | .72 |
| Underlying diseasea | |||
| Diabetes mellitus | 1 (16.7) | 3 (17.6) | 1.00 |
| Hypertension | 1 (16.7) | 6 (35.3) | .62 |
| Coronary artery disease | 0 | 2 (11.8) | 1.00 |
| Cancer | 1 (16.7) | 3 (17.6) | 1.00 |
| Chronic lung disease | 0 | 1 (5.9) | 1.00 |
| Clinical variables and laboratory data | |||
| Proven cryptococcosis | 6 (100.0) | 16 (94.1) | 1.00 |
| Probable cryptococcosis | 0 | 1 (5.9) | 1.00 |
| Pulmonary involvement | 5 (83.3) | 11 (64.7) | .62 |
| CNS involvement | 2 (33.3) | 7 (41.2) | 1.00 |
| Other sites | 1 (16.7) | 1 (5.9) | .46 |
| Culture provenb | 5 (83.3) | 9 (52.9) | .34 |
| WBC, mean ± SD | 7538.3 ± 3972.8 | 7075.3 ± 2781.6 | .76 |
| ANC, mean ± SD | 5359.5 ± 4128.5 | 5041.3 ± 2186.8 | .81 |
| ALC, mean ± SD | 1496.3 ± 493.5 | 1373.4 ± 799.9 | .73 |
| CD4, mean ± SD | 447.5 ± 158.5 (n = 4) | 634.4 ± 351.7 (n = 11) | .33 |
| IgG, mean ± SD | 1365.0 ± 232.5 (n = 4) | 1212.6 ± 250.2 (n = 12) | .30 |
| IgA, mean ± SD | 269.3 ± 77.8 (n = 4) | 244.4 ± 191.0 (n = 8) | .81 |
| IgM, mean ± SD | 86.4 ± 32.9 (n = 4) | 94.5 ± 28.9 (n = 8) | .67 |
| Mycology | |||
| Initial serum CrAg ≥512 | 3 (75.0), n = 4 | 5 (33.3), n = 15 | .26 |
| Initial CSF CrAg ≥512 | 0, n = 2 | 4 (57.1), n = 7 | .44 |
| Peak serum CrAg ≥512 | 4 (100), n = 4 | 7 (46.7), n = 15 | .10 |
| Persistent serum CrAg after 12 mo | 5 (100), n = 5 | 3 (33.3), n = 9 | .03 |
| C. gattii | 5 (100), n = 5 | 0 | 0 |
| Treatment and prognosis | |||
| Amphotericin B included in regimen | 3 (50.0) | 10 (58.8) | 1.00 |
| Treatment duration for complete treatment, d, mean ± SDc | 571.0 ± 277.5 (n = 4) | 445.9 ± 283.9 (n = 14) | .45 |
| Mortality | 0 | 1 (5.9) | 1.00 |
| Demographic and Clinical Variables . | AAb Positive (n = 6) . | AAb Negative (n = 17) . | P Value . |
|---|---|---|---|
| Demographics | |||
| Sex, male | 5 (83.3) | 14 (82.3) | 1.00 |
| Age, y, median (IQR) | 50.5 (46–61) | 50.0 (36–61.5) | .72 |
| Underlying diseasea | |||
| Diabetes mellitus | 1 (16.7) | 3 (17.6) | 1.00 |
| Hypertension | 1 (16.7) | 6 (35.3) | .62 |
| Coronary artery disease | 0 | 2 (11.8) | 1.00 |
| Cancer | 1 (16.7) | 3 (17.6) | 1.00 |
| Chronic lung disease | 0 | 1 (5.9) | 1.00 |
| Clinical variables and laboratory data | |||
| Proven cryptococcosis | 6 (100.0) | 16 (94.1) | 1.00 |
| Probable cryptococcosis | 0 | 1 (5.9) | 1.00 |
| Pulmonary involvement | 5 (83.3) | 11 (64.7) | .62 |
| CNS involvement | 2 (33.3) | 7 (41.2) | 1.00 |
| Other sites | 1 (16.7) | 1 (5.9) | .46 |
| Culture provenb | 5 (83.3) | 9 (52.9) | .34 |
| WBC, mean ± SD | 7538.3 ± 3972.8 | 7075.3 ± 2781.6 | .76 |
| ANC, mean ± SD | 5359.5 ± 4128.5 | 5041.3 ± 2186.8 | .81 |
| ALC, mean ± SD | 1496.3 ± 493.5 | 1373.4 ± 799.9 | .73 |
| CD4, mean ± SD | 447.5 ± 158.5 (n = 4) | 634.4 ± 351.7 (n = 11) | .33 |
| IgG, mean ± SD | 1365.0 ± 232.5 (n = 4) | 1212.6 ± 250.2 (n = 12) | .30 |
| IgA, mean ± SD | 269.3 ± 77.8 (n = 4) | 244.4 ± 191.0 (n = 8) | .81 |
| IgM, mean ± SD | 86.4 ± 32.9 (n = 4) | 94.5 ± 28.9 (n = 8) | .67 |
| Mycology | |||
| Initial serum CrAg ≥512 | 3 (75.0), n = 4 | 5 (33.3), n = 15 | .26 |
| Initial CSF CrAg ≥512 | 0, n = 2 | 4 (57.1), n = 7 | .44 |
| Peak serum CrAg ≥512 | 4 (100), n = 4 | 7 (46.7), n = 15 | .10 |
| Persistent serum CrAg after 12 mo | 5 (100), n = 5 | 3 (33.3), n = 9 | .03 |
| C. gattii | 5 (100), n = 5 | 0 | 0 |
| Treatment and prognosis | |||
| Amphotericin B included in regimen | 3 (50.0) | 10 (58.8) | 1.00 |
| Treatment duration for complete treatment, d, mean ± SDc | 571.0 ± 277.5 (n = 4) | 445.9 ± 283.9 (n = 14) | .45 |
| Mortality | 0 | 1 (5.9) | 1.00 |
Abbreviations: AAb, autoantibodies; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CNS, central nervous system; CrAg, cryptococcal antigen; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; SD, standard deviation; WBC, white blood count.
None of the enrolled patients had chronic liver disease, liver cirrhosis, or cerebrovascular disease.
One patient with positive AAb had bone lesions only, and 1 patient with negative AAb had antigenemia only.
Patients with ongoing treatment or mortality were not included for analysis of treatment duration.
Characteristics of Disseminated or Unusual Cryptococcosis in Patients With and Those Without Anti–Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies
| Demographic and Clinical Variables . | AAb Positive (n = 6) . | AAb Negative (n = 17) . | P Value . |
|---|---|---|---|
| Demographics | |||
| Sex, male | 5 (83.3) | 14 (82.3) | 1.00 |
| Age, y, median (IQR) | 50.5 (46–61) | 50.0 (36–61.5) | .72 |
| Underlying diseasea | |||
| Diabetes mellitus | 1 (16.7) | 3 (17.6) | 1.00 |
| Hypertension | 1 (16.7) | 6 (35.3) | .62 |
| Coronary artery disease | 0 | 2 (11.8) | 1.00 |
| Cancer | 1 (16.7) | 3 (17.6) | 1.00 |
| Chronic lung disease | 0 | 1 (5.9) | 1.00 |
| Clinical variables and laboratory data | |||
| Proven cryptococcosis | 6 (100.0) | 16 (94.1) | 1.00 |
| Probable cryptococcosis | 0 | 1 (5.9) | 1.00 |
| Pulmonary involvement | 5 (83.3) | 11 (64.7) | .62 |
| CNS involvement | 2 (33.3) | 7 (41.2) | 1.00 |
| Other sites | 1 (16.7) | 1 (5.9) | .46 |
| Culture provenb | 5 (83.3) | 9 (52.9) | .34 |
| WBC, mean ± SD | 7538.3 ± 3972.8 | 7075.3 ± 2781.6 | .76 |
| ANC, mean ± SD | 5359.5 ± 4128.5 | 5041.3 ± 2186.8 | .81 |
| ALC, mean ± SD | 1496.3 ± 493.5 | 1373.4 ± 799.9 | .73 |
| CD4, mean ± SD | 447.5 ± 158.5 (n = 4) | 634.4 ± 351.7 (n = 11) | .33 |
| IgG, mean ± SD | 1365.0 ± 232.5 (n = 4) | 1212.6 ± 250.2 (n = 12) | .30 |
| IgA, mean ± SD | 269.3 ± 77.8 (n = 4) | 244.4 ± 191.0 (n = 8) | .81 |
| IgM, mean ± SD | 86.4 ± 32.9 (n = 4) | 94.5 ± 28.9 (n = 8) | .67 |
| Mycology | |||
| Initial serum CrAg ≥512 | 3 (75.0), n = 4 | 5 (33.3), n = 15 | .26 |
| Initial CSF CrAg ≥512 | 0, n = 2 | 4 (57.1), n = 7 | .44 |
| Peak serum CrAg ≥512 | 4 (100), n = 4 | 7 (46.7), n = 15 | .10 |
| Persistent serum CrAg after 12 mo | 5 (100), n = 5 | 3 (33.3), n = 9 | .03 |
| C. gattii | 5 (100), n = 5 | 0 | 0 |
| Treatment and prognosis | |||
| Amphotericin B included in regimen | 3 (50.0) | 10 (58.8) | 1.00 |
| Treatment duration for complete treatment, d, mean ± SDc | 571.0 ± 277.5 (n = 4) | 445.9 ± 283.9 (n = 14) | .45 |
| Mortality | 0 | 1 (5.9) | 1.00 |
| Demographic and Clinical Variables . | AAb Positive (n = 6) . | AAb Negative (n = 17) . | P Value . |
|---|---|---|---|
| Demographics | |||
| Sex, male | 5 (83.3) | 14 (82.3) | 1.00 |
| Age, y, median (IQR) | 50.5 (46–61) | 50.0 (36–61.5) | .72 |
| Underlying diseasea | |||
| Diabetes mellitus | 1 (16.7) | 3 (17.6) | 1.00 |
| Hypertension | 1 (16.7) | 6 (35.3) | .62 |
| Coronary artery disease | 0 | 2 (11.8) | 1.00 |
| Cancer | 1 (16.7) | 3 (17.6) | 1.00 |
| Chronic lung disease | 0 | 1 (5.9) | 1.00 |
| Clinical variables and laboratory data | |||
| Proven cryptococcosis | 6 (100.0) | 16 (94.1) | 1.00 |
| Probable cryptococcosis | 0 | 1 (5.9) | 1.00 |
| Pulmonary involvement | 5 (83.3) | 11 (64.7) | .62 |
| CNS involvement | 2 (33.3) | 7 (41.2) | 1.00 |
| Other sites | 1 (16.7) | 1 (5.9) | .46 |
| Culture provenb | 5 (83.3) | 9 (52.9) | .34 |
| WBC, mean ± SD | 7538.3 ± 3972.8 | 7075.3 ± 2781.6 | .76 |
| ANC, mean ± SD | 5359.5 ± 4128.5 | 5041.3 ± 2186.8 | .81 |
| ALC, mean ± SD | 1496.3 ± 493.5 | 1373.4 ± 799.9 | .73 |
| CD4, mean ± SD | 447.5 ± 158.5 (n = 4) | 634.4 ± 351.7 (n = 11) | .33 |
| IgG, mean ± SD | 1365.0 ± 232.5 (n = 4) | 1212.6 ± 250.2 (n = 12) | .30 |
| IgA, mean ± SD | 269.3 ± 77.8 (n = 4) | 244.4 ± 191.0 (n = 8) | .81 |
| IgM, mean ± SD | 86.4 ± 32.9 (n = 4) | 94.5 ± 28.9 (n = 8) | .67 |
| Mycology | |||
| Initial serum CrAg ≥512 | 3 (75.0), n = 4 | 5 (33.3), n = 15 | .26 |
| Initial CSF CrAg ≥512 | 0, n = 2 | 4 (57.1), n = 7 | .44 |
| Peak serum CrAg ≥512 | 4 (100), n = 4 | 7 (46.7), n = 15 | .10 |
| Persistent serum CrAg after 12 mo | 5 (100), n = 5 | 3 (33.3), n = 9 | .03 |
| C. gattii | 5 (100), n = 5 | 0 | 0 |
| Treatment and prognosis | |||
| Amphotericin B included in regimen | 3 (50.0) | 10 (58.8) | 1.00 |
| Treatment duration for complete treatment, d, mean ± SDc | 571.0 ± 277.5 (n = 4) | 445.9 ± 283.9 (n = 14) | .45 |
| Mortality | 0 | 1 (5.9) | 1.00 |
Abbreviations: AAb, autoantibodies; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CNS, central nervous system; CrAg, cryptococcal antigen; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; SD, standard deviation; WBC, white blood count.
None of the enrolled patients had chronic liver disease, liver cirrhosis, or cerebrovascular disease.
One patient with positive AAb had bone lesions only, and 1 patient with negative AAb had antigenemia only.
Patients with ongoing treatment or mortality were not included for analysis of treatment duration.
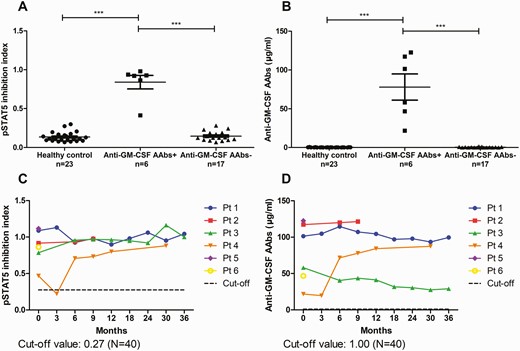
A, Inhibition index of pSTAT5. B, Anti–granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibody (AAb) concentration in healthy controls and 23 patients (Pt) with unusual or disseminated cryptococcosis. C, Dynamic changes of pSTAT5 inhibition index (performed in the same experiment using peripheral blood mononuclear cells from a single healthy volunteer). D, Anti–GM-CSF AAb concentrations in case patients over time (all assays were performed in the same plate). ∗∗∗P value <.001.
Notably, only 2 case patients had CNS cryptococcosis (Table 2). Both of them had pulmonary mass lesions. Three patients had exclusively pulmonary involvement, and 1 patient had only musculoskeletal lesions (Figure 3). None had CD4 lymphocytopenia or abnormal immunoglobulin levels at the time of enrollment. All patients demonstrated favorable clinical response to antifungal therapy, including the 3 patients who received fluconazole therapy without combination of polyenes. All 4 patients who discontinued antifungal therapy after a mean treatment duration of 571 days remained alive without recurrence of cryptococcosis, with a median follow-up duration of 1120 (range, 672–2347) days (Table 1 and Supplementary Table 1). None of the 6 case patients had reported new respiratory symptoms or exhibited any radiographic features of PAP during the follow-up period.
Major Features of Cryptococcosis Associated With Anti–Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies in the Current and Previous Cases
| Age/Sex . | Country/Ethnicity . | Underlying Condition(s) . | Infection Site . | Serum CrAg . | CSF CrAg . | Species . | Genotype . | CD4 Counta . | ALCb . | Treatment . | PAP . | Outcome . | Ref. . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 49/M | Taiwan/Asian | Hypertension | Lung | 1:320 | NA | NA | NA | NA | 1870 | FLU | No | Ongoing treatment | |
| 52/M | Taiwan/Asian | Smoker | CNS, lung | >1:1280 | 1:320 | C. gattii | VGII | 283 | 1424 | L-AmB + 5FC then L-AmB + FLU then FLU | No | Ongoing treatment | |
| 61/M | Taiwan/Asian | Nil | CNS, lung | >1:512 | 1:256 | C. gattii | VGII | 371 | 1162 | AmB-d + 5FC then FLU | No | Recovery | |
| 71/F | Taiwan/Asian | Cancer, DM | Bone | >1:512 | Neg | C. gattii | VGII | NA | 1249 | FLU | No | Recovery | |
| 39/M | Taiwan/Asian | Nil | Lung | >1:512 | Neg | C. gattii | VGII | 650 | 2299 | FLU + 5FC then FLU | No | Recovery | |
| 46/M | Taiwan/Asian | Nil | Lung | >1:512 | Neg | C. gattii | VGII | 486 | 975 | AmB-d + 5FC then FLU | No | Recovery | |
| 26/M | USA/White | Nil | CNS, lung, bone | >1:1 100 000 | 1:4 | C. neoformans | NA | 606 | 2088 | L-AmB + 5FC then L-AmB + FLU then FLU | No | Recovery | [12] |
| 41/F | France/African | Nil | CNS, lung | 1:15 | 1:10 | C. grubii | IGS1 | 2244 | 3803 | L-AmB + 5FC then FLU | No | Recovery | [11] |
| 69/M | USA/White | Nil | CNS | 1:1280 | 1:40 | C. gattii | VGII | 228 | 600 | AmB-d + 5FC then FLU | No | Recovery | [10] |
| 48/M | Australia/NA | Nil | CNS, lung | 1:64 | Neg | C. gattii | NA | NA | NA | AmB-d + 5FC then FLU, with recurrence after 1 y, L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | 4 y later | Recovery | [9] |
| 43/M | Australia/Asian | Nil | CNS, lung | 1:1280 | NA | C. gattii | NA | NA | NA | AMB-d + 5FC then FLU, VRC, and POS | No | Recovery | [9] |
| 42/M | France/White | Prior smoker | CNS, lung | NA | Pos | C. gattii | NA | NA | NA | AmB-d then FLU then VRC | 3 y later | Hearing loss, anosmia, and blindness | [8] |
| 73/M | USA/White | ICL | CNS | 1:256 | 1:4 | C. neoformans | NA | 18 | 370 | Antifungals | No | Responded well | [7] |
| 42/M | USA/White | Nil | OM, epidural abscess, lung | 1:5 | NA | C. gattii | VGII | 548 | NA | L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | No | Recovery | [6] |
| 34/M | USA/Hispanic | Nil | CNS, lung, skin | 1:2 | Neg | C. gattii | NA | 675 | NA | L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | No | Recovery | [6] |
| 37/M | Taiwan/Asian | Nil | CNS, lung, skin | >1:1024 | >1:1024 | NA | NA | 375 | 999 | AmB-d + 5FC | No | Death | [5] |
| 40/M | Taiwan/Asian | Asthma | CNS, lung | 1:512 | NA | NA | NA | NA | 2215 | AmB-d + 5FC then FLU | No | Hydrocephalus, seizure, and left hemiplegia | [5] |
| 59/F | Taiwan/Asian | Nil | Eye | 1:64 | NA | NA | NA | 514 | 1547 | AmB IO then VRC, 2nd prophylaxis with VRC | No | Impaired vision | [5] |
| 37/M | Taiwan/Asian | HCV | CNS, lung | 1:256 | 1:256 | NA | NA | 595 | 1579 | AmB-d + 5FC then FLU | No | Recovery | [5] |
| 49/F | China/Asian | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/M | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/Aborigine | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/Asian | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/M | Australia/White | NA | CNS | NA | NA | C. gattii | VGII | NA | NA | NA | NA | NA | [4] |
| 20/F | USA/White | Nil | CNS, lung | 1:128 | 1:256 | C. neoformans | NA | 1399 | 795 | L-AmB + 5FC then FLU | 2 y later | Recovery | [3] |
| 31/F | USA/White | Nil | CNS, lung | 1:256 | 1:8 | C. gattii | NA | 950 | 800 | L-AmB + 5FC then FLU + 5FC | No | Recovery | [3] |
| 48/M | USA/Asian | Nil | CNS, lung | 1:1024 | Pos | C. neoformans | NA | 817 | 1714 | AmB-d then FLU | No | Recovery | [3] |
| 47/M | USA/Mexican | Nil | CNS, lung, skin | 1:512 | 1:16 | C. neoformans | NA | 773 | 1870 | AmB-d then FLU | 4 y later | Recovery | [3] |
| 26/M | USA/African American | NA | CNS | NA | NA | C. gattii | VGII | NA | 1071 | AmB + 5FC | No | Recovery | [3] |
| 34/M | USA/White | NA | CNS, lung | NA | NA | C. gattii | VGII | NA | 972 | AmB IT/IV + 5FC | No | Seizures, homonymous, hemianopsia, NPH | [3] |
| 32/M | USA/White | NA | CNS, lung | NA | NA | C. gattii | VGI | NA | 1250 | AmB IT/IV + 5FC | No | Seizures, neurogenic bladder | [3] |
| Age/Sex . | Country/Ethnicity . | Underlying Condition(s) . | Infection Site . | Serum CrAg . | CSF CrAg . | Species . | Genotype . | CD4 Counta . | ALCb . | Treatment . | PAP . | Outcome . | Ref. . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 49/M | Taiwan/Asian | Hypertension | Lung | 1:320 | NA | NA | NA | NA | 1870 | FLU | No | Ongoing treatment | |
| 52/M | Taiwan/Asian | Smoker | CNS, lung | >1:1280 | 1:320 | C. gattii | VGII | 283 | 1424 | L-AmB + 5FC then L-AmB + FLU then FLU | No | Ongoing treatment | |
| 61/M | Taiwan/Asian | Nil | CNS, lung | >1:512 | 1:256 | C. gattii | VGII | 371 | 1162 | AmB-d + 5FC then FLU | No | Recovery | |
| 71/F | Taiwan/Asian | Cancer, DM | Bone | >1:512 | Neg | C. gattii | VGII | NA | 1249 | FLU | No | Recovery | |
| 39/M | Taiwan/Asian | Nil | Lung | >1:512 | Neg | C. gattii | VGII | 650 | 2299 | FLU + 5FC then FLU | No | Recovery | |
| 46/M | Taiwan/Asian | Nil | Lung | >1:512 | Neg | C. gattii | VGII | 486 | 975 | AmB-d + 5FC then FLU | No | Recovery | |
| 26/M | USA/White | Nil | CNS, lung, bone | >1:1 100 000 | 1:4 | C. neoformans | NA | 606 | 2088 | L-AmB + 5FC then L-AmB + FLU then FLU | No | Recovery | [12] |
| 41/F | France/African | Nil | CNS, lung | 1:15 | 1:10 | C. grubii | IGS1 | 2244 | 3803 | L-AmB + 5FC then FLU | No | Recovery | [11] |
| 69/M | USA/White | Nil | CNS | 1:1280 | 1:40 | C. gattii | VGII | 228 | 600 | AmB-d + 5FC then FLU | No | Recovery | [10] |
| 48/M | Australia/NA | Nil | CNS, lung | 1:64 | Neg | C. gattii | NA | NA | NA | AmB-d + 5FC then FLU, with recurrence after 1 y, L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | 4 y later | Recovery | [9] |
| 43/M | Australia/Asian | Nil | CNS, lung | 1:1280 | NA | C. gattii | NA | NA | NA | AMB-d + 5FC then FLU, VRC, and POS | No | Recovery | [9] |
| 42/M | France/White | Prior smoker | CNS, lung | NA | Pos | C. gattii | NA | NA | NA | AmB-d then FLU then VRC | 3 y later | Hearing loss, anosmia, and blindness | [8] |
| 73/M | USA/White | ICL | CNS | 1:256 | 1:4 | C. neoformans | NA | 18 | 370 | Antifungals | No | Responded well | [7] |
| 42/M | USA/White | Nil | OM, epidural abscess, lung | 1:5 | NA | C. gattii | VGII | 548 | NA | L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | No | Recovery | [6] |
| 34/M | USA/Hispanic | Nil | CNS, lung, skin | 1:2 | Neg | C. gattii | NA | 675 | NA | L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | No | Recovery | [6] |
| 37/M | Taiwan/Asian | Nil | CNS, lung, skin | >1:1024 | >1:1024 | NA | NA | 375 | 999 | AmB-d + 5FC | No | Death | [5] |
| 40/M | Taiwan/Asian | Asthma | CNS, lung | 1:512 | NA | NA | NA | NA | 2215 | AmB-d + 5FC then FLU | No | Hydrocephalus, seizure, and left hemiplegia | [5] |
| 59/F | Taiwan/Asian | Nil | Eye | 1:64 | NA | NA | NA | 514 | 1547 | AmB IO then VRC, 2nd prophylaxis with VRC | No | Impaired vision | [5] |
| 37/M | Taiwan/Asian | HCV | CNS, lung | 1:256 | 1:256 | NA | NA | 595 | 1579 | AmB-d + 5FC then FLU | No | Recovery | [5] |
| 49/F | China/Asian | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/M | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/Aborigine | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/Asian | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/M | Australia/White | NA | CNS | NA | NA | C. gattii | VGII | NA | NA | NA | NA | NA | [4] |
| 20/F | USA/White | Nil | CNS, lung | 1:128 | 1:256 | C. neoformans | NA | 1399 | 795 | L-AmB + 5FC then FLU | 2 y later | Recovery | [3] |
| 31/F | USA/White | Nil | CNS, lung | 1:256 | 1:8 | C. gattii | NA | 950 | 800 | L-AmB + 5FC then FLU + 5FC | No | Recovery | [3] |
| 48/M | USA/Asian | Nil | CNS, lung | 1:1024 | Pos | C. neoformans | NA | 817 | 1714 | AmB-d then FLU | No | Recovery | [3] |
| 47/M | USA/Mexican | Nil | CNS, lung, skin | 1:512 | 1:16 | C. neoformans | NA | 773 | 1870 | AmB-d then FLU | 4 y later | Recovery | [3] |
| 26/M | USA/African American | NA | CNS | NA | NA | C. gattii | VGII | NA | 1071 | AmB + 5FC | No | Recovery | [3] |
| 34/M | USA/White | NA | CNS, lung | NA | NA | C. gattii | VGII | NA | 972 | AmB IT/IV + 5FC | No | Seizures, homonymous, hemianopsia, NPH | [3] |
| 32/M | USA/White | NA | CNS, lung | NA | NA | C. gattii | VGI | NA | 1250 | AmB IT/IV + 5FC | No | Seizures, neurogenic bladder | [3] |
Abbreviations: 5FC, flucytosine; AmB, amphotericin B; ALC, absolute lymphocyte count; AmB-d, amphotericin B deoxycholate; CNS, central nervous system; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; DM, diabetes mellitus, F, female; FLU, fluconazole; HCV, hepatitis C virus; ICL, idiopathic CD4+ lymphopenia; IO, intraocular; IT, intrathecal; IV, intravenous; L-AmB, liposomal amphotericin B; M, male; NA, not available; NPH, normal pressure hydrocephalus; OM, osteomyelitis; PAP, pulmonary alveolar proteinosis; POS, posaconazole; USA, United States; VRC, voriconazole.
Cells/μL.
Cells/μL.
Major Features of Cryptococcosis Associated With Anti–Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies in the Current and Previous Cases
| Age/Sex . | Country/Ethnicity . | Underlying Condition(s) . | Infection Site . | Serum CrAg . | CSF CrAg . | Species . | Genotype . | CD4 Counta . | ALCb . | Treatment . | PAP . | Outcome . | Ref. . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 49/M | Taiwan/Asian | Hypertension | Lung | 1:320 | NA | NA | NA | NA | 1870 | FLU | No | Ongoing treatment | |
| 52/M | Taiwan/Asian | Smoker | CNS, lung | >1:1280 | 1:320 | C. gattii | VGII | 283 | 1424 | L-AmB + 5FC then L-AmB + FLU then FLU | No | Ongoing treatment | |
| 61/M | Taiwan/Asian | Nil | CNS, lung | >1:512 | 1:256 | C. gattii | VGII | 371 | 1162 | AmB-d + 5FC then FLU | No | Recovery | |
| 71/F | Taiwan/Asian | Cancer, DM | Bone | >1:512 | Neg | C. gattii | VGII | NA | 1249 | FLU | No | Recovery | |
| 39/M | Taiwan/Asian | Nil | Lung | >1:512 | Neg | C. gattii | VGII | 650 | 2299 | FLU + 5FC then FLU | No | Recovery | |
| 46/M | Taiwan/Asian | Nil | Lung | >1:512 | Neg | C. gattii | VGII | 486 | 975 | AmB-d + 5FC then FLU | No | Recovery | |
| 26/M | USA/White | Nil | CNS, lung, bone | >1:1 100 000 | 1:4 | C. neoformans | NA | 606 | 2088 | L-AmB + 5FC then L-AmB + FLU then FLU | No | Recovery | [12] |
| 41/F | France/African | Nil | CNS, lung | 1:15 | 1:10 | C. grubii | IGS1 | 2244 | 3803 | L-AmB + 5FC then FLU | No | Recovery | [11] |
| 69/M | USA/White | Nil | CNS | 1:1280 | 1:40 | C. gattii | VGII | 228 | 600 | AmB-d + 5FC then FLU | No | Recovery | [10] |
| 48/M | Australia/NA | Nil | CNS, lung | 1:64 | Neg | C. gattii | NA | NA | NA | AmB-d + 5FC then FLU, with recurrence after 1 y, L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | 4 y later | Recovery | [9] |
| 43/M | Australia/Asian | Nil | CNS, lung | 1:1280 | NA | C. gattii | NA | NA | NA | AMB-d + 5FC then FLU, VRC, and POS | No | Recovery | [9] |
| 42/M | France/White | Prior smoker | CNS, lung | NA | Pos | C. gattii | NA | NA | NA | AmB-d then FLU then VRC | 3 y later | Hearing loss, anosmia, and blindness | [8] |
| 73/M | USA/White | ICL | CNS | 1:256 | 1:4 | C. neoformans | NA | 18 | 370 | Antifungals | No | Responded well | [7] |
| 42/M | USA/White | Nil | OM, epidural abscess, lung | 1:5 | NA | C. gattii | VGII | 548 | NA | L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | No | Recovery | [6] |
| 34/M | USA/Hispanic | Nil | CNS, lung, skin | 1:2 | Neg | C. gattii | NA | 675 | NA | L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | No | Recovery | [6] |
| 37/M | Taiwan/Asian | Nil | CNS, lung, skin | >1:1024 | >1:1024 | NA | NA | 375 | 999 | AmB-d + 5FC | No | Death | [5] |
| 40/M | Taiwan/Asian | Asthma | CNS, lung | 1:512 | NA | NA | NA | NA | 2215 | AmB-d + 5FC then FLU | No | Hydrocephalus, seizure, and left hemiplegia | [5] |
| 59/F | Taiwan/Asian | Nil | Eye | 1:64 | NA | NA | NA | 514 | 1547 | AmB IO then VRC, 2nd prophylaxis with VRC | No | Impaired vision | [5] |
| 37/M | Taiwan/Asian | HCV | CNS, lung | 1:256 | 1:256 | NA | NA | 595 | 1579 | AmB-d + 5FC then FLU | No | Recovery | [5] |
| 49/F | China/Asian | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/M | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/Aborigine | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/Asian | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/M | Australia/White | NA | CNS | NA | NA | C. gattii | VGII | NA | NA | NA | NA | NA | [4] |
| 20/F | USA/White | Nil | CNS, lung | 1:128 | 1:256 | C. neoformans | NA | 1399 | 795 | L-AmB + 5FC then FLU | 2 y later | Recovery | [3] |
| 31/F | USA/White | Nil | CNS, lung | 1:256 | 1:8 | C. gattii | NA | 950 | 800 | L-AmB + 5FC then FLU + 5FC | No | Recovery | [3] |
| 48/M | USA/Asian | Nil | CNS, lung | 1:1024 | Pos | C. neoformans | NA | 817 | 1714 | AmB-d then FLU | No | Recovery | [3] |
| 47/M | USA/Mexican | Nil | CNS, lung, skin | 1:512 | 1:16 | C. neoformans | NA | 773 | 1870 | AmB-d then FLU | 4 y later | Recovery | [3] |
| 26/M | USA/African American | NA | CNS | NA | NA | C. gattii | VGII | NA | 1071 | AmB + 5FC | No | Recovery | [3] |
| 34/M | USA/White | NA | CNS, lung | NA | NA | C. gattii | VGII | NA | 972 | AmB IT/IV + 5FC | No | Seizures, homonymous, hemianopsia, NPH | [3] |
| 32/M | USA/White | NA | CNS, lung | NA | NA | C. gattii | VGI | NA | 1250 | AmB IT/IV + 5FC | No | Seizures, neurogenic bladder | [3] |
| Age/Sex . | Country/Ethnicity . | Underlying Condition(s) . | Infection Site . | Serum CrAg . | CSF CrAg . | Species . | Genotype . | CD4 Counta . | ALCb . | Treatment . | PAP . | Outcome . | Ref. . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 49/M | Taiwan/Asian | Hypertension | Lung | 1:320 | NA | NA | NA | NA | 1870 | FLU | No | Ongoing treatment | |
| 52/M | Taiwan/Asian | Smoker | CNS, lung | >1:1280 | 1:320 | C. gattii | VGII | 283 | 1424 | L-AmB + 5FC then L-AmB + FLU then FLU | No | Ongoing treatment | |
| 61/M | Taiwan/Asian | Nil | CNS, lung | >1:512 | 1:256 | C. gattii | VGII | 371 | 1162 | AmB-d + 5FC then FLU | No | Recovery | |
| 71/F | Taiwan/Asian | Cancer, DM | Bone | >1:512 | Neg | C. gattii | VGII | NA | 1249 | FLU | No | Recovery | |
| 39/M | Taiwan/Asian | Nil | Lung | >1:512 | Neg | C. gattii | VGII | 650 | 2299 | FLU + 5FC then FLU | No | Recovery | |
| 46/M | Taiwan/Asian | Nil | Lung | >1:512 | Neg | C. gattii | VGII | 486 | 975 | AmB-d + 5FC then FLU | No | Recovery | |
| 26/M | USA/White | Nil | CNS, lung, bone | >1:1 100 000 | 1:4 | C. neoformans | NA | 606 | 2088 | L-AmB + 5FC then L-AmB + FLU then FLU | No | Recovery | [12] |
| 41/F | France/African | Nil | CNS, lung | 1:15 | 1:10 | C. grubii | IGS1 | 2244 | 3803 | L-AmB + 5FC then FLU | No | Recovery | [11] |
| 69/M | USA/White | Nil | CNS | 1:1280 | 1:40 | C. gattii | VGII | 228 | 600 | AmB-d + 5FC then FLU | No | Recovery | [10] |
| 48/M | Australia/NA | Nil | CNS, lung | 1:64 | Neg | C. gattii | NA | NA | NA | AmB-d + 5FC then FLU, with recurrence after 1 y, L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | 4 y later | Recovery | [9] |
| 43/M | Australia/Asian | Nil | CNS, lung | 1:1280 | NA | C. gattii | NA | NA | NA | AMB-d + 5FC then FLU, VRC, and POS | No | Recovery | [9] |
| 42/M | France/White | Prior smoker | CNS, lung | NA | Pos | C. gattii | NA | NA | NA | AmB-d then FLU then VRC | 3 y later | Hearing loss, anosmia, and blindness | [8] |
| 73/M | USA/White | ICL | CNS | 1:256 | 1:4 | C. neoformans | NA | 18 | 370 | Antifungals | No | Responded well | [7] |
| 42/M | USA/White | Nil | OM, epidural abscess, lung | 1:5 | NA | C. gattii | VGII | 548 | NA | L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | No | Recovery | [6] |
| 34/M | USA/Hispanic | Nil | CNS, lung, skin | 1:2 | Neg | C. gattii | NA | 675 | NA | L-AmB + 5FC then FLU then 2nd prophylaxis with FLU | No | Recovery | [6] |
| 37/M | Taiwan/Asian | Nil | CNS, lung, skin | >1:1024 | >1:1024 | NA | NA | 375 | 999 | AmB-d + 5FC | No | Death | [5] |
| 40/M | Taiwan/Asian | Asthma | CNS, lung | 1:512 | NA | NA | NA | NA | 2215 | AmB-d + 5FC then FLU | No | Hydrocephalus, seizure, and left hemiplegia | [5] |
| 59/F | Taiwan/Asian | Nil | Eye | 1:64 | NA | NA | NA | 514 | 1547 | AmB IO then VRC, 2nd prophylaxis with VRC | No | Impaired vision | [5] |
| 37/M | Taiwan/Asian | HCV | CNS, lung | 1:256 | 1:256 | NA | NA | 595 | 1579 | AmB-d + 5FC then FLU | No | Recovery | [5] |
| 49/F | China/Asian | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/M | Australia/White | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/Aborigine | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/F | Australia/Asian | NA | CNS | NA | NA | C. gattii | VGI | NA | NA | NA | NA | NA | [4] |
| NA/M | Australia/White | NA | CNS | NA | NA | C. gattii | VGII | NA | NA | NA | NA | NA | [4] |
| 20/F | USA/White | Nil | CNS, lung | 1:128 | 1:256 | C. neoformans | NA | 1399 | 795 | L-AmB + 5FC then FLU | 2 y later | Recovery | [3] |
| 31/F | USA/White | Nil | CNS, lung | 1:256 | 1:8 | C. gattii | NA | 950 | 800 | L-AmB + 5FC then FLU + 5FC | No | Recovery | [3] |
| 48/M | USA/Asian | Nil | CNS, lung | 1:1024 | Pos | C. neoformans | NA | 817 | 1714 | AmB-d then FLU | No | Recovery | [3] |
| 47/M | USA/Mexican | Nil | CNS, lung, skin | 1:512 | 1:16 | C. neoformans | NA | 773 | 1870 | AmB-d then FLU | 4 y later | Recovery | [3] |
| 26/M | USA/African American | NA | CNS | NA | NA | C. gattii | VGII | NA | 1071 | AmB + 5FC | No | Recovery | [3] |
| 34/M | USA/White | NA | CNS, lung | NA | NA | C. gattii | VGII | NA | 972 | AmB IT/IV + 5FC | No | Seizures, homonymous, hemianopsia, NPH | [3] |
| 32/M | USA/White | NA | CNS, lung | NA | NA | C. gattii | VGI | NA | 1250 | AmB IT/IV + 5FC | No | Seizures, neurogenic bladder | [3] |
Abbreviations: 5FC, flucytosine; AmB, amphotericin B; ALC, absolute lymphocyte count; AmB-d, amphotericin B deoxycholate; CNS, central nervous system; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; DM, diabetes mellitus, F, female; FLU, fluconazole; HCV, hepatitis C virus; ICL, idiopathic CD4+ lymphopenia; IO, intraocular; IT, intrathecal; IV, intravenous; L-AmB, liposomal amphotericin B; M, male; NA, not available; NPH, normal pressure hydrocephalus; OM, osteomyelitis; PAP, pulmonary alveolar proteinosis; POS, posaconazole; USA, United States; VRC, voriconazole.
Cells/μL.
Cells/μL.
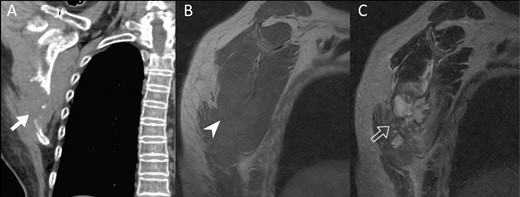
Image findings of case patient with exclusively musculoskeletal cryptococcosis. A, Non–contrast-enhanced computed tomography image shows a destructive osteolytic lesion (solid arrow) in the right lower scapular body. B, Sagittal oblique magnetic resonance imaging of right shoulder reveals an osteolytic isointense soft tissue lesion (arrowhead) on T1-weighted magnetic resonance imaging (MRI). C, A lobulated hyperintense lesion (open arrow) is shown on T2-weighted MRI.
Correlation Between Anti–GM-CSF AAb Titers and Clinical Outcomes
Patients with extrapulmonary cryptococcosis (patients 1, 2, and 5) had a higher serum concentration of anti–GM-CSF AAbs than had those with exclusively pulmonary cryptococcosis (patients 3, 4, and 6; Figure 2C and 2D). Although all patients had favorable clinical outcomes at the end of completion of antifungal therapy, their serum concentrations of anti–GM-CSF AAbs and pSTAT5 inhibition index did not return to normal levels. Among the 4 patients with serial follow-up data, a positive correlation between anti–GM-CSF AAbs and pSTAT5 inhibition index was observed in 2 patients, negative correlation in 1 patient, and no correlation in 1 patient (Supplementary Figures 1 and 2).
Comparisons Between Patients With and Those Without Anti–GM-CSF AAbs
Both groups of patients shared similar demographic features, including male predominance, age at disease onset, and proportion with chronic medical conditions. No significant differences in laboratory data at disease onset, including white blood cell count, CD4 lymphocyte count, and immunoglobulin levels, were observed between the 2 groups (Table 1). Cryptococcus gattii was only isolated from patients with anti–GM-CSF AAbs (case group), not from those without anti–GM-CSF AAbs (control group).
Case patients had higher (although not statistically significant) initial and peak levels of serum CrAg compared with control patients (Table 1). After 12 months of antifungal therapy, a larger proportion of the case group (100%) had positive serum CrAg compared with the control group (33.3%) (P value = .03; Figure 4). No statistical difference in treatment duration and mortality was observed between the 2 groups.
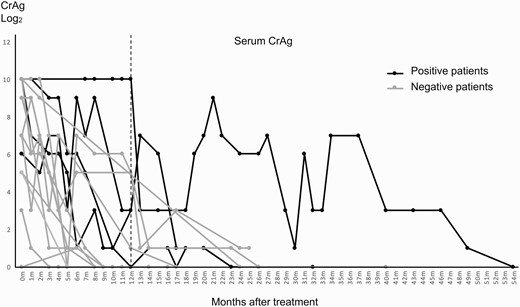
Kinetics of serum cryptococcal antigen (CrAg) between patients with and those without neutralizing anti–granulocyte-macrophage colony-stimulating factor autoantibodies (AAbs).
Literature Review
A total of 33 cases of cryptococcosis associated with anti–GM-CSF AAbs (including our 6 cases) have now been reported in Australia, the United States, and Asia since 2013 (Table 2). All of the 27 previously reported cases involved the CNS, and only 1 was characterized by CD4 lymphopenia.
The majority of the previous cases (63%) involved C. gattii infection. Although genotyping was not universally performed, the susceptibilities of the reported cases seemed not to be limited to a particular genotype of C. gattii.
Among the 19 reported cases with available outcomes, 68% fully recovered from cryptococcosis. Approximately one-third of the patients had neurological sequelae, and 1 patient (5%) succumbed to the infection. Four patients (21%) developed PAP during the follow-up period, which ranged from 2 to 4 years after the diagnosis of cryptococcosis. Most (95%) patients received standard induction therapy, including amphotericin B deoxycholate or liposomal amphotericin B. Four patients (21%) received prolonged secondary prophylaxis (3 patients with fluconazole and 1 patient with voriconazole).
DISCUSSION
This is the first study to demonstrate that apparently or previously immunocompetent patients with neutralizing AAbs to GM-CSF can present with not only CNS cryptococcosis but also localized and pulmonary cryptococcosis. This study also revealed that although patients with CNS cryptococcosis exhibited a higher serum concentration of neutralizing anti–GM-CSF AAbs compared with the concentration of those with extraneural cryptococcosis, no clear correlation exists between the clinical outcomes and concentrations or inhibitory activity of the AAbs.
GM-CSF was initially characterized as a hematopoietic growth factor that induces the maturation of macrophages and neutrophils and was later observed to modulate the complex inflammatory system. Derangement in GM-CSF may lead to inflammatory or autoimmune diseases [17]. Abnormalities in GM-CSF caused by the presence of AAbs result in impaired macrophage function and decreased surfactant clearance, eventually leading to PAP. Patients with PAP are more susceptible to opportunistic infections, including nocardiosis, aspergillosis, and cryptococcosis [18].
Rosen et al in 2013 reported the first case series describing the association between neutralizing anti–GM-CSF AAbs and cryptococcal meningitis [3]. Since then, additional case series and case reports have been published, totaling 27 cases in the literature so far. The actual prevalence of anti–GM-CSF AAbs in patients with cryptococcosis is probably underrecognized owing to inaccessibility to diagnostic tests in clinical settings and insufficient knowledge regarding the disease spectrum.
Cryptococcus neoformans and C. gattii are the 2 main pathogenic species causing human cryptococcosis. These 2 species are each characterized by distinct pathogenesis, ecology, epidemiology, and clinical features. They react and adapt differently to host defense mechanisms in several aspects, including temperature tolerance, oxidative and nitrosative stress, and osmotic balance [19]. Most patients infected with C. neoformans have underlying conditions or immunocompromised status, including HIV infection, prolonged use of corticosteroids, solid organ transplantation, and cirrhosis [20, 21]; however C. gattii causes disease predominantly in people with apparently healthy immune systems [22]. In Taiwan, C. gattii infection constitutes only 4.1% of all proven cryptococcosis cases [16]. Specifically, in our study, all patients positive for neutralizing anti–GM-CSF AAbs were infected with the VGII genotype of C. gattii. This observation of species-specific susceptibility is consistent with the findings of previous studies in which the vast majority of patients with anti–GM-CSF AAbs were more susceptible to C. gattii than to C. neoformans. In fact, all of the C. neoformans isolates from previous cases have been postulated to be C. gattii because clinical laboratories commonly report the etiologic agent for cryptococcosis as C. neoformans without attempting to distinguish between the 2 pathogens [4]. The reason why susceptibility to each pathogen differs among patients with anti–GM-CSF AAbs, however, remains elusive. Other factors than the presence of anti–GM-CSF antibodies may have played some role in these apparently immunocompetent patients who developed C. gattii infections [22]. Three of the 6 case patients (1 fruit vendor, 1 wood factory worker, and 1 farmer) might have exposed to C. gattii during their occupational activities. However, none of them had ever traveled to any international C. gattii–endemic area before illness onset.
Although routine follow-up of cerebrospinal fluid and serum CrAg for therapeutic monitoring is generally unnecessary because of the absence of a definite correlation between the kinetics of CrAg and disease severity [23, 24], patients with anti–GM-CSF AAbs seemed to have high serum CrAg at diagnosis, which then fluctuated over time and remained positive after 12 months of antifungal therapy. Similarly, at the end of antifungal treatment, a high level of neutralizing anti–GM-CSF AAbs could still be detected in the sera of all case patients. This suggests that the kinetics of anti–GM-CSF AAbs is not a suitable indicator for guiding treatment duration or predicting clinical outcomes.
Patients with anti–GM-CSF AAbs generally had favorable clinical outcomes after completing treatment without recurrence, which was substantially different from the clinical course of patients with immunodeficiency associated with anti–IFN-γ AAbs. A large proportion of patients with anti–IFN-γ AAbs frequently experience treatment failure and disease recurrence despite prolonged antimicrobial therapy [25]. Thus, adjunctive immunotherapies such as rituximab, cyclophosphamide, and daratumumab have been used to control disease activity [26]. By contrast, none of the reported cases involving anti–GM-CSF AAb required adjunctive immunomodulatory agents, in addition to antifungal therapy. Although prolonged secondary antifungal prophylaxis has been proposed in the context of persistent anti–GM-CSF AAb in patients’ sera [6], such practice may not be universally necessary considering the nonrecurrence of cryptococcosis in our 4 case patients who had a minimal follow-up duration of 2 years. Notably, while none of the cases in our study had PAP, 4 of the 27 reported cases (14.8%) developed PAP in the follow-up period. An extended period of monitoring is thus necessary to determine the actual risk of PAP in patients with cryptococcosis associated with anti–GM-CSF AAbs.
This study has several limitations. First, this was a single-center study, thus limiting the generalizability of the findings to patients of different ethnicities or from different geographic regions. Furthermore, although no overt immunodeficiencies were identified among the control patients, who all tested negative for anti–GM-CSF AAb, these patients probably represented a heterogeneous group with various unrecognized immune defects. Thus, with a small sample size, we were underpowered to detect the difference in clinical features and outcomes between patients with and without anti–GM-CSF AAbs. Given that all case patients had C. gattii infection, whether the differences in serum CrAg levels and sustainability of cryptococcal antigenemia between cases and controls were attributed to the species per se or their underlying immunodeficiencies remains to be elucidated. Nevertheless, this is the first prospective, longitudinal study to provide deeper understanding of the disease spectrum, natural course, outcomes, and kinetics of anti–GM-CSF AAbs in patients with CNS and localized cryptococcosis.
CONCLUSIONS
Neutralizing anti–GM-CSF AAbs can predispose otherwise healthy individuals to pulmonary and localized cryptococcosis. Hence, testing for anti–GM-CSF AAbs should be included in the immunological profiling for not only patients with severe CNS cryptococcosis but also those with localized cryptococcosis, particularly in patients with a positive serum CrAg or unusual presentations. The rarity of recurrence of cryptococcal infection in this population, despite the persistence of anti–GM-CSF AAbs, indicates that secondary prophylaxis may not be necessary after completion of primary antifungal therapy.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This study was partially funded by the Ministry of Science and Technology, Taiwan (grant numbers MOST 106–2314-B-002–214, MOST 107–2314-B-002–209-MY3, and 110–2314-B-002–247-).
References
Author notes
Potential conflicts of interest. The authors: No reported conflicts of interest. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.




