-
PDF
- Split View
-
Views
-
Cite
Cite
Shabnam Naseer, Edward A Weinstein, Daniel B Rubin, Kalavati Suvarna, Xiaohui Wei, Karen Higgins, Avery Goodwin, Seong H Jang, Dmitri Iarikov, John Farley, Sumathi Nambiar, US Food and Drug Administration (FDA): Benefit-Risk Considerations for Cefiderocol (Fetroja®), Clinical Infectious Diseases, Volume 72, Issue 12, 15 June 2021, Pages e1103–e1111, https://doi.org/10.1093/cid/ciaa1799
Close - Share Icon Share
Abstract
In November 2019, the Food and Drug Administration (FDA) approved cefiderocol for the treatment of complicated urinary tract infections (cUTI) including pyelonephritis caused by susceptible gram-negative bacteria in adults with limited to no alternative treatment options based on a randomized, double-blind, noninferiority cUTI trial (APEKS-cUTI). In a randomized, open-label trial (CREDIBLE-CR) in patients with cUTI, nosocomial pneumonia, bloodstream infections, or sepsis due to carbapenem-resistant gram-negative bacteria, an increase in all-cause mortality was observed in patients treated with cefiderocol as compared to best available therapy. The cause of the increased mortality was not established, but some deaths were attributed to treatment failure. Preliminary data from a randomized, double-blind trial (APEKS-NP) in patients with nosocomial pneumonia due to carbapenem-susceptible gram-negative bacteria showed a similar rate of mortality as compared to meropenem. We describe the uncertainties and challenges in the interpretation of the CREDIBLE-CR trial and some benefit-risk considerations for the use of cefiderocol in clinical practice.
Clinical Trials Registration: NCT02321800.
(See the Editorial Commentary by Powers III on pages e1112–4.)
Between 2000 and 2009, the frequency of hospitalizations in the United States for cUTI increased by about 50% for multidrug resistant Pseudomonas aeruginosa and by about 300% for extended-spectrum beta-lactamase (ESBL)-producing organisms [1]. In December 2019, Shionogi, Inc., submitted a new drug application (NDA) to the Food and Drug Administration (FDA) for cefiderocol for the treatment of cUTI, including pyelonephritis due to susceptible gram-negative bacteria.
The clinical data package submitted to the NDA included a randomized, active controlled noninferiority (NI) trial in cUTI comparing cefiderocol to imipenem-cilastatin (IMP), a descriptive study (CREDIBLE-CR) comparing cefiderocol to best available therapy (BAT) in patients with infections due to carbapenem-resistant gram-negative organisms and top-line results from a recently completed active controlled NI trial (APEKS-NP) in hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia/healthcare-associated bacterial pneumonia (HABP/VABP/HCABP) comparing cefiderocol to meropenem (results were not verified by FDA).
Cefiderocol is a modified cephalosporin with a catechol moiety that is hypothesized to facilitate entry into the bacterial cell. The principal mechanism of action is the inhibition of cell wall synthesis by binding to penicillin-binding proteins and inhibiting peptidoglycan synthesis [2].
The approved dosing regimen of cefiderocol for the treatment of cUTI is 2 grams every 8 hours by intravenous (IV) infusion over 3 hours in adults with a creatinine clearance (CLcr) of 60–119 mL/min. This dosing regimen was evaluated in CREDIBLE-CR and APEKS-NP trials. In APEKS-cUTI, the same dosing regimen was used with 1-hour infusion.
The treatment duration is 7–14 days. Cefiderocol is primarily eliminated by urinary excretion. The free drug area under the curve (AUC) ratio between epithelial lining fluid and plasma is approximately 40%.
TRIAL IN CUTI (APEKS-CUTI, NCT02321800)
Study Design
Patients were randomized in a 2:1 ratio to either cefiderocol or IMP. Baseline demographic and clinical characteristics were similar between the treatment groups, and the trial was predominantly conducted in Eastern Europe [3]. The primary endpoint was a composite of clinical response (resolution or improvement of the symptoms of cUTI at study entry and no new symptoms) and microbiological eradication (reduction of the baseline bacterial pathogen to <104 colony-forming units [CFUs]/mL) on urine culture at the test of cure visit (TOC; 7 ± 2 days from end of treatment) in the microbiological intent-to-treat (mITT population using an NI margin of 15%. The mITT population was defined as all randomized patients who received at least 1 dose of study drug and had a baseline gram-negative pathogen in urine culture (≥105 CFUs/mL) or blood. The NI margin of 15% was considered acceptable to support a limited use indication.
Efficacy Results
Of 452 patients randomized, 448 received treatment (n = 300 in the cefiderocol group and n = 148 in the IMP group). The response rates for the primary composite endpoint fulfilled criteria for NI: 183/252 (72.6%) in the cefiderocol group and 65/119 (54.6%) in the IMP group; the difference in success rates was 18.6% (95% confidence interval [CI], 8.2% to 28.9%). Statistical superiority of cefiderocol was shown; however, the result was primarily driven by the microbiologic endpoint at TOC. The clinical response rates were similar between the treatment groups. In other words, although a greater proportion of cefiderocol-treated patients had suppressed pathogen growth after the end of treatment, the correlation between reduction in colony count and clinical benefit is unclear. This is further supported by the finding that among those patients with microbiological failure at TOC, 48/53 (90.6%) in the cefiderocol group and 44/44 (100%) in the IMP group did not receive additional IV antibacterial drugs on or after the TOC visit.
In both treatment groups, the predominant pathogen was Escherichia coli (over 60%), followed by Klebsiella pneumoniae (approximately 20%), Proteus mirabilis, P. aeruginosa, and others. Baseline pathogens in this trial were generally susceptible to IMP, based on in vitro susceptibility testing.
Safety Results
Most adverse events (AEs) that occurred in the cefiderocol group were generally consistent with those of the cephalosporin-class, including hepatotoxicity, hypersensitivity reactions, Clostridioides difficile colitis, and seizures. The most common AEs in cefiderocol-treated patients were diarrhea, hypertension, infusion site reactions, constipation, rash, elevated liver tests, headache, and nausea. In the trial, there was 1 death in the cefiderocol group and none in the IMP group; the death was due to cardiac arrest and considered unrelated to the study drug by the investigator.
TRIAL IN CARBAPENEM-RESISTANT INFECTIONS (CREDIBLE-CR; NCT02714595)
Study Design
CREDIBLE-CR was a randomized, open-label, multicenter trial of cefiderocol versus BAT for the treatment of severe infections caused by carbapenem-resistant gram-negative pathogens.
Trial methods have been reported previously [4], but the results are yet unpublished. Both the cUTI and CREDIBLE-CR trials were discussed at the Antimicrobial Drugs Advisory Committee (AMDAC) meeting convened on 16 October 2019 [5]. Patients were randomized 2:1 to receive cefiderocol or BAT. Cefiderocol (2 grams IV every 8 hours with dosing adjustment for renal impairment) was given with or without adjunctive gram-negative therapy. A single systemic antibacterial drug with gram-negative activity (other than a polymyxin or a cephalosporin/ carbapenem including combination with a β-lactamase inhibitor) was permitted for use as concomitant therapy in the cefiderocol group for patients with HABP/VABP/HCABP or BSI/sepsis. The BAT regimen (1–3 antibacterial drugs to treat the carbapenem-resistant gram-negative infection) in the control group was the standard of care treatment as determined by the investigator. The duration of treatment was 7–14 days, with extension to 21 days if necessary.
Randomization was stratified by infection site (HABP/VABP/HCABP, cUTI, and BSI/sepsis), APACHE II score (≤15 and ≥16), and geographic region. CREDIBLE-CR was designed to be a descriptively analyzed study without formal testing of statistical hypotheses and open-label due to the difficulty in blinding multiple potential BAT regimens in the control group.
The study enrolled patients at least 18 years of age with clinically documented HABP/VABP/HCABP, cUTI, or BSI/sepsis caused by a suspected or confirmed carbapenem resistant gram-negative pathogen. Patients previously treated with an empiric antibacterial regimen were eligible if there was clinical and microbiological treatment failure. Potentially effective prior antibacterial therapy was allowed for ≤36 hours for HABP/VABP/HCABP and BSI/sepsis and ≤24 hours for cUTI.
The primary efficacy endpoint at the TOC visit (7 ± 2 days) was defined as the following: clinical outcome for patients with HABP/VABP/HCABP or BSI/sepsis and microbiological outcome for patients with cUTI. The primary efficacy analysis population was the carbapenem- resistant microbiological ITT (CR mITT), composed of patients who had a baseline carbapenem- resistant pathogen from an appropriate specimen. The safety population coincided with the ITT population and consisted of all randomized patients who received at least 1 dose of study drug.
Survival status for each patient in the safety population was to be captured through the end-of-study (EOS) visit 28 ± 3 days after the end of treatment. Mortality status was known for all patients through day 28 but was censored for 50% of patients by day 49. The day 49 all-cause mortality was analyzed because it included more events and because there were numerical trends with higher cefiderocol mortality at later times.
The Medical Dictionary for Regulatory Activities (MedDRA) system was used to classify AEs, and each death was coded by specific fatal AEs. An independent adjudication committee consisting of 3 external physicians appointed by Shionogi, Inc., reviewed blinded mortality data to determine the cause of death.
RESULTS
Patient Characteristics
Of 152 patients randomized, 150 were treated (n = 101 in the cefiderocol group and n = 49 in the BAT group); 118 were included in the CR mITT population (n = 80 in cefiderocol group and n = 38 in the BAT group). The baseline characteristics for both the safety and the CR mITT efficacy analysis populations were relatively similar, as noted in Table 1. There were 45%, 31%, and 24% of patients with diagnoses of HABP/VABP/HCABP, BSI/sepsis, and cUTI, respectively. Patients were predominantly male and either white or Asian. Slightly less than half of patients had a baseline APACHE II score ≥16, and over one third of patients had estimated CLcr ≤50 mL/min. The most common baseline pathogens were Acinetobacter baumannii, K. pneumoniae, and P. aeruginosa. In the CR mITT population, over 80% of patients in the cefiderocol group received cefiderocol as monotherapy, and approximately two-thirds of patients in the BAT group were treated with colistin-based regimens. There was significant variability in the investigator-chosen BAT regimens.
Baseline Characteristics (Safety Population and Carbapenem-resistant [CR] Microbiological Intent-to-Treat [mITT] Population), CREDIBLE-CR Trial
| . | Safety Population . | CR mITT Population . | ||
|---|---|---|---|---|
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | Cefiderocol (N = 80) . | BAT (N = 38) . |
| . | n (%) . | n (%) . | n (%) . | n (%) . |
| Age | ||||
| Mean | 63.1 | 63.0 | 63.1 | 62.1 |
| Median | 69.0 | 62.0 | 69.0 | 62.0 |
| Age group | ||||
| <65 years | 37 (36.6) | 27 (55.1) | 30 (37.5) | 21 (55.3) |
| ≥65 years | 64 (63.4) | 22 (44.9) | 50 (62.5) | 17 (44.7) |
| Gender | ||||
| Male | 66 (65.3) | 35 (71.4) | 55 (68.8) | 29 (76.3) |
| Female | 35 (34.7) | 14 (28.6) | 25 (31.2) | 9 (23.7) |
| Race | ||||
| White | 63 (62.4) | 32 (65.3) | 48 (60.0) | 27 (71.1) |
| Asian | 29 (28.7) | 14 (28.6) | 24 (30.0) | 9 (23.7) |
| Other | 9 (8.9) | 3 (6.1) | 8 (10.0) | 2 (5.3) |
| Body mass index kg/m2 (mean) | 25.5 | 25.3 | 25.4 | 25.1 |
| Region | ||||
| North America | 6 (5.9) | 3 (6.1) | 4 (5.0) | 3 (7.9) |
| South America | 9 (8.9) | 4 (8.2) | 7 (8.8) | 3 (7.9) |
| Europe | 57 (56.4) | 28 (57.1) | 45 (56.2) | 23 (60.5) |
| Asia-Pacific | 29 (28.7) | 14 (28.6) | 24 (30.0) | 9 (23.7) |
| Clinical diagnosis | ||||
| HABP/VABP/HCABP | 45 (44.6) | 22 (44.9) | 40 (50.0) | 19 (50.0) |
| BSI/sepsis | 30 (29.7) | 17 (34.7) | 23 (28.8) | 14 (36.8) |
| cUTI | 26 (25.7) | 10 (20.4) | 17 (21.2) | 5 (13.2) |
| Baseline pathogen | ||||
| A. baumannii | 39 (38.6) | 17 (34.7) | 37 (46.2) | 17 (44.7) |
| K. pneumoniae | 34 (33.7) | 16 (32.7) | 32 (40.0) | 12 (31.6) |
| P. aeruginosa | 17 (16.8) | 12 (24.5) | 17 (21.2) | 11 (28.9) |
| S. maltophilia | 5 (5.0) | 0 (0.0) | 5 (6.2) | 0 (0.0) |
| APACHE II group | ||||
| ≤15 | 55 (54.5) | 27 (55.1) | 41 (51.2) | 21 (55.3) |
| ≥16 | 46 (45.5) | 22 (44.9) | 39 (48.8) | 17 (44.7) |
| SOFA score (mean) | 5.1 | 5.1 | 5.6 | 5.6 |
| Ventilation at randomization (in HABP/VABP/HCABP) | 32/45 (71.1) | 18/22 (81.8) | 29/40 (72.5) | 15/19 (78.9) |
| Receipt of prior antibacterial therapy | 93 (92.1) | 49 (100.0) | 73 (91.3) | 38 (100.0) |
| Creatinine clearance (mL/min) | ||||
| Mean | 85.8 | 88.9 | 90.3 | 98.5 |
| Creatinine clearance (mL/min) group | ||||
| <30 (severe) | 20 (19.8) | 7 (14.3) | 15 (18.8) | 3 (7.9) |
| 30 to 50 (moderate) | 23 (22.8) | 8 (16.3) | 18 (22.5) | 6 (15.8) |
| >50 to 80 (mild) | 20 (19.8) | 12 (24.5) | 15 (18.8) | 9 (23.7) |
| >80 to <120 (normal) | 18 (17.8) | 10 (20.4) | 15 (18.8) | 9 (23.7) |
| ≥120 (ARC) | 20 (19.8) | 12 (24.5) | 17 (21.2) | 11 (28.9) |
| Presence of bacteremia with carbapenem-resistant pathogen(s) | N/A | N/A | 22/80 (27.5) | 13/38 (34.2) |
| . | Safety Population . | CR mITT Population . | ||
|---|---|---|---|---|
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | Cefiderocol (N = 80) . | BAT (N = 38) . |
| . | n (%) . | n (%) . | n (%) . | n (%) . |
| Age | ||||
| Mean | 63.1 | 63.0 | 63.1 | 62.1 |
| Median | 69.0 | 62.0 | 69.0 | 62.0 |
| Age group | ||||
| <65 years | 37 (36.6) | 27 (55.1) | 30 (37.5) | 21 (55.3) |
| ≥65 years | 64 (63.4) | 22 (44.9) | 50 (62.5) | 17 (44.7) |
| Gender | ||||
| Male | 66 (65.3) | 35 (71.4) | 55 (68.8) | 29 (76.3) |
| Female | 35 (34.7) | 14 (28.6) | 25 (31.2) | 9 (23.7) |
| Race | ||||
| White | 63 (62.4) | 32 (65.3) | 48 (60.0) | 27 (71.1) |
| Asian | 29 (28.7) | 14 (28.6) | 24 (30.0) | 9 (23.7) |
| Other | 9 (8.9) | 3 (6.1) | 8 (10.0) | 2 (5.3) |
| Body mass index kg/m2 (mean) | 25.5 | 25.3 | 25.4 | 25.1 |
| Region | ||||
| North America | 6 (5.9) | 3 (6.1) | 4 (5.0) | 3 (7.9) |
| South America | 9 (8.9) | 4 (8.2) | 7 (8.8) | 3 (7.9) |
| Europe | 57 (56.4) | 28 (57.1) | 45 (56.2) | 23 (60.5) |
| Asia-Pacific | 29 (28.7) | 14 (28.6) | 24 (30.0) | 9 (23.7) |
| Clinical diagnosis | ||||
| HABP/VABP/HCABP | 45 (44.6) | 22 (44.9) | 40 (50.0) | 19 (50.0) |
| BSI/sepsis | 30 (29.7) | 17 (34.7) | 23 (28.8) | 14 (36.8) |
| cUTI | 26 (25.7) | 10 (20.4) | 17 (21.2) | 5 (13.2) |
| Baseline pathogen | ||||
| A. baumannii | 39 (38.6) | 17 (34.7) | 37 (46.2) | 17 (44.7) |
| K. pneumoniae | 34 (33.7) | 16 (32.7) | 32 (40.0) | 12 (31.6) |
| P. aeruginosa | 17 (16.8) | 12 (24.5) | 17 (21.2) | 11 (28.9) |
| S. maltophilia | 5 (5.0) | 0 (0.0) | 5 (6.2) | 0 (0.0) |
| APACHE II group | ||||
| ≤15 | 55 (54.5) | 27 (55.1) | 41 (51.2) | 21 (55.3) |
| ≥16 | 46 (45.5) | 22 (44.9) | 39 (48.8) | 17 (44.7) |
| SOFA score (mean) | 5.1 | 5.1 | 5.6 | 5.6 |
| Ventilation at randomization (in HABP/VABP/HCABP) | 32/45 (71.1) | 18/22 (81.8) | 29/40 (72.5) | 15/19 (78.9) |
| Receipt of prior antibacterial therapy | 93 (92.1) | 49 (100.0) | 73 (91.3) | 38 (100.0) |
| Creatinine clearance (mL/min) | ||||
| Mean | 85.8 | 88.9 | 90.3 | 98.5 |
| Creatinine clearance (mL/min) group | ||||
| <30 (severe) | 20 (19.8) | 7 (14.3) | 15 (18.8) | 3 (7.9) |
| 30 to 50 (moderate) | 23 (22.8) | 8 (16.3) | 18 (22.5) | 6 (15.8) |
| >50 to 80 (mild) | 20 (19.8) | 12 (24.5) | 15 (18.8) | 9 (23.7) |
| >80 to <120 (normal) | 18 (17.8) | 10 (20.4) | 15 (18.8) | 9 (23.7) |
| ≥120 (ARC) | 20 (19.8) | 12 (24.5) | 17 (21.2) | 11 (28.9) |
| Presence of bacteremia with carbapenem-resistant pathogen(s) | N/A | N/A | 22/80 (27.5) | 13/38 (34.2) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; ARC, augmented renal clearance; BAT, best available therapy; BSI, bloodstream infections; cUTI, complicated urinary tract infections; HCABP/VABP/HCABP, hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia/healthcare-associated bacterial pneumonia; SOFA, sequential organ failure assessment.
Baseline Characteristics (Safety Population and Carbapenem-resistant [CR] Microbiological Intent-to-Treat [mITT] Population), CREDIBLE-CR Trial
| . | Safety Population . | CR mITT Population . | ||
|---|---|---|---|---|
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | Cefiderocol (N = 80) . | BAT (N = 38) . |
| . | n (%) . | n (%) . | n (%) . | n (%) . |
| Age | ||||
| Mean | 63.1 | 63.0 | 63.1 | 62.1 |
| Median | 69.0 | 62.0 | 69.0 | 62.0 |
| Age group | ||||
| <65 years | 37 (36.6) | 27 (55.1) | 30 (37.5) | 21 (55.3) |
| ≥65 years | 64 (63.4) | 22 (44.9) | 50 (62.5) | 17 (44.7) |
| Gender | ||||
| Male | 66 (65.3) | 35 (71.4) | 55 (68.8) | 29 (76.3) |
| Female | 35 (34.7) | 14 (28.6) | 25 (31.2) | 9 (23.7) |
| Race | ||||
| White | 63 (62.4) | 32 (65.3) | 48 (60.0) | 27 (71.1) |
| Asian | 29 (28.7) | 14 (28.6) | 24 (30.0) | 9 (23.7) |
| Other | 9 (8.9) | 3 (6.1) | 8 (10.0) | 2 (5.3) |
| Body mass index kg/m2 (mean) | 25.5 | 25.3 | 25.4 | 25.1 |
| Region | ||||
| North America | 6 (5.9) | 3 (6.1) | 4 (5.0) | 3 (7.9) |
| South America | 9 (8.9) | 4 (8.2) | 7 (8.8) | 3 (7.9) |
| Europe | 57 (56.4) | 28 (57.1) | 45 (56.2) | 23 (60.5) |
| Asia-Pacific | 29 (28.7) | 14 (28.6) | 24 (30.0) | 9 (23.7) |
| Clinical diagnosis | ||||
| HABP/VABP/HCABP | 45 (44.6) | 22 (44.9) | 40 (50.0) | 19 (50.0) |
| BSI/sepsis | 30 (29.7) | 17 (34.7) | 23 (28.8) | 14 (36.8) |
| cUTI | 26 (25.7) | 10 (20.4) | 17 (21.2) | 5 (13.2) |
| Baseline pathogen | ||||
| A. baumannii | 39 (38.6) | 17 (34.7) | 37 (46.2) | 17 (44.7) |
| K. pneumoniae | 34 (33.7) | 16 (32.7) | 32 (40.0) | 12 (31.6) |
| P. aeruginosa | 17 (16.8) | 12 (24.5) | 17 (21.2) | 11 (28.9) |
| S. maltophilia | 5 (5.0) | 0 (0.0) | 5 (6.2) | 0 (0.0) |
| APACHE II group | ||||
| ≤15 | 55 (54.5) | 27 (55.1) | 41 (51.2) | 21 (55.3) |
| ≥16 | 46 (45.5) | 22 (44.9) | 39 (48.8) | 17 (44.7) |
| SOFA score (mean) | 5.1 | 5.1 | 5.6 | 5.6 |
| Ventilation at randomization (in HABP/VABP/HCABP) | 32/45 (71.1) | 18/22 (81.8) | 29/40 (72.5) | 15/19 (78.9) |
| Receipt of prior antibacterial therapy | 93 (92.1) | 49 (100.0) | 73 (91.3) | 38 (100.0) |
| Creatinine clearance (mL/min) | ||||
| Mean | 85.8 | 88.9 | 90.3 | 98.5 |
| Creatinine clearance (mL/min) group | ||||
| <30 (severe) | 20 (19.8) | 7 (14.3) | 15 (18.8) | 3 (7.9) |
| 30 to 50 (moderate) | 23 (22.8) | 8 (16.3) | 18 (22.5) | 6 (15.8) |
| >50 to 80 (mild) | 20 (19.8) | 12 (24.5) | 15 (18.8) | 9 (23.7) |
| >80 to <120 (normal) | 18 (17.8) | 10 (20.4) | 15 (18.8) | 9 (23.7) |
| ≥120 (ARC) | 20 (19.8) | 12 (24.5) | 17 (21.2) | 11 (28.9) |
| Presence of bacteremia with carbapenem-resistant pathogen(s) | N/A | N/A | 22/80 (27.5) | 13/38 (34.2) |
| . | Safety Population . | CR mITT Population . | ||
|---|---|---|---|---|
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | Cefiderocol (N = 80) . | BAT (N = 38) . |
| . | n (%) . | n (%) . | n (%) . | n (%) . |
| Age | ||||
| Mean | 63.1 | 63.0 | 63.1 | 62.1 |
| Median | 69.0 | 62.0 | 69.0 | 62.0 |
| Age group | ||||
| <65 years | 37 (36.6) | 27 (55.1) | 30 (37.5) | 21 (55.3) |
| ≥65 years | 64 (63.4) | 22 (44.9) | 50 (62.5) | 17 (44.7) |
| Gender | ||||
| Male | 66 (65.3) | 35 (71.4) | 55 (68.8) | 29 (76.3) |
| Female | 35 (34.7) | 14 (28.6) | 25 (31.2) | 9 (23.7) |
| Race | ||||
| White | 63 (62.4) | 32 (65.3) | 48 (60.0) | 27 (71.1) |
| Asian | 29 (28.7) | 14 (28.6) | 24 (30.0) | 9 (23.7) |
| Other | 9 (8.9) | 3 (6.1) | 8 (10.0) | 2 (5.3) |
| Body mass index kg/m2 (mean) | 25.5 | 25.3 | 25.4 | 25.1 |
| Region | ||||
| North America | 6 (5.9) | 3 (6.1) | 4 (5.0) | 3 (7.9) |
| South America | 9 (8.9) | 4 (8.2) | 7 (8.8) | 3 (7.9) |
| Europe | 57 (56.4) | 28 (57.1) | 45 (56.2) | 23 (60.5) |
| Asia-Pacific | 29 (28.7) | 14 (28.6) | 24 (30.0) | 9 (23.7) |
| Clinical diagnosis | ||||
| HABP/VABP/HCABP | 45 (44.6) | 22 (44.9) | 40 (50.0) | 19 (50.0) |
| BSI/sepsis | 30 (29.7) | 17 (34.7) | 23 (28.8) | 14 (36.8) |
| cUTI | 26 (25.7) | 10 (20.4) | 17 (21.2) | 5 (13.2) |
| Baseline pathogen | ||||
| A. baumannii | 39 (38.6) | 17 (34.7) | 37 (46.2) | 17 (44.7) |
| K. pneumoniae | 34 (33.7) | 16 (32.7) | 32 (40.0) | 12 (31.6) |
| P. aeruginosa | 17 (16.8) | 12 (24.5) | 17 (21.2) | 11 (28.9) |
| S. maltophilia | 5 (5.0) | 0 (0.0) | 5 (6.2) | 0 (0.0) |
| APACHE II group | ||||
| ≤15 | 55 (54.5) | 27 (55.1) | 41 (51.2) | 21 (55.3) |
| ≥16 | 46 (45.5) | 22 (44.9) | 39 (48.8) | 17 (44.7) |
| SOFA score (mean) | 5.1 | 5.1 | 5.6 | 5.6 |
| Ventilation at randomization (in HABP/VABP/HCABP) | 32/45 (71.1) | 18/22 (81.8) | 29/40 (72.5) | 15/19 (78.9) |
| Receipt of prior antibacterial therapy | 93 (92.1) | 49 (100.0) | 73 (91.3) | 38 (100.0) |
| Creatinine clearance (mL/min) | ||||
| Mean | 85.8 | 88.9 | 90.3 | 98.5 |
| Creatinine clearance (mL/min) group | ||||
| <30 (severe) | 20 (19.8) | 7 (14.3) | 15 (18.8) | 3 (7.9) |
| 30 to 50 (moderate) | 23 (22.8) | 8 (16.3) | 18 (22.5) | 6 (15.8) |
| >50 to 80 (mild) | 20 (19.8) | 12 (24.5) | 15 (18.8) | 9 (23.7) |
| >80 to <120 (normal) | 18 (17.8) | 10 (20.4) | 15 (18.8) | 9 (23.7) |
| ≥120 (ARC) | 20 (19.8) | 12 (24.5) | 17 (21.2) | 11 (28.9) |
| Presence of bacteremia with carbapenem-resistant pathogen(s) | N/A | N/A | 22/80 (27.5) | 13/38 (34.2) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; ARC, augmented renal clearance; BAT, best available therapy; BSI, bloodstream infections; cUTI, complicated urinary tract infections; HCABP/VABP/HCABP, hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia/healthcare-associated bacterial pneumonia; SOFA, sequential organ failure assessment.
There were some baseline imbalances between the cefiderocol and BAT groups in both the safety and the CR mITT population. Some possibly disfavored the cefiderocol group due to an increased frequency of the following parameters: number of patients in the age ≥65 years subgroup, moderate to severe renal impairment, and S. maltophilia infection. However, other age subgroups (≥50 years or ≥75 years) were well balanced between groups. Other baseline factors possibly disfavored the BAT group due to the increased frequency of the following parameters: ventilation in the HABP/VABP/HCABP subgroup, prior antibacterial therapy in the last 2 weeks prior to randomization, and bacteremia (only relevant to the CR mITT population).
Efficacy Results
Clinical cure rates were approximately 50% in each treatment group in the CR mITT population. The clinical cure rates by baseline pathogen in the cefiderocol and BAT groups were as follows: 65.6% versus 50.0% in patients with K. pneumoniae, 52.9% versus 54.5% in patients with P. aeruginosa, and 43.2% versus 52.9% in patients with A. baumannii, respectively. Assessments of microbiological eradication rates were limited because approximately 50% of patients in each treatment group were classified as having an indeterminate outcome. Efficacy assessments within the cUTI subgroup were limited by the small sample size in the BAT group (5 patients).
The cefiderocol group had a higher rate of all-cause mortality than the BAT group at day 14 (19/101 [18.8%] vs 6/49 [12.2%], difference = 6.6%, 95% CI: −5.4% to 18.5%), at day 28 (25/101 [24.8%] vs 9/49 [18.4%], difference = 6.4%, 95% CI: −7.3% to 20.1%), and at day 49 (34/101 [33.7%] vs 10/49 [20.4%], difference = 13.3%, 95% CI: −1.3% to 27.8%). A Kaplan-Meier plot of all-cause mortality in the safety population is displayed in Figure 1. The mortality curves separated at an early time and cefiderocol mortality numerically exceeded the BAT mortality through day 49. The hazard ratio of time-to-death through day 49 in the safety population was 1.77 (95% CI: .87 to 3.57).
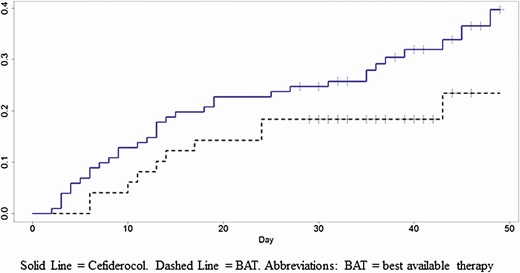
Kaplan-Meier plot of all-cause mortality by treatment group through day 49, CREDIBLE-CR trial. Cefiderocol (solid line). BAT (dashed line). Abbreviation: BAT, best available therapy.
All-cause mortality at day 14, day 28, and day 49 by time and infection site was analyzed. The greatest mortality imbalance disfavoring cefiderocol was noted in the HABP/VABP/HCABP subgroup at day 49 (42.2% in cefiderocol group and 18.2% in BAT group, difference of 24.0% [95% CI: 2.4% to 45.7%]), followed by the BSI/Sepsis subgroup at day 49 (36.7% in cefiderocol group and 23.5% in BAT group, difference of 13.1% [95 CI: −13.4% to 39.7%]). Numerically, the cUTI subgroup had a higher mortality in the BAT group at day 49 compared to the cefiderocol group, but the difference was difficult to interpret with a small sample size and wide confidence intervals (15.4% in cefiderocol group, 20% in BAT group, difference −4.6 [95% CI: −33.0% to 23.8%]).
The majority of deaths occurred within 15 days of the start of study treatment in both groups, and 9 additional deaths occurred past 30 days in the cefiderocol group as compared to 1 in the BAT group. Most of the deaths were associated with infections, and the frequency was higher in the cefiderocol group than in the BAT group (20.8% vs 6.1%, respectively). In general, the infection-related fatal AEs that occurred in the cefiderocol group (septic shock, pneumonia, sepsis, bacteremia) represented worsening of the original infection. The following subgroups were associated with an absolute 10% higher mortality rate in the cefiderocol as compared to the BAT group at day 49: clinical diagnosis of HABP/VABP/HCABP or BSI/Sepsis, male or female gender, age group ≤65 years or ≥75 years, Asian and “other” race, A. baumannii or P. aeruginosa baseline pathogen, APACHE II Score of ≥16, Asia-Pacific or South American region, as noted in Table 2.
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | . | . |
|---|---|---|---|---|
| . | n/N (%) . | n/N (%) . | Difference (%) . | 95% CI . |
| Age group | ||||
| <65 years | 10/37 (27.0) | 3/27 (11.1) | 15.9 | −2.7 to 34.5 |
| ≥65 years | 24/64 (37.5) | 7/22 (31.8) | 5.7 | −17.1 to 28.5 |
| ≥75 years | 13/29 (44.8) | 4/14 (28.6) | 16.3 | −13.5 to 46.0 |
| Gender | ||||
| Male | 23/66 (34.8) | 8/35 (22.9) | 12.0 | −6.1 to 30.0 |
| Female | 11/35 (31.4) | 2/14 (14.3) | 17.1 | −6.8 to 41.1 |
| Race | ||||
| White | 18/63 (28.6) | 7/32 (21.9) | 6.7 | −11.5 to 24.9 |
| Asian | 14/29 (48.3) | 3/14 (21.4) | 26.8 | −1.3 to 55.0 |
| Other | 2/9 (22.2) | 0/3 (0.0) | 22.2 | |
| Region | ||||
| North America | 0/6 (0.0) | 0/3 (0.0) | 0.0 | |
| South America | 1/9 (11.1) | 0/4 (0.0) | 11.1 | |
| Europe | 19/57 (33.3) | 7/28 (25.0) | 8.3 | −11.8 to 28.5 |
| Asia-Pacific | 14/29 (48.3) | 3/14 (21.4) | 26.8 | −1.3 to 55.0 |
| Clinical diagnosis | ||||
| HABP/VABP/HCABP | 19/45 (42.2) | 4/22 (18.2) | 24.0 | 2.4 to 45.7 |
| BSI/Sepsis | 11/30 (36.7) | 4/17 (23.5) | 13.1 | −13.4 to 39.7 |
| cUTI | 4/26 (15.4) | 2/10 (20.0) | −4.6 | −33.0 to 23.8 |
| Baseline pathogen | ||||
| A. baumannii | 19/39 (48.7) | 4/17 (23.5) | 25.2 | −.4 to 50.7 |
| K. pneumoniae | 8/34 (23.5) | 4/16 (25.0) | −1.5 | −27.0 to 24.1 |
| P. aeruginosa | 6/17 (35.3) | 2/12 (16.7) | 18.6 | −12.4 to 49.6 |
| S. maltophilia | 4/5 (80.0) | 0/0 | ||
| APACHE II score | ||||
| ≤15 | 13/55 (23.6) | 5/27 (18.5) | 5.1 | −13.3 to 23.6 |
| ≥16 | 21/46 (45.7) | 5/22 (22.7) | 22.9 | .3 to 45.6 |
| Creatinine clearance (mL/min) group | ||||
| <30 (severe) | 8/20 (40.0) | 2/7 (28.6) | 11.4 | |
| 30 to 50 (moderate) | 10/23 (43.5) | 2/8 (25.0) | 18.5 | |
| >50 to 80 (mild) | 9/20 (45.0) | 3/12 (25.0) | 20.0 | −12.8 to 52.8 |
| >80 to <120 (normal) | 4/18 (22.2) | 1/10 (10.0) | 12.2 | −14.5 to 39.0 |
| ≥120 (ARC) | 3/20 (15.0) | 2/12 (16.7) | −1.7 | −27.9 to 24.6 |
| Bacteremia statusa | ||||
| Bacteremia | 10/24 (41.7) | 4/13 (30.8) | 10.9 | −21.0 to 42.8 |
| No bacteremia | 22/62 (35.5) | 5/31 (16.1) | 19.4 | 1.8 to 36.9 |
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | . | . |
|---|---|---|---|---|
| . | n/N (%) . | n/N (%) . | Difference (%) . | 95% CI . |
| Age group | ||||
| <65 years | 10/37 (27.0) | 3/27 (11.1) | 15.9 | −2.7 to 34.5 |
| ≥65 years | 24/64 (37.5) | 7/22 (31.8) | 5.7 | −17.1 to 28.5 |
| ≥75 years | 13/29 (44.8) | 4/14 (28.6) | 16.3 | −13.5 to 46.0 |
| Gender | ||||
| Male | 23/66 (34.8) | 8/35 (22.9) | 12.0 | −6.1 to 30.0 |
| Female | 11/35 (31.4) | 2/14 (14.3) | 17.1 | −6.8 to 41.1 |
| Race | ||||
| White | 18/63 (28.6) | 7/32 (21.9) | 6.7 | −11.5 to 24.9 |
| Asian | 14/29 (48.3) | 3/14 (21.4) | 26.8 | −1.3 to 55.0 |
| Other | 2/9 (22.2) | 0/3 (0.0) | 22.2 | |
| Region | ||||
| North America | 0/6 (0.0) | 0/3 (0.0) | 0.0 | |
| South America | 1/9 (11.1) | 0/4 (0.0) | 11.1 | |
| Europe | 19/57 (33.3) | 7/28 (25.0) | 8.3 | −11.8 to 28.5 |
| Asia-Pacific | 14/29 (48.3) | 3/14 (21.4) | 26.8 | −1.3 to 55.0 |
| Clinical diagnosis | ||||
| HABP/VABP/HCABP | 19/45 (42.2) | 4/22 (18.2) | 24.0 | 2.4 to 45.7 |
| BSI/Sepsis | 11/30 (36.7) | 4/17 (23.5) | 13.1 | −13.4 to 39.7 |
| cUTI | 4/26 (15.4) | 2/10 (20.0) | −4.6 | −33.0 to 23.8 |
| Baseline pathogen | ||||
| A. baumannii | 19/39 (48.7) | 4/17 (23.5) | 25.2 | −.4 to 50.7 |
| K. pneumoniae | 8/34 (23.5) | 4/16 (25.0) | −1.5 | −27.0 to 24.1 |
| P. aeruginosa | 6/17 (35.3) | 2/12 (16.7) | 18.6 | −12.4 to 49.6 |
| S. maltophilia | 4/5 (80.0) | 0/0 | ||
| APACHE II score | ||||
| ≤15 | 13/55 (23.6) | 5/27 (18.5) | 5.1 | −13.3 to 23.6 |
| ≥16 | 21/46 (45.7) | 5/22 (22.7) | 22.9 | .3 to 45.6 |
| Creatinine clearance (mL/min) group | ||||
| <30 (severe) | 8/20 (40.0) | 2/7 (28.6) | 11.4 | |
| 30 to 50 (moderate) | 10/23 (43.5) | 2/8 (25.0) | 18.5 | |
| >50 to 80 (mild) | 9/20 (45.0) | 3/12 (25.0) | 20.0 | −12.8 to 52.8 |
| >80 to <120 (normal) | 4/18 (22.2) | 1/10 (10.0) | 12.2 | −14.5 to 39.0 |
| ≥120 (ARC) | 3/20 (15.0) | 2/12 (16.7) | −1.7 | −27.9 to 24.6 |
| Bacteremia statusa | ||||
| Bacteremia | 10/24 (41.7) | 4/13 (30.8) | 10.9 | −21.0 to 42.8 |
| No bacteremia | 22/62 (35.5) | 5/31 (16.1) | 19.4 | 1.8 to 36.9 |
Abbreviations: APACHE II, APACHE, Acute Physiology and Chronic Health Evaluation II; ARC, augmented renal clearance; BAT, best available therapy; BSI, bloodstream infections; CI, confidence interval; cUTI, complicated urinary tract infections; HCABP/VABP/HCABP, hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia/healthcare-associated bacterial pneumonia; SOFA, sequential organ failure assessment.
aPrimary or concomitant bacteremia in any clinical diagnosis subgroup. Bacteremia subgroups defined within the CR mITT Population rather than the safety population.
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | . | . |
|---|---|---|---|---|
| . | n/N (%) . | n/N (%) . | Difference (%) . | 95% CI . |
| Age group | ||||
| <65 years | 10/37 (27.0) | 3/27 (11.1) | 15.9 | −2.7 to 34.5 |
| ≥65 years | 24/64 (37.5) | 7/22 (31.8) | 5.7 | −17.1 to 28.5 |
| ≥75 years | 13/29 (44.8) | 4/14 (28.6) | 16.3 | −13.5 to 46.0 |
| Gender | ||||
| Male | 23/66 (34.8) | 8/35 (22.9) | 12.0 | −6.1 to 30.0 |
| Female | 11/35 (31.4) | 2/14 (14.3) | 17.1 | −6.8 to 41.1 |
| Race | ||||
| White | 18/63 (28.6) | 7/32 (21.9) | 6.7 | −11.5 to 24.9 |
| Asian | 14/29 (48.3) | 3/14 (21.4) | 26.8 | −1.3 to 55.0 |
| Other | 2/9 (22.2) | 0/3 (0.0) | 22.2 | |
| Region | ||||
| North America | 0/6 (0.0) | 0/3 (0.0) | 0.0 | |
| South America | 1/9 (11.1) | 0/4 (0.0) | 11.1 | |
| Europe | 19/57 (33.3) | 7/28 (25.0) | 8.3 | −11.8 to 28.5 |
| Asia-Pacific | 14/29 (48.3) | 3/14 (21.4) | 26.8 | −1.3 to 55.0 |
| Clinical diagnosis | ||||
| HABP/VABP/HCABP | 19/45 (42.2) | 4/22 (18.2) | 24.0 | 2.4 to 45.7 |
| BSI/Sepsis | 11/30 (36.7) | 4/17 (23.5) | 13.1 | −13.4 to 39.7 |
| cUTI | 4/26 (15.4) | 2/10 (20.0) | −4.6 | −33.0 to 23.8 |
| Baseline pathogen | ||||
| A. baumannii | 19/39 (48.7) | 4/17 (23.5) | 25.2 | −.4 to 50.7 |
| K. pneumoniae | 8/34 (23.5) | 4/16 (25.0) | −1.5 | −27.0 to 24.1 |
| P. aeruginosa | 6/17 (35.3) | 2/12 (16.7) | 18.6 | −12.4 to 49.6 |
| S. maltophilia | 4/5 (80.0) | 0/0 | ||
| APACHE II score | ||||
| ≤15 | 13/55 (23.6) | 5/27 (18.5) | 5.1 | −13.3 to 23.6 |
| ≥16 | 21/46 (45.7) | 5/22 (22.7) | 22.9 | .3 to 45.6 |
| Creatinine clearance (mL/min) group | ||||
| <30 (severe) | 8/20 (40.0) | 2/7 (28.6) | 11.4 | |
| 30 to 50 (moderate) | 10/23 (43.5) | 2/8 (25.0) | 18.5 | |
| >50 to 80 (mild) | 9/20 (45.0) | 3/12 (25.0) | 20.0 | −12.8 to 52.8 |
| >80 to <120 (normal) | 4/18 (22.2) | 1/10 (10.0) | 12.2 | −14.5 to 39.0 |
| ≥120 (ARC) | 3/20 (15.0) | 2/12 (16.7) | −1.7 | −27.9 to 24.6 |
| Bacteremia statusa | ||||
| Bacteremia | 10/24 (41.7) | 4/13 (30.8) | 10.9 | −21.0 to 42.8 |
| No bacteremia | 22/62 (35.5) | 5/31 (16.1) | 19.4 | 1.8 to 36.9 |
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | . | . |
|---|---|---|---|---|
| . | n/N (%) . | n/N (%) . | Difference (%) . | 95% CI . |
| Age group | ||||
| <65 years | 10/37 (27.0) | 3/27 (11.1) | 15.9 | −2.7 to 34.5 |
| ≥65 years | 24/64 (37.5) | 7/22 (31.8) | 5.7 | −17.1 to 28.5 |
| ≥75 years | 13/29 (44.8) | 4/14 (28.6) | 16.3 | −13.5 to 46.0 |
| Gender | ||||
| Male | 23/66 (34.8) | 8/35 (22.9) | 12.0 | −6.1 to 30.0 |
| Female | 11/35 (31.4) | 2/14 (14.3) | 17.1 | −6.8 to 41.1 |
| Race | ||||
| White | 18/63 (28.6) | 7/32 (21.9) | 6.7 | −11.5 to 24.9 |
| Asian | 14/29 (48.3) | 3/14 (21.4) | 26.8 | −1.3 to 55.0 |
| Other | 2/9 (22.2) | 0/3 (0.0) | 22.2 | |
| Region | ||||
| North America | 0/6 (0.0) | 0/3 (0.0) | 0.0 | |
| South America | 1/9 (11.1) | 0/4 (0.0) | 11.1 | |
| Europe | 19/57 (33.3) | 7/28 (25.0) | 8.3 | −11.8 to 28.5 |
| Asia-Pacific | 14/29 (48.3) | 3/14 (21.4) | 26.8 | −1.3 to 55.0 |
| Clinical diagnosis | ||||
| HABP/VABP/HCABP | 19/45 (42.2) | 4/22 (18.2) | 24.0 | 2.4 to 45.7 |
| BSI/Sepsis | 11/30 (36.7) | 4/17 (23.5) | 13.1 | −13.4 to 39.7 |
| cUTI | 4/26 (15.4) | 2/10 (20.0) | −4.6 | −33.0 to 23.8 |
| Baseline pathogen | ||||
| A. baumannii | 19/39 (48.7) | 4/17 (23.5) | 25.2 | −.4 to 50.7 |
| K. pneumoniae | 8/34 (23.5) | 4/16 (25.0) | −1.5 | −27.0 to 24.1 |
| P. aeruginosa | 6/17 (35.3) | 2/12 (16.7) | 18.6 | −12.4 to 49.6 |
| S. maltophilia | 4/5 (80.0) | 0/0 | ||
| APACHE II score | ||||
| ≤15 | 13/55 (23.6) | 5/27 (18.5) | 5.1 | −13.3 to 23.6 |
| ≥16 | 21/46 (45.7) | 5/22 (22.7) | 22.9 | .3 to 45.6 |
| Creatinine clearance (mL/min) group | ||||
| <30 (severe) | 8/20 (40.0) | 2/7 (28.6) | 11.4 | |
| 30 to 50 (moderate) | 10/23 (43.5) | 2/8 (25.0) | 18.5 | |
| >50 to 80 (mild) | 9/20 (45.0) | 3/12 (25.0) | 20.0 | −12.8 to 52.8 |
| >80 to <120 (normal) | 4/18 (22.2) | 1/10 (10.0) | 12.2 | −14.5 to 39.0 |
| ≥120 (ARC) | 3/20 (15.0) | 2/12 (16.7) | −1.7 | −27.9 to 24.6 |
| Bacteremia statusa | ||||
| Bacteremia | 10/24 (41.7) | 4/13 (30.8) | 10.9 | −21.0 to 42.8 |
| No bacteremia | 22/62 (35.5) | 5/31 (16.1) | 19.4 | 1.8 to 36.9 |
Abbreviations: APACHE II, APACHE, Acute Physiology and Chronic Health Evaluation II; ARC, augmented renal clearance; BAT, best available therapy; BSI, bloodstream infections; CI, confidence interval; cUTI, complicated urinary tract infections; HCABP/VABP/HCABP, hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia/healthcare-associated bacterial pneumonia; SOFA, sequential organ failure assessment.
aPrimary or concomitant bacteremia in any clinical diagnosis subgroup. Bacteremia subgroups defined within the CR mITT Population rather than the safety population.
The cause of death was categorized by the adjudication committee and the results are summarized through day 49 in Table 3. A greater percentage of patients in the cefiderocol group than in the BAT group had infection-related death with treatment failure (15.8% vs 8.2%) but also death due to underlying comorbidity (9.9% vs 4.1%). In a few cases where causality was not readily apparent, the committee seemed to attribute the death to “underlying comorbidity” as it did not readily fit into another category. In general, the contribution of the infection to underlying comorbidity as a cause of death could not be excluded. In 10 cefiderocol-treated patients in whom death was attributed to an underlying comorbidity, 2 patients’ reports did not specify which comorbidities contributed to death, 2 involved a 4-fold MIC increase to cefiderocol (suggesting a potential for development of resistance), and 3 reports involved an extensive discussion by the committee to arrive at a unanimous decision. Overall, the FDA accepted the adjudication of deaths as noted by the committee.
Adjudication Committee Analyses of Causes of Death Through Day 49 Visit in the CREDIBLE-CR Trial
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | . |
|---|---|---|---|
| Cause of Death . | n (%) . | n (%) . | Difference (%) . |
| Overall mortality | 34 (33.7) | 10 (20.4) | 13.3 |
| Directly related to original gram-negative infection | |||
| Failure of study drug treatment | 16 (15.8) | 4 (8.2) | 7.7 |
| No failure of study drug treatment | 1 (1.0) | 1 (2.0) | −1.0 |
| No unanimous vote | 1 (1.0) | 0 | 1.0 |
| Unrelated to original gram-negative infection | |||
| Underlying comorbidity | 10 (9.9) | 2 (4.1) | 5.8 |
| Other infection | 4 (4.0) | 2 (4.1) | −0.1 |
| No unanimous vote | 2 (2.0) | 0 | 2.0 |
| Not assessed by committeea | 0 (0) | 1 (2.0) | −2.0 |
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | . |
|---|---|---|---|
| Cause of Death . | n (%) . | n (%) . | Difference (%) . |
| Overall mortality | 34 (33.7) | 10 (20.4) | 13.3 |
| Directly related to original gram-negative infection | |||
| Failure of study drug treatment | 16 (15.8) | 4 (8.2) | 7.7 |
| No failure of study drug treatment | 1 (1.0) | 1 (2.0) | −1.0 |
| No unanimous vote | 1 (1.0) | 0 | 1.0 |
| Unrelated to original gram-negative infection | |||
| Underlying comorbidity | 10 (9.9) | 2 (4.1) | 5.8 |
| Other infection | 4 (4.0) | 2 (4.1) | −0.1 |
| No unanimous vote | 2 (2.0) | 0 | 2.0 |
| Not assessed by committeea | 0 (0) | 1 (2.0) | −2.0 |
Abbreviation: BAT, best available therapy.
Source: Adapted from Table 1 of “Cefiderocol Adjudication Committee Review and Shionogi’s Assessment – Final.”
aCommittee assessed all deaths until end of study (EOS); 1 additional death occurred after EOS, but prior to day 49 in the BAT group which was not assessed.
Adjudication Committee Analyses of Causes of Death Through Day 49 Visit in the CREDIBLE-CR Trial
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | . |
|---|---|---|---|
| Cause of Death . | n (%) . | n (%) . | Difference (%) . |
| Overall mortality | 34 (33.7) | 10 (20.4) | 13.3 |
| Directly related to original gram-negative infection | |||
| Failure of study drug treatment | 16 (15.8) | 4 (8.2) | 7.7 |
| No failure of study drug treatment | 1 (1.0) | 1 (2.0) | −1.0 |
| No unanimous vote | 1 (1.0) | 0 | 1.0 |
| Unrelated to original gram-negative infection | |||
| Underlying comorbidity | 10 (9.9) | 2 (4.1) | 5.8 |
| Other infection | 4 (4.0) | 2 (4.1) | −0.1 |
| No unanimous vote | 2 (2.0) | 0 | 2.0 |
| Not assessed by committeea | 0 (0) | 1 (2.0) | −2.0 |
| . | Cefiderocol (N = 101) . | BAT (N = 49) . | . |
|---|---|---|---|
| Cause of Death . | n (%) . | n (%) . | Difference (%) . |
| Overall mortality | 34 (33.7) | 10 (20.4) | 13.3 |
| Directly related to original gram-negative infection | |||
| Failure of study drug treatment | 16 (15.8) | 4 (8.2) | 7.7 |
| No failure of study drug treatment | 1 (1.0) | 1 (2.0) | −1.0 |
| No unanimous vote | 1 (1.0) | 0 | 1.0 |
| Unrelated to original gram-negative infection | |||
| Underlying comorbidity | 10 (9.9) | 2 (4.1) | 5.8 |
| Other infection | 4 (4.0) | 2 (4.1) | −0.1 |
| No unanimous vote | 2 (2.0) | 0 | 2.0 |
| Not assessed by committeea | 0 (0) | 1 (2.0) | −2.0 |
Abbreviation: BAT, best available therapy.
Source: Adapted from Table 1 of “Cefiderocol Adjudication Committee Review and Shionogi’s Assessment – Final.”
aCommittee assessed all deaths until end of study (EOS); 1 additional death occurred after EOS, but prior to day 49 in the BAT group which was not assessed.
Certain trends were apparent in the characteristics of patients who died due to treatment failure, as summarized in Table 4. Factors included baseline pathogen, severity of illness, and underlying diagnosis. Deaths due to treatment failure in the cefiderocol group generally occurred early (<day 15) in the course of treatment and were more frequent in HABP/VABP and in patients infected with Acinetobacter spp. Of the 16 deaths due treatment failure, 13 involved Acinetobacter spp. (9 with only A. baumannii or A. nosocomialis and 4 with mixed pathogens including Acinetobacter spp.).
Characteristics of Patients Who Died due to Treatment Failure, CREDIBLE-CR Trial
| Parameter . | Cefiderocol (N = 101) n (%) . | BAT (N = 49) n (%) . |
|---|---|---|
| Failure of study drug treatment | 16 (15.8) | 4 (8.2) |
| Timing of death | ||
| <Day 15 | 11 (10.9) | 1 (2.1) |
| Day 15–30 | 3 (3.0) | 3 (6.1) |
| ≥Day 30 | 2 (2.0) | 0 |
| Baseline pathogen | ||
| A. baumannii or A. nosocomialis | 9 (8.9) | 1 (2.1) |
| Mixed (≥2 pathogens) | 4 (4.0)a | 0 |
| P. aeruginosa | 0 | 1 (2.1) |
| S. maltophilia | 1 (1.0) | 0 |
| Enterobacteriaceae (K. pneumoniae or E. cloacae) | 2 (2.0) | 2 (4.1) |
| APACHE II score group | ||
| ≥16 | 11 (10.9) | 3 (6.1) |
| ≤15 | 5 (5.0) | 1 (2.1) |
| Mean | 19 | 19 |
| Baseline clinical diagnosis group | ||
| HABP/VABP | 13 (12.9) | 2 (4.1) |
| BSI | 2 (2.0) | 2 (4.1) |
| cUTI | 1 (1.0) | 0 |
| Parameter . | Cefiderocol (N = 101) n (%) . | BAT (N = 49) n (%) . |
|---|---|---|
| Failure of study drug treatment | 16 (15.8) | 4 (8.2) |
| Timing of death | ||
| <Day 15 | 11 (10.9) | 1 (2.1) |
| Day 15–30 | 3 (3.0) | 3 (6.1) |
| ≥Day 30 | 2 (2.0) | 0 |
| Baseline pathogen | ||
| A. baumannii or A. nosocomialis | 9 (8.9) | 1 (2.1) |
| Mixed (≥2 pathogens) | 4 (4.0)a | 0 |
| P. aeruginosa | 0 | 1 (2.1) |
| S. maltophilia | 1 (1.0) | 0 |
| Enterobacteriaceae (K. pneumoniae or E. cloacae) | 2 (2.0) | 2 (4.1) |
| APACHE II score group | ||
| ≥16 | 11 (10.9) | 3 (6.1) |
| ≤15 | 5 (5.0) | 1 (2.1) |
| Mean | 19 | 19 |
| Baseline clinical diagnosis group | ||
| HABP/VABP | 13 (12.9) | 2 (4.1) |
| BSI | 2 (2.0) | 2 (4.1) |
| cUTI | 1 (1.0) | 0 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; BAT, best available therapy; BSI, bloodstream infection; cUTI, complicated urinary tract infection; HABP, hospital-acquired bacterial pneumonia; HCABP, healthcare-associated bacterial pneumonia; VABP, ventilator-associated bacterial pneumonia.
aMixed: (1) A. baumannii and S. maltophilia; (2) A. baumannii, K. pneumoniae, P. aeruginosa; (3) A. baumannii, E. coli, K. pneumoniae; (4) A. baumannii, P. aeruginosa.
Characteristics of Patients Who Died due to Treatment Failure, CREDIBLE-CR Trial
| Parameter . | Cefiderocol (N = 101) n (%) . | BAT (N = 49) n (%) . |
|---|---|---|
| Failure of study drug treatment | 16 (15.8) | 4 (8.2) |
| Timing of death | ||
| <Day 15 | 11 (10.9) | 1 (2.1) |
| Day 15–30 | 3 (3.0) | 3 (6.1) |
| ≥Day 30 | 2 (2.0) | 0 |
| Baseline pathogen | ||
| A. baumannii or A. nosocomialis | 9 (8.9) | 1 (2.1) |
| Mixed (≥2 pathogens) | 4 (4.0)a | 0 |
| P. aeruginosa | 0 | 1 (2.1) |
| S. maltophilia | 1 (1.0) | 0 |
| Enterobacteriaceae (K. pneumoniae or E. cloacae) | 2 (2.0) | 2 (4.1) |
| APACHE II score group | ||
| ≥16 | 11 (10.9) | 3 (6.1) |
| ≤15 | 5 (5.0) | 1 (2.1) |
| Mean | 19 | 19 |
| Baseline clinical diagnosis group | ||
| HABP/VABP | 13 (12.9) | 2 (4.1) |
| BSI | 2 (2.0) | 2 (4.1) |
| cUTI | 1 (1.0) | 0 |
| Parameter . | Cefiderocol (N = 101) n (%) . | BAT (N = 49) n (%) . |
|---|---|---|
| Failure of study drug treatment | 16 (15.8) | 4 (8.2) |
| Timing of death | ||
| <Day 15 | 11 (10.9) | 1 (2.1) |
| Day 15–30 | 3 (3.0) | 3 (6.1) |
| ≥Day 30 | 2 (2.0) | 0 |
| Baseline pathogen | ||
| A. baumannii or A. nosocomialis | 9 (8.9) | 1 (2.1) |
| Mixed (≥2 pathogens) | 4 (4.0)a | 0 |
| P. aeruginosa | 0 | 1 (2.1) |
| S. maltophilia | 1 (1.0) | 0 |
| Enterobacteriaceae (K. pneumoniae or E. cloacae) | 2 (2.0) | 2 (4.1) |
| APACHE II score group | ||
| ≥16 | 11 (10.9) | 3 (6.1) |
| ≤15 | 5 (5.0) | 1 (2.1) |
| Mean | 19 | 19 |
| Baseline clinical diagnosis group | ||
| HABP/VABP | 13 (12.9) | 2 (4.1) |
| BSI | 2 (2.0) | 2 (4.1) |
| cUTI | 1 (1.0) | 0 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; BAT, best available therapy; BSI, bloodstream infection; cUTI, complicated urinary tract infection; HABP, hospital-acquired bacterial pneumonia; HCABP, healthcare-associated bacterial pneumonia; VABP, ventilator-associated bacterial pneumonia.
aMixed: (1) A. baumannii and S. maltophilia; (2) A. baumannii, K. pneumoniae, P. aeruginosa; (3) A. baumannii, E. coli, K. pneumoniae; (4) A. baumannii, P. aeruginosa.
The steady-state cefiderocol Cmax and AUC0-8h in patients who survived (N = 57) were comparable with those in patients who died (N = 22), indicating that there was no apparent association between cefiderocol exposure and death.
Development of Resistance to Study Drug
In 15 cefiderocol-treated patients, there was a 4-fold increase in cefiderocol MIC for the baseline pathogen(s) as shown in Table 5. Ten of these patients had outcomes of either clinical failure or death by TOC. Eight deaths involved baseline infection with carbapenem-resistant lactose nonfermenting species (A. baumannii, S. maltophilia, and P. aeruginosa). Despite the 4-fold increase in cefiderocol MIC, the Enterobacteriaceae (E. coli and K. pneumoniae) isolates remained susceptible to cefiderocol post-baseline with the current FDA susceptibility breakpoint of ≤2 mcg/mL. Three P. aeruginosa isolates became nonsusceptible to cefiderocol post-baseline with the current FDA susceptibility breakpoint of ≤1 mcg/mL. FDA susceptibility breakpoints have not been established for A. baumannii or S. maltophilia. Molecular characterization of the isolates was not available.
| Subject ID/Diagnosis . | Pathogen . | MIC (mcg/mL) at Baseline . | MIC (mcg/mL)/Study Day . | Outcome by TOC . | Day of Death . | Fatal PT . |
|---|---|---|---|---|---|---|
| 1/VABP | A. baumannii | 0.25 | 1.0 (day 3) | Death | 9 | Nonresolved sepsis |
| 2/VABP | A. baumanniia | 1.0 | 8 (day 10) | Death | 13 | Nonresolved sepsis |
| 3/VABP | S. maltophiliaa | 0.06 | 0.25 (day 8) | Death | 8 | Septic shock, hepatic failure |
| 4/HABP | A. baumanniia | 1.0 | 4.0 (day 11) | Death | 13 | Aggravated pneumonia |
| P. aeruginosa | 0.25 | 2.0 (day 11)c | ||||
| 5/VABP | S. maltophilia | 0.06 | 0.25 (day 14) | Death | 15 | Septic shock, cardiac arrest |
| 6/Sepsis | A. baumannii | 2 | >64 (day 16) | Cure | 27 | Secretion obstruction, hemoptysis |
| 7/VABP | A. baumannii | 0.25 | 4.0 (day 14) | Failureb | 39 | Septic shock |
| 8/VABP | A. baumanniia | 1.0 | 8.0 (day 15) | Failureb | 45 | Septic shock |
| 9/HABP | K. pneumoniaea | 0.25 | 2 (day 23) | Failureb | 31 | Septic shock |
| 10/VABP | P. aeruginosaa | 0.5 | 2.0 (day 16)c | Failureb | Survived | NA |
| 11/BSI | E. colia | 0.5 | 2 (day 11) | Failureb | Survived | NA |
| 12/cUTI | K. pneumoniae | 0.12 | 0.5 (day 17) | Cure | Survived | NA |
| 13/cUTI | P. aeruginosa | 0.12 | 2.0 (day 22)c | Cure | Survived | NA |
| 14/VABP | A. baumannii | 0.06 | 1.0 (day 3) | Cure | Survived | NA |
| 15/VABP | K. pneumoniaea | 0.06 | 0.5 (day 8) | Cure | Survived | NA |
| Subject ID/Diagnosis . | Pathogen . | MIC (mcg/mL) at Baseline . | MIC (mcg/mL)/Study Day . | Outcome by TOC . | Day of Death . | Fatal PT . |
|---|---|---|---|---|---|---|
| 1/VABP | A. baumannii | 0.25 | 1.0 (day 3) | Death | 9 | Nonresolved sepsis |
| 2/VABP | A. baumanniia | 1.0 | 8 (day 10) | Death | 13 | Nonresolved sepsis |
| 3/VABP | S. maltophiliaa | 0.06 | 0.25 (day 8) | Death | 8 | Septic shock, hepatic failure |
| 4/HABP | A. baumanniia | 1.0 | 4.0 (day 11) | Death | 13 | Aggravated pneumonia |
| P. aeruginosa | 0.25 | 2.0 (day 11)c | ||||
| 5/VABP | S. maltophilia | 0.06 | 0.25 (day 14) | Death | 15 | Septic shock, cardiac arrest |
| 6/Sepsis | A. baumannii | 2 | >64 (day 16) | Cure | 27 | Secretion obstruction, hemoptysis |
| 7/VABP | A. baumannii | 0.25 | 4.0 (day 14) | Failureb | 39 | Septic shock |
| 8/VABP | A. baumanniia | 1.0 | 8.0 (day 15) | Failureb | 45 | Septic shock |
| 9/HABP | K. pneumoniaea | 0.25 | 2 (day 23) | Failureb | 31 | Septic shock |
| 10/VABP | P. aeruginosaa | 0.5 | 2.0 (day 16)c | Failureb | Survived | NA |
| 11/BSI | E. colia | 0.5 | 2 (day 11) | Failureb | Survived | NA |
| 12/cUTI | K. pneumoniae | 0.12 | 0.5 (day 17) | Cure | Survived | NA |
| 13/cUTI | P. aeruginosa | 0.12 | 2.0 (day 22)c | Cure | Survived | NA |
| 14/VABP | A. baumannii | 0.06 | 1.0 (day 3) | Cure | Survived | NA |
| 15/VABP | K. pneumoniaea | 0.06 | 0.5 (day 8) | Cure | Survived | NA |
Subject ID has been de-identified for confidentiality.
Abbreviations: BSI, bloodstream infection; cUTI, complicated urinary tract infection; HABP, hospital-acquired bacterial pneumonia; MIC, minimum inhibitory concentration; NA, not applicable; PT, preferred term; TOC, test of cure; VABP, ventilator-associated bacterial pneumonia
a More than 1 pathogen at baseline (other pathogens without 4-fold MIC increase).
b Treatment with rescue intravenous (IV) antibacterial drugs for initial gram-negative infection.
c Nonsusceptible to cefiderocol (P. aeruginosa MIC >1 mcg/mL).
| Subject ID/Diagnosis . | Pathogen . | MIC (mcg/mL) at Baseline . | MIC (mcg/mL)/Study Day . | Outcome by TOC . | Day of Death . | Fatal PT . |
|---|---|---|---|---|---|---|
| 1/VABP | A. baumannii | 0.25 | 1.0 (day 3) | Death | 9 | Nonresolved sepsis |
| 2/VABP | A. baumanniia | 1.0 | 8 (day 10) | Death | 13 | Nonresolved sepsis |
| 3/VABP | S. maltophiliaa | 0.06 | 0.25 (day 8) | Death | 8 | Septic shock, hepatic failure |
| 4/HABP | A. baumanniia | 1.0 | 4.0 (day 11) | Death | 13 | Aggravated pneumonia |
| P. aeruginosa | 0.25 | 2.0 (day 11)c | ||||
| 5/VABP | S. maltophilia | 0.06 | 0.25 (day 14) | Death | 15 | Septic shock, cardiac arrest |
| 6/Sepsis | A. baumannii | 2 | >64 (day 16) | Cure | 27 | Secretion obstruction, hemoptysis |
| 7/VABP | A. baumannii | 0.25 | 4.0 (day 14) | Failureb | 39 | Septic shock |
| 8/VABP | A. baumanniia | 1.0 | 8.0 (day 15) | Failureb | 45 | Septic shock |
| 9/HABP | K. pneumoniaea | 0.25 | 2 (day 23) | Failureb | 31 | Septic shock |
| 10/VABP | P. aeruginosaa | 0.5 | 2.0 (day 16)c | Failureb | Survived | NA |
| 11/BSI | E. colia | 0.5 | 2 (day 11) | Failureb | Survived | NA |
| 12/cUTI | K. pneumoniae | 0.12 | 0.5 (day 17) | Cure | Survived | NA |
| 13/cUTI | P. aeruginosa | 0.12 | 2.0 (day 22)c | Cure | Survived | NA |
| 14/VABP | A. baumannii | 0.06 | 1.0 (day 3) | Cure | Survived | NA |
| 15/VABP | K. pneumoniaea | 0.06 | 0.5 (day 8) | Cure | Survived | NA |
| Subject ID/Diagnosis . | Pathogen . | MIC (mcg/mL) at Baseline . | MIC (mcg/mL)/Study Day . | Outcome by TOC . | Day of Death . | Fatal PT . |
|---|---|---|---|---|---|---|
| 1/VABP | A. baumannii | 0.25 | 1.0 (day 3) | Death | 9 | Nonresolved sepsis |
| 2/VABP | A. baumanniia | 1.0 | 8 (day 10) | Death | 13 | Nonresolved sepsis |
| 3/VABP | S. maltophiliaa | 0.06 | 0.25 (day 8) | Death | 8 | Septic shock, hepatic failure |
| 4/HABP | A. baumanniia | 1.0 | 4.0 (day 11) | Death | 13 | Aggravated pneumonia |
| P. aeruginosa | 0.25 | 2.0 (day 11)c | ||||
| 5/VABP | S. maltophilia | 0.06 | 0.25 (day 14) | Death | 15 | Septic shock, cardiac arrest |
| 6/Sepsis | A. baumannii | 2 | >64 (day 16) | Cure | 27 | Secretion obstruction, hemoptysis |
| 7/VABP | A. baumannii | 0.25 | 4.0 (day 14) | Failureb | 39 | Septic shock |
| 8/VABP | A. baumanniia | 1.0 | 8.0 (day 15) | Failureb | 45 | Septic shock |
| 9/HABP | K. pneumoniaea | 0.25 | 2 (day 23) | Failureb | 31 | Septic shock |
| 10/VABP | P. aeruginosaa | 0.5 | 2.0 (day 16)c | Failureb | Survived | NA |
| 11/BSI | E. colia | 0.5 | 2 (day 11) | Failureb | Survived | NA |
| 12/cUTI | K. pneumoniae | 0.12 | 0.5 (day 17) | Cure | Survived | NA |
| 13/cUTI | P. aeruginosa | 0.12 | 2.0 (day 22)c | Cure | Survived | NA |
| 14/VABP | A. baumannii | 0.06 | 1.0 (day 3) | Cure | Survived | NA |
| 15/VABP | K. pneumoniaea | 0.06 | 0.5 (day 8) | Cure | Survived | NA |
Subject ID has been de-identified for confidentiality.
Abbreviations: BSI, bloodstream infection; cUTI, complicated urinary tract infection; HABP, hospital-acquired bacterial pneumonia; MIC, minimum inhibitory concentration; NA, not applicable; PT, preferred term; TOC, test of cure; VABP, ventilator-associated bacterial pneumonia
a More than 1 pathogen at baseline (other pathogens without 4-fold MIC increase).
b Treatment with rescue intravenous (IV) antibacterial drugs for initial gram-negative infection.
c Nonsusceptible to cefiderocol (P. aeruginosa MIC >1 mcg/mL).
Safety Results
In the CREDIBLE-CR trial, a slightly higher proportion of patients who received cefiderocol compared to BAT experienced serious adverse events (SAEs) (49.5% vs 46.9%), and treatment discontinuations due to AEs (9.9% vs 6.1%, respectively). The most common AEs in the cefiderocol-treated patients were diarrhea, elevated liver tests, pneumonia, candidiasis, anemia, fluid overload, pleural effusion, dyspnea, and chest pain.
TRIAL IN NOSOCOMIAL PNEUMONIA (APEKS-NP; NCT02714595)
APEKS-NP was a randomized, prospective, double-blind, active-controlled NI trial comparing cefiderocol to meropenem for the treatment of HABP/VABP/HCABP. The trial completed enrollment during the NDA review, and only top-line mortality results were submitted to the FDA. In the ITT population, the rates reported for the primary endpoint of day 14 mortality were 12.8% in the cefiderocol group and 11.4% in the meropenem group (treatment difference 1.4% and 95% CI: −6.0% to 8.7%). Thus, the estimated mortality rates were similar in the 2 groups, but the confidence interval could not rule out increased mortality for cefiderocol. The data had not been reviewed or verified by the FDA.
DISCUSSION
The FDA approval of cefiderocol for the treatment of cUTI, including pyelonephritis, was based on an adequate and well-controlled trial demonstrating safety and effectiveness of cefiderocol compared to IMP. A single trial was considered adequate to support the cUTI indication for cefiderocol in adults who have limited or no alternative treatment options [6, 7]. Supportive information was provided by results from in vitro microbiology studies and animal models of infection demonstrating the activity of cefiderocol against a range of gram-negative bacteria and the probability of pharmacokinetics/pharmacodynamics target attainment analyses [8]. One limitation of the cUTI trial was that carbapenem-resistant pathogens were not evaluated (because the comparator was a carbapenem) [9].
Notwithstanding the mortality imbalance observed in the CREDIBLE-CR trial, the overall benefit-risk profile of cefiderocol was considered favorable for a limited use indication given the limitations in interpreting the findings of the CREDIBLE-CR trial. Additionally, communicating the potential mortality risk in labeling was considered an adequate risk mitigation strategy for a product to be used in patients with limited or no treatment options. The preliminary results of the APEKS-NP trial, where an increased mortality risk was not observed also provided some reassurance in the overall benefit-risk assessment.
Although the CREDIBLE-CR trial objective was to evaluate cefiderocol for the treatment of carbapenem-resistant pathogens of clinical concern, it was not an adequate and well-controlled trial. There were several critical limitations: a relatively small sample size of 150 patients, a descriptive analysis without formal statistical testing, the inclusion of cUTI with more serious infection types, an open-label assessment of a subjective primary endpoint (clinical outcome), and continuous monitoring of unblinded results by treatment group. In addition, there were imbalances in the baseline characteristics in this relatively small trial, despite randomization. As the CREDIBLE-CR trial was not considered adequate and well controlled, its results are not described in the Clinical Studies section of the cefiderocol prescribing information.
Although the cause of the increased mortality was uncertain due to the limitations of the data, treatment failure progressing to sepsis and death occurred more frequently in the cefiderocol treatment group. Treatment failures appeared to share certain characteristics, such as infection with Acinetobacter spp., pulmonary infection at baseline, and increases in cefiderocol MIC while on therapy. Mortality generally occurred in patients with poor prognostic factors.
The FDA convened an AMDAC meeting to discuss the overall risk benefit assessment of cefiderocol with respect to the mortality imbalance observed in the CREDIBLE-CR trial. Although the majority of the committee (14 yes, 2 no) voted that there was substantial evidence of the efficacy and sufficient evidence of the safety of cefiderocol for the treatment of cUTI including pyelonephritis in patients with limited or no alternative treatment options, the committee voiced concern regarding the increased mortality observed in the cefiderocol treatment group of the CREDIBLE-CR trial. It was recommended that the prescribing information contain a warning for use in indications other than cUTI.
BENEFIT-RISK CONSIDERATIONS FOR THE USE OF CEFIDEROCOL IN CLINICAL PRACTICE
Although cefiderocol provides a treatment option for some patients, it is important to consider the limited clinical data available and the associated uncertainties. The FDA product labeling states that cefiderocol should be reserved for use in patients who have limited or no alternative treatment options for the treatment of cUTI and also includes a warning regarding the increase in all-cause mortality observed in patients with carbapenem-resistant gram-negative bacterial infections treated with cefiderocol compared to those treated with BAT [10]. The results from the CREDIBLE-CR trial raise concern regarding the potential for reduced efficacy with cefiderocol in patients with HABP/VABP and BSI/sepsis, particularly due to certain carbapenem-resistant bacteria such as P. aeruginosa, A. baumannii, and S. maltophilia. The safety and efficacy of cefiderocol has not been established for the treatment of nosocomial pneumonia, BSI, or sepsis.
Notes
Disclaimer. This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




