-
PDF
- Split View
-
Views
-
Cite
Cite
Pranita D Tamma, Shanan Immel, Sara M Karaba, Caitlin L Soto, Rick Conzemius, Emily Gisriel, Tsigereda Tekle, Haley Stambaugh, Emily Johnson, Jeffrey A Tornheim, Patricia J Simner, Successful Treatment of Carbapenem-Resistant Acinetobacter baumannii Meningitis With Sulbactam-Durlobactam, Clinical Infectious Diseases, Volume 79, Issue 4, 15 October 2024, Pages 819–825, https://doi.org/10.1093/cid/ciae210
Close - Share Icon Share
Abstract
The treatment of carbapenem-resistant Acinetobacter baumannii/calcoaceticus complex (CRAB) presents significant treatment challenges.
We report the case of a 42-year-old woman with CRAB meningitis who experienced persistently positive cerebrospinal fluid (CSF) cultures for 13 days despite treatment with high-dose ampicillin-sulbactam and cefiderocol. On day 13, she was transitioned to sulbactam-durlobactam and meropenem; 4 subsequent CSF cultures remained negative. After 14 days of sulbactam-durlobactam, she was cured of infection. Whole genome sequencing investigations identified putative mechanisms that contributed to the reduced cefiderocol susceptibility observed during cefiderocol therapy. Blood and CSF samples were collected pre-dose and 3-hours post initiation of a sulbactam-durlobactam infusion.
The CRAB isolate belonged to sequence type 2. An acquired blaOXA-23 and an intrinsic blaOXA-51-like (ie, blaOXA-66) carbapenemase gene were identified. The paradoxical effect (ie, no growth at lower cefiderocol dilutions but growth at higher dilutions) was observed by broth microdilution after 8 days of cefiderocol exposure but not by disk diffusion. Potential markers of resistance to cefiderocol included mutations in the start codon of piuA and piuC iron transport genes and an A515V substitution in PBP3, the primary target of cefiderocol. Sulbactam and durlobactam were detected in CSF at both timepoints, indicating CSF penetration.
This case describes successful treatment of refractory CRAB meningitis with the administration of sulbactam-durlobactam and meropenem and highlights the need to be cognizant of the paradoxical effect that can be observed with broth microdilution testing of CRAB isolates with cefiderocol.
Carbapenem-resistant Acinetobacter baumannii/calcoaceticus complex (CRAB) remains an international public health crisis [1]. CRAB are notorious for their ability to survive in intensive care units, infect vulnerable populations, and accumulate antimicrobial resistance markers [2]. Moreover, unlike other problematic antimicrobial resistant pathogens, until recently, no antibiotic treatment options have been consistently associated with improved patient outcomes for CRAB infections [2, 3]. To this point, across clinical trials, mortality associated with CRAB infections is upwards of 40% [2].
Sulbactam-durlobactam was approved by the United States Food and Drug Administration in May 2023. This novel β-lactam-β-lactamase inhibitor combination capitalizes on sulbactam's ability to inhibit essential penicillin binding proteins (PBP1 and PBP3) in Acinetobacter species [4, 5]. Although sulbactam is subject to degradation by a variety of β-lactamases in the periplasmic space of CRAB isolates—most notably Class D carbapenemases—combining sulbactam with a broad-spectrum serine β-lactamase inhibitor (ie, durlobactam) enables sulbactam to successfully reach its PBP targets [6].
Herein we report the case of a patient with refractory CRAB meningitis. Whole genome sequencing (WGS) was used to identify putative resistance mechanisms. Plasma and cerebrospinal fluid (CSF) concentrations of sulbactam and durlobactam were assessed.
METHODS
Clinical Case Presentation
A 42-year-old woman with congenital hydrocephalus and ventriculoperitoneal (VP) shunt dependency since infancy underwent an elective admission for an ileostomy reversal. One year prior she had undergone an emergency exploratory laparotomy with a left hemicolectomy after bowel perforation. She had a complex clinical course and received approximately 8 months of antibiotic therapy. When she returned to the hospital for the ileostomy reversal (Day −6) she underwent additional intra-abdominal procedures to repair bowel fistulae, remove adhesions, and drain abscesses. Due to its distal proximity with potentially infected fluid, she returned to the operating room for a planned removal of her VP shunt (Day −2) and replacement with an external ventricular drain. Prompted by a fever to 39.1° Celsius, headache, and lethargy (Day 1), CSF was obtained and indicated 41 white blood cell (WBC)/cu mm (71% neutrophils), glucose of 12 mg/mL, and protein of 130 mg/dL. Gram-negative bacilli were identified on Gram stain, later identified on CSF culture as Acinetobacter baumannii/calcoaceticus complex. The isolate was carbapenem resistant (ie, CRAB) and not susceptible to any routinely tested antibiotics (Table 1). However, the CRAB isolate was susceptible to cefiderocol by disk diffusion. The external ventricular drain was removed on Day 1. From Day 2 to Day 13 the patient received high-dose ampicillin-sulbactam 3 grams IV every 4 hours (6 grams total daily dose of sulbactam), over 30 minutes and cefiderocol 2 grams intravenously (IV) every 6 hours, over 3 hours. Almost daily Gram stains and cultures were obtained during this period through a new external ventricular drain. All cultures grew CRAB and all isolates remained susceptible to cefiderocol by standard of care disk diffusion testing (Table 1). On Day 13, ampicillin-sulbactam and cefiderocol were discontinued and the patient was transitioned to sulbactam-durlobactam 1 gram/1 gram every 6 hours and meropenem 2 grams every 8 hours, both over 3 hours. The patient had a creatinine clearance (CrCl) of 213.7 mL/minute on the day blood and CSF samples were collected to evaluate CSF concentrations of sulbactam and durlobactam (ie, Day 19). Despite her augmented renal clearance, dosing frequency was not increased given vascular access constraints. Since initiating the sulbactam-durlobactam and meropenem regimen she experienced clinical improvement. At the end of the 14-day treatment course of sulbactam-durlobactam and meropenem (ie, Days 13–26), she was cured of her CRAB infection; 4 subsequent CSF Gram stain and culture results remained negative. No antibiotic-associated adverse events were identified. A new VP shunt was placed on Day 26 without complications. She remains free of CRAB infections since the completion of sulbactam-durlobactam and meropenem therapy (ie, more than 6 months after completing antibiotic therapy).
Antimicrobial Susceptibility Testing Results Using Broth Microdilution for 10 Acinetobacter baumannii Isolates Recovered From the Cerebrospinal Fluid of a Patient Over a 13-day Period Before and During Treatment With Cefiderocol
| Isolate Number . | Day of Culture . | Days of FDC Exposure . | AMI MICa . | AMP-SUL MIC . | CAZ MIC . | CIP MIC . | CST MIC . | FDC DD (mm)b . | FDC MIC . | FEP MIC . | GEN MIC . | MER MIC . | MIN MIC . | SUL-DUR DD (mm)c . | TOB MIC . | TMP-SMX MIC . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Day 1 | 0 | >32 | >16/8 | >16 | >2 | 1 | 22 | ≤0.25 | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
| 2 | Day 3 | 2 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 22 | >8 | >2/38 |
| 3 | Day 5 | 4 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 24 | >8 | >2/38 |
| 4 | Day 5 | 4 | >32 | >16/8 | >16 | >2 | 1 | 19 | ≤0.25 | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
| 5 | Day 7 | 6 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 22 | >8 | >2/38 |
| 6 | Day 9 | 8 | >32 | >16/8 | >16 | >2 | 1 | 22 | PEd | >16 | >8 | 64 | 8 | 21 | >8 | >2/38 |
| 7 | Day 11 | 10 | >32 | >16/8 | >16 | >2 | 1 | 21 | PEd | >16 | >8 | 64 | 8 | 24 | >8 | >2/38 |
| 8 | Day 11 | 10 | >32 | >16/8 | >16 | >2 | 1 | 23 | PEd | >16 | >8 | 64 | 8 | 21 | >8 | >2/38 |
| 9 | Day 12 | 11 | >32 | >16/8 | >16 | >2 | 1 | 22 | PEd | >16 | >8 | 64 | 8 | 23 | >8 | >2/38 |
| 10 | Day 13 | 12 | >32 | >16/8 | >16 | >2 | 2 | 23 | PEd | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
| Isolate Number . | Day of Culture . | Days of FDC Exposure . | AMI MICa . | AMP-SUL MIC . | CAZ MIC . | CIP MIC . | CST MIC . | FDC DD (mm)b . | FDC MIC . | FEP MIC . | GEN MIC . | MER MIC . | MIN MIC . | SUL-DUR DD (mm)c . | TOB MIC . | TMP-SMX MIC . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Day 1 | 0 | >32 | >16/8 | >16 | >2 | 1 | 22 | ≤0.25 | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
| 2 | Day 3 | 2 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 22 | >8 | >2/38 |
| 3 | Day 5 | 4 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 24 | >8 | >2/38 |
| 4 | Day 5 | 4 | >32 | >16/8 | >16 | >2 | 1 | 19 | ≤0.25 | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
| 5 | Day 7 | 6 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 22 | >8 | >2/38 |
| 6 | Day 9 | 8 | >32 | >16/8 | >16 | >2 | 1 | 22 | PEd | >16 | >8 | 64 | 8 | 21 | >8 | >2/38 |
| 7 | Day 11 | 10 | >32 | >16/8 | >16 | >2 | 1 | 21 | PEd | >16 | >8 | 64 | 8 | 24 | >8 | >2/38 |
| 8 | Day 11 | 10 | >32 | >16/8 | >16 | >2 | 1 | 23 | PEd | >16 | >8 | 64 | 8 | 21 | >8 | >2/38 |
| 9 | Day 12 | 11 | >32 | >16/8 | >16 | >2 | 1 | 22 | PEd | >16 | >8 | 64 | 8 | 23 | >8 | >2/38 |
| 10 | Day 13 | 12 | >32 | >16/8 | >16 | >2 | 2 | 23 | PEd | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
Abbreviations: AMI, amikacin; AMP-SUL, ampicillin-sulbactam; CAZ, ceftazidime; CIP, ciprofloxacin; CST, colistin; FDC, cefiderocol; FEP, cefepime; GEN, gentamicin; MER, meropenem; MIN, minocycline; PD, paradoxical effect; SUL-DUR, sulbactam-durlobactam; TOB, tobramycin; TMP-SMX, trimethoprim-sulfamethoxazole.
aMIC, Minimum inhibitory concentration.
bBased on disk zone diameters with susceptibility defined as ≥15 mm according to the Clinical and Laboratory Standards Institute.
cBased on disk zone diameters with susceptible defined as ≥17 mm according to the Clinical and Laboratory Standards Institute.
dThe paradoxical effect was observed by reference broth microdilution. For isolates 6–10, button growth was observed at lower dilutions (0.25–0.5 µg/mL) with no growth observed at dilutions of 1–2 µg/mL. Regrowth was observed starting at 4 µg/mL with increasing growth as the MIC increased up to 128 µg/mL.
Antimicrobial Susceptibility Testing Results Using Broth Microdilution for 10 Acinetobacter baumannii Isolates Recovered From the Cerebrospinal Fluid of a Patient Over a 13-day Period Before and During Treatment With Cefiderocol
| Isolate Number . | Day of Culture . | Days of FDC Exposure . | AMI MICa . | AMP-SUL MIC . | CAZ MIC . | CIP MIC . | CST MIC . | FDC DD (mm)b . | FDC MIC . | FEP MIC . | GEN MIC . | MER MIC . | MIN MIC . | SUL-DUR DD (mm)c . | TOB MIC . | TMP-SMX MIC . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Day 1 | 0 | >32 | >16/8 | >16 | >2 | 1 | 22 | ≤0.25 | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
| 2 | Day 3 | 2 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 22 | >8 | >2/38 |
| 3 | Day 5 | 4 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 24 | >8 | >2/38 |
| 4 | Day 5 | 4 | >32 | >16/8 | >16 | >2 | 1 | 19 | ≤0.25 | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
| 5 | Day 7 | 6 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 22 | >8 | >2/38 |
| 6 | Day 9 | 8 | >32 | >16/8 | >16 | >2 | 1 | 22 | PEd | >16 | >8 | 64 | 8 | 21 | >8 | >2/38 |
| 7 | Day 11 | 10 | >32 | >16/8 | >16 | >2 | 1 | 21 | PEd | >16 | >8 | 64 | 8 | 24 | >8 | >2/38 |
| 8 | Day 11 | 10 | >32 | >16/8 | >16 | >2 | 1 | 23 | PEd | >16 | >8 | 64 | 8 | 21 | >8 | >2/38 |
| 9 | Day 12 | 11 | >32 | >16/8 | >16 | >2 | 1 | 22 | PEd | >16 | >8 | 64 | 8 | 23 | >8 | >2/38 |
| 10 | Day 13 | 12 | >32 | >16/8 | >16 | >2 | 2 | 23 | PEd | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
| Isolate Number . | Day of Culture . | Days of FDC Exposure . | AMI MICa . | AMP-SUL MIC . | CAZ MIC . | CIP MIC . | CST MIC . | FDC DD (mm)b . | FDC MIC . | FEP MIC . | GEN MIC . | MER MIC . | MIN MIC . | SUL-DUR DD (mm)c . | TOB MIC . | TMP-SMX MIC . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Day 1 | 0 | >32 | >16/8 | >16 | >2 | 1 | 22 | ≤0.25 | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
| 2 | Day 3 | 2 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 22 | >8 | >2/38 |
| 3 | Day 5 | 4 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 24 | >8 | >2/38 |
| 4 | Day 5 | 4 | >32 | >16/8 | >16 | >2 | 1 | 19 | ≤0.25 | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
| 5 | Day 7 | 6 | >32 | >16/8 | >16 | >2 | 1 | 21 | ≤0.25 | >16 | >8 | 64 | 8 | 22 | >8 | >2/38 |
| 6 | Day 9 | 8 | >32 | >16/8 | >16 | >2 | 1 | 22 | PEd | >16 | >8 | 64 | 8 | 21 | >8 | >2/38 |
| 7 | Day 11 | 10 | >32 | >16/8 | >16 | >2 | 1 | 21 | PEd | >16 | >8 | 64 | 8 | 24 | >8 | >2/38 |
| 8 | Day 11 | 10 | >32 | >16/8 | >16 | >2 | 1 | 23 | PEd | >16 | >8 | 64 | 8 | 21 | >8 | >2/38 |
| 9 | Day 12 | 11 | >32 | >16/8 | >16 | >2 | 1 | 22 | PEd | >16 | >8 | 64 | 8 | 23 | >8 | >2/38 |
| 10 | Day 13 | 12 | >32 | >16/8 | >16 | >2 | 2 | 23 | PEd | >16 | >8 | 64 | 8 | 20 | >8 | >2/38 |
Abbreviations: AMI, amikacin; AMP-SUL, ampicillin-sulbactam; CAZ, ceftazidime; CIP, ciprofloxacin; CST, colistin; FDC, cefiderocol; FEP, cefepime; GEN, gentamicin; MER, meropenem; MIN, minocycline; PD, paradoxical effect; SUL-DUR, sulbactam-durlobactam; TOB, tobramycin; TMP-SMX, trimethoprim-sulfamethoxazole.
aMIC, Minimum inhibitory concentration.
bBased on disk zone diameters with susceptibility defined as ≥15 mm according to the Clinical and Laboratory Standards Institute.
cBased on disk zone diameters with susceptible defined as ≥17 mm according to the Clinical and Laboratory Standards Institute.
dThe paradoxical effect was observed by reference broth microdilution. For isolates 6–10, button growth was observed at lower dilutions (0.25–0.5 µg/mL) with no growth observed at dilutions of 1–2 µg/mL. Regrowth was observed starting at 4 µg/mL with increasing growth as the MIC increased up to 128 µg/mL.
Antimicrobial Susceptibility Testing
The 10 CRAB isolates obtained over a 13 day period underwent identical microbial identification and antimicrobial susceptibility testing (AST). Matrix-assisted laser-desorption ionization time-of-flight mass spectrometry (Bruker Daltonics Inc., Billerica, Massachusetts) was used for bacterial genus and species identification. Initial AST results were determined using the gram-negative Emerge panel (NMIC-306) on the BD Phoenix Automated System (Becton Dickinson Diagnostics, Sparks, Maryland). Cefiderocol susceptibility was determined using disk diffusion (Hardy Diagnostics, Santa Maria, California), with results reported in the medical record. Repeat cefiderocol testing was performed by frozen reference broth microdilution (BMD) panels for research purposes and were not reported to clinicians [7]. Colistin susceptibility was determined by reference BMD [7]. Sulbactam-durlobactam disks (Hardy Diagnostics) were used to test susceptibility to sulbactam-durlobactam; a disk zone diameter of ≥17 mm was applied to determine sulbactam-durlobactam susceptibility [8]. For all AST studies, quality control organisms were prepared weekly or each day of testing, as appropriate. Clinical and Laboratory Standards Institute (CLSI) interpretive criteria were applied to all agents to interpret susceptibility [8].
Whole Genome Sequencing and Analysis
WGS of the index (Day 1) and final (Day 13) CRAB isolate was conducted using the Clear Dx™ (Clear Labs, San Carlo, California) fully automated sequencing platform with integrated Illumina iSeq short-read sequencing (Illumina, San Diego, California). Sequence alignment to reference protein sequences was performed to identify missense mutations resulting in changes to amino acid composition that may have contributed to elevated cefiderocol minimum inhibitory concentrations (MICs) in the final isolate compared to the index isolate. Insertions, deletions, and frameshift mutations in ftsI (gene encoding PBP3, the target of cefiderocol) were also investigated. Sequencing results were interrogated for known markers previously identified in Acinetobacter spp. that translate to relevant enzymatic changes in TonB-dependent receptors which enable the uptake of siderophore-iron complexes across the bacterial outer membrane (eg, piuA, piuC, pirA, exbD3, tonB) [9–18]. Bioinformatics analyses were performed by Ares Genetics. Sequencing reads and de novo whole genome assemblies were deposited to NCBI under BioProject PRJNA1061068.
Sulbactam-Durlobactam Pharmacokinetic Evaluation
On Day 19 (7 days into the initiation of sulbactam-durlobactam therapy), plasma and CSF samples were obtained prior to the start of infusion (pre-dose) and 3 hours after the start of infusion. A detailed description of the specimen collection and analytic approach used is described in the Supplementary Material including Supplementary Tables 1 and 2.
RESULTS
Antimicrobial Susceptibility Testing
AST results for the 10 isolates are displayed in Table 1. As previously stated, MICs for antibiotics commonly considered in treatment regimens for A. baumannii infections were all in the non-susceptible range. All 10 isolates were susceptible to sulbactam-durlobactam with zone diameters ranging between 20 and 24 mm. Notably, all cefiderocol disk zone diameters were reported as susceptible to clinicians by a verified disk diffusion method. However, testing by reference BMD to further investigate the potential failure of cefiderocol revealed that after 8 days of exposure to cefiderocol therapy, isolates demonstrated a paradoxical effect. More specifically, for isolates 6–10, button growth was observed at lower dilutions of 0.25–0.5 µg/mL, no growth was observed at dilutions of 1–2 µg/mL, but regrowth was observed starting at 2–4 µg/mL with increasing growth as the MIC increased to 128 µg/mL. This observation was repeated by triplicate BMD testing with all results indicating a paradoxical effect (Figure 1). Disk diffusion results were repeated with clear zones of inhibition observed for all isolates, as initially reported.
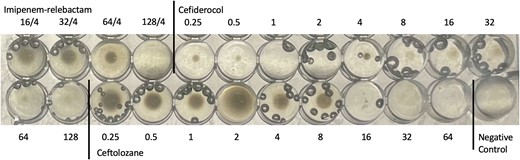
Acinetobacter baumannii complex isolate #9 demonstrating the paradoxical effect with cefiderocol by reference broth microdilution testing. Growth is observed at 0.25–0.5 µg/mL with no growth at 1–2 µg/mL and the reappearance of growth at 4 µg/mL, with increasing strength of growth as the doubling dilutions increase up to 128 µg/mL. Growth at 64 µg/mL and 128 µg/mL are diffuse throughout the wells. This observation was repeated by triplicate testing. Bubbles are observed in most wells and should be ignored.
WGS analysis illumina reads yielded high quality assemblies with an average sequencing depth of 25× coverage. The CRAB isolates belonged to sequence type 2 and contained three non-typeable plasmids. Antimicrobial resistance markers were identical in both isolates. Isolates harbored multiple aminoglycoside modifying genes (ant(3'’)-IIa, aph(3'’)-Ib, aph(6)-Id), and the 16S rRNA methyltransferase gene, armA, mediating aminoglycoside resistance. gyrA (S81L) and parC (S83L) mutations conferred fluoroquinolone resistance. sul2 was associated with sulfamethoxazole resistance. An acquired blaOXA-23 and chromosomally-encoded blaOXA-51-like (ie, blaOXA-66; absence of ISAba1 upstream) carbapenemase genes were identified—contributing to beta-lactam resistance. Furthermore, a PBP3 mutation was observed leading to an alanine to valine substitution at position 515 (ie, A515V). Mutations were identified in the start codons of piuC (M1V) and pirA (M1A, I262V) encoding TonB-dependent siderophore receptors.
Sulbactam and Durlobactam Concentrations
Pre-dose plasma sample revealed concentration of 3.56 µg/mL and 6.20 µg/mL for sulbactam and durlobactam, respectively. At the 3-hour timepoint, which coincided with the end of infusion, the concentration was 13.1 µg/mL and 18.4 µg/mL, for sulbactam and durlobactam, respectively. These plasma concentrations are lower than the expected maximum concentration (Cmax) following a 1 g/1 g sulbactam-durlobactam q6h dosing [19], reflecting the patient's augmented renal clearance status (CrCL of 213.7 mL/minute). Sulbactam and durlobactam were also detected in CSF. Sulbactam CSF concentrations were 1.32 µg/mL and 1.36 µg/mL prior to infusion and 3-hour post-start of infusion, respectively. Durlobactam CSF concentrations were 1.58 µg/mL and 1.62 µg/mL prior to infusion and 3-hour post-start of infusion, respectively.
DISCUSSION
We describe the case of a 42-year-old woman with CRAB meningitis with positive CSF cultures over 13 days despite source control measures (ie, removal of central nervous system hardware) and the administration of high-dose ampicillin-sulbactam in combination with cefiderocol. She experienced microbiological clearance and clinical cure only after the initiation of sulbactam-durlobactam and meropenem. This case highlights the role of sulbactam-durlobactam as a long-awaited addition to the antibiotic armamentarium for treating CRAB infections, particularly given the underwhelming clinical outcomes data with alternate regimens (eg, ampicillin-sulbactam, polymyxins, minocycline, cefiderocol) [2].
Sulbactam is a competitive, irreversible β-lactamase inhibitor that, in high doses, saturates PBP1a/1b and PBP3 of A. baumannii isolates [4, 5]. Sulbactam's unique activity against A. baumannii isolates has been observed through in vitro studies [20–22], animal models [23], and clinical outcomes data [3, 24–28]. Durlobactam is a diazabicyclooctane β-lactamase inhibitor with potent inhibition of class A (eg, TEM-1), class C (eg, Acinetobacter-derived cephalosporinases), and class D beta-lactamases (eg, OXA-23 carbapenemases). Durlobactam binds to and inhibits these serine β-lactamases, enabling sulbactam to successfully reach its PBP targets [6]. Although comprehensive investigations into mechanisms of resistance to sulbactam-durlobactam since its clinical availability have not yet been conducted, available data suggest CRAB resistance to sulbactam-durlobactam remains rare and is most commonly mediated by the presence of metallo-β-lactamase enzymes or PBP3 mutations [29]. Sulbactam-durlobactam was investigated in a clinical trial of patients with CRAB pneumonia or bloodstream infections [3]. Patients were randomized to sulbactam-durlobactam or colistin; all patients also received imipenem-cilastatin as adjunctive therapy to cover other potential pathogens in cases of polymicrobial infection. Mortality by day 28 occurred in 19% (12/63) of patients in the sulbactam-durlobactam group and 32% (20/62) in the colistin group, underscoring its niche as a promising agent for CRAB infections.
The additive benefit of imipenem-cilastatin to sulbactam-durlobactam remains unclear. In vitro data have had mixed results; some studies suggest the combination of sulbactam-durlobactam and imipenem-cilastatin lowers the sulbactam-durlobactam MIC by approximately 2-fold [30–32]. Pharmacodynamic models incorporating clinical exposures of sulbactam-durlobactam and a carbapenem suggest further reduction of bacterial burden relative to sulbactam-durlobactam alone [30]. The potential incremental benefit of a carbapenem is not entirely understood. It is possible the carbapenem serves as a “decoy” subjecting itself to hydrolysis from OXA enzymes that have escaped durlobactam inhibition, diverting attention from sulbactam. Alternatively, as imipenem—and meropenem—preferentially target PBP2, it is plausible that under the protection of durlobactam, multiple PBPs are being targeted [32–34]. Robust clinical data investigating the benefit of sulbactam-durlobactam for the treatment of CRAB infections in the absence of carbapenem therapy are not available.
Data are lacking to provide insight into central nervous system (CNS) penetration of sulbactam-durlobactam. Sulbactam CNS penetration estimates can be derived from ampicillin-sulbactam data, which indicate adequate CSF penetration for the treatment of meningitis [35–37]. As durlobactam is very structurally similar to avibactam [38], data on the potential role of durlobactam for CNS infections can likely be extrapolated from experiences with avibactam. Avibactam is hydrophilic, has <10% plasma protein binding, and is a substrate of OAT3 transporters [39]. Translating these features into clinical success is challenging; the hydrophilic nature of avibactam may impede penetration across the lipid-enriched CNS, whereas the low protein binding is favorable for CSF penetration [40, 41]. In the alternative, avibactam being a substrate for active transporters may enhance drug removal from the CNS. In a rabbit meningitis model, the mean CSF penetration of avibactam was 38% in the setting of inflamed meninges [42], which is comparable to what has been observed with meropenem during meningeal inflammation [43–45]. Furthermore, with the understanding there is some likely publication bias, there have been over 50 individual cases of the successful use of ceftazidime-avibactam for the treatment of CNS infections; however, most of these cases did not include CSF measurements, and many involved adjunctive use of intraventricular or intrathecal antibiotics [46–65]. From clinical reports where plasma and CSF concentrations were available, the CSF penetration of avibactam ranged from 10%–41% [46–48, 60, 62]. Collectively, these preclinical and clinical data suggest avibactam has adequate CSF penetration to successfully treat CNS infections.
For our patient, sulbactam and durlobactam were detected in CSF, albeit at low concentrations. By the time CSF sampling was performed, the CSF WBC and protein normalized; had CSF concentrations been investigated earlier in the patient's clinical course when greater meningeal inflammation was present, CSF concentrations of sulbactam and durlobactam would have been expected to be higher. Additionally, the plasma Cmax with a 1 gram/1 gram every 6 hours dosing regimen was lower than anticipated, likely as a result of the patient's augmented renal clearance. The package insert recommends increasing the sulbactam-durlobactam dosing frequency to every 4 hours for CrCl over 130 mL/minute [19]. Our patient had a CrCl of approximately 213 mL/minute, but continued to receive sulbactam-durlobactam every 6 hours despite her increased renal clearance due to competing needs for intravenous medication infusions. If the administration frequency was increased to every 4 hours, it is plausible that CSF concentrations would have been higher. At the 3-hour timepoint (end of infusion) CSF concentrations were approximately 10% (for sulbactam) and 7% (for durlobactam) of the plasma concentrations at the same timepoint. Penetration as estimated by a ratio of the CSF AUC to plasma AUC was not possible given the limited CSF sampling. As sulbactam-durlobactam ASTs were determined by disk diffusion and MICs were not available, estimations of CSF exposure relative to pharmacokinetic-pharmacodynamic exposures were also not possible. Despite limitations in our ability to adequately assess sulbactam and durlobactam CSF concentrations, the swift and sustained clinical improvement of our patient and the rapid CSF clearance of A. baumannii are arguably the most important data supporting the effectiveness of sulbactam-durlobactam for meningitis.
Cefiderocol AST interpretations, in particular for A. baumannii complex, pose challenges as they have been associated with both reproducibility and accuracy issues [66]. In our patient's case, CRAB isolates collected after at least 5 days of exposure to cefiderocol demonstrated a paradoxical effect by reference BMD testing. More specifically, paradoxical regrowth refers to the inhibition of bacterial growth at low antibiotic concentrations but regrowth at higher concentrations [67]. Mechanisms mediating the paradoxical effect are not well understood but one prevailing hypothesis is that beta-lactamase expression may be enhanced in the setting of increasing antibiotic concentrations [68]. Interestingly, a previous report of the paradoxical effect was observed for a siderophore-antibiotic conjugate against an Escherichia coli isolate [67]. Notably, the paradoxical effect was only demonstrated in the iron-depleted state. Unlike BMD which requires iron-depleted cation-adjusted Mueller Hinton broth, disk diffusion methods do not require iron depleted agar [7]. The lack of recognition of the potential for a paradoxical effect by disk diffusion for cefiderocol in our case is concerning as it led to a false reassurance that cefiderocol susceptibility would translate to clinical success.
There have been a number of reports of the emergence of cefiderocol-resistance due to mutations in the TonB-dependent iron transporter pathway [69]. Often, individual mutations in iron binding proteins are associated with gradual increases in cefiderocol MICs rather than frank resistance. Mutations identified in the start codons of two iron transport proteins, piuC and pirA factored into the gradual reduction in cefiderocol susceptibility that likely became more pronounced under the selective pressure of cefiderocol therapy [16]. Moreover, the A515V substitution in PBP3, the target site of cefiderocol may have also contributed, as previously reported [16, 70].
In conclusion, we describe a case of the successful use of sulbactam-durlobactam with adjunctive carbapenem therapy for the treatment of refractory CRAB meningitis. The benefit of adjunctive carbapenem therapy warrants further investigation. Moreover, our case highlights the need to be cautious about false reassurances with disk diffusion methods for cefiderocol susceptibility testing for CRAB isolates as the paradoxical effect may not be recognized with this approach.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors would like to thank April Chen from Innoviva Specialty Therapeutics for her assistance with the pharmacokinetic work.
Financial support. This work was supported by the National Institutes of Health (grant number R21-AI173475 to P. D. T). Innoviva Specialty Therapeutics did not provide funding to the authors for this work.
References
WHO publishes list of bacteria for which new antibiotics are urgently needed. Available at: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed 13 January 2024.
United States Food and Drug Administration Sulbactam AND Durlobactam package insert. Available at: https://xacduro-assets.s3.amazonaws.com/prescribing-information.pdf. Accessed 2 January 2024.
Author notes
Potential conflicts of interest. S. M. K. and P. J. S. served on an Advisory Board for Entasis/Innoviva Specialty Therapeutics. P. J. S. reports grants from T2 Diagnostics and Accelerate Diagnostics, grants and personal fees from bioMérieux, Inc., and Qiagen Sciences Inc.; and personal fees from BD Diagnostics, Shionogi, Inc., GeneCapture, Day Zero Diagnostics, Next Gen Diagnostics, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




