-
PDF
- Split View
-
Views
-
Cite
Cite
Roger Echols, Mari Ariyasu, Tsutae Den Nagata, Pathogen-focused Clinical Development to Address Unmet Medical Need: Cefiderocol Targeting Carbapenem Resistance, Clinical Infectious Diseases, Volume 69, Issue Supplement_7, 1 December 2019, Pages S559–S564, https://doi.org/10.1093/cid/ciz829
Close - Share Icon Share
Abstract
Historically, the regulatory requirements of the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for developing new antibiotics have not addressed pathogen-focused indications for drug approval. The design of the necessary randomized controlled trials traditionally involves the enrollment of patients with site-specific infections caused by susceptible as well as resistant pathogens. Cefiderocol has undergone a streamlined clinical development program to address serious carbapenem-resistant infections. The regulatory approach, and the pivotal clinical trials, differed between the FDA and EMA. In the United States, the APEKS-cUTI (Acinetobacter, Pseudomonas, Escherichia coli, Klebsiella, Stenotrophomonas–complicated urinary tract infection) study was conducted to provide the basis for FDA approval of a site-specific cUTI indication. The EMA, however, preferred the CREDIBLE-CR (A MultiCenter, RandomizED, Open-label ClInical Study of S-649266 or Best AvailabLE Therapy for the Treatment of Severe Infections Caused by Carbapenem-Resistant Gram-negative Pathogens) study, in which patients with nosocomial pneumonia, bloodstream infections, or cUTIs were enrolled if they had a carbapenem-resistant pathogen. The resulting European label will be pathogen focused rather than infection site specific (ie, treatment of gram-negative infection in patients with limited treatment options). The implications and limitations of these different regulatory processes are discussed.
CEFIDEROCOL: “PATHOGEN-FOCUSED” DEVELOPMENT
With the increasing prevalence of carbapenem-resistant pathogens and lack of efficacious antibiotics, a great unmet medical need has emerged in the treatment of carbapenem-resistant gram-negative organisms, including the nonfermenters Pseudomonas aeruginosa and Acinetobacter baumannii [1]. Despite the public health focus on Klebsiella pneumoniae carbapenemase (KPC)–producing carbapenem-resistant Enterobacteriaceae (CRE), it is the nonfermenting species that represent the majority of the disease burden for infections in the United States (US) caused by carbapenem-resistant organisms [1, 2]. Similarly, in Europe and Asia-Pacific, the prevalence of carbapenem-resistant nonfermenters has been reported to be several times greater than that of Enterobacteriaceae species [2, 3]. Thus, antibiotic research addressing these important pathogens is urgently needed [4]. Shionogi & Co, Ltd, has designed and developed cefiderocol (previously known as S-649266), the first siderophore cephalosporin, which has a molecular profile that addresses all major carbapenem resistance mechanisms [5], and has successfully progressed it into late clinical development.
Current guidance published by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) most often requires randomized controlled clinical studies with inferential testing (ie, statistical hypothesis testing). This has to be complemented by the enrollment of a large number of patients to support the marketing application (New Drug Application [NDA] or Marketing Authorization Application [MAA], respectively) for 1 or more infection site–specific indication (eg, complicated urinary tract infection [cUTI] or complicated intra-abdominal infection), based on the drug’s clinical efficacy and safety. The bacterial pathogens relevant to the indication listed in the prescribing information are a secondary consideration based on the spectrum of activity of the investigational antibiotic and the microbiological efficacy data extracted from the clinical trials. Historically, prior to the Kefauver-Harris amendment to the Food and Drug Act in 1962 in the US, most of the antibiotics developed and approved were “pathogen focused” [6]. Clinicians’ prescribing also focuses on identifying the bacterial pathogen responsible for a patient’s infection, combined with consideration of the antibiotic’s ability to reach adequate concentrations at the infection site; the treatment choice is therefore determined by the infecting pathogen but not necessarily limited to a specific infection site.
Shionogi understood that cefiderocol would be restricted in its use through the tenets of antibiotic stewardship and not used empirically where carbapenem resistance was not identified or suspected. But following the traditional development pathway for infection-site specific indications, enrolling patients with carbapenem-susceptible pathogens, would not provide information to prescribers about its efficacy in the treatment of carbapenem-resistant infections. Instead, opting for a pathogen-focused approach, where the objective was the inclusion of carbapenem-resistant gram-negative pathogens and not the site of infection, seemed a more useful approach for the clinical development. The strategy was to develop the drug for how it will be used in clinical practice, which meant that the clinical trials should include patients who were infected by carbapenem-resistant gram-negative pathogens regardless of the infection site. However, there were no regulatory guidelines for pathogen-focused clinical development (Figure 1).
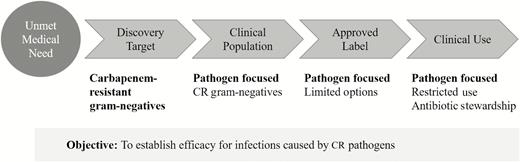
Pathogen-focused clinical development program. This is a theoretical strategic concept and not an approved regulatory pathway in the United States. Abbreviation: CR, carbapenem resistant.
Discussions with the FDA regarding the development of a new type of antibiotic that broadly addressed the problem of carbapenem resistance among gram-negative bacteria, both Enterobacteriaceae and nonfermenters, took place in 2011 and 2012. The FDA, and subsequently representative authorities in Europe, were very supportive and encouraged a collaborative effort to efficiently bring promising new antibiotics into clinical use. The Generating Antibiotic Incentives Now (GAIN) Act and other FDA programs provided “pull” incentives of extending marketing exclusivity as well as flexibility in the clinical development requirements to shorten the development and regulatory review time needed to approve a new antibiotic that addresses a significant unmet medical need [7]. Importantly, in the draft guidance in 2013, the FDA outlined a “streamlined development” strategy that stated: “The [FDA] has determined that it is appropriate to exercise the broadest flexibility in applying the statutory standards, while preserving appropriate guarantees for safety and effectiveness. These procedures reflect the recognition that physicians and patients are generally willing to accept greater risks or side effects from drugs that treat life-threatening and severely debilitating illnesses than they would accept from drugs that treat less serious illnesses” [8]. Details regarding pathogen-specific or pathogen-focused development were lacking; however, a minimum sample size of 300 patients treated with the approved dose was stated.
In essence, the FDA had difficulty with Shionogi’s pathogen-focused plan for 2 reasons. First, the plan did not fit their infection-site indication rules, in which the infection site, and not the specific bacterial pathogen, was the labeled indication. In other words, identifying a target pathogen by its resistance to other drugs (eg, carbapenem resistance) did not meet the requirements for their labeling construct. Second, the FDA was concerned that any pathogen-focused study that included multiple sites of infection would be difficult to interpret using a noninferiority study design, although a superiority design would be acceptable [9, 10]. What became clear was that the development strategy, which Shionogi considered to be “pathogen focused” based on the pathogen resistance profile, did not fit the FDA definition of a pathogen-focused or targeted development, which by their definition was limited to a single genus and species [11].
Discussions with the FDA regarding a pathogen-focused clinical trial that could lead to a pathogen-focused label continued as Shionogi tried to address the FDA’s concerns. But during 2014, it was clear that the FDA’s need for interpretable data meant a randomized clinical study with inferential testing with an acceptable endpoint, which in a study population with life-threatening carbapenem-resistant infections would have to be all-cause mortality. Other unresolvable points included how to determine a noninferiority margin and what could be an acceptable control antibiotic or antibiotic combination.
The EMA was similarly trying to facilitate rapid antibiotic development for drugs addressing carbapenem resistance. In 2013, the EMA issued an addendum to their general guidance for new antibiotics, which specifically addressed any new treatment for highly resistant pathogens for which there were no or only limited approved treatment options. Importantly, they discussed pathogen-focused clinical development: “It is not expected that such a study would be powered for inferential testing. There is no rationale for determining a noninferiority margin based on clinical success rates. In addition, it is not expected to be feasible to demonstrate superiority for the new agent over BAT [best available therapy] based on the usual endpoints” (eg, mortality) [12]. Thus, the EMA, in contrast to the FDA, did not require robust inferential testing and was receptive to the idea that the cefiderocol indication included in the summary of product characteristics could be pathogen based (eg, the treatment of aerobic gram-negative bacterial infections where patients have limited treatment options). Table 1 identifies the 3 pivotal efficacy and safety clinical studies to support both the NDA and MAA submissions (Table 1).
| . | APEKS-cUTI . | APEKS-NP . | CREDIBLE-CR . |
|---|---|---|---|
| . | (N = 450) . | (N = 300) . | (N = 152) . |
| Feature | Site/indication focus | Site/indication focus | Pathogen-focused |
| US Pivotal | Supplemental NDA | Europe Pivotal | |
| Patients | cUTI/AUP | HAP/VAP/HCAP | cUTI, HAP/VAP/HCAP, BSI/sepsis due to CR GNB |
| Design | Randomized 2:1 | Randomized 1:1 | Randomized 2:1 |
| Double blind | Double blind | Open label | |
| Comparator | Imipenem/cilastatin | Meropenem | “Best available therapy” |
| Status | Completed | Ongoing | Ongoing |
| NCT02321800 | NCT03032380 | NCT02714595 |
| . | APEKS-cUTI . | APEKS-NP . | CREDIBLE-CR . |
|---|---|---|---|
| . | (N = 450) . | (N = 300) . | (N = 152) . |
| Feature | Site/indication focus | Site/indication focus | Pathogen-focused |
| US Pivotal | Supplemental NDA | Europe Pivotal | |
| Patients | cUTI/AUP | HAP/VAP/HCAP | cUTI, HAP/VAP/HCAP, BSI/sepsis due to CR GNB |
| Design | Randomized 2:1 | Randomized 1:1 | Randomized 2:1 |
| Double blind | Double blind | Open label | |
| Comparator | Imipenem/cilastatin | Meropenem | “Best available therapy” |
| Status | Completed | Ongoing | Ongoing |
| NCT02321800 | NCT03032380 | NCT02714595 |
Abbreviations: APEKS, Acinetobacter, Pseudomonas, Escherichia coli, Klebsiella, Stenotrophomonas; AUP, acute uncomplicated pyelonephritis; BSI, bloodstream infection; CR, carbapenem-resistant; CREDIBLE-CR, A MultiCenter, RandomizED, Open-label ClInical Study of S-649266 or Best AvailabLE Therapy for the Treatment of Severe Infections Caused by Carbapenem-Resistant Gram-negative Pathogens; cUTI, complicated urinary tract infection; GNB, gram-negative bacteria; HAP, hospital-acquired pneumonia; HCAP, healthcare-associated pneumonia; NP, nosocomial pneumonia; VAP, ventilator-associated pneumonia.
| . | APEKS-cUTI . | APEKS-NP . | CREDIBLE-CR . |
|---|---|---|---|
| . | (N = 450) . | (N = 300) . | (N = 152) . |
| Feature | Site/indication focus | Site/indication focus | Pathogen-focused |
| US Pivotal | Supplemental NDA | Europe Pivotal | |
| Patients | cUTI/AUP | HAP/VAP/HCAP | cUTI, HAP/VAP/HCAP, BSI/sepsis due to CR GNB |
| Design | Randomized 2:1 | Randomized 1:1 | Randomized 2:1 |
| Double blind | Double blind | Open label | |
| Comparator | Imipenem/cilastatin | Meropenem | “Best available therapy” |
| Status | Completed | Ongoing | Ongoing |
| NCT02321800 | NCT03032380 | NCT02714595 |
| . | APEKS-cUTI . | APEKS-NP . | CREDIBLE-CR . |
|---|---|---|---|
| . | (N = 450) . | (N = 300) . | (N = 152) . |
| Feature | Site/indication focus | Site/indication focus | Pathogen-focused |
| US Pivotal | Supplemental NDA | Europe Pivotal | |
| Patients | cUTI/AUP | HAP/VAP/HCAP | cUTI, HAP/VAP/HCAP, BSI/sepsis due to CR GNB |
| Design | Randomized 2:1 | Randomized 1:1 | Randomized 2:1 |
| Double blind | Double blind | Open label | |
| Comparator | Imipenem/cilastatin | Meropenem | “Best available therapy” |
| Status | Completed | Ongoing | Ongoing |
| NCT02321800 | NCT03032380 | NCT02714595 |
Abbreviations: APEKS, Acinetobacter, Pseudomonas, Escherichia coli, Klebsiella, Stenotrophomonas; AUP, acute uncomplicated pyelonephritis; BSI, bloodstream infection; CR, carbapenem-resistant; CREDIBLE-CR, A MultiCenter, RandomizED, Open-label ClInical Study of S-649266 or Best AvailabLE Therapy for the Treatment of Severe Infections Caused by Carbapenem-Resistant Gram-negative Pathogens; cUTI, complicated urinary tract infection; GNB, gram-negative bacteria; HAP, hospital-acquired pneumonia; HCAP, healthcare-associated pneumonia; NP, nosocomial pneumonia; VAP, ventilator-associated pneumonia.
APEKS-cUTI STUDY
While discussions continued regarding the design of a pathogen-focused clinical trial, Shionogi and the FDA agreed to first conduct a clinical study in cUTI, which would not enroll patients infected with carbapenem-resistant organisms. The primary objective of this study was to establish the safety profile of cefiderocol [13, 14] when used at the maximum dose intended for the treatment of systemic carbapenem-resistant infections and in a patient population “at risk” for multidrug resistant (MDR) infections. Thus, the APEKS (Acinetobacter, Pseudomonas, Escherichia coli, Klebsiella, Stenotrophomonas)–cUTI study was initiated in 2014, as a phase 2, randomized, double-blind study of cUTIs, including acute pyelonephritis, and the comparator regimen was high-dose imipenem-cilastatin (1 g of each every 8 hours) [14]. The high dose of imipenem was selected to allow for the inclusion of patients with P. aeruginosa infection. The dose of cefiderocol was 2 g every 8 hours, and both treatments were administered intravenously over 1 hour to maintain blinding of the study. To minimize the proportion of enrolled patients with acute uncomplicated pyelonephritis (ie, patients who would not be at risk of MDR infections), the study did not allow for switching to oral therapy or outpatient therapy and the proportion of enrolled patients with acute uncomplicated pyelonephritis was limited to 30%. The usual exclusion criteria for immunosuppression (eg, renal transplant patients) were not applied and the resulting study population was older with a more equal gender distribution, and with more comorbidities than other contemporary studies [15]. The initial noninferiority margin for this phase 2 study was 20% and the sample size was 300 patients randomized 2:1.
After initiation of the APEKS-cUTI study, the FDA suggested that this study could be the sole clinical study for FDA regulatory approval of cefiderocol if the noninferiority margin was decreased to 15% and the sample size increased accordingly. The remaining requirement for this streamlined development was a safety population of at least 300 patients receiving the 2 g dosing regimen of cefiderocol. The APEKS-cUTI study protocol was formally amended to include these changes, and the enrollment was completed in 2016 with a total of 450 patients, providing the requisite 300 patients treated with cefiderocol. The results of the APEKS-cUTI study have recently been published [13].
Although this study was designed as a noninferiority study, a post hoc analysis showed that the observed treatment difference between cefiderocol and imipenem-cilastatin demonstrated superiority of cefiderocol for both the primary composite endpoint of clinical and microbiological response as well as the secondary endpoint of microbiological eradication [13]. This difference in microbiologic eradication was not the result of baseline resistance to imipenem nor other potential imbalances in the patient populations. Importantly, the study also demonstrated a favorable safety profile compared with that of imipenem-cilastatin; the most frequently reported adverse events were gastrointestinal side effects in both treatment arms, and these were mainly mild or moderate in severity [13]. Serious adverse events occurred in a numerically lower proportion of cefiderocol-treated patients (5%) than of imipenem-cilastatin–treated patients (8%), and only 2% of patients in each treatment arm discontinued therapy. Numerically, there were fewer cases of Clostridioides difficile infection in the cefiderocol arm, which may reflect the narrower spectrum of cefiderocol [13]. The study has established the safety profile of cefiderocol, which is very similar to that of other β-lactam antibiotics.
CREDIBLE-CR STUDY
Meanwhile, for Europe, the plan for a pathogen-focused study went through Scientific Advice with a combined Committee for Medicinal Products for Human Use and Health Technology Assessment meeting in early 2015. Three key elements were discussed. The first was the suitability of a pathogen-focused study where all patients would have evidence of infection caused by a carbapenem-resistant pathogen prior to randomization (Figure 2).
![CREDIBLE-CR (Clinical Trials.gov identifier NCT02714595): Diagnostic pathways for enrollment of patients with carbapenem-resistant infections within a restricted time window of ≤24 hours for patients with complicated urinary tract infection and ≤36 hours for other diagnoses. A, Rapid diagnostics. Cepheid GeneXpert CARBA-R polymerase chain reaction instrument was provided to study sites to identify the presence of various carbapenemase genes (eg, Klebsiella pneumoniae carbapenemase [KPC], New Delhi metallo-β-lactamase [NDM], Verona integron-encoded metallo-β-lactamase [VIM], imipenemase metallo-β-lactamase [IMP], oxacillinase [OXA]-48) from samples. Testing of either direct clinical specimens (endotracheal aspirates, bronchoalveolar lavage, or urine) or cultures was allowed. B, Patients could have failed treatment on empirical therapy, and the primary culture and susceptibility confirmed the presence of a pathogen resistant or nonsusceptible to carbapenems. C, Confirmed carbapenem resistance from a surveillance culture from same anatomical site within 72 hours of enrollment. *To cover gram-negative bacteria. **To cover gram-positive bacteria. Abbreviations: CR-GNB, carbapenem-resistant gram-negative bacteria; Rx, therapy.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/69/Supplement_7/10.1093_cid_ciz829/1/m_ciz829f0002.jpeg?Expires=1750321357&Signature=HpsF0IrRbV-aLpeMF~eAjRRtl5eEHWJMS6rIJ3No32RgiqI80M4uP2yUk5oOUl33fWo8p0wJrttDFjKvoVTkYEd5ssDFxVvpkfH-F61m1GmV0bosRiDDmZJgfWg269nxRAy2TUy5LPY7eXGclpiAe-q~vAHbgzChDBcGsl8BzxMQAgGNnAYNNsTS8utYhHOZliMj8oMcom7LIMhMfUmmTJ704C~XIoNzoHrmOlNcYmy28h77GK2ydKumSg5d6rgKP6TZfXnaS49C5jp9wDgCGpkIO05myRff8TSsojpp7mmkQGdzyeN6IHUtWzBOgX9k3K2vxzwYa6O1zmyw3Fu8PQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
CREDIBLE-CR (Clinical Trials.gov identifier NCT02714595): Diagnostic pathways for enrollment of patients with carbapenem-resistant infections within a restricted time window of ≤24 hours for patients with complicated urinary tract infection and ≤36 hours for other diagnoses. A, Rapid diagnostics. Cepheid GeneXpert CARBA-R polymerase chain reaction instrument was provided to study sites to identify the presence of various carbapenemase genes (eg, Klebsiella pneumoniae carbapenemase [KPC], New Delhi metallo-β-lactamase [NDM], Verona integron-encoded metallo-β-lactamase [VIM], imipenemase metallo-β-lactamase [IMP], oxacillinase [OXA]-48) from samples. Testing of either direct clinical specimens (endotracheal aspirates, bronchoalveolar lavage, or urine) or cultures was allowed. B, Patients could have failed treatment on empirical therapy, and the primary culture and susceptibility confirmed the presence of a pathogen resistant or nonsusceptible to carbapenems. C, Confirmed carbapenem resistance from a surveillance culture from same anatomical site within 72 hours of enrollment. *To cover gram-negative bacteria. **To cover gram-positive bacteria. Abbreviations: CR-GNB, carbapenem-resistant gram-negative bacteria; Rx, therapy.
It was acceptable for EMA that these infections could include different clinical diagnoses from different infection sites but the predominant infection type should be hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP) followed by bloodstream infection (BSI)/sepsis and cUTI. The second element discussed was the comparator agent; as cefiderocol has a broad spectrum of activity that would allow enrollment of CREs as well as carbapenem-resistant nonfermenters such as P. aeruginosa and A. baumannii, there was no single control drug or drug combination regimen that could be applied. Furthermore, different countries have different antibiotics that are available for treatment and which would constitute current standard of care. Therefore, BAT was accepted for this multinational study of infections potentially caused by all species of gram-negative pathogens. The third element of the discussion was that although this would be a randomized study, it would concentrate on capturing details of individual clinical cases qualitatively as well as the microbiological findings in the 3 main indications. Furthermore, the study would only provide descriptive statistics of clinical and microbiological outcomes for a sample size of 150 (2:1 ratio) without specifying or providing inferential testing of a hypothesis.
The resulting CREDIBLE-CR (A MultiCenter, RandomizED, Open-label ClInical Study of S-649266 or Best AvailabLE Therapy for the Treatment of Severe Infections Caused by Carbapenem-Resistant Gram-negative Pathogens) study, which is a randomized, open-label, controlled study, is ongoing in countries in the Americas, Europe, and the Asia-Pacific region [16]. It represents the only cefiderocol clinical study being conducted that specifically provides clinical and microbiological evidence of efficacy and safety for the treatment of carbapenem-resistant infections, including both CREs and nonfermenters.
APEKS-NP
With the APEKS-cUTI study serving as the pivotal and only clinical study to support a streamlined development “limited use” label in the US, Shionogi considered the labeled indication problematic because epidemiologic research had shown that the medical need was greatest in other types of infection (eg, HAP/VAP) [1]. Subsequently, discussions with the FDA took place regarding a clinical study in hospital-acquired and ventilator-associated bacterial pneumonia that would be adequate to support a second limited use indication in the US. The APEKS-NP study in nosocomial pneumonia (NP) is similar to APEKS-cUTI in that both are randomized, double-blinded, monotherapy studies in which the comparator antibiotic is a carbapenem (ie, meropenem and imipenem-cilastatin, respectively; Table 1). The use of a carbapenem as a control requires exclusion of patients known to have carbapenem-resistant organisms at the time of randomization. The primary endpoint in this nosocomial pneumonia study is all-cause mortality [17]. Cefiderocol and the meropenem active control are both dosed at 2 g every 8 hours, and both treatments are administered over 3 hours instead of the 1 hour used for the cUTI study. This APEKS-NP study has recently been completed and aims to provide data for a supplemental NDA [17].
DISCUSSION AND CONCLUSIONS
Cefiderocol is a unique β-lactam antibiotic offering a new approach to broadly overcome β-lactam resistance, including carbapenem resistance among gram-negative pathogens. As a siderophore antibiotic conjugate, it uses a “Trojan horse” approach to gain entry into the bacterial cell through the active iron transporters and does not require passage through porin channels; thus, it is unaffected by porin mutations. Furthermore, overexpression of efflux pumps also does not have an impact on the activity of cefiderocol. Cefiderocol does not require the addition of a β-lactamase inhibitor as it is stable to all classes of β-lactamases including both serine-carbapenemases (eg, KPC and oxacillin carbapenemases) and metallo-carbapenemases (eg, New Delhi, Verona integron-encoded, and imipenemase metallo-β-lactamases). This unique siderophore cephalosporin is active in vitro against all gram-negative species including less common nonfermenters such as Stenotrophomonas maltophilia, Burkholderia cepacia, Burkholderia pseudomallei, and Achromobacter species [18]. Although cefiderocol has broad potent gram-negative activity against both carbapenem-susceptible and carbapenem-resistant bacteria, its activity against gram-positive or anaerobic bacteria is quite limited [19, 20]; in fact, with this “gram-negative only” spectrum, one could consider cefiderocol a narrow-spectrum antibiotic. The use of cefiderocol should be reserved for patients with limited treatment options, meaning patients with documented or suspected antibiotic-resistant pathogens.
Both the FDA and EMA greatly facilitated the clinical development of cefiderocol by accepting the reduced number and size of the clinical studies required for NDA/MAA review. This streamlined development pathway recognizes that cefiderocol does indeed have the potential to treat carbapenem-resistant gram-negative pathogens, including the nonfermenters, regardless of the type of β-lactamase production and/or the presence of porin channel mutations or efflux pump upregulation. The initially targeted indication in the US is cUTI, in contrast to the pathogen-focused label anticipated in Europe. The limited-use indication in the US has implications beyond simply the indication. In the US, the labeled indication includes not only the specific infection type (cUTI) but also identifies specific species of bacteria, such as E. coli, K. pneumoniae, and P. aeruginosa, based on the organisms that were included in the APEKS-cUTI clinical trial. Furthermore, only those species named in the treatment indication of the label can have FDA interpretive criteria or breakpoints for antibiotic susceptibility testing (AST). Without these breakpoints, clinical microbiology laboratories in the US are not allowed to report the results of AST as susceptible or resistant for nonindicated species of bacteria. What this means is that organisms such as A. baumannii and S. maltophilia, which are more common in respiratory tract infections or BSIs and therefore not generally captured in cUTI studies, may not be tested and reported for use in patient management decisions [21]. In US hospitals with strong antibiotic stewardship programs, treatment guidance may be possible. This restriction in AST laboratory reporting is not an issue in Europe, where the indication would be broadly pathogen-focused (aerobic gram-negative bacteria) and the use of devices for AST is regulated differently. Fortunately, in the US, the FDA has responded to this dilemma by potentially accepting the Clinical and Laboratory Standards Institute (CLSI) or other authorized groups outside of the FDA to determine clinical breakpoints for organisms not included in the product label indication [22]. In June 2018, the CLSI approved provisional breakpoints for cefiderocol for Enterobacteriaceae as well as P. aeruginosa, A. baumannii, and S. maltophilia [23]. These CLSI breakpoints (ie, susceptible ≤4 μg/mL, intermediate 8 μg/mL, resistant ≥16 μg/mL) [23], especially for A. baumannii and S. maltophilia, will need to be accepted by the FDA before being added to the FDA website for antibiotic breakpoints [24].
Both regulatory agencies should be commended for the way they have responded to the challenges faced by industry, providing incentives and flexibility for the discovery and development of new antibiotics. It is still a daunting task, particularly when the target population is defined by the infecting pathogen rather than the site of infection. Clinical trial enrollment of very sick patients who are infected with the actual drug-resistant pathogens for which clinical efficacy data are desired is difficult for many reasons. Fortunately, the expanded use of rapid diagnostic tests to identify markers of bacterial resistance should facilitate the selection of the appropriate patient population in a timely way. Particularly for new drugs developed to address the problem of antimicrobial resistance, evaluating the new antibiotic in the target population is essential and best performed in randomized clinical studies, although conducting such trials may be hindered by ethical dilemmas due to lack of appropriate comparators and require thorough discussions with regulatory authorities, as was the case for cefiderocol.
Notes
Acknowledgments. Editorial support was provided by Highfield (Oxford, United Kingdom), sponsored by Shionogi Inc (Florham Park, New Jersey).
Financial support. This review article was sponsored by Shionogi & Co, Ltd (Osaka, Japan), but R. E. did not receive any fee for his authorship.
Supplement sponsorship. This supplement is sponsored by Shionogi & Co., Ltd.
Potential conflicts of interest. R. E. is a consultant for Shionogi Inc. T. D. N. and M. A. are employees of Shionogi & Co, Ltd. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




