-
PDF
- Split View
-
Views
-
Cite
Cite
Yuqi You, Angeles Correas, R Joanne Jao Keehn, Laura C Wagner, Burke Q Rosen, Lauren E Beaton, Yangfeifei Gao, William T Brocklehurst, Inna Fishman, Ralph-Axel Müller, Ksenija Marinkovic, MEG Theta during Lexico-Semantic and Executive Processing Is Altered in High-Functioning Adolescents with Autism, Cerebral Cortex, Volume 31, Issue 2, February 2021, Pages 1116–1130, https://doi.org/10.1093/cercor/bhaa279
Close - Share Icon Share
Abstract
Neuroimaging studies have revealed atypical activation during language and executive tasks in individuals with autism spectrum disorders (ASD). However, the spatiotemporal stages of processing associated with these dysfunctions remain poorly understood. Using an anatomically constrained magnetoencephalography approach, we examined event-related theta oscillations during a double-duty lexical decision task that combined demands on lexico-semantic processing and executive functions. Relative to typically developing peers, high-functioning adolescents with ASD had lower performance accuracy on trials engaging selective semantic retrieval and cognitive control. They showed an early overall theta increase in the left fusiform cortex followed by greater activity in the left-lateralized temporal (starting at ~250 ms) and frontal cortical areas (after ~450 ms) known to contribute to language processing. During response preparation and execution, the ASD group exhibited elevated theta in the anterior cingulate cortex, indicative of greater engagement of cognitive control. Simultaneously increased activity in the ipsilateral motor cortex may reflect a less lateralized and suboptimally organized motor circuitry. Spanning early sensory-specific and late response selection stages, the higher event-related theta responsivity in ASD may indicate compensatory recruitment to offset inefficient lexico-semantic retrieval under cognitively demanding conditions. Together, these findings provide further support for atypical language and executive functions in high-functioning ASD.
Introduction
Autism spectrum disorders (ASD) are neurodevelopmental disorders defined by deficits in social communication, as well as restricted interests and repetitive behaviors (American Psychiatric Association 2013). Although not a formal diagnostic criterion, language impairments or atypical language use are common in individuals with ASD (for reviews, see Rapin and Dunn 2003; Groen et al. 2008; Mody and Belliveau 2013). Language delay is a frequent first symptom of ASD, and once established, language is often characterized by echolalia, i.e., parroting of speech, or repetitiveness, idiosyncratic vocabulary and patterns of speech, and atypical prosody (Luyster et al. 2008; Eigsti et al. 2011). Even high-functioning individuals with ASD and otherwise typical language skills often show subtle impairments in pragmatics (e.g., turn-taking) and nonverbal communication (e.g., gesturing, facial expression; Boucher 2003; Howlin 2003; Tager-Flusberg 2003; Kelley 2011; Naigles and Tek 2017).
Extensive functional magnetic resonance imaging (fMRI) evidence indicates that left inferior prefrontal (iPFC) and temporal cortices are essential for language processing in neurotypical, right-handed individuals (Howard et al. 1992; Wagner et al. 2001; Bookheimer 2002; Noppeney and Price 2004; Vigneau et al. 2006; ). Atypical activation in these areas has been reported in both children and adults with ASD during semantic processing of single words (Harris et al. 2006; Gaffrey et al. 2007) and sentences (Müller et al. 1998; Müller et al. 1999; Just et al. 2004; Kana et al. 2006; Knaus et al. 2008; Tesink et al. 2011). Task-related functional connectivity studies further show decreased connectivity between frontal and temporal language regions in ASD (Just et al. 2004; Knaus et al. 2008). However, fMRI relies on slow hemodynamic effects that reflect neural activity indirectly, and therefore could not shed light on neural activity changes that occur at much faster time scales (Canolty et al. 2007).
Studies using electro- and magnetoencephalography (EEG/MEG), which can better capture brain dynamics, have examined alterations of semantic processing in ASD by relying primarily on event-related potentials (ERP) or fields (ERF). As an established marker of attempts to access and integrate semantic representations, the N400 deflection or its magnetic equivalent, N400m, is attenuated in children and adults with ASD (Dunn et al. 1999; Braeutigam et al. 2008; McCleery et al. 2010; Pijnacker et al. 2010; Ribeiro et al. 2013), consistent with lexico-semantic impairments. However, ERPs and ERFs reflect only the signal that is phase-locked to the stimulus and ignore the majority of oscillatory activity, which is essential for forming long-range functional networks that subserve lexico-semantic processing (Bastiaansen and Hagoort 2006; Marinkovic et al. 2012; Halgren et al. 2015). Neural oscillations in the theta band (4–7 Hz) are of particular relevance to high-level cognitive processes, as they are primarily generated in superficial cortical layers and are implicated in the integration of task-relevant representations into the current context across distinct cortical areas (Wang et al. 2005; Halgren et al. 2015). Given its involvement in the formation and retrieval of long-term memory (Klimesch et al. 2001; Guderian and Duzel 2005; Fell et al. 2011; Hasselmo and Stern 2014), event-related theta power may constitute a neural signature for the retrieval of lexico-semantic information (Bastiaansen et al. 2005; Bastiaansen et al. 2008; Halgren et al. 2015). Consistent with these findings, we have previously reported increased left-lateralized event-related theta power to real words relative to pseudowords in a lexical decision task in neurotypical adults (Marinkovic et al. 2012). The anterior cingulate cortex (ACC) was additionally activated by words evoking response conflict. Indeed, the ACC and the lateral prefrontal cortex are the primary generators of theta oscillations that are considered to be an index of cognitive control engagement in decision-making tasks (Wang et al. 2005; Kovacevic et al. 2012; Cavanagh and Frank 2014; Rosen et al. 2016; Marinkovic et al. 2019).
Because of their favorable spatiotemporal features, MEG-based methods have contributed to our understanding of neurodevelopmental features of cognition in ASD (Mody et al. 2013; Taylor et al. 2014; Kikuchi et al. 2016). Some MEG indices have been proposed as potential biomarkers for ASD that could be useful for diagnostic accuracy and prognostic specificity (Port et al. 2015). Nonetheless, neurophysiological studies on oscillatory dynamics in ASD are surprisingly scant in both language and executive domains, despite fMRI reports of atypical activation in tasks probing cognitive control (Schmitz et al. 2006; Kana et al. 2007; Shafritz et al. 2008; Thakkar et al. 2008). To address this research gap, the present study aimed to investigate event-related theta oscillations during performance on a visual word paradigm in high-functioning adolescents with ASD and typically developing (TD) peers. We employed a double-duty lexical decision task (Marinkovic et al. 2012; Marinkovic et al. 2014), which combines demands on lexico-semantic processing and cognitive control. A time-frequency analysis of theta oscillations within an anatomically constrained MEG (aMEG) approach made it possible to examine “where” ASD-specific oscillatory changes occur, and the temporal sequence (“when”) of these processes (Marinkovic et al. 2019). We hypothesized that participants with ASD would: 1) perform with lower accuracy and longer response times (RTs) than their TD peers on the lexical decision task and 2) demonstrate atypical theta power in the left-lateralized frontotemporal language network and medial frontal executive areas spanning successive temporal windows.
Materials and Methods
Participants
In total, 30 adolescents with ASD and 24 TD peers were recruited. Five participants (ASD: 4; TD: 1) dropped out during the course of the experiment. Five participants (ASD: 4; TD: 1) were excluded due to low scores on the WIAT-III screener (<6th grade reading level) or the practice test (<60% accuracy). Four additional participants (ASD: 2; TD: 2) were excluded due to technical difficulties or excessive motion during MEG or magnetic resonance imaging (MRI) scans, resulting in a final sample of 20 adolescents with ASD and 20 TD peers. Diagnoses of ASD based on Diagnostic and Statistical Manual of Mental Disorders, 5th Edition criteria (American Psychiatric Association 2013) were established using the Autism Diagnostic Observation Schedule, 2nd Edition (ADOS-2; Lord et al. 2012), the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al. 2003), and expert clinical judgment. ASD participants had no reported history of neurological or other autism-related medical conditions (e.g., epilepsy, Fragile-X syndrome, tuberous sclerosis). Eight (out of 20) ASD participants reported taking psychoactive medications. Based on parent reports, seven participants with ASD had co-occurring attention-deficit/hyperactivity (n = 4), depression (n = 1), and anxiety (n = 3), with one participant reporting more than one comorbid condition. Given the high prevalence of such conditions and medication use in ASD, these participants were not excluded to avoid a sample that would be unrepresentative of the broader ASD population (Simonoff et al. 2008; Siegel and Beaulieu 2012; Spencer et al. 2013; Schubart et al. 2014). Exclusionary criteria for TD participants were personal or family history of autism, or other developmental, neurological, or psychiatric conditions. The two groups did not differ on gender, handedness, age, or verbal and nonverbal IQ (Table 1). Informed consent was obtained from all participants and their caregivers in accordance with the University of California, San Diego (UCSD) and the San Diego State University Institutional Review Boards.
| . | ASD (n = 20) . | TD (n = 20) . | Group comparisons . | ||||
|---|---|---|---|---|---|---|---|
| . | Mean ± SD . | Range . | Mean ± SD . | Range . | χ2 or t . | P value . | Cohen’s d . |
| Gender (M/F)a | 17/3 | 15/5 | 0.6 | 0.43 | – | ||
| Handedness (R/L)a | 18/2 | 18/2 | 0.0 | 1.0 | – | ||
| Age (year) | 15.0 ± 2.4 | 12.5–20.0 | 15.3 ± 2.0 | 11.8–20.0 | −0.4 | 0.68 | 0.13 |
| Full Scale IQ (WASI-II) | 104.8 ± 18.0 | 59–136 | 111.2 ± 14.1 | 88–135 | −1.3 | 0.22 | 0.40 |
| Verbal IQ (WASI-II) | 104.1 ± 16.0 | 68–131 | 110.9 ± 13.6 | 85–135 | −1.5 | 0.15 | 0.46 |
| Nonverbal IQ (WASI-II) | 106.9 ± 22.7 | 54–156 | 108.8 ± 14.0 | 80–128 | −0.3 | 0.75 | 0.10 |
| ADOS-2 Total | 10.9 ± 3.3 | 6–20 | – | – | – | – | – |
| Social Affect | 8.9 ± 3.4 | 3–19 | |||||
| Repetitive Behaviors | 2.6 ± 2.1 | 0–9 | – | – | – | – | – |
| ADI-R Social Interaction | 17.9 ± 4.7 | 9–25 | – | – | – | – | – |
| Communication | 14.1 ± 4.1 | 8–21 | – | – | – | – | – |
| Repetitive Behaviors | 5.7 ± 2.3 | 1–9 | – | – | – | – | – |
| WIAT-III Word Reading | 106.4 ± 13.5 | 80–128 | 110.8 ± 8.4 | 97–129 | −1.2 | 0.23 | 0.39 |
| CELF-5 Core Language | 102.4 ± 15.7 | 62–135 | 108.9 ± 13.2 | 76–127 | −1.4 | 0.17 | 0.46 |
| SRS-2 Total | 72.7 ± 9.7 | 52–90 | 45.3 ± 6.0 | 38–58 | 10.7 | <.001 | 3.4 |
| SCQ | 17.1 ± 7.1 | 3–35 | 3.3 ± 4.2 | 0–18 | 7.5 | <.001 | 2.4 |
| BRIEF-2 Global Executive Composite | 66.9 ± 7.8 | 54–84 | 47.6 ± 9.1 | 36–68 | 7.2 | <.001 | 2.3 |
| . | ASD (n = 20) . | TD (n = 20) . | Group comparisons . | ||||
|---|---|---|---|---|---|---|---|
| . | Mean ± SD . | Range . | Mean ± SD . | Range . | χ2 or t . | P value . | Cohen’s d . |
| Gender (M/F)a | 17/3 | 15/5 | 0.6 | 0.43 | – | ||
| Handedness (R/L)a | 18/2 | 18/2 | 0.0 | 1.0 | – | ||
| Age (year) | 15.0 ± 2.4 | 12.5–20.0 | 15.3 ± 2.0 | 11.8–20.0 | −0.4 | 0.68 | 0.13 |
| Full Scale IQ (WASI-II) | 104.8 ± 18.0 | 59–136 | 111.2 ± 14.1 | 88–135 | −1.3 | 0.22 | 0.40 |
| Verbal IQ (WASI-II) | 104.1 ± 16.0 | 68–131 | 110.9 ± 13.6 | 85–135 | −1.5 | 0.15 | 0.46 |
| Nonverbal IQ (WASI-II) | 106.9 ± 22.7 | 54–156 | 108.8 ± 14.0 | 80–128 | −0.3 | 0.75 | 0.10 |
| ADOS-2 Total | 10.9 ± 3.3 | 6–20 | – | – | – | – | – |
| Social Affect | 8.9 ± 3.4 | 3–19 | |||||
| Repetitive Behaviors | 2.6 ± 2.1 | 0–9 | – | – | – | – | – |
| ADI-R Social Interaction | 17.9 ± 4.7 | 9–25 | – | – | – | – | – |
| Communication | 14.1 ± 4.1 | 8–21 | – | – | – | – | – |
| Repetitive Behaviors | 5.7 ± 2.3 | 1–9 | – | – | – | – | – |
| WIAT-III Word Reading | 106.4 ± 13.5 | 80–128 | 110.8 ± 8.4 | 97–129 | −1.2 | 0.23 | 0.39 |
| CELF-5 Core Language | 102.4 ± 15.7 | 62–135 | 108.9 ± 13.2 | 76–127 | −1.4 | 0.17 | 0.46 |
| SRS-2 Total | 72.7 ± 9.7 | 52–90 | 45.3 ± 6.0 | 38–58 | 10.7 | <.001 | 3.4 |
| SCQ | 17.1 ± 7.1 | 3–35 | 3.3 ± 4.2 | 0–18 | 7.5 | <.001 | 2.4 |
| BRIEF-2 Global Executive Composite | 66.9 ± 7.8 | 54–84 | 47.6 ± 9.1 | 36–68 | 7.2 | <.001 | 2.3 |
Group comparisons were conducted with independent samples t-tests, except for categorical variablesa, which were performed using χ2 tests. Cohen’s d was calculated as a standardized mean difference between two groups of independent observations for the sample. SD, standard deviation; F = female; M = male; L = left; R = right; Values for IQ (WASI-II), WIAT-III, and CELF-5 are standard scores with a normative mean of 100 and SD of 15; values for SRS-2 and BRIEF-2 are T-scores with a mean of 50 and SD of 10.
| . | ASD (n = 20) . | TD (n = 20) . | Group comparisons . | ||||
|---|---|---|---|---|---|---|---|
| . | Mean ± SD . | Range . | Mean ± SD . | Range . | χ2 or t . | P value . | Cohen’s d . |
| Gender (M/F)a | 17/3 | 15/5 | 0.6 | 0.43 | – | ||
| Handedness (R/L)a | 18/2 | 18/2 | 0.0 | 1.0 | – | ||
| Age (year) | 15.0 ± 2.4 | 12.5–20.0 | 15.3 ± 2.0 | 11.8–20.0 | −0.4 | 0.68 | 0.13 |
| Full Scale IQ (WASI-II) | 104.8 ± 18.0 | 59–136 | 111.2 ± 14.1 | 88–135 | −1.3 | 0.22 | 0.40 |
| Verbal IQ (WASI-II) | 104.1 ± 16.0 | 68–131 | 110.9 ± 13.6 | 85–135 | −1.5 | 0.15 | 0.46 |
| Nonverbal IQ (WASI-II) | 106.9 ± 22.7 | 54–156 | 108.8 ± 14.0 | 80–128 | −0.3 | 0.75 | 0.10 |
| ADOS-2 Total | 10.9 ± 3.3 | 6–20 | – | – | – | – | – |
| Social Affect | 8.9 ± 3.4 | 3–19 | |||||
| Repetitive Behaviors | 2.6 ± 2.1 | 0–9 | – | – | – | – | – |
| ADI-R Social Interaction | 17.9 ± 4.7 | 9–25 | – | – | – | – | – |
| Communication | 14.1 ± 4.1 | 8–21 | – | – | – | – | – |
| Repetitive Behaviors | 5.7 ± 2.3 | 1–9 | – | – | – | – | – |
| WIAT-III Word Reading | 106.4 ± 13.5 | 80–128 | 110.8 ± 8.4 | 97–129 | −1.2 | 0.23 | 0.39 |
| CELF-5 Core Language | 102.4 ± 15.7 | 62–135 | 108.9 ± 13.2 | 76–127 | −1.4 | 0.17 | 0.46 |
| SRS-2 Total | 72.7 ± 9.7 | 52–90 | 45.3 ± 6.0 | 38–58 | 10.7 | <.001 | 3.4 |
| SCQ | 17.1 ± 7.1 | 3–35 | 3.3 ± 4.2 | 0–18 | 7.5 | <.001 | 2.4 |
| BRIEF-2 Global Executive Composite | 66.9 ± 7.8 | 54–84 | 47.6 ± 9.1 | 36–68 | 7.2 | <.001 | 2.3 |
| . | ASD (n = 20) . | TD (n = 20) . | Group comparisons . | ||||
|---|---|---|---|---|---|---|---|
| . | Mean ± SD . | Range . | Mean ± SD . | Range . | χ2 or t . | P value . | Cohen’s d . |
| Gender (M/F)a | 17/3 | 15/5 | 0.6 | 0.43 | – | ||
| Handedness (R/L)a | 18/2 | 18/2 | 0.0 | 1.0 | – | ||
| Age (year) | 15.0 ± 2.4 | 12.5–20.0 | 15.3 ± 2.0 | 11.8–20.0 | −0.4 | 0.68 | 0.13 |
| Full Scale IQ (WASI-II) | 104.8 ± 18.0 | 59–136 | 111.2 ± 14.1 | 88–135 | −1.3 | 0.22 | 0.40 |
| Verbal IQ (WASI-II) | 104.1 ± 16.0 | 68–131 | 110.9 ± 13.6 | 85–135 | −1.5 | 0.15 | 0.46 |
| Nonverbal IQ (WASI-II) | 106.9 ± 22.7 | 54–156 | 108.8 ± 14.0 | 80–128 | −0.3 | 0.75 | 0.10 |
| ADOS-2 Total | 10.9 ± 3.3 | 6–20 | – | – | – | – | – |
| Social Affect | 8.9 ± 3.4 | 3–19 | |||||
| Repetitive Behaviors | 2.6 ± 2.1 | 0–9 | – | – | – | – | – |
| ADI-R Social Interaction | 17.9 ± 4.7 | 9–25 | – | – | – | – | – |
| Communication | 14.1 ± 4.1 | 8–21 | – | – | – | – | – |
| Repetitive Behaviors | 5.7 ± 2.3 | 1–9 | – | – | – | – | – |
| WIAT-III Word Reading | 106.4 ± 13.5 | 80–128 | 110.8 ± 8.4 | 97–129 | −1.2 | 0.23 | 0.39 |
| CELF-5 Core Language | 102.4 ± 15.7 | 62–135 | 108.9 ± 13.2 | 76–127 | −1.4 | 0.17 | 0.46 |
| SRS-2 Total | 72.7 ± 9.7 | 52–90 | 45.3 ± 6.0 | 38–58 | 10.7 | <.001 | 3.4 |
| SCQ | 17.1 ± 7.1 | 3–35 | 3.3 ± 4.2 | 0–18 | 7.5 | <.001 | 2.4 |
| BRIEF-2 Global Executive Composite | 66.9 ± 7.8 | 54–84 | 47.6 ± 9.1 | 36–68 | 7.2 | <.001 | 2.3 |
Group comparisons were conducted with independent samples t-tests, except for categorical variablesa, which were performed using χ2 tests. Cohen’s d was calculated as a standardized mean difference between two groups of independent observations for the sample. SD, standard deviation; F = female; M = male; L = left; R = right; Values for IQ (WASI-II), WIAT-III, and CELF-5 are standard scores with a normative mean of 100 and SD of 15; values for SRS-2 and BRIEF-2 are T-scores with a mean of 50 and SD of 10.
Experimental Procedure
Neuropsychological assessments were administered during an initial session in which participants also practiced the behavioral task and were acclimated to the MRI environment in a mock MRI scanner. The Word Reading subtest of the Wechsler Individual Achievement Test, 3rd ed. (WIAT-III; Wechsler 2009) was administered as a screener to ensure that all participants were able to read at a 6th grade reading level. Participants were then administered the Wechsler Abbreviated Scale of Intelligence–2nd ed. (WASI-II; Wechsler 1999) and the Clinical Evaluation of Language Fundamentals–5th ed. (CELF-5; Semel et al. 2013). Hand preference was assessed using the Edinburgh Handedness Inventory (Oldfield 1971). Parent-report measures including the Social Responsiveness Scale–2nd ed. (SRS-2; Constantino and Gruber 2012), a measure of reciprocal social behavior and social impairments associated with ASD, and Behavior Rating Inventory of Executive Function–2nd ed. (BRIEF-2; Gioia et al. 2015), a measure of executive function in daily environments, were also obtained. Rare incidences of missing data points were excluded from group means shown in Table 1.
All participants completed an MEG and structural MRI scan in separate sessions. During the MEG session, participants were familiarized with the MEG scanner and with the task using a set of practice trials prior to performing the experimental task. During structural MRI scans, participants were instructed to lie still, and were allowed to watch a movie of their choice.
Experimental Task
The lexical decision task was adapted from our earlier studies (Marinkovic et al. 2012; Marinkovic et al. 2014), which indicated sensitivity to task conditions in participant groups of comparable size. Participants were instructed to press a button to visually presented real, standard words (SW) with their left index finger, and to animal words (AN) with their left middle finger. They were asked to withhold responses to pseudowords (PW), which were orthographically and phonologically legal letter strings with no meaning (e.g., “stigor”). In the initial prescan session, a practice test with trial-by-trial feedback was administered to ensure that participants fully understood the task instructions. A pretest was then administered immediately following the practice test. A minimum overall accuracy of 60% was required in order to be eligible for the MEG scan. The practice and pretest trials contained words that were different from those presented during the experiment.
During the lexical decision task, stimuli were displayed in a randomized order with the Presentation software (Neurobehavioral Systems) in white lower-case letters on a black background. The same stimulus list was used for all participants. Letter strings were centrally presented for 500 ms with an overall trial duration of 2.5 s. They were preceded and followed by a fixation string (xxxxxx) subtending a visual angle of 6.2° × 1.1°. Participants’ responses were recorded with a fiberoptic button box (Current Designs, Philadelphia, PA). The number of letters did not differ between the three conditions (SW: 5.9 ± 1.5; AN: 6.0 ± 1.7; PW: 6.0 ± 1.5). Furthermore, the SW and AN conditions did not differ in terms of the number of syllables (SW: 1.8 ± 0.7; AN: 1.9 ± 0.7), frequency of occurrence based on the Zipf scale (SW: 3.5 ± 0.6; AN: 3.4 ± 0.6; (Brysbaert and New 2009; van Heuven et al. 2014), or age of acquisition (SW: 6.5 ± 1.9 years; AN: 6.6 ± 1.7; (Kuperman et al. 2012). One hundred trials were presented and analyzed for each condition. A prepotent response tendency was established by presenting additional 180 standard word fillers, which made it possible to probe cognitive control. More specifically, AN words elicited a robust response conflict, whereas PW stimuli engaged response inhibition. Short breaks were given approximately every 4 min.
Data Acquisition and Analysis
Magnetic Resonance Imaging
Structural MRI scans were acquired at the UCSD Center for fMRI on a General Electric Discovery MR750 3.0 Tesla Scanner (GE Healthcare, Milwaukee, WI), using a Nova Medical 32 channel head coil. Whole-head structural images were acquired using a standard Fast Spoiled Gradient Recalled T1-weighted sequence (TR = 8.136 ms; TE = 3.172 ms; flip angle = 8°; FOV = 25.6 cm; acquisition matrix = 256 × 256; voxel size = 1 mm3; slices: 172; duration: 5 min). Each participant’s cortical surface was reconstructed using Freesurfer (Dale et al. 1999; Fischl et al. 1999a) and served to constrain inverse solution estimates. Inner skull surface was derived from segmented MRI data and used for a boundary element model of the volume conductor in the forward calculations. For the purpose of group analysis, the reconstructed individual surfaces were morphed into an average representation by aligning their sulcal-gyral patterns (Fischl et al. 1999b) and decimated, defining the solution space with 5124 free-rotating dipoles spaced ~7 mm apart.
Magnetoencephalography
High-density MEG data were acquired from 204 planar gradiometers (102 pairs) with a whole-head Neuromag Vectorview system (Elekta AB, Stockholm, Sweden) in a magnetically and electrically shielded room at the UCSD Radiology Imaging Laboratory. The signals were recorded continuously with a 1000 Hz sampling rate and minimal filtering (0.1–300 Hz). To achieve precise coregistration with structural MRI images, head position indicator coils, the main fiducial points including the nasion and preauricular points, and numerous random points covering the scalp were digitized with a 3Space Isotrak II system (Polhemus Inc., Colchester, VT).
Data were analyzed with custom-made Matlab routines (Kovacevic et al. 2012; Rosen et al. 2016; Beaton et al. 2018; Correas et al. 2018), partly relying on publicly available packages including Fieldtrip (Oostenveld et al. 2011), EEGLab (Delorme and Makeig 2004) and MNE (Gramfort et al. 2014). Continuous data were band-pass filtered from 0.1 to 100 Hz, downsampled to 250 Hz, epoched from −300 to 1100 ms relative to stimulus onset for the stimulus-locked analysis, and baseline-corrected using the 300 ms prestimulus period as the baseline. Independent component analysis was used to remove eye blinks and heart beat artifacts (Delorme and Makeig 2004). Any remaining artifacts were removed by careful visual inspection and threshold rejection (Oostenveld et al. 2011). Only artifact-free trials with correct responses were included in the final analysis. For each participant, the number of included trials was equated across all three conditions to minimize potential statistical bias. This was accomplished with an automatic algorithm that excluded superfluous trials at random in the conditions with relatively more correct trials.
Complex power spectra were calculated across all epochs using convolution with Morlet wavelets (Lachaux et al. 1999) in 1 Hz increments from 4 to 7 Hz for theta band, with wavelet width varying from 2 to 3.5 cycles to ensure a constant frequency resolution of 2 Hz and time resolution of 80 ms. Padding (300 ms) was added to the two ends of each epoch for wavelet analysis and subsequently discarded to remove edge artifacts. Wavelet results were visually inspected across all epochs for any additional artifacts. Source power estimates were calculated with an aMEG method (Dale and Sereno 1993; Dale et al. 2000). This model is based on the assumption that the synaptic currents giving rise to the summated MEG signals are generated in the cortical gray matter, which can be reconstructed from each individual’s anatomical MRI. Using a realistic brain model, each participant’s cortical surface was parcellated into a large number of small cortical patches that represent a distributed solution space for inverse estimates. Estimated source power thusly constrained to cortical surface was calculated for each location at each time point by applying a minimum norm estimation procedure (Dale et al. 2000), within the spectral dynamic statistical parametric approach (Lin et al. 2004; Kovacevic et al. 2012; Marinkovic et al. 2012). To prevent biasing the inverse solution against spontaneous brain oscillations, the noise covariance matrix was estimated by pooling empty room data across sessions. Empty room data were band-pass filtered between 3 and 50 Hz and a signal-to-noise ratio of 5 was used for scaling the noise covariance matrix in calculation of the inverse operator. An identity matrix was used for noise-sensitivity normalization of the source-space solution with a purpose of correcting the (inverse) depth bias. The noise-sensitivity normalized estimates of total source power were obtained for each frequency step at each location on the cortical surface. For each subject, a map of total theta source power was estimated by averaging across theta frequency (4–7 Hz) and across all artifact-free, correct trials for each condition. Finally, total event-related theta power estimates were expressed as percent signal change from the 300 ms prestimulus baseline. For the group-level analysis, each individual’s reconstructed cortical surface was first inflated and mapped onto a sphere using a maximally isometric transformation (Fischl et al. 1999a), followed by aligning each individual’s cortical sulcal-gyral pattern with an average folding pattern of a canonical surface (Fischl et al. 1999b). Based on this transformation into a unified surface-based coordinate system, group averages were computed by averaging individual source power estimates (Kovacevic et al. 2012). These estimates are presented as spatial activity maps on inflated average surfaces to ensure better visualization of sulcal estimates.
Regions of interest (ROIs) were created to represent groups of dipoles along the cortical surface with most notable theta source power. They were based on the overall grand average source power estimates across all participants and conditions, resulting in unbiased, orthogonal contrasts (Friston et al. 2006). Importantly, the same set of ROIs was used for all participants in a manner blind to their individual activations, as the grand average-based ROIs are translated across all surfaces with an automatic morphing procedure (Fischl et al. 1999b). Within each ROI, for each participant and condition, time courses were extracted by averaging across the cortical dipoles comprised in the ROI, and were presented as percent change from the baseline. This ROI approach permits further statistical exploration of possible interactions of Group and Condition, by testing for differences in a 2 (Group)-by-3 (Condition) model while controlling for intersubject variability analogous to the “random effects” fMRI analysis (Friston et al. 1999). See Marinkovic et al. (2019) for technical details and a step-by-step visualization of this methodology. To represent the spatiotemporal stages of processing, ROIs encompassed early visual cortex (VisCx) in the calcarine fissure and the fusiform cortex (FusCx) along the ventral visual stream. A frontotemporal network associated with language and cognitive/executive functions included the lateral temporal cortex (LTC), anteroventral prefrontal cortex (avPFC), iPFC, and the ACC. The motor cortex (MotCx) ROI was centered on the hand area.
Behavioral and MEG data were analyzed with mixed model analyses of variance (ANOVAs) with the between-subject factor of Group (ASD, TD), and within-subject factor of Condition (SW, AN, and PW). Greenhouse–Geisser corrections were applied. We inspected grand-averaged time courses of estimated source activity across different ROIs and followed recommended practices for analyzing temporally sensitive signal such as ERPs (Kappenman and Luck 2016). We used time windows that captured prominent peaks and were similar to time windows in prior research using the same experimental task (Marinkovic et al. 2012; Marinkovic et al. 2014), presumably reflecting shared underlying cognitive processes. The ANOVAs were performed for each ROI on event-related theta power estimates averaged over time points in five time windows, which reflect successive processing stages across different ROIs: early visual processing in VisCx (T1: 110–170 ms), visual word form processing in the FusCx (T2: 150–200 ms), lexical access in the LTC (T3: 250–350 ms), lexico-semantic retrieval in prefrontal and temporal cortices (T4: 450–650 ms), and motor preparation and execution in MotCx (T5: 700–1000 ms). As no Group-by-Condition interaction was observed for language-processing regions in the right hemisphere, we focused on reporting results in the left hemisphere. However, because responses were made with the left hand, MotCx activity was additionally examined in the right hemisphere in the T5 window. Effects of Group were examined by planned contrasts between ASD and TD for each condition using independent samples t-tests (two-tailed), whereas paired-sample t-tests (two-tailed) were used for Condition contrasts (SW vs. PW, AN vs. SW, and AN vs. PW) within each group, respectively. Tukey’s Honestly Significant Difference test was used to correct for post hoc comparisons for all Condition contrasts, with bolded contrasts in Table 2 reflecting a corrected P < 0.05. Pearson correlations were computed between significant theta effects and neuropsychological measures (i.e., ADOS-2 Total, ADI-R Social Interaction, Communication, and Repetitive Behaviors scores, WASI-II Full Scale IQ, CELF-5 Core Language, SRS-2 Total, Social Communication Questionnaire (SCQ), BRIEF-2 Global Executive Composite) within ASD and TD groups, respectively. Multiple correlations were corrected with a false discovery rate (FDR)-based procedure of Benjamini and Hochberg (1995). Analysis of the raw theta power in the baseline (−300 to 0 ms) revealed no group effects, confirming that the observed effects were not conflated with systematic group differences.
| Time . | ROI . | Overall effects . | Group (ASD > TD) contrast . | . | Condition contrast . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Cond. x Group F(2,76)/P . | Group F(1,38)/P . | Cond. F(2,76)/P . | SW t(38)/P . | AN t(38)/P . | PW t(38)/P . | ASD AN > SW t(19)/P . | ASD SW > PW t(19)/P . | ASD AN > PW t(19)/P . | TD AN > SW t(19)/P . | TD SW > PW t(19)/P . | TD AN > PW t(19)/P . |
| 110–170 | L VisCx | 0.1/.93 | 0.2/0.68 | 0.4/0.69 | −0.5/0.64 | −0.2/0.87 | −0.5/0.61 | −0.3/0.79 | 0.6/.59 | 0.3/0.75 | −0.7/0.51 | 0.6/0.58 | −0.0/0.96 |
| 150–200 | L FusCx | 0.4/0.67 | 6.7/0.01 | 0.1/0.99 | 2.7/0.01 | 2.2/0.03 | 2.2/0.04 | −0.4/0.66 | 0.7/0.49 | 0.1/0.89 | 0.5/0.64 | −0.6/0.56 | −0.1/0.96 |
| 250–350 | L LTC | 3.8/0.03 | 2.0/0.17 | 2.1/0.13 | 0.2/0.88 | 2.7/0.01 | 0.5/0.61 | 2.5/0.02 | −1/0.34 | 2.3/0.03 | −0.6/0.56 | −0.3/0.77 | −0.7/0.50 |
| 450–650 | L avPFC | 3.3/0.05 | 2.1/0.15 | 18.5/<0.001 | 0.9/0.37 | 2.2/0.04 | 0.7/0.50 | 3.3/0.004 | 2.2/0.04 | 4.3/<0.001 | 1.4/0.16 | 2.3/0.04 | 2.8/0.01 |
| L iPFC | 3.9/0.03 | 4.2/0.05 | 24.4/<0.001 | 1.4/0.17 | 2.7/0.01 | 0.9/0.37 | 3.3/0.003 | 3.0/0.01 | 4.7/<0.001 | 2.6/0.02 | 2.3/0.03 | 3.5/0.003 | |
| L LTC | 3.2/0.05 | 1.0/0.33 | 9.8/<0.001 | 0.5/0.60 | 2.0/0.05 | 0.03/0.98 | 2.9/0.01 | 1.5/0.16 | 4.7/<0.001 | 0.4/0.67 | 1.1/0.30 | 1.8/0.09 | |
| 700–1000 | R Acc | 7.3/<0.001 | 9.5/<0.001 | 1.9/.16 | 4.3/<0.001 | 2.5/0.02 | −1.0/0.33 | −1.1/0.27 | 3.6/0.002 | 2.2/0.04 | 1.5/0.14 | 1.9/0.07 | −0.4/0.70 |
| R MotCx | 0.0/0.91 | 0.3/0.59 | 26.5/<0.001 | 0.4/0.66 | 0.2/0.83 | 1.0/0.32 | 0.4/0.71 | 4.3/<0.001 | 3.5/0.002 | 1.4/0.18 | 3.6/0.002 | 4.1/0.001 | |
| L MotCx | 4.6/0.02 | 7.6/0.01 | 1.4/0.24 | 2.5/0.02 | 3.0/0.01 | 0.5/0.64 | 0.2/0.84 | 0.7/0.49 | 0.7/0.49 | −1.8/0.09 | −2.4/0.03 | −2.8/0.01 | |
| L avPFC | 7.1/<0.001 | 14.6/<0.001 | 1.7/0.19 | 4.0/<0.001 | 3.9/<0.001 | 1.2/0.25 | −0.1/0.91 | 3.8/0.001 | 3.2/0.004 | −0.4/0.71 | −1.3/0.21 | −1.2/0.26 | |
| L iPFC | 8.4/<0.001 | 10.6/<0.001 | 4.0/0.03 | 3.5/0.001 | 3.8/<0.001 | 0.2/0.82 | 1.0/0.33 | 4.4/<0.001 | 4.1/0.001 | −0.3/0.76 | −.6/0.53 | −0.7/0.51 | |
| Time . | ROI . | Overall effects . | Group (ASD > TD) contrast . | . | Condition contrast . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Cond. x Group F(2,76)/P . | Group F(1,38)/P . | Cond. F(2,76)/P . | SW t(38)/P . | AN t(38)/P . | PW t(38)/P . | ASD AN > SW t(19)/P . | ASD SW > PW t(19)/P . | ASD AN > PW t(19)/P . | TD AN > SW t(19)/P . | TD SW > PW t(19)/P . | TD AN > PW t(19)/P . |
| 110–170 | L VisCx | 0.1/.93 | 0.2/0.68 | 0.4/0.69 | −0.5/0.64 | −0.2/0.87 | −0.5/0.61 | −0.3/0.79 | 0.6/.59 | 0.3/0.75 | −0.7/0.51 | 0.6/0.58 | −0.0/0.96 |
| 150–200 | L FusCx | 0.4/0.67 | 6.7/0.01 | 0.1/0.99 | 2.7/0.01 | 2.2/0.03 | 2.2/0.04 | −0.4/0.66 | 0.7/0.49 | 0.1/0.89 | 0.5/0.64 | −0.6/0.56 | −0.1/0.96 |
| 250–350 | L LTC | 3.8/0.03 | 2.0/0.17 | 2.1/0.13 | 0.2/0.88 | 2.7/0.01 | 0.5/0.61 | 2.5/0.02 | −1/0.34 | 2.3/0.03 | −0.6/0.56 | −0.3/0.77 | −0.7/0.50 |
| 450–650 | L avPFC | 3.3/0.05 | 2.1/0.15 | 18.5/<0.001 | 0.9/0.37 | 2.2/0.04 | 0.7/0.50 | 3.3/0.004 | 2.2/0.04 | 4.3/<0.001 | 1.4/0.16 | 2.3/0.04 | 2.8/0.01 |
| L iPFC | 3.9/0.03 | 4.2/0.05 | 24.4/<0.001 | 1.4/0.17 | 2.7/0.01 | 0.9/0.37 | 3.3/0.003 | 3.0/0.01 | 4.7/<0.001 | 2.6/0.02 | 2.3/0.03 | 3.5/0.003 | |
| L LTC | 3.2/0.05 | 1.0/0.33 | 9.8/<0.001 | 0.5/0.60 | 2.0/0.05 | 0.03/0.98 | 2.9/0.01 | 1.5/0.16 | 4.7/<0.001 | 0.4/0.67 | 1.1/0.30 | 1.8/0.09 | |
| 700–1000 | R Acc | 7.3/<0.001 | 9.5/<0.001 | 1.9/.16 | 4.3/<0.001 | 2.5/0.02 | −1.0/0.33 | −1.1/0.27 | 3.6/0.002 | 2.2/0.04 | 1.5/0.14 | 1.9/0.07 | −0.4/0.70 |
| R MotCx | 0.0/0.91 | 0.3/0.59 | 26.5/<0.001 | 0.4/0.66 | 0.2/0.83 | 1.0/0.32 | 0.4/0.71 | 4.3/<0.001 | 3.5/0.002 | 1.4/0.18 | 3.6/0.002 | 4.1/0.001 | |
| L MotCx | 4.6/0.02 | 7.6/0.01 | 1.4/0.24 | 2.5/0.02 | 3.0/0.01 | 0.5/0.64 | 0.2/0.84 | 0.7/0.49 | 0.7/0.49 | −1.8/0.09 | −2.4/0.03 | −2.8/0.01 | |
| L avPFC | 7.1/<0.001 | 14.6/<0.001 | 1.7/0.19 | 4.0/<0.001 | 3.9/<0.001 | 1.2/0.25 | −0.1/0.91 | 3.8/0.001 | 3.2/0.004 | −0.4/0.71 | −1.3/0.21 | −1.2/0.26 | |
| L iPFC | 8.4/<0.001 | 10.6/<0.001 | 4.0/0.03 | 3.5/0.001 | 3.8/<0.001 | 0.2/0.82 | 1.0/0.33 | 4.4/<0.001 | 4.1/0.001 | −0.3/0.76 | −.6/0.53 | −0.7/0.51 | |
Summary of ANOVA main effects and interactions (F/P-value), as well as follow-up Group and Condition contrasts (t/P-value) within successive time windows. Significant contrasts are marked in bold font (corrected for multiple comparisons for Condition contrasts).
| Time . | ROI . | Overall effects . | Group (ASD > TD) contrast . | . | Condition contrast . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Cond. x Group F(2,76)/P . | Group F(1,38)/P . | Cond. F(2,76)/P . | SW t(38)/P . | AN t(38)/P . | PW t(38)/P . | ASD AN > SW t(19)/P . | ASD SW > PW t(19)/P . | ASD AN > PW t(19)/P . | TD AN > SW t(19)/P . | TD SW > PW t(19)/P . | TD AN > PW t(19)/P . |
| 110–170 | L VisCx | 0.1/.93 | 0.2/0.68 | 0.4/0.69 | −0.5/0.64 | −0.2/0.87 | −0.5/0.61 | −0.3/0.79 | 0.6/.59 | 0.3/0.75 | −0.7/0.51 | 0.6/0.58 | −0.0/0.96 |
| 150–200 | L FusCx | 0.4/0.67 | 6.7/0.01 | 0.1/0.99 | 2.7/0.01 | 2.2/0.03 | 2.2/0.04 | −0.4/0.66 | 0.7/0.49 | 0.1/0.89 | 0.5/0.64 | −0.6/0.56 | −0.1/0.96 |
| 250–350 | L LTC | 3.8/0.03 | 2.0/0.17 | 2.1/0.13 | 0.2/0.88 | 2.7/0.01 | 0.5/0.61 | 2.5/0.02 | −1/0.34 | 2.3/0.03 | −0.6/0.56 | −0.3/0.77 | −0.7/0.50 |
| 450–650 | L avPFC | 3.3/0.05 | 2.1/0.15 | 18.5/<0.001 | 0.9/0.37 | 2.2/0.04 | 0.7/0.50 | 3.3/0.004 | 2.2/0.04 | 4.3/<0.001 | 1.4/0.16 | 2.3/0.04 | 2.8/0.01 |
| L iPFC | 3.9/0.03 | 4.2/0.05 | 24.4/<0.001 | 1.4/0.17 | 2.7/0.01 | 0.9/0.37 | 3.3/0.003 | 3.0/0.01 | 4.7/<0.001 | 2.6/0.02 | 2.3/0.03 | 3.5/0.003 | |
| L LTC | 3.2/0.05 | 1.0/0.33 | 9.8/<0.001 | 0.5/0.60 | 2.0/0.05 | 0.03/0.98 | 2.9/0.01 | 1.5/0.16 | 4.7/<0.001 | 0.4/0.67 | 1.1/0.30 | 1.8/0.09 | |
| 700–1000 | R Acc | 7.3/<0.001 | 9.5/<0.001 | 1.9/.16 | 4.3/<0.001 | 2.5/0.02 | −1.0/0.33 | −1.1/0.27 | 3.6/0.002 | 2.2/0.04 | 1.5/0.14 | 1.9/0.07 | −0.4/0.70 |
| R MotCx | 0.0/0.91 | 0.3/0.59 | 26.5/<0.001 | 0.4/0.66 | 0.2/0.83 | 1.0/0.32 | 0.4/0.71 | 4.3/<0.001 | 3.5/0.002 | 1.4/0.18 | 3.6/0.002 | 4.1/0.001 | |
| L MotCx | 4.6/0.02 | 7.6/0.01 | 1.4/0.24 | 2.5/0.02 | 3.0/0.01 | 0.5/0.64 | 0.2/0.84 | 0.7/0.49 | 0.7/0.49 | −1.8/0.09 | −2.4/0.03 | −2.8/0.01 | |
| L avPFC | 7.1/<0.001 | 14.6/<0.001 | 1.7/0.19 | 4.0/<0.001 | 3.9/<0.001 | 1.2/0.25 | −0.1/0.91 | 3.8/0.001 | 3.2/0.004 | −0.4/0.71 | −1.3/0.21 | −1.2/0.26 | |
| L iPFC | 8.4/<0.001 | 10.6/<0.001 | 4.0/0.03 | 3.5/0.001 | 3.8/<0.001 | 0.2/0.82 | 1.0/0.33 | 4.4/<0.001 | 4.1/0.001 | −0.3/0.76 | −.6/0.53 | −0.7/0.51 | |
| Time . | ROI . | Overall effects . | Group (ASD > TD) contrast . | . | Condition contrast . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Cond. x Group F(2,76)/P . | Group F(1,38)/P . | Cond. F(2,76)/P . | SW t(38)/P . | AN t(38)/P . | PW t(38)/P . | ASD AN > SW t(19)/P . | ASD SW > PW t(19)/P . | ASD AN > PW t(19)/P . | TD AN > SW t(19)/P . | TD SW > PW t(19)/P . | TD AN > PW t(19)/P . |
| 110–170 | L VisCx | 0.1/.93 | 0.2/0.68 | 0.4/0.69 | −0.5/0.64 | −0.2/0.87 | −0.5/0.61 | −0.3/0.79 | 0.6/.59 | 0.3/0.75 | −0.7/0.51 | 0.6/0.58 | −0.0/0.96 |
| 150–200 | L FusCx | 0.4/0.67 | 6.7/0.01 | 0.1/0.99 | 2.7/0.01 | 2.2/0.03 | 2.2/0.04 | −0.4/0.66 | 0.7/0.49 | 0.1/0.89 | 0.5/0.64 | −0.6/0.56 | −0.1/0.96 |
| 250–350 | L LTC | 3.8/0.03 | 2.0/0.17 | 2.1/0.13 | 0.2/0.88 | 2.7/0.01 | 0.5/0.61 | 2.5/0.02 | −1/0.34 | 2.3/0.03 | −0.6/0.56 | −0.3/0.77 | −0.7/0.50 |
| 450–650 | L avPFC | 3.3/0.05 | 2.1/0.15 | 18.5/<0.001 | 0.9/0.37 | 2.2/0.04 | 0.7/0.50 | 3.3/0.004 | 2.2/0.04 | 4.3/<0.001 | 1.4/0.16 | 2.3/0.04 | 2.8/0.01 |
| L iPFC | 3.9/0.03 | 4.2/0.05 | 24.4/<0.001 | 1.4/0.17 | 2.7/0.01 | 0.9/0.37 | 3.3/0.003 | 3.0/0.01 | 4.7/<0.001 | 2.6/0.02 | 2.3/0.03 | 3.5/0.003 | |
| L LTC | 3.2/0.05 | 1.0/0.33 | 9.8/<0.001 | 0.5/0.60 | 2.0/0.05 | 0.03/0.98 | 2.9/0.01 | 1.5/0.16 | 4.7/<0.001 | 0.4/0.67 | 1.1/0.30 | 1.8/0.09 | |
| 700–1000 | R Acc | 7.3/<0.001 | 9.5/<0.001 | 1.9/.16 | 4.3/<0.001 | 2.5/0.02 | −1.0/0.33 | −1.1/0.27 | 3.6/0.002 | 2.2/0.04 | 1.5/0.14 | 1.9/0.07 | −0.4/0.70 |
| R MotCx | 0.0/0.91 | 0.3/0.59 | 26.5/<0.001 | 0.4/0.66 | 0.2/0.83 | 1.0/0.32 | 0.4/0.71 | 4.3/<0.001 | 3.5/0.002 | 1.4/0.18 | 3.6/0.002 | 4.1/0.001 | |
| L MotCx | 4.6/0.02 | 7.6/0.01 | 1.4/0.24 | 2.5/0.02 | 3.0/0.01 | 0.5/0.64 | 0.2/0.84 | 0.7/0.49 | 0.7/0.49 | −1.8/0.09 | −2.4/0.03 | −2.8/0.01 | |
| L avPFC | 7.1/<0.001 | 14.6/<0.001 | 1.7/0.19 | 4.0/<0.001 | 3.9/<0.001 | 1.2/0.25 | −0.1/0.91 | 3.8/0.001 | 3.2/0.004 | −0.4/0.71 | −1.3/0.21 | −1.2/0.26 | |
| L iPFC | 8.4/<0.001 | 10.6/<0.001 | 4.0/0.03 | 3.5/0.001 | 3.8/<0.001 | 0.2/0.82 | 1.0/0.33 | 4.4/<0.001 | 4.1/0.001 | −0.3/0.76 | −.6/0.53 | −0.7/0.51 | |
Summary of ANOVA main effects and interactions (F/P-value), as well as follow-up Group and Condition contrasts (t/P-value) within successive time windows. Significant contrasts are marked in bold font (corrected for multiple comparisons for Condition contrasts).
Results
Task Performance
Performance accuracy was lower overall in ASD compared to TD participants, as shown by a main effect of Group [F(1,38) = 7.8, P = 0.008]. Group x Condition interaction was not significant [F(1.6, 59.9) = 0.86, P = 0.41], but the effect of Group was examined for each word condition separately. Performance was lower in the ASD group particularly on AN trials [t(32.9) = −2.5, P = 0.018], and marginally so on PW trials [t(22.1) = −2.0, P = 0.054], with no group difference on SW trials [t(38) = −1.6, P = 0.11] (Fig. 1A). A main effect of Condition was also evident [F(1.6,59.9) = 6.3, P = 0.006]. Across both groups, performance accuracy was significantly higher for SW than AN [F(1,38) = 19.9, P < 0.001], marginally higher for PW than AN [F(1,38) = 4.1, P = 0.05], and not significantly different between SW and PW trials [F(1,38) = 1.9, P = 0.18].
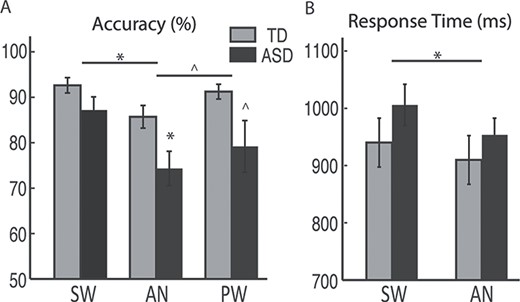
Behavioral performance for TD and ASD groups. A) percent accuracy and B) RTs (mean ± standard errors) are shown for each word condition. Only SW and AN required a response, whereas responses were withheld to PW. *P < 0.05; ^P < 0.07.
The two groups did not differ in their RTs overall [F(1,38) = 1.1, P = 0.31], nor was there a Group x Condition interaction [F(1, 38) = 0.96, P = 0.33; Fig. 1B]. A main effect of Condition [F(1,38) = 13.5, P = 0.001] emerged, however, due to faster RTs to AN [931.6 ± 162.7 ms] than SW [973.0 ± 177.7 ms].
Anatomically Constrained MEG
The overall spatiotemporal sequence of event-related changes in theta power is consistent with the left-lateralized activity progression from sensory-specific to supramodal regions observed with the aMEG method during visual word processing (Marinkovic et al. 2003; Marinkovic 2004; Marinkovic et al. 2012; Marinkovic et al. 2014). The earliest activity in VisCx was similar for the two groups. It was followed by activity in the left FusCx, which was greater in ASD participants for all three conditions (Fig. 2). In the subsequent time windows, greater theta activity was observed in the left-lateralized temporal and frontal cortices to the more cognitively demanding AN words in ASD (Fig. 3). In the final stage of motor preparation and response execution, the two groups showed similar activity in MotCx contralateral to the responding hand; however, the ACC activity to SW and AN words was greater in ASD (Fig. 4). Table 2 lists ANOVA results for each time window including interactions, main effects, contrasts between the ASD and TD groups for each word condition, and Condition contrasts for each group.
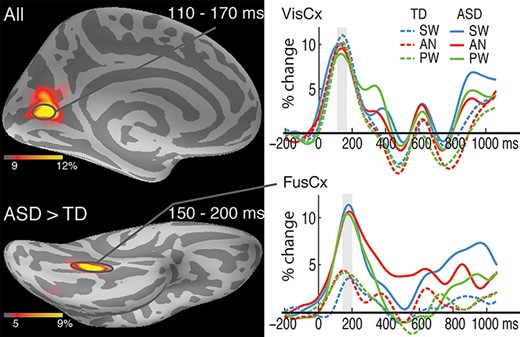
Group average maps and time courses of event-related theta source power during 110–200 ms. The top panel shows similar overall theta activity in the left visual cortex (VisCx) across the ASD and TD groups. The bottom map (left) shows group differences in theta activity in the left fusiform cortex (FusCx). The associated time courses (bottom right) indicate greater theta for all three word conditions in the ASD compared to the TD group. The vertical light-shaded bars denote the time window used in the ROI statistical analysis.
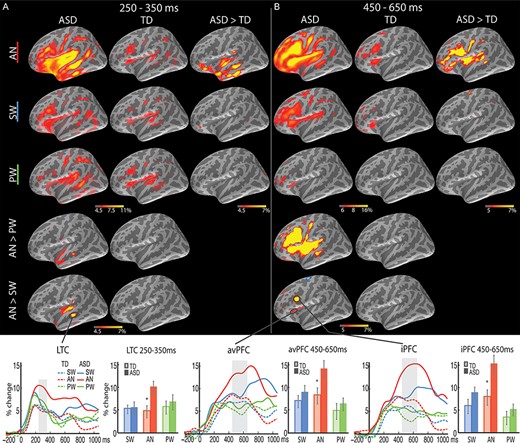
Group average maps and time courses of event-related theta source power during 250–650 ms. In (A) 250–350 ms and (B) 450–650 ms time windows, greater theta was observed to the cognitively demanding AN words in the ASD than TD group in the LTC and left avPFC/iPFC, respectively. The vertical light-shaded bars denote the time window applied to the ROI statistical analysis. *P < 0.05.
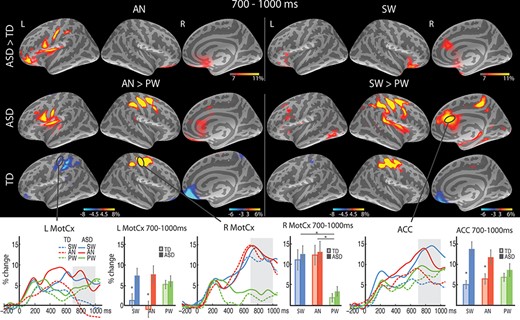
Group average maps and time courses of event-related theta source power in the 700–1000 ms time window. The top row shows greater theta to SW (right) and AN (left) in the ASD than TD group in the ACC. The bottom row demonstrates similar response-related theta enhancement to SW (vs. PW) and AN (vs. PW) in the right motor cortex (R MotCx) across the groups, and less suppressed theta to SW and AN in the ASD than TD group in the L MotCx. The vertical light-shaded bars denote the time window applied to the ROI statistical analysis. *P < 0.05.
T1/T2 (110–200 ms)
The earliest event-related theta power deflection estimated to the VisCx peaked at approximately 140 ms. It did not differ as a function of the Group or Condition factors (Fig. 2). A main effect of Group emerged in the left FusCx in the subsequent time window (150–200 ms) along the ventral visual stream, with stronger theta power in the ASD than TD group [F(1,38) = 6.7, P = 0.01]. However, no effect of Condition was observed at this early processing stage.
T3 (250–350 ms)
The left LTC was the principal source of theta power within this time window, which showed a Group x Condition interaction [F(2.0, 75.9) = 3.8, P = 0.03; Fig. 3A]. On AN trials, the ASD group showed stronger theta power than the TD group [t(38) = 2.7, P = 0.01], while no group difference was observed for SW or PW. In ASD participants, theta power estimated to the left LTC was stronger to AN relative to SW [t(19) = 2.5, P = 0.02] and PW [t(19) = 2.3, P = 0.03], whereas no difference among the three word types was observed for TD participants.
T4 (450–650 ms)
Left prefrontal regions were extensively recruited for further semantic processing during this time window (Fig. 3B), with ASD participants continuing to show elevated theta power to AN in the left LTC. Group x Condition interactions were observed in both the left avPFC [F(1.7, 64.0) = 3.3, P = 0.05] and iPFC [F(1.7, 62.7) = 3.9, P = 0.03], due to stronger theta power to AN in the ASD than the TD group [avPFC: t(38) = 2.2, P = 0.04; iPFC: t(38) = 2.7, P = 0.01]. There were no Group differences for SW or PW conditions.
Examination of the Condition effects for each group separately revealed the following: For the ASD group, theta power was stronger to AN than SW in the left avPFC [t(19) = 3.3, P = 0.004] and iPFC [t(19) = 3.3, P = 0.003]; theta power was also stronger to SW than PW in both left PFC regions [avPFC: t(19) = 2.2, P = 0.038; iPFC: t(19) = 3.0, P = 0.007]. For the TD group, while the iPFC similarly showed stronger theta power to AN than SW [t(19) = 2.6, P = 0.019], and to SW than PW [t(19) = 2.3, P = 0.032], avPFC only showed stronger theta to SW than PW [t(19) = 2.3, P = 0.036], with no difference between AN and SW.
T5 (700–1000 ms)
As expected, left-hand motor responses to SW and AN (vs. none to PW) were associated with enhanced theta power in the right MotCx, resulting in a main effect of Condition [F(1.3,50.2) = 26.5, P < 0.001; Fig. 4]. The two groups did not differ in theta activity in the right MotCx, indicating similar levels of motor preparation and execution. Interestingly, in the left MotCx, ipsilateral to the responding left hand, there was a significant Group x Condition interaction, [F(1.5, 57.1) = 4.6, P = 0.02]. It reflected greater theta power for response-relevant AN [t(37.9) = 3.0, P = 0.01] and SW [t(38) = 2.5, P = 0.02] conditions in ASD participants, possibly indicating compromised motor inhibition in the ipsilateral MotCx in ASD. A Group x Condition interaction was also observed in the right ACC during this time window [F(1.9, 73.0) = 7.3, P < 0.001] due to much stronger theta power for SW [t(29.9) = 4.3, P < 0.001] and AN [t(38) = 2.5, P = 0.02] in the ASD than TD group.
Discussion
Combining a time-sensitive multimodal imaging approach with a double-duty lexical decision task, this study examined the spatiotemporal dynamics of theta oscillations during lexico-semantic and cognitive control processing in high-functioning adolescents with ASD and their TD peers. The most notable findings are summarized here and are subsequently discussed seriatim in greater detail.
Performance accuracy was robustly lower on AN trials and marginally lower on PW trials in ASD participants compared to the TD group, suggesting deficits in selective semantic retrieval and cognitive control. Following activation of the early visual cortex, which was equivalent across both groups, ASD participants showed greater theta activity in the FusCx overall (Fig. 2). The subsequent activation pattern followed a left-dominant posterior-to-anterior temporofrontal stream that characterizes lexico-semantic processing. The most striking group difference was reflected in greater theta activity in the ASD group on AN trials in the lateral temporal and prefrontal cortices (Fig. 3). Along with deficient task performance on the demanding AN condition in the ASD group, the enhanced theta may be suggestive of a compensatory recruitment of the distributed cortical areas. During the final, motor preparation stage, both groups showed comparable engagement of the response-related right motor cortex (Fig. 4). However, greater activation of the ipsilateral MotCx in ASD participants indicates reduced lateralization during motor response, which may be reflective of suboptimal organization of the motor executive network. Furthermore, in comparison to the TD group, the ASD participants exhibited increased theta activity in the ACC, suggesting greater engagement of cognitive control.
Task Performance: Demands on Cognitive Control Compromise Lexico-Semantic Retrieval in ASD
Our dual-contingency task was designed to engage lexico-semantic processing as participants were instructed to press a button to real, standard words (SW, Go trials) with their left index finger and withhold responding to meaningless letter strings (PW, NoGo trials). If a word denoted an animal, they were instructed to respond with their left middle finger instead. This resulted in a response conflict which, combined with lower presentation frequency of the AN trials, required greater engagement of cognitive control. Accordingly, the AN trials were the most difficult in the present task (AN accuracy: 80.0 ± 15.3%), consistent with previous reports in young, healthy adults (Marinkovic et al. 2012; Marinkovic et al. 2014). Compared to the TD group, performance accuracy in ASD participants was reliably lower only for AN, whereas group differences for SW did not reach significance. This impaired performance to AN but not SW may reflect a deficit in targeted semantic retrieval in ASD. While lexico-semantic abilities are less impaired than pragmatics in ASD (Eigsti et al. 2011), they are often affected even in high-functioning individuals with ASD who do not show clinically relevant language impairment (Boucher 2012). In addition, this selective impairment could reflect impaired executive function in ASD, as this double-duty task imposes heavy demands on working memory, response selection, and cognitive control. Indeed, this challenging lexical decision task engages lexico-semantic and executive processing simultaneously. For PW, adolescents with ASD performed marginally worse than TD peers. ASD participants tended to have more false alarms on PW trials, which may be attributed to either reduced semantic memory storage or lower capacity to inhibit prepotent responses. The overall pattern of group differences in performance accuracy indicates that adolescents with ASD may have compromised targeted semantic retrieval and/or executive functions, which is in line with the previous evidence of such deficits (Hill 2004; Solomon et al. 2008; Boucher 2012; Poljac and Bekkering 2012; Inokuchi and Kamio 2013; Yeung et al. 2019). Indeed, our ASD participants had distinctly higher scores (indicating greater impairment) on a parent-report measure of executive functions (BRIEF-2 Global Executive Composite, Table 1).
Early Processing: Greater Theta Power in the Fusiform Cortex in ASD
Inspection of event-related theta activity maps and time courses (Fig. 2 and Fig. 3) revealed that overall the neural substrates and patterns of spatiotemporal progression associated with visual word processing are largely shared by ASD and TD groups. Upon reading a word, cortical theta activity starts in the visual cortex. It then proceeds anteriorly along the ventral visual stream and engages the left-dominant frontotemporal language network subserving semantic access and retrieval (Marinkovic et al. 2003; Pylkkanen and Marantz 2003; Marinkovic 2004; Van Petten and Luka 2006; Pulvermüller 2007; Marinkovic et al. 2012; Hulten et al. 2019). The finding of comparable spatial activation patterns across both groups is not unexpected (Sahyoun et al. 2010), given that our high-functioning adolescents with ASD demonstrated relatively spared semantic retrieval of SW and had similar language test scores as their TD peers.
The earliest theta activity was observed in the calcarine cortex at approximately 140 ms indicating the first cortical response to visually presented words (Fig. 2). No group differences were observed at this time, consistent with other MEG findings focused on activity in the early visual cortex (Orekhova et al. 2019). Between-group differences emerged in the first subsequent processing stage, however, with the ASD group showing greater theta in the FusCx overall, which peaked at approximately 180 ms (Fig. 2). The latency and spatial estimate of this effect are suggestive of a greater engagement of the fusiform visual word form area (Price 2000; McCandliss et al. 2003; Marinkovic 2004). This enhanced fusiform theta power is broadly consistent with previous studies reporting elevated activity of the associative visual cortex during linguistic processing in ASD (Kana et al. 2006; Gaffrey et al. 2007; Sahyoun et al. 2010). A meta-analysis of studies implementing a variety of visual word tasks confirmed higher activity in the fusiform gyrus and extrastriate visual cortex in ASD participants in the absence of group differences in behavioral performance between ASD and TD groups (Samson et al. 2012). It has been postulated that recruitment of visual cortices during semantic processing may reflect a perceptually based lexico-semantic strategy in ASD that relies more heavily on mental imagery and visualization (Just et al. 2004; Kana et al. 2006; Gaffrey et al. 2007). Indeed, a successful reading intervention in children with ASD was accompanied by increased activation of the visual cortices including the left fusiform gyrus during word comprehension (Murdaugh et al. 2017). In further potential support, Shen et al. (2012) reported atypically increased functional connectivity between left inferior frontal cortex and extensive occipitotemporal regions, including left FusCx, in a study of lexico-semantic processing in adolescents and young adults with ASD. Such an alternative perceptually based strategy may compare to strategies observed in preteen TD children who show high levels of visual cortical activation during lexical processing (Brown et al. 2005).
Event-Related Theta Is Greater in ASD during Lexico-Semantic Retrieval and Cognitive Control
The earliest condition-based group differentiation was observed in the LTC starting at approximately 250 ms (Fig. 3), with greater theta power to AN in the ASD relative to the TD group, which extended to the avPFC/iPFC 450–650 ms after stimulus onset. The left LTC and avPFC/iPFC together constitute a well-established left-lateralized frontotemporal network underlying lexical access and semantic memory retrieval (Noppeney and Price 2004; Binder et al. 2009; Price 2010; Visser et al. 2010; Liakakis et al. 2011). These regions are also known to be sensitive to increased task difficulty in studies employing semantic, as well as nonsemantic, paradigms (Philiastides et al. 2006; Binder et al. 2009; Rosen et al. 2016). The AN trials were cognitively demanding because they imposed multiple constraints: they captured attention, taxed vocabulary knowledge by requiring category-selective retrieval, and evoked response conflict by necessitating a response switch. As expected, this was reflected in lower accuracy, especially in ASD participants. Greater theta in ASD participants may thus indicate increased prefrontal and temporal recruitment needed to perform the task, consistent with its sensitivity to cognitive effort (Sasaki et al. 1996; Mitchell et al. 2008; Kovacevic et al. 2012; Cavanagh and Frank 2014; Wascher et al. 2014; Rosen et al. 2016; Beaton et al. 2018; Marinkovic et al. 2019). In light of the evidence that theta reflects long-range synchronization needed for retrieval and cognitive control (Halgren et al. 2015; Halgren et al. 2018), increased event-related theta activity observed in the present study may indicate inefficient processing and compensatory cortical activation in ASD participants.
Furthermore, theta is associated with orienting of attention (Basar et al. 2001; Dugue et al. 2016), which was engaged by AN trials. An alternative, though not mutually exclusive interpretation suggests that a greater increase in theta in ASD participants may be related to alterations in physiological circuits subserving heightened arousal during orienting. Indeed, there is considerable evidence of differences in the neurochemical circuitry in individuals with ASD. For instance, extensive evidence indicates that norepinephrine plays an important role in cognition, with an emphasis on attentional capture and arousal (Berridge and Waterhouse 2003; Sara and Bouret 2012). Individuals with ASD commonly show increased arousal (Anderson and Colombo 2009; Keehn et al. 2013) and anxiety (White et al. 2009). Higher plasma levels of norepinephrine in persons with ASD (Lam et al. 2006) are consistent with hyperarousal resulting from dysregulated homeostasis (London 2018) which could, in turn, be reflected in excitation/inhibition imbalance (Port et al. 2015). Pharmacological manipulations indicate that greater norepinephrine availability enhances theta oscillations (Hajos et al. 2003). Taken together, the present findings suggest that the selective retrieval and response switching on AN trials were particularly challenging to our sample of high-functioning ASD adolescents, which may have enhanced phasic norepinephrine release associated with orienting and arousal, resulting in greater event-related theta oscillations.
In the current study, PW were presented with equal probability as AN words. Serving as NoGo trials, PW required response inhibition and elicited response conflict. Even though nonsemantic NoGo trials typically elicit much greater theta than frequent Go trials (Holcomb et al. 2019), in this study, PW elicited the lowest theta power overall. This observation is consistent with the notion that in a language context, theta is primarily sensitive to the outcome of semantic retrieval (Bastiaansen and Hagoort 2006; Marinkovic et al. 2012; Halgren et al. 2015) rather than to response inhibition per se.
ASD and TD groups did not differ reliably in the frontotemporal theta power to SW, which is consistent with the absence of group differences in the behavioral indices of the basic lexico-semantic processing. Some functional imaging studies have reported equivalent or increased activations in the left prefrontal (Gaffrey et al. 2007; Kleinhans et al. 2008; Knaus et al. 2008) or temporal cortices (Just et al. 2004; Harris et al. 2006; Gaffrey et al. 2007) during semantic tasks in ASD participants, often without concurrent behavioral impairments (Just et al. 2004; Harris et al. 2006; Knaus et al. 2008). However, other studies have observed reduced left prefrontal or temporal activations in individuals with ASD during semantic processing of words or sentences (Müller et al. 1998; Kana et al. 2006; Groen et al. 2010; Tesink et al. 2011). These inconsistencies may be due to differences in task requirements (Kleinhans et al. 2008; Knaus et al. 2008), age range (Knaus et al. 2008), or language ability (Dawson et al. 1986; Herbert et al. 2005; Coffey-Corina et al. 2008).
Response Preparation and Execution: Greater Activation of the ACC and Motor Cortex in ASD
During the final stage (700–1000 ms), the most notable activation was observed in the areas subserving response preparation and execution (Fig. 4). As expected, the response-relevant right MotCx exhibited greater theta power for SW and AN in comparison to PW, which required no response. The right MotCx was activated similarly by both groups. In contrast, group differences were detected in the left MotCx, ipsilateral to the responding hand. While the TD participants showed lateralized motor activation with dampened event-related theta in the ipsilateral (left) MotCx, adolescents with ASD showed higher levels of theta power in the left MotCx, which may indicate reduced motor lateralization. In healthy cohorts, activation of the ipsilateral MotCx during the execution of a unilateral motor task has been mostly observed in children (Muller et al. 1997), in adults after stroke (Butefisch et al. 2005), and in tasks demanding complex movements (Verstynen and Ivry 2011; Buetefisch et al. 2014). As such, additional recruitment of the ipsilateral MotCx during left-hand button presses in participants with ASD indicates a less lateralized motor cortical network (Jansiewicz et al. 2006; Mostofsky and Ewen 2011; Gowen and Hamilton 2013; Nebel et al. 2014). Consistent with the notion of diffuse, poorly specified motor activations in ASD, studies have reported increased activations in regions beyond those directly involved in motor execution (Müller et al. 2001; Allen et al. 2004; Mostofsky et al. 2009). A more recent study (Carper et al. 2015) showed atypical motor asymmetries in children and adolescents with ASD, both anatomically with respect to the corticospinal tract and functionally based on connectivity of primary motor cortices, in line with our current aMEG findings.
Compared to TD peers, participants with ASD showed elevated theta power in the ACC for conditions requiring a response (i.e., SW and AN). As part of the frontal executive network, the ACC is a major generator of frontomidline theta reflecting increased demands on cognitive control (Wang et al. 2005; Botvinick 2007; Hanslmayr et al. 2008; Kovacevic et al. 2012; Cavanagh and Frank 2014; Marinkovic et al. 2019; ). The ACC is involved in motor planning, response selection, and execution, particularly under demanding conditions (Picard and Strick 1996; Paus 2001; Nachev 2006; Kovacevic et al. 2012; Rosen et al. 2016).
The increased ACC theta activity to AN/SW relative to PW observed in participants with ASD suggests that their ACC showed greater event-related theta power to response selection/execution than response inhibition. Greater ACC theta activity to SW was positively correlated with SCQ score (r = 0.63, P = 0.003, FDR-corrected) and ADI-R Communication score (r = 0.44, P = 0.05, uncorrected), both scores indicative of lower social functioning in adolescents with ASD. This lends support to the idea that the ACC over-recruitment may constitute a compensatory mechanism in adolescents with more significant social communication challenges, as greater ACC activation might be required for cognitive control and response selection in complex social situations. This association also appears to be specific to adolescents with ASD, as the same ACC theta to SW was predictive of better global execution functioning (as indicated by lower BRIEF-2 Global Executive Composite score, r = −.45, P = 0.046, uncorrected) in TD peers. Furthermore, no differential ACC activity to AN/SW and PW was observed for TD peers, indicating similar ACC engagement to response selection and inhibition. Overall, these preliminary findings are consistent with abundant evidence of atypical executive functions in ASD (Demetriou et al. 2018). Although not a core diagnostic feature of ASD, difficulties with inhibition and self-regulation are commonly reported in children and adults with ASD (Corbett et al. 2009; Craig et al. 2016).
Some limitations to the current study should be noted. First, given the demanding nature of the modified lexical decision task, our sample consists only of high-functioning adolescents with ASD, who had passed the WIAT-III screener and could read at a 6th grade reading level. As such, the generalizability of our findings to lower-functioning individuals with ASD, especially those with compromised language abilities, is limited. Second, we studied language and executive functions among adolescents of within a wide age range (12–20 years), during which asynchronous development may occur, with continued development in executive relative to lexico-semantic abilities (Sigman and McGovern 2005; Blakemore and Choudhury 2006). Finally, in addition to theta, gamma-band oscillations have also been implicated in language comprehension and unification (Bastiaansen and Hagoort 2006). Studies on oscillatory activity in other frequency bands would shed further light on neural mechanisms of atypical language and executive processes in ASD.
Conclusions
We used aMEG to examine spatiotemporal profiles of event-related theta power indexing lexico-semantic and cognitive control processes in high-functioning adolescents with ASD and age-matched TD peers. While no group differences were observed in the early responsivity of the visual cortex, participants with ASD showed atypically greater theta in the fusiform cortex. This may inform fMRI findings of increased early occipitotemporal activation in ASD during language tasks, which could previously not be attributed to specific processing stages due to fMRI’s low temporal resolution. Relative to TD participants, adolescents with ASD showed lower accuracy to AN words, which imposed demands on cognitive control and which elicited greater theta power in frontotemporal cortices during selective lexico-semantic retrieval. During the response preparation and execution stage, ASD participants exhibited increased activation of the ipsilateral MotCx, which may reflect a less lateralized and poorly differentiated motor cortical network. Simultaneously, increased activity in the ACC in ASD participants is consistent with greater engagement of cognitive control. Together, these findings provide further support for atypical language processing in ASD. The widespread activity increases in high-functioning individuals with ASD possibly indicate compensatory recruitment to offset inefficient lexico-semantic retrieval under cognitively demanding conditions.
Notes
We thank members of the Spatio-Temporal Brain Imaging Lab and the Brain Development Imaging Laboratories for assistance. Conflict of Interest: None declared.
Funding
The US National Institute of Mental Health (Research Grant R01 MH101173 to R.A.M.).

