-
PDF
- Split View
-
Views
-
Cite
Cite
Sten Madsbad, Jens J Holst, Cardiovascular effects of incretins: focus on glucagon-like peptide-1 receptor agonists, Cardiovascular Research, Volume 119, Issue 4, April 2023, Pages 886–904, https://doi.org/10.1093/cvr/cvac112
Close - Share Icon Share
Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have been used to treat patients with type 2 diabetes since 2005 and have become popular because of the efficacy and durability in relation to glycaemic control in combination with weight loss in most patients. Today in 2022, seven GLP-1 RAs, including oral semaglutide are available for treatment of type 2 diabetes. Since the efficacy in relation to reduction of HbA1c and body weight as well as tolerability and dosing frequency vary between agents, the GLP-1 RAs cannot be considered equal. The short acting lixisenatide showed no cardiovascular benefits, while once daily liraglutide and the weekly agonists, subcutaneous semaglutide, dulaglutide, and efpeglenatide, all lowered the incidence of cardiovascular events. Liraglutide, oral semaglutide and exenatide once weekly also reduced mortality. GLP-1 RAs reduce the progression of diabetic kidney disease. In the 2019 consensus report from European Association for the Study of Diabetes/American Diabetes Association, GLP-1 RAs with demonstrated cardio-renal benefits (liraglutide, semaglutide and dulaglutide) are recommended after metformin to patients with established cardiovascular diseases or multiple cardiovascular risk factors. European Society of Cardiology suggests starting with a sodium-glucose cotransprter-2 inhibitor or a GLP-1 RA in drug naïve patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (CVD) or high CV Risk. However, the results from cardiovascular outcome trials (CVOT) are very heterogeneous suggesting that some GLP-1RAs are more suitable to prevent CVD than others. The CVOTs provide a basis upon which individual treatment decisions for patients with T2D and CVD can be made.
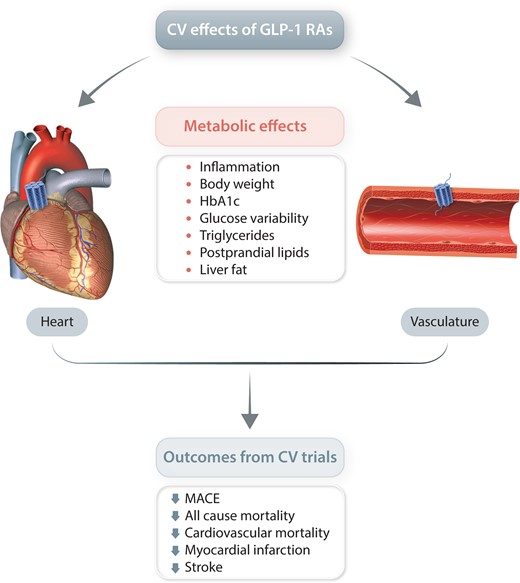
1. Introduction
In patients with type 2 diabetes (T2D) cardiovascular disease (CVD) is the leading cause of death.1 CVD is associated to central obesity, physical inactivity, blood pressure, lipid disturbances, platelet/clotting factor changes, hypoglycaemia as well as hyperglycaemia and some, more poorly described, risk factors like inflammation.1,2 Today, the treatment of T2DM therefore focuses not only on treatment of hyperglycaemia but also on reduction of additional cardiovascular risk factors.2–4
The use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) in people with T2D has become popular because of their efficacy and durability in relation to glycaemic control combined with a low risk of hypoglycaemia, as well as weight loss, resulting from decreased appetite and increasing satiety in most patients.5,6 However, the effects on glycaemic control and body weight differ between the GLP-1RAs.
GLP-1 RAs mimic the effects of native GLP-1, which increases insulin secretion, inhibits glucagon secretion, increases satiety and slows gastric emptying.5,6 Notably, the insulinotropic and glucagonostatic effects are glucose dependent, and therefore the risk of hypoglycaemia is very low during treatment with a GLP-1 RA, unless it is combined with sulfonylurea or insulin.5–7 Furthermore, during hypoglycaemia treatment with a GLP-1 RA does not, for unexplained reasons, impair the glucagon response or the general hypoglycaemic counter-regulation.8 The effect on gastric emptying is primarily observed with the short acting GLP-1 RAs, since tachyphylaxis for this effect develops after few days’ treatment with the long acting GLP-1 RAs.9,10 The postprandial glucose control exerted by the short acting GLP-1 RA seems to be primarily explained through the delaying effect on gastric emptying rather than the effect on insulin and glucagon secretion, while the long acting GLP-1 RAs reduce the 24 h glucose profile including fasting plasma glucose primarily by enhancing beta cell sensitivity to glucose and decreasing glucagon secretion.10 In addition, GLP-1 RAs reduce blood pressure during chronic treatment, increase pulse rate, reduce postprandial triglyceride concentrations and modify low-grade inflammation as discussed thoroughly in.11–13 The most common adverse events of GLP-1 RAs are nausea, vomiting and other gastrointestinal discomfort.5,6 Other drawbacks of the GLP-1 RAs include the subcutaneous administration (which may now be avoided with oral semaglutide) and cost.5
Their use as second-line therapy after first-line metformin and as third or fourth agents (in combination with metformin and sulphonylurea/thiazolidinedione/sodium-glucose cotransprter-2 (SGLT-2) inhibitor or insulin) is covered in the 2018 American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) consensus report for treatment of people with T2D.3 The ADA/EASD updated consensus report of 2019 also recommends the use of a GLP-1 RA with proven positive effect on CVD in patients with T2D and established atherosclerotic CVD or in high risk for cardiovascular events (age > 55 years and coronary, carotid or lower extremity artery stenosis > 50%, or left ventricular hypertrophy), independent of actual HbA1c.14 An SGLT-2 inhibitor is recommended to patients with heart failure and reduced ejection fraction or diabetic kidney disease (DKD) (microalbuminuria and estimated glomerular filtration rate (eGFR) < 60 mL/min or macroalbuminuria).14
The effect of the GLP-1 RAs on cardiovascular disease is an area of major interest and will be discussed in this review. First, the differences between GLP-1 RAs will be discussed. Thereafter, the effect of GLP-1 and GLP-1 RAs on cardiovascular risk factors and GLP-1 RAs effects on major adverse cardiovascular events (MACEs), heart failure and cardiovascular and total mortality will be updated. Lastly, the cardiovascular effects of glucose-dependent insulinotropic polypeptide (GIP) and the dual agonist (GIP/GLP-1) tirzepatide will be discussed. The present review will primarily focus on human studies, but in the discussion of potential mechanisms underlying the cardioprotective effects of GLP-1 and GLP-1 RAs, preclinical studies will be briefly included. During the last few years, several excellent reviews have summarized different aspects of treatment with GLP-1 RAs and of the cardiovascular and renal findings with GLP-1 RAs.11,15–30
2. Differences between GLP-1 RAs
At present, seven GLP-1 RAs are (2022) approved in Europe and the US. GLP-1 RAs differ substantially in their molecular structures, molecular sizes, other chemical and physiological properties, as well as duration of action.23,31–33 Partly because of this, they also differ with respect to clinical outcomes.6,3,34 Based on duration of action, the GLP1- RAs can be classified as short acting [few hours daily, this group comprises lixisenatide once daily (OD) and exenatide twice daily (BID)] or long-acting, providing more or less constant exposure [liraglutide, once daily, and dulaglutide, semaglutide, exenatide and efpeglanitide with weekly (OW) administration]. Semaglutide has also been developed as a tablet for daily administration, but when absorbed into the blood it has the half-life of the injectable semaglutide once weekly.35 The GLP-1 RAs can also be subdivided into those based on human GLP-1 (liraglutide, semaglutide, dulaglutide, albiglutide) and the exendin-based agonists (lixisenatide, exenatide, and efpeglenatide).
3. Head-to Head comparisons of GLP-1 RAs
The effects of the GLP-1 receptor agonists differ with respect to reductions in HbA1c and body weight. The effect on body weight seems to be dose dependent, and with the greatest mean weight loss observed in subjects with the highest body mass index. Valid comparisons between the GLP-1 RAs require head-to-head studies in controlled, randomised trials. Effects on HbA1c and body weight are illustrated in Table 1. In total, 17 head-to-head trials have compared the GLP-1 RAs.36–50
| Study . | Comparators . | Study size and duration . | Basal HbA1c (%) . | Basal body weight (kg) . | Changes in HbA1c (%) . | Changes in body weight (kg) . |
|---|---|---|---|---|---|---|
| LEAD-6 (Reference38) | Exenatide 10 mcg BID Liraglutide 1.8 mg OD | N = 444 26 weeks | 8.1 | 93 | −0.79a −1.12 | −2.87 −3.24 |
| DURATION-1 (Reference41) | Exenatide 10 mcg BID Exenatide 2 mg OW | N = 295 30 weeks | 8.3 | 102 | −1.5a −1.9 | −3.6 −3.7 |
| DURATION-5 (Reference37) | Exenatide 10 mcgBID Exenatide 2 mg OW | N = 252 24 weeks | 8.4 | 96 | −0.9a −1.6 | −1.4 −2.3 |
| DURATION-6 (Reference39) | Exenatide 2 mg OW Liraglutide 1.8 mg OD | N = 911 26 weeks | 8.5 | 91 | −1.28a −1.48 | −2.68a −3.57 |
| GetGOAL-X (Reference47) | Lixisenatide 20 mcg OD Exenatide 10 mcg BID | N = 634 24 weeks | 8.0 | 95 | −0.79a −0.96 | −2.96a −3.98 |
| LIRA-LIXI (Reference43) | Liraglutide1.8 mg OD Lixisenatide 20 mcg OD | N = 404 26 weeks | 8.4 | 101 | −1.8a −1.2 | −4.3 −3.7 |
| HARMONY-7 (Reference45) | Albiglutide 50 mg OW Liraglutide 1.8 mg OD | N= 841 32 weeks | 8.2 | 93 | −1.2a −1.8 | −0.6a −2.2 |
| AWARD-1 (Reference48) | Dulaglutide 1.5 mg OW Exenatide 10 mcg BID | N = 978 52 weeks | 8.1 | 96 | −1.51a −0.99 | −1.3 −1.1 |
| AWARD-6 (Reference42) | Dulaglutide 1.5 mg OW Liraglutide 1.8 mg OD | N = 599 26 weeks | 8.1 | 94 | −1.42 −1.36 | −2.9a −3.6 |
| SUSTAIN-3 (Reference36) | Semaglutide 1 mg OW Exenatide 2 mg OW | N = 813 56 weeks | 8.3 | 96 | −1.5a −0.9 | −5.6a −1.9 |
| SUSTAIN-7 (Reference46) | Semaglutide 1 mg OW Dulaglutide 1.5 mg OW | N = 1201 40 weeks | 8.2 | 93 | −1.8a −1.4 | −6.5a −3.0 |
| SUSTAIN-10 (Reference40) | Semaglutide 1.0 mg OW Liraglutide 1.2 mg OD | N = 577 30 weeks | 8.2 | 97 | −1.7a −1.0 | −5.8a −1.9 |
| PIONEER-4 (Reference44) | Oral Semaglutide 14 mg OD Liraglutide 1.8 mg OD | N = 711 52 weeks | 8.0 | 94 | −1.3a −1.1 | −4.4a −3.1 |
| PIONEER-9 (Reference50) | Oral semaglutide 14 mg OD Liraglutide 0.9 mg OD | N = 243 52 weeks | 8.2 | 71 | −1.5 −1.1 | −2.0 0 |
| PIONEER-10 (Reference49) | Oral semaglutide 14 mg OD Dulaglutide 0.75 mg OW | N = 458 57 weeks | 8.3 | 72 | −1.7a −1.4 | −1.6a + 1.0 |
| SUSTAIN FORTE (Reference51) | Semaglutide 1 mg OW Semaglutide 2 mf OW | N = 961 40 weeks | 8.9 | 99 | −1.9a −2.1 | −5.6a −6.4 |
| AWARD-11 (Reference52) | Dulaglutide 1.5 mg OW Dulaglutide 3.0 mg OW Dulaglutide 4.5 mg OW | N= 1842 36 week | 8.6 | 96 | −1.5 −1.6 −1.8a | −3.0 −3.8 −4.6a |
| Study . | Comparators . | Study size and duration . | Basal HbA1c (%) . | Basal body weight (kg) . | Changes in HbA1c (%) . | Changes in body weight (kg) . |
|---|---|---|---|---|---|---|
| LEAD-6 (Reference38) | Exenatide 10 mcg BID Liraglutide 1.8 mg OD | N = 444 26 weeks | 8.1 | 93 | −0.79a −1.12 | −2.87 −3.24 |
| DURATION-1 (Reference41) | Exenatide 10 mcg BID Exenatide 2 mg OW | N = 295 30 weeks | 8.3 | 102 | −1.5a −1.9 | −3.6 −3.7 |
| DURATION-5 (Reference37) | Exenatide 10 mcgBID Exenatide 2 mg OW | N = 252 24 weeks | 8.4 | 96 | −0.9a −1.6 | −1.4 −2.3 |
| DURATION-6 (Reference39) | Exenatide 2 mg OW Liraglutide 1.8 mg OD | N = 911 26 weeks | 8.5 | 91 | −1.28a −1.48 | −2.68a −3.57 |
| GetGOAL-X (Reference47) | Lixisenatide 20 mcg OD Exenatide 10 mcg BID | N = 634 24 weeks | 8.0 | 95 | −0.79a −0.96 | −2.96a −3.98 |
| LIRA-LIXI (Reference43) | Liraglutide1.8 mg OD Lixisenatide 20 mcg OD | N = 404 26 weeks | 8.4 | 101 | −1.8a −1.2 | −4.3 −3.7 |
| HARMONY-7 (Reference45) | Albiglutide 50 mg OW Liraglutide 1.8 mg OD | N= 841 32 weeks | 8.2 | 93 | −1.2a −1.8 | −0.6a −2.2 |
| AWARD-1 (Reference48) | Dulaglutide 1.5 mg OW Exenatide 10 mcg BID | N = 978 52 weeks | 8.1 | 96 | −1.51a −0.99 | −1.3 −1.1 |
| AWARD-6 (Reference42) | Dulaglutide 1.5 mg OW Liraglutide 1.8 mg OD | N = 599 26 weeks | 8.1 | 94 | −1.42 −1.36 | −2.9a −3.6 |
| SUSTAIN-3 (Reference36) | Semaglutide 1 mg OW Exenatide 2 mg OW | N = 813 56 weeks | 8.3 | 96 | −1.5a −0.9 | −5.6a −1.9 |
| SUSTAIN-7 (Reference46) | Semaglutide 1 mg OW Dulaglutide 1.5 mg OW | N = 1201 40 weeks | 8.2 | 93 | −1.8a −1.4 | −6.5a −3.0 |
| SUSTAIN-10 (Reference40) | Semaglutide 1.0 mg OW Liraglutide 1.2 mg OD | N = 577 30 weeks | 8.2 | 97 | −1.7a −1.0 | −5.8a −1.9 |
| PIONEER-4 (Reference44) | Oral Semaglutide 14 mg OD Liraglutide 1.8 mg OD | N = 711 52 weeks | 8.0 | 94 | −1.3a −1.1 | −4.4a −3.1 |
| PIONEER-9 (Reference50) | Oral semaglutide 14 mg OD Liraglutide 0.9 mg OD | N = 243 52 weeks | 8.2 | 71 | −1.5 −1.1 | −2.0 0 |
| PIONEER-10 (Reference49) | Oral semaglutide 14 mg OD Dulaglutide 0.75 mg OW | N = 458 57 weeks | 8.3 | 72 | −1.7a −1.4 | −1.6a + 1.0 |
| SUSTAIN FORTE (Reference51) | Semaglutide 1 mg OW Semaglutide 2 mf OW | N = 961 40 weeks | 8.9 | 99 | −1.9a −2.1 | −5.6a −6.4 |
| AWARD-11 (Reference52) | Dulaglutide 1.5 mg OW Dulaglutide 3.0 mg OW Dulaglutide 4.5 mg OW | N= 1842 36 week | 8.6 | 96 | −1.5 −1.6 −1.8a | −3.0 −3.8 −4.6a |
In AWARD-11, significant differences in HbA1c and body weight reduction were observed between 1.5 mg vs. 4.5 mg OW.
Indicates statistically significant difference between comparators in relation to reduction in HbA1c in % percent point or in body weight in kg.
| Study . | Comparators . | Study size and duration . | Basal HbA1c (%) . | Basal body weight (kg) . | Changes in HbA1c (%) . | Changes in body weight (kg) . |
|---|---|---|---|---|---|---|
| LEAD-6 (Reference38) | Exenatide 10 mcg BID Liraglutide 1.8 mg OD | N = 444 26 weeks | 8.1 | 93 | −0.79a −1.12 | −2.87 −3.24 |
| DURATION-1 (Reference41) | Exenatide 10 mcg BID Exenatide 2 mg OW | N = 295 30 weeks | 8.3 | 102 | −1.5a −1.9 | −3.6 −3.7 |
| DURATION-5 (Reference37) | Exenatide 10 mcgBID Exenatide 2 mg OW | N = 252 24 weeks | 8.4 | 96 | −0.9a −1.6 | −1.4 −2.3 |
| DURATION-6 (Reference39) | Exenatide 2 mg OW Liraglutide 1.8 mg OD | N = 911 26 weeks | 8.5 | 91 | −1.28a −1.48 | −2.68a −3.57 |
| GetGOAL-X (Reference47) | Lixisenatide 20 mcg OD Exenatide 10 mcg BID | N = 634 24 weeks | 8.0 | 95 | −0.79a −0.96 | −2.96a −3.98 |
| LIRA-LIXI (Reference43) | Liraglutide1.8 mg OD Lixisenatide 20 mcg OD | N = 404 26 weeks | 8.4 | 101 | −1.8a −1.2 | −4.3 −3.7 |
| HARMONY-7 (Reference45) | Albiglutide 50 mg OW Liraglutide 1.8 mg OD | N= 841 32 weeks | 8.2 | 93 | −1.2a −1.8 | −0.6a −2.2 |
| AWARD-1 (Reference48) | Dulaglutide 1.5 mg OW Exenatide 10 mcg BID | N = 978 52 weeks | 8.1 | 96 | −1.51a −0.99 | −1.3 −1.1 |
| AWARD-6 (Reference42) | Dulaglutide 1.5 mg OW Liraglutide 1.8 mg OD | N = 599 26 weeks | 8.1 | 94 | −1.42 −1.36 | −2.9a −3.6 |
| SUSTAIN-3 (Reference36) | Semaglutide 1 mg OW Exenatide 2 mg OW | N = 813 56 weeks | 8.3 | 96 | −1.5a −0.9 | −5.6a −1.9 |
| SUSTAIN-7 (Reference46) | Semaglutide 1 mg OW Dulaglutide 1.5 mg OW | N = 1201 40 weeks | 8.2 | 93 | −1.8a −1.4 | −6.5a −3.0 |
| SUSTAIN-10 (Reference40) | Semaglutide 1.0 mg OW Liraglutide 1.2 mg OD | N = 577 30 weeks | 8.2 | 97 | −1.7a −1.0 | −5.8a −1.9 |
| PIONEER-4 (Reference44) | Oral Semaglutide 14 mg OD Liraglutide 1.8 mg OD | N = 711 52 weeks | 8.0 | 94 | −1.3a −1.1 | −4.4a −3.1 |
| PIONEER-9 (Reference50) | Oral semaglutide 14 mg OD Liraglutide 0.9 mg OD | N = 243 52 weeks | 8.2 | 71 | −1.5 −1.1 | −2.0 0 |
| PIONEER-10 (Reference49) | Oral semaglutide 14 mg OD Dulaglutide 0.75 mg OW | N = 458 57 weeks | 8.3 | 72 | −1.7a −1.4 | −1.6a + 1.0 |
| SUSTAIN FORTE (Reference51) | Semaglutide 1 mg OW Semaglutide 2 mf OW | N = 961 40 weeks | 8.9 | 99 | −1.9a −2.1 | −5.6a −6.4 |
| AWARD-11 (Reference52) | Dulaglutide 1.5 mg OW Dulaglutide 3.0 mg OW Dulaglutide 4.5 mg OW | N= 1842 36 week | 8.6 | 96 | −1.5 −1.6 −1.8a | −3.0 −3.8 −4.6a |
| Study . | Comparators . | Study size and duration . | Basal HbA1c (%) . | Basal body weight (kg) . | Changes in HbA1c (%) . | Changes in body weight (kg) . |
|---|---|---|---|---|---|---|
| LEAD-6 (Reference38) | Exenatide 10 mcg BID Liraglutide 1.8 mg OD | N = 444 26 weeks | 8.1 | 93 | −0.79a −1.12 | −2.87 −3.24 |
| DURATION-1 (Reference41) | Exenatide 10 mcg BID Exenatide 2 mg OW | N = 295 30 weeks | 8.3 | 102 | −1.5a −1.9 | −3.6 −3.7 |
| DURATION-5 (Reference37) | Exenatide 10 mcgBID Exenatide 2 mg OW | N = 252 24 weeks | 8.4 | 96 | −0.9a −1.6 | −1.4 −2.3 |
| DURATION-6 (Reference39) | Exenatide 2 mg OW Liraglutide 1.8 mg OD | N = 911 26 weeks | 8.5 | 91 | −1.28a −1.48 | −2.68a −3.57 |
| GetGOAL-X (Reference47) | Lixisenatide 20 mcg OD Exenatide 10 mcg BID | N = 634 24 weeks | 8.0 | 95 | −0.79a −0.96 | −2.96a −3.98 |
| LIRA-LIXI (Reference43) | Liraglutide1.8 mg OD Lixisenatide 20 mcg OD | N = 404 26 weeks | 8.4 | 101 | −1.8a −1.2 | −4.3 −3.7 |
| HARMONY-7 (Reference45) | Albiglutide 50 mg OW Liraglutide 1.8 mg OD | N= 841 32 weeks | 8.2 | 93 | −1.2a −1.8 | −0.6a −2.2 |
| AWARD-1 (Reference48) | Dulaglutide 1.5 mg OW Exenatide 10 mcg BID | N = 978 52 weeks | 8.1 | 96 | −1.51a −0.99 | −1.3 −1.1 |
| AWARD-6 (Reference42) | Dulaglutide 1.5 mg OW Liraglutide 1.8 mg OD | N = 599 26 weeks | 8.1 | 94 | −1.42 −1.36 | −2.9a −3.6 |
| SUSTAIN-3 (Reference36) | Semaglutide 1 mg OW Exenatide 2 mg OW | N = 813 56 weeks | 8.3 | 96 | −1.5a −0.9 | −5.6a −1.9 |
| SUSTAIN-7 (Reference46) | Semaglutide 1 mg OW Dulaglutide 1.5 mg OW | N = 1201 40 weeks | 8.2 | 93 | −1.8a −1.4 | −6.5a −3.0 |
| SUSTAIN-10 (Reference40) | Semaglutide 1.0 mg OW Liraglutide 1.2 mg OD | N = 577 30 weeks | 8.2 | 97 | −1.7a −1.0 | −5.8a −1.9 |
| PIONEER-4 (Reference44) | Oral Semaglutide 14 mg OD Liraglutide 1.8 mg OD | N = 711 52 weeks | 8.0 | 94 | −1.3a −1.1 | −4.4a −3.1 |
| PIONEER-9 (Reference50) | Oral semaglutide 14 mg OD Liraglutide 0.9 mg OD | N = 243 52 weeks | 8.2 | 71 | −1.5 −1.1 | −2.0 0 |
| PIONEER-10 (Reference49) | Oral semaglutide 14 mg OD Dulaglutide 0.75 mg OW | N = 458 57 weeks | 8.3 | 72 | −1.7a −1.4 | −1.6a + 1.0 |
| SUSTAIN FORTE (Reference51) | Semaglutide 1 mg OW Semaglutide 2 mf OW | N = 961 40 weeks | 8.9 | 99 | −1.9a −2.1 | −5.6a −6.4 |
| AWARD-11 (Reference52) | Dulaglutide 1.5 mg OW Dulaglutide 3.0 mg OW Dulaglutide 4.5 mg OW | N= 1842 36 week | 8.6 | 96 | −1.5 −1.6 −1.8a | −3.0 −3.8 −4.6a |
In AWARD-11, significant differences in HbA1c and body weight reduction were observed between 1.5 mg vs. 4.5 mg OW.
Indicates statistically significant difference between comparators in relation to reduction in HbA1c in % percent point or in body weight in kg.
From the head-to-head comparisons, the most effective GLP-1 RA is semaglutide SC OW or oral OD both with respect to reduction in HbA1c and body weight; followed by liraglutide 1.8 mg OD and dulaglutide 1.5 mg OW, Table 1.36–50 Semaglutide 2 mg OW compared with semaglutide 1 mg OW recently showed additional reductions in HbA1c and body weight (Table 1), and in another study, dulaglutide, escalating from 1.5 mg to 3.0 mg or 4.5 mg OW provided dose-dependent reduction in HbA1c and body weight (Table 1).51,52 However, a network meta-regression analysis suggested significantly greater reductions from baseline in HbA1c and body weight with semaglutide 2.0 mg versus dulaglutide 3.0 mg and 4.5 mg OW.53 Currently, (March 2022) the higher doses of semaglutide and dulaglutide are not yet available for treatment of T2D.
Especially the different duration of diabetes and their background antidiabetic medication hinder comparisons between the different trials, since these differences are related to different secretory capacities of insulin, which critically govern the glycaemic response to GLP-1RA therapy.54
4. Effects of GLP-1 RAs on CVD risk factors
The classical cardiovascular risk factors include hyperglycaemia, blood pressure, lipoproteins, and increased body weight, and a reduction in these factors is supposed to decrease the risk of developing a cardiovascular event.2 Improvements of these risk factors and additional mechanisms that may underlie the beneficial CV effect of the GLP-1 RAs such as effect on inflammation, endothelial function, liver fat, epicardial fat, kidney function and heart effects will be reviewed below.
4.1 Hyperglycaemia
The importance of glucose control for reducing cardiovascular diseases has been difficult to demonstrate, not the least because of the short duration (< 10 years) of most studies, but in the UK Prospective Diabetes Study study with very long follow-up, hyperglycaemia increased the risk of cardiovascular disease in patients with T2D and furthermore a legacy or memory effect was noted regarding the effect of early strict glycaemic control on the risk of development of late diabetic complications.55,56 In contrast, in patients with long duration of diabetes, both the Action to Control Cardiovascular risk in Diabetes (ACCORD) and the Action in Diabetes and Vascular disease: preterax and Diamicron MR controlled Evaluation trial demonstrated that attempts at reaching near- or normo-glycaemia for about 3–5 years did not reduce CVD cardiovascular events.56 Rather, the ACCORD, based on intensification with thiazolidineones, sulfonylureas, metformin and insulin, was associated with a significantly increased risk of cardiovascular and total mortality.56 The Veterans Affairs Diabetes Trial also reported that intensive glycaemic control did not reduce the rate of cardiovascular events.56 How glycaemic control per se affects risk of CVD is unclear, partly because groups in strict glycaemic control have a higher risk of severe hypoglycaemia, explained by an aggressive use of insulin to reach unrealistic targets of glycaemic control.56
A major breakthrough in this area was the demonstration that the SGLT-2 inhibitor empagliflozin and the GLP-1 receptor agonist liraglutide significantly reduced risk of MACEs including cardiovascular mortality in patients with established CVD.57,58 The benefit of these agents probably cannot be explained solely by the improvement in glycaemic control in the active treated groups compared with placebo, although a meta-regression analysis and an exploratory mediation analysis of the Liraglutide Effect and Action in Diabetes (LEADER) trial showed a linear relation between the degree of lowering of HbA1c and the hazard of MACE or stroke.59,60
4.2 Body weight
The effect of GLP-1 RAs on body weight is illustrated in Table 1 but notably, the body weight reducing effects of the GLP-1 RAs show considerable individual variability. The weight loss in the CVOT will be discussed below, but the most potent GLP-1 RA is semaglutide once weekly with a mean weight loss of about 5–6 kg in the phase 3 programme using 1 mg weekly, which is nearly 2-fold more than seen with the other GLP-1 RAs.33,36,46 A similar weight loss was associated with a reduced risk of CVD in the Look AHEAD study.61 In addition, it is difficult not to assign an important role of weight loss for the beneficial effects of weight loss after bariatric surgery.62
4.3 Blood pressure
In a meta-analysis of six trials, exenatide twice daily decreased systolic blood pressure by 2–4 mm Hg compared with insulin or placebo.63 In the CVOT trials, systolic blood pressure was reduced from 0.6–4 mm Hg as discussed in detail below.64,57,65,66–69
Notably, in a study involving 24 h ambulatory blood pressure monitoring, the effect of liraglutide on blood pressure was minimal or absent ,70 and a meta-analysis of 4 studies using 24-h ambulatory blood pressure revealed no clinically relevant differences in blood pressure between liraglutide versus placebo.70 In a recent meta-analysis of 18 randomized clinical trials including both subjects with T2D as well as glucose-tolerant people with obesity, liraglutide reduced systolic blood pressure by 3.2 mm Hg compared with placebo, but had no effect on diastolic blood pressure.71 Moreover, the effect of liraglutide on blood pressure was dose dependent up to 3.0 mg. The difference was no longer significant when the intervention lasted more than 1 year.71 Liraglutide did not reduce blood pressure in normotensive people and has not been associated with development of hypotension.72
The mechanisms behind the effect of liraglutide on blood pressure are poorly understood. Acute infusion of GLP-1 produced natriuresis in healthy individuals providing that the extracellular volume is expanded, but the effect seems to vanish over time.73,74 GLP-1 does not increase atrial natriuretic hormone in humans, but was reported to do so in mice.75 Acute experiments in human have indicated that GLP-1 infusions are accompanied by lower angiotensin II concentration.76,77 In contrast, 3 weeks treatment with dulaglutide 1.5 mg OW reduced systolic blood pressure but with no effect on plasma renin, angiotensin II, aldosterone and urinary sodium excretion levels compared with placebo.78 Systemic vascular resistance appears to be unchanged, although experiments have indicated that GLP-1 may have a vasodilatory effect.79–81
4.4 Lipoproteins
The effect of GLP-1 RAs on fasting lipids has been minimal in the cardiovascular outcome trials (CVOTs).57,64–69,82 A small elevation in HDL cholesterol in combination with a minor reduction in triglycerides and LDL cholesterol have been reported in a meta-analysis of studies investigating liraglutide.83
Liraglutide and semaglutide in patients with T2DM significantly and markedly reduce postprandial excursions of triglyceride and apolipoprotein B48 after a fat-rich meal, independently of gastric emptying rate,12,84 possibly by attenuating intestinal lipoprotein production and by increasing ApoB48 clearance.85,86
4.5 Miscellaneous cardiovascular risk markers including inflammation
Elevated levels of certain circulating biomarkers are consistently found in people with T2D. Treatment with GLP-1RAs or native GLP-1 generally reduce levels of PAI-1, B-type natriuretic peptide, intercellular adhesion molecule-1, monocyte chemoattractant protein-1 (MCP-1), tumour necrosis factor (TNF)- alfa, interleukin (IL)-1 beta, IL-6, and C-reactive protein levels are reduced, while adiponectin increases, also when compared with other antidiabetic treatments,15,28,87,88 all of which may play a role in CV protection.89,90 GLP-1 also reduces expression of matrix metalloproteinase-2 (MMP2), which is produced in vascular cells in response to mechanical injury and which is increased in T2DM patients. A reduction in MPP2 concentrations may be a mechanism for preventing vascular damage and plaque rupture.91
Treatment with GLP-1 decreased monocyte/macrophage accumulation and RNA expression of inflammatory markers as TNF-alfa and MCP-1 in the arterial wall of ApoE−/− mice.92 Accordingly, infusion of native GLP-1 decreased the development of atherosclerotic lesions in the aortic wall in the ApoE knockout mice, and this effect could be blocked by the exendin 9–39, a specific GLP-1 receptor antagonist.87 In the same model liraglutide has been shown to inhibit the progression of vascular disease by effects on atherogenesis, plaque stability and endothelial function93 and to reduce monocyte/macrophage infiltration in and to suppress foam cell formation.94 Lastly, GLP-1 treatment has been shown to suppress oxidized LDL-induced foam cell formation in a receptor dependent manner in experimental animals.95
Liraglutide also reduced the expression of the inflammatory macrophage activation molecule, CD163, independent of changes in HbA1c and weight, supporting a direct role of liraglutide on innate, immune cell driven inflammation in individuals with T2D.96 In contrast to these positive studies, Koska et al.97 reported that the GLP-1 RA, exenatide OW, had no effect on carotid plaque volume and composition in 163 patients with T2D during a 18 months follow-up. Another study (without a placebo group) showed that liraglutide significantly reduced carotid intima-media thickness.98 In a recent study, it was investigated whether liraglutide (vs. placebo) has a direct anti-inflammatory effect in the coronary arteries using positron emission tomography and a radioactive tracer targeting activated macrophages in the coronary vessel wall.99 After 26 weeks of treatment, liraglutide caused a significant reduction in tracer uptake in the coronary arteries whereas this was not seen in the placebo group. The mean changes in uptake values between the two groups was borderline significant. In theory, an effect of GLP-1 RAs on inflammation may stabilize the atherogenic plaque and reduce the risk of acute cardiovascular events and attenuate development of arteriosclerosis. It has been shown that most acute AMIs occur in people with unstable plaques.100
4.6 Epicardial fat
Epicardial adipose tissue, the metabolically active visceral fat depot of the heart which appears to have anatomical and functional contiguity with the myocardium and coronary arteries has been suggested to represent a modifiable cardiovascular risk factor.101 In a 6 months randomized study, liraglutide reduced epicardial adipose tissue by 36%, compared with no reduction in the control group.102 The authors speculate that this could represent a mechanism behind the cardioprotective effect of liraglutide.102
4.7 Effect on endothelial function
Excessive formation of reactive oxygen species has been identified as a central mechanism of endothelial dysfunction, which contributes to macro- and micro-vascular complications. GLP-1 RAs have been reported to increase nitric oxide synthesis in human endothelial cells,103,104 and to improve endothelial function and acetylcholine-induced vasodilation.105 Furthermore, liraglutide treatment was reported to reduce intima media thickness or slow the progression of widening when administered over a period of 18 months. This would support a beneficial effect on atherosclerosis.98 In a mouse model of restenosis, liraglutide reduced vascular inflammation and suppressed neointima hyperplasia via a mechanism that appeared to involve improvements in nitric oxide bioavailability.106
On the other hand, administration of GLP-1 into the brachial artery with or without sitagliptin had no effect on forearm blood flow.107 Additional negative results with liraglutide with respect to improving endothelial dysfunction have also been reported.105,108 Thus, there was no effect on capillary perfusion or vasomotor activity of administration of liraglutide for 12 weeks in subjects with T2D.109 Similarly, there was no effect of liraglutide on coronary or peripheral blood flow in a randomized placebo controlled study.110 However, intravenous exenatide has been reported to increase myocardial blood flow.111 Thus, the effect of GLP-1 on vascular function remains unclear.
4.8 Effect on liver
Patients with non-alcoholic steatohepatitis have an increased risk of cardiovascular disease, which increases with nonalcoholic fatty liver disease progression, specifically fibrosis.112 Hepatocyte do not express the GLP-1 receptors,113 and any effect of GLP-1 RAs on non-alcoholic steatohepatitis may be secondary to weight loss, metabolic effects or effects on inflammation. Liraglutide and semaglutide treatment for 1 to 1.5 years improved liver histology and decrease the rate of progression to fibrosis and non-alcoholic steatohepatitis but with no effect on fibrosis stage.114,115
4.9 Effect on the kidney
DKD is one of the most common complications in T2DM and a major cause of morbidity and mortality in diabetes, and about 40% of patients with T2D are affected by DKD.116 The CVOTs with GLP-1 show significant renoprotective effects including reduced risk of macroalbuminuria and a composite kidney endpoint including macroalbuminuria and kidney function based on eGFR.117,118 The change in the composite endpoint was driven mainly by effects on new persistent macroalbuminuria with no significant effect on the components representing more later stage progression.119,120 This is a definite difference to findings with SGLT-2 inhibitors.121 However, recent data from AWARD 7 and also accumulated data obtained with semaglutide indicate that the decline in eGFR of DKD may be dampened by the GLP-1 RAs.122,123 Note, that the renal outcomes in the CVOTs discussed below were included as secondary outcomes or explorative analyses.
In a mediation analysis of LEADER and The preapproval Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN 6) trials, HbA1c improvement was the only significant factor, but attributable to only 24% of the effect of the renal outcome.124 Changes in body weight and blood pressure had no effect. Other mechanisms could be effects on natriuresis, reduction in hyperfiltration and the renin-angiotensin-aldosterone system activity and anti-inflammatory properties.125,126 The renoprotection by semaglutide is currently being investigated in the dedicated FLOW (A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease) study (NCT03819153) expected to report in 2024.
4.10 Direct effect on the heart and heart failure
GLP-1 receptors have been localized in the human sinoatrial node, while its expression in human ventricular tissue has been controversial.28 However, in a careful study of 15 human hearts, full-length transcripts of GLP-1 receptors were detected in all four heart chambers at levels comparable to those of pancreatic tissue, whereas transcripts were neither detected in RNA from human cardiac fibroblast, nor in coronary artery endothelial, or in vascular smooth muscle cells.127 Definite cellular localization of the GLP-1 receptor mRNA transcripts or immunoreactive GLP-1R protein within cardiomyocytes or cardiac blood vessels remained elusive.127
In preclinical studies involving left anterior coronary occlusion, GLP-1 RAs demonstrated clear cardioprotection associated with reduced infarct size, and improved survival.29,80,128
In humans, 6 h infusion of exenatide at the time of revascularization procedures reduced infarct size,129 and a 72 h infusion of GLP- 1 after percutaneous coronary intervention reduced impairment in ventricular systolic function.130 Longer and larger trials are needed to evaluate whether GLP-1 therapy has a place in the treatment of acute coronary syndrome.
Patients with established heart failure and reduced LVEF (median LVEF of 25%) were randomized to liraglutide 1.8 mg (n = 154) or placebo (n = 146) for 180 days in a double-blind, placebo-controlled randomized clinical trial.131 Liraglutide had no significant effect compared with placebo on left ventricular function or the number of deaths or re-hospitalizations for heart failure.131 In two additional studies, there were no effects of liraglutide treatment for 12 to 24 weeks on left ventricular function in patients with or without T2D and stable heart failure.132,133 On the contrary, there was a tendency to an increased frequency of adverse cardiovascular events.132 These findings do not support the use of liraglutide for the treatment in patients with severe heart failure. Notably, in the The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA), LEADER, SUSTAIN 6, PIONEEER 6, Exenatide Study of Cardiovascular Event Lowering (EXSCEL) A Long Term, Randomised, Double Blind, Placebo-controlled Study to Determine the Effect of Albiglutide, When Added to Standard Blood Glucose Lowering Therapies, on Major Cardiovascular Events in Patients With Type 2 Diabetes Mellitus (HARMONY OUTCOMES), Researching cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) and Effect of Efpeglenatide on Cardiovascular Outcomes (AMPLITUDE-O) studies, the risk of hospitalization because of heart failure in the GLP-1 RAs treated groups was not increased, but patients with New York Heart Association IV heart failure were excluded from the trials.118 In recent meta-analysis of the above mentioned CVOT, the risk of hospital admission for heart failure was significantly reduced by 11% (95% CI 0.82–0.98, number needed to treat 258, median follow-up 3.0 years).118
4.11 Conclusion regarding effects of GLP-1 RAs on cardiovascular risk factors
In addition to weight loss and improved glycaemic control, the suggested indirect and direct effects of GLP-1 RA on CVD risk factors comprise increases in natriuresis, reductions in blood pressure, reduced inflammation, reduced ischaemic injury, increased heart rate, increased plaque stabilization and decreased smooth muscle proliferation. GLP-1RAs also reduce postprandial hyperlipidaemia including chylomicrons and number of low-density LDL particles to be oxidized as well as liver fat. Taken together, it seems that the positive results on cardiovascular disease of the GLP-1 RAs may be due to a delaying effect on the arteriosclerotic process. At least there is no evidence for a hemodynamic benefit. A metabolic, anti-atherosclerotic, and anti-inflammatory effect would be in accordance with the slow onset of significant reductions in cardiovascular events, which generally first occur after months of treatment.57,118,134
5. GLP-1 RAs and CVOTs
In 2008, the Food and Drug Administration recommended that all drugs investigated for diabetes should be evaluated for cardiovascular effects in large and long-term trials. Although generally designed to show non-inferiority versus placebo, a prespecified test for superiority is sometimes included. The CVOTs are generally parallel-group, double-blinded studies with a mean duration from 1.3 to 5.4 years. In the trials, the blinded investigators have been encouraged to improve glycaemia control equally in the two groups (equipoise), which means that the placebo groups typically have received more additional antidiabetic treatment that the GLP-1RA groups. The metabolic effects of the GLP-1RAs in the trials therefore must evaluate in view of this.
The primary outcome has been reductions in MACEs in all trials. The populations studied range from 3297 to 14752 (Table 2). The GLP-1 RA was added to standard care and compared with placebo. The eight CVOTs with GLP-1 RAs show variable results indicating that GLP-1 RAs not can be considered a homogenous class of drugs as illustrated in Table 2.
| GLP-1 RA . | Trial . | N . | Follow-up (years) . | Baseline HbA1c (%) . | MACE HR (95% CI) . | CVD mortalitet HR (95% CI) . | Fatal or non-fatal myocardial infarction HR (95% CI) . | Fatal or non-fatal stroke HR (95% CI) . | All-cause mortality HR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| Lixisenatide | ELIXA (Reference64) | 6068 | 2.1 | 7.7% | 1.02(0.89–1.17) P = 0.776 | 0.98(0.78–1.22) P = 0.85 | 1.03(0.87–1.22) P = 0.71 | 1.12(0.79–1.58) P = 0.54 | 0.94(0.78–1.13) P = 0.50 |
| Liraglutide | LEADER (reference57) | 9340 | 3.8 | 8.7% | 0.87(0.78–0.97) P = 0.015 | 0.78(0.66–0.93) P = 0.007 | 0.86(0.75–1.00) P = 0.046 | 0.86 (0.71–1.06) P = 0.16 | 0.85(0.74–0.97) P = 0.02 |
| Semaglutide OW | SUSTAIN-6 (Reference65) | 3297 | 2.1 | 8.7% | 0.74(0.58–0.95) P = 0.016 | 0.98(0.65–1.48) P = 0.92 | 0.81(0.57–1.16) P = 0.26 | 0.65(0.41–1.03) P = 0.066 | 1.05(0.74–1.50) P = 0.79 |
| Oral semaglutide | PIONEER-6 (Reference82) | 3183 | 1.3 | 8.2% | 0.79(0.57–1.11) P = 0.17 | 0.49(0.27–0.92) P = 0.02 | 1.04(0.66–1.66) P = 0.49 | 0.76(0.37–1.56) P = 0.43 | 0.51(0.31–0.84) P = 0.008 |
| Exenatide OW | EXSCEL (Reference69) | 14752 | 3.2 | 8.0% | 0.91(0.83–1.00) P = 0.061 | 0.88(0.76–1.02) P = 0.096 | 0.97(0.85–1.10) P = 0.62 | 0.85(0.70–1.03) P = 0.095 | 0.86(0.77–0.97) P = 0.016 |
| Albiglutide OW | HARMONY OUTCOMES (Reference68) | 9463 | 1.6 | 8.7% | 0.78(0.68–0.90) P = 0.0006 | 0.93 (0.79–1.19) P = 0.58 | 0.75(0.61–0.90) P = 0.003 | 0.86(0.66–1.14) P = 0.30 | 0.95(0.79–1.01) P = 0.64 |
| Dulaglutide OW | REWIND (Reference66) | 9901 | 5.4 | 7.2% | 0.88(0.79–0.99) P = 0.03 | 0.91(0.78–1.06) P = 0.21 | 0.96(0.79–1.16) P = 0.63 | 0.76(0.62–0.94) P = 0.01 | 0.90 (0.80–2.02) P = 0.067 |
| Efpeglenatide | AMPLITUDE-O (reference67) | 4075 | 1.8 | 8.9% | 0.73 (0.58–0.92) P = 0.007 | 0.72 (0.50–1.03) P = 0.07 | 0.75 (0.54–1.05) P = 0.09 | 0.74 (0.47–1.17) P = 0.19 | 0.78 (0.58–1.06) P = 0.11 |
| GLP-1 RA . | Trial . | N . | Follow-up (years) . | Baseline HbA1c (%) . | MACE HR (95% CI) . | CVD mortalitet HR (95% CI) . | Fatal or non-fatal myocardial infarction HR (95% CI) . | Fatal or non-fatal stroke HR (95% CI) . | All-cause mortality HR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| Lixisenatide | ELIXA (Reference64) | 6068 | 2.1 | 7.7% | 1.02(0.89–1.17) P = 0.776 | 0.98(0.78–1.22) P = 0.85 | 1.03(0.87–1.22) P = 0.71 | 1.12(0.79–1.58) P = 0.54 | 0.94(0.78–1.13) P = 0.50 |
| Liraglutide | LEADER (reference57) | 9340 | 3.8 | 8.7% | 0.87(0.78–0.97) P = 0.015 | 0.78(0.66–0.93) P = 0.007 | 0.86(0.75–1.00) P = 0.046 | 0.86 (0.71–1.06) P = 0.16 | 0.85(0.74–0.97) P = 0.02 |
| Semaglutide OW | SUSTAIN-6 (Reference65) | 3297 | 2.1 | 8.7% | 0.74(0.58–0.95) P = 0.016 | 0.98(0.65–1.48) P = 0.92 | 0.81(0.57–1.16) P = 0.26 | 0.65(0.41–1.03) P = 0.066 | 1.05(0.74–1.50) P = 0.79 |
| Oral semaglutide | PIONEER-6 (Reference82) | 3183 | 1.3 | 8.2% | 0.79(0.57–1.11) P = 0.17 | 0.49(0.27–0.92) P = 0.02 | 1.04(0.66–1.66) P = 0.49 | 0.76(0.37–1.56) P = 0.43 | 0.51(0.31–0.84) P = 0.008 |
| Exenatide OW | EXSCEL (Reference69) | 14752 | 3.2 | 8.0% | 0.91(0.83–1.00) P = 0.061 | 0.88(0.76–1.02) P = 0.096 | 0.97(0.85–1.10) P = 0.62 | 0.85(0.70–1.03) P = 0.095 | 0.86(0.77–0.97) P = 0.016 |
| Albiglutide OW | HARMONY OUTCOMES (Reference68) | 9463 | 1.6 | 8.7% | 0.78(0.68–0.90) P = 0.0006 | 0.93 (0.79–1.19) P = 0.58 | 0.75(0.61–0.90) P = 0.003 | 0.86(0.66–1.14) P = 0.30 | 0.95(0.79–1.01) P = 0.64 |
| Dulaglutide OW | REWIND (Reference66) | 9901 | 5.4 | 7.2% | 0.88(0.79–0.99) P = 0.03 | 0.91(0.78–1.06) P = 0.21 | 0.96(0.79–1.16) P = 0.63 | 0.76(0.62–0.94) P = 0.01 | 0.90 (0.80–2.02) P = 0.067 |
| Efpeglenatide | AMPLITUDE-O (reference67) | 4075 | 1.8 | 8.9% | 0.73 (0.58–0.92) P = 0.007 | 0.72 (0.50–1.03) P = 0.07 | 0.75 (0.54–1.05) P = 0.09 | 0.74 (0.47–1.17) P = 0.19 | 0.78 (0.58–1.06) P = 0.11 |
Statistically significant outcomes are marked with bold type. In total, 60 079 patients have been included in the studies. Mean age was 60–66 years, 54–69% were men, 75–83% were white. Mean duration of diabetes was from 9 to 15 years. Number of patients with primary outcome varied from 254 participants (SUSTAIN-6) to 1744 participants (EXSCEL). Reduction in HbA1c, body weight and blood pressure are given in the main text. No increase in pancreatitis or pancreatic cancer and thyroid C-cell adenoma as well as medullary thyroid carcinoma was reported between the GLP-1 RAs and placebo groups. Prior cardiovascular disease at baseline was 100% in ELIXA, 81% in LEADER, 60% in SUSTAIN-6, 73% in EXSCEL, 100% in HARMONY OUTCOMES, 85% in PIONEER, 31% in REWIND and 90% in AMPLITUDE-O trials. History of heart failure range from 8.6% to 20.3% and eGFR < 60 ml/min from 21.6% to 31.6%.
| GLP-1 RA . | Trial . | N . | Follow-up (years) . | Baseline HbA1c (%) . | MACE HR (95% CI) . | CVD mortalitet HR (95% CI) . | Fatal or non-fatal myocardial infarction HR (95% CI) . | Fatal or non-fatal stroke HR (95% CI) . | All-cause mortality HR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| Lixisenatide | ELIXA (Reference64) | 6068 | 2.1 | 7.7% | 1.02(0.89–1.17) P = 0.776 | 0.98(0.78–1.22) P = 0.85 | 1.03(0.87–1.22) P = 0.71 | 1.12(0.79–1.58) P = 0.54 | 0.94(0.78–1.13) P = 0.50 |
| Liraglutide | LEADER (reference57) | 9340 | 3.8 | 8.7% | 0.87(0.78–0.97) P = 0.015 | 0.78(0.66–0.93) P = 0.007 | 0.86(0.75–1.00) P = 0.046 | 0.86 (0.71–1.06) P = 0.16 | 0.85(0.74–0.97) P = 0.02 |
| Semaglutide OW | SUSTAIN-6 (Reference65) | 3297 | 2.1 | 8.7% | 0.74(0.58–0.95) P = 0.016 | 0.98(0.65–1.48) P = 0.92 | 0.81(0.57–1.16) P = 0.26 | 0.65(0.41–1.03) P = 0.066 | 1.05(0.74–1.50) P = 0.79 |
| Oral semaglutide | PIONEER-6 (Reference82) | 3183 | 1.3 | 8.2% | 0.79(0.57–1.11) P = 0.17 | 0.49(0.27–0.92) P = 0.02 | 1.04(0.66–1.66) P = 0.49 | 0.76(0.37–1.56) P = 0.43 | 0.51(0.31–0.84) P = 0.008 |
| Exenatide OW | EXSCEL (Reference69) | 14752 | 3.2 | 8.0% | 0.91(0.83–1.00) P = 0.061 | 0.88(0.76–1.02) P = 0.096 | 0.97(0.85–1.10) P = 0.62 | 0.85(0.70–1.03) P = 0.095 | 0.86(0.77–0.97) P = 0.016 |
| Albiglutide OW | HARMONY OUTCOMES (Reference68) | 9463 | 1.6 | 8.7% | 0.78(0.68–0.90) P = 0.0006 | 0.93 (0.79–1.19) P = 0.58 | 0.75(0.61–0.90) P = 0.003 | 0.86(0.66–1.14) P = 0.30 | 0.95(0.79–1.01) P = 0.64 |
| Dulaglutide OW | REWIND (Reference66) | 9901 | 5.4 | 7.2% | 0.88(0.79–0.99) P = 0.03 | 0.91(0.78–1.06) P = 0.21 | 0.96(0.79–1.16) P = 0.63 | 0.76(0.62–0.94) P = 0.01 | 0.90 (0.80–2.02) P = 0.067 |
| Efpeglenatide | AMPLITUDE-O (reference67) | 4075 | 1.8 | 8.9% | 0.73 (0.58–0.92) P = 0.007 | 0.72 (0.50–1.03) P = 0.07 | 0.75 (0.54–1.05) P = 0.09 | 0.74 (0.47–1.17) P = 0.19 | 0.78 (0.58–1.06) P = 0.11 |
| GLP-1 RA . | Trial . | N . | Follow-up (years) . | Baseline HbA1c (%) . | MACE HR (95% CI) . | CVD mortalitet HR (95% CI) . | Fatal or non-fatal myocardial infarction HR (95% CI) . | Fatal or non-fatal stroke HR (95% CI) . | All-cause mortality HR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| Lixisenatide | ELIXA (Reference64) | 6068 | 2.1 | 7.7% | 1.02(0.89–1.17) P = 0.776 | 0.98(0.78–1.22) P = 0.85 | 1.03(0.87–1.22) P = 0.71 | 1.12(0.79–1.58) P = 0.54 | 0.94(0.78–1.13) P = 0.50 |
| Liraglutide | LEADER (reference57) | 9340 | 3.8 | 8.7% | 0.87(0.78–0.97) P = 0.015 | 0.78(0.66–0.93) P = 0.007 | 0.86(0.75–1.00) P = 0.046 | 0.86 (0.71–1.06) P = 0.16 | 0.85(0.74–0.97) P = 0.02 |
| Semaglutide OW | SUSTAIN-6 (Reference65) | 3297 | 2.1 | 8.7% | 0.74(0.58–0.95) P = 0.016 | 0.98(0.65–1.48) P = 0.92 | 0.81(0.57–1.16) P = 0.26 | 0.65(0.41–1.03) P = 0.066 | 1.05(0.74–1.50) P = 0.79 |
| Oral semaglutide | PIONEER-6 (Reference82) | 3183 | 1.3 | 8.2% | 0.79(0.57–1.11) P = 0.17 | 0.49(0.27–0.92) P = 0.02 | 1.04(0.66–1.66) P = 0.49 | 0.76(0.37–1.56) P = 0.43 | 0.51(0.31–0.84) P = 0.008 |
| Exenatide OW | EXSCEL (Reference69) | 14752 | 3.2 | 8.0% | 0.91(0.83–1.00) P = 0.061 | 0.88(0.76–1.02) P = 0.096 | 0.97(0.85–1.10) P = 0.62 | 0.85(0.70–1.03) P = 0.095 | 0.86(0.77–0.97) P = 0.016 |
| Albiglutide OW | HARMONY OUTCOMES (Reference68) | 9463 | 1.6 | 8.7% | 0.78(0.68–0.90) P = 0.0006 | 0.93 (0.79–1.19) P = 0.58 | 0.75(0.61–0.90) P = 0.003 | 0.86(0.66–1.14) P = 0.30 | 0.95(0.79–1.01) P = 0.64 |
| Dulaglutide OW | REWIND (Reference66) | 9901 | 5.4 | 7.2% | 0.88(0.79–0.99) P = 0.03 | 0.91(0.78–1.06) P = 0.21 | 0.96(0.79–1.16) P = 0.63 | 0.76(0.62–0.94) P = 0.01 | 0.90 (0.80–2.02) P = 0.067 |
| Efpeglenatide | AMPLITUDE-O (reference67) | 4075 | 1.8 | 8.9% | 0.73 (0.58–0.92) P = 0.007 | 0.72 (0.50–1.03) P = 0.07 | 0.75 (0.54–1.05) P = 0.09 | 0.74 (0.47–1.17) P = 0.19 | 0.78 (0.58–1.06) P = 0.11 |
Statistically significant outcomes are marked with bold type. In total, 60 079 patients have been included in the studies. Mean age was 60–66 years, 54–69% were men, 75–83% were white. Mean duration of diabetes was from 9 to 15 years. Number of patients with primary outcome varied from 254 participants (SUSTAIN-6) to 1744 participants (EXSCEL). Reduction in HbA1c, body weight and blood pressure are given in the main text. No increase in pancreatitis or pancreatic cancer and thyroid C-cell adenoma as well as medullary thyroid carcinoma was reported between the GLP-1 RAs and placebo groups. Prior cardiovascular disease at baseline was 100% in ELIXA, 81% in LEADER, 60% in SUSTAIN-6, 73% in EXSCEL, 100% in HARMONY OUTCOMES, 85% in PIONEER, 31% in REWIND and 90% in AMPLITUDE-O trials. History of heart failure range from 8.6% to 20.3% and eGFR < 60 ml/min from 21.6% to 31.6%.
5.1 ELIXA: a CVOT of lixisenatide
The short acting GLP-1 RA lixisenatide (o.d.) was assessed vs. placebo with respect to cardiovascular outcomes in the ELIXA trial in patients with T2D, who had had a recent (< 180 days) acute coronary event.64 The primary endpoint, comprising cardiovascular death, myocardial infarction, stroke or hospitalization for unstable angina did not differ between the lixisenatide and placebo groups.64 There was no difference in heart failure or death. Lixisenatide reduced HbA1c by 0.27%, systolic blood pressure by 0.8 mmHg, body weight by −0.7 kg.64 Lixisenatide reduced progression of urine albumin-to-creatinine ratio in macroalbuminuric patients and was associated with a lower risk of new-onset macroalbuminuria but had no effect on progression of kidney function evaluated out from eGFR.135
5.2 LEADER: a CVOT of liraglutide
The safety of liraglutide was evaluated in the double-blinded LEADER trial.57 Patients included had cardiovascular or kidney disease (81%) or were at high risk for developing cardiovascular disease. MACE was reduced by 13% (P < 0.001), CV mortality by 22% (P = 0.007) and death of any course by 15%, (P = 0.002).57 In a subgroup analysis liraglutide had a neutral effect on MACE (+8%) in patients without established cardiovascular disease at baseline,57 whereas in patients with polyvascular disease (n = 1536) MACE was reduced by 18%, similar to the reduction in total trial population.136 In elderly patients (75 years or older; n = 836) liraglutide reduced MACE by 34% and mortality by 35%.137
There were fewer myocardial infarcts in the liraglutide group than with placebo (359 vs. 421, P = 0.022) and the number of CV deaths was lower (17 vs. 28, P = 0.28).138 In a post-hoc analysis of patients with myocardial infarction during the study, there was a trend of lower troponin levels in patients with liraglutide compared with placebo, which may be suggestive of reduced infarct severity.139,140
Liraglutide reduced HbA1c by 0.4%, systolic blood pressure by 1.2 mmHg, body weight by 2.3 kg and increased heart rate by 3.0 beats per minute relative to placebo (who had intensified alternative antidiabetic therapy).57 Patients, who experienced severe hypoglycaemia (liraglutide 114 vs. placebo 153, a 31% reduction), were more likely to experience MACE, CV death and all-cause death, with higher risk shortly after hypoglycaemia, although causality remains unclear.141
Lraglutide resulted in lower rates of development and progression of DKD than placebo.142 This result was driven primarily by reduced risk of new onset of persistent macroalbuminuria (-36%).
5.3 SUSTAIN 6: a CVOT of semaglutide once weekly
In the preapproval Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6), semaglutide (once weekly) was evaluated in two doses (0.5 mg or 1.0 mg sc) versus placebo.65 At baseline, 83% had established cardiovascular disease, chronic kidney disease or both. After 104 weeks follow-up, the primary outcome: MACE was reduced by 26%, P < 0.001, nonfatal myocardial infarction by 26%, P = 0.12 and nonfatal stroke by 39%, P = 0.04).65 Rates of death, including cardiovascular death, were low and similar in the two groups. Revascularization surgery rates were also greatly reduced (35%) by semaglutide compared with placebo and rates of new or worsening of nephropathy were 36% lower. Surprisingly, rates of retinopathy were significantly higher with semaglutide,65 but may be associated with fast and large reductions in HbA1c during the first 16 weeks of treatment in analogy with the findings in the DCCT trials of intensive insulin therapy in patients with Type 1 Diabetes. Worsened retinopathy was only observed in patients already having retinopathy and mostly treated with insulin.143 In metanalyses of the actions of the GLP-1RAs, worsening of retinopathy has not appeared as a significant problem. Om the contrary, GLP-1RA treatment may be associated with a tendency to improvements in retinopathy but the occurrence illustrates the importance of eye control in patients with T2DM. Semaglutide 1 mg reduced HbA1c by 1.0%, systolic blood pressure by 2.6 mmHg, body weight by 4.3 kg and increased heart rate by 2.5 beat per minute, all compared with placebo.65
5.4 PIONEER 6: a CVOT of Oral semaglutide
Semaglutide is also available in a once-daily oral formulation and its safety was tested in the Peptide Innovation for Early Diabetes Treatment (PIONEER 6) study, which is the smallest and shortest of the GLP-1 RA CVOTs.82 Patients were randomized to oral semaglutide OD or placebo. Inclusion criteria were ≥ 50 years with established cardiovascular or chronic kidney disease (85%) or age ≥ 60 years with cardiovascular risk factors (15%). The primary endpoint MACE (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) was reduced by 21% (P< 0.001 for noninferiority) with oral semaglutide OD. Death from cardiovascular disease was significantly reduced (-51%) with oral semaglutide, whereas nonfatal myocardial infarction (+18%), or nonfatal stroke (−26%) did not differ significantly between groups. Death from any course was significantly reduced with oral semaglutide (−49%).
HbA1c and body weight decreased more with oral semaglutide OD compared with placebo (−1.0% vs. 0.3%) and −4.2 kg vs. −0.8 kg, respectively.82 Blood pressure decreased more with oral semaglutide (2.6 mm Hg), however, mean pulse rate increased by 4 beats per minutes compared with the placebo group.
Notably, when oral semaglutide is absorbed, its pharmacokinetic properties with a half-life of about one week and its effects are similar to those of injectable semaglutide OW.144
5.5 EXSCEL: a CVOT of exenatide once weekly
In the EXSCEL, 73% of participants had previous cardiovascular disease and were randomized to treatment with exenatide once weekly or placebo as add-on to usual therapy.69 MACE occurred in 839 vs. 905 participants in the exenatide and placebo groups (−9%, P = 0.06 for superiority). Cardiovascular death did not differ between exenatide and placebo groups, but exenatide significantly reduced total mortality by 14%.69 Exenatide once weekly reduced HbA1c by 0.53%, blood pressure by 1.57 mmHg, body weight by 1.27 kg and increased heart rate by 2.5 beat per min.69 Notably, 43% of participants discontinued the study and overall compliance to study drug was only 76% of the participants finishing the study.
5.6 HARMONY OUTCOMES: a CVOT of albiglutide
In the HARMONY OUTCOMES trial patients with cardiovascular disease were randomised to receive albiglutide or placebo once weekly.68 The relative risk of MACE was reduced by 22% (P = 0.0006), myocardial infarction by 25% (P = 0.003) and heart failure by 29% (P = 0.019). The number of patients with stroke, CVD mortality, and total mortality did not differ significantly between groups. The reduction in HbA1c was 0.52% (baseline HbA1c 8.7%), in body weight 0.83 kg, in systolic blood pressure 0.67 mmHg, and the increase in heart rate was 1.4 beats per min after 16 months compared with placebo. The results may indicate that the beneficial effect of albiglutide on cardiovascular disease is not mediated by changes in plasma glucose or body weight, and that the beneficial CV effects of the GLP-1RAs are not directly associated with the hypoglycaemic and body weight lowering effects. This is of importance for our understanding of mechanisms of action of GLP-1 RAs. Albiglutide is no longer promoted for clinical use.
5.7 REWIND: a CVOT of dulaglutide
In the REWIND subjects with T2D aged at least 50 years, who had either previous cardiovascular events (31.5%) or cardiovascular risk factors were randomized to once-weekly injection of dulaglutide (1.5 mg) SC or placebo.66 MACE was significantly reduced (−12%). Non-fatal myocardial infarction (−4%), Cardiovascular (−9%) and total mortality (−10%), heart failure (−7%) were numerically, but not significantly reduced. Non-fatal stroke was reduced by 24% (P = 0.017), with no effect on haemorrhagic stroke or stroke severity.145 The effect on the primary endpoint was similar in participants with and without cardiovascular disease at baseline [for both groups: hazard ratio (HR) 0.87, 95% CI: 0.74–1.02] and failed to reach statistical significance in either subgroup.
The relative risk of reaching the composite renal endpoint (development of albumin-to-creatinine ratio > 33.9 mg/mmol, a sustained 30% or greater decline in eGFR, or chronic renal replacement therapy) was significantly reduced with dulaglutide (−15%).66,146
HbA1c was reduced with 0.61%, body weight with 1.46 kg systolic blood pressure 1.70 mm Hg with dulaglutide compared with placebo. Heart rate increased 1.9 beats per min.
The REWIND trial differs from other trials with a GLP-1 RA since only about 31% had previous cardiovascular disease, there was a higher proportion of women and lower baseline HbA1c (7.2%).66 The greatest between-groups difference was seen in the number of non-fatal strokes.66,145 The REWIND trial also has the longest follow-up of 5.4 years among the GLP-1RAs CVOT, but it is a limitation that 25% of the participants were not taken study drug at last visit.
The protective effect of dulaglutide on renal outcomes is consistent with the other trials in which renal outcome is reported, with greatest effect on reducing the risk of incident macroalbuminuria.57,65,69,146
5.8 AMPLITUDE-O: a CVOT of efpeglenatide
Once-weekly subcutaneous dosing of 4 or 6 mg of the GLP-1 RA efpeglenatide, which is an exendin-4 based long-acting agonist was tested against placebo.67 At baseline 15% were reated with an SGLT2 inhibitor and at end of trial, 21 and 15% received SGLT2 inhibitors in the placebo and the efpeglenatide groups. The is of course important in view of the CV effects of the SGLT2 inhibitors.
The risk of MACE was significantly reduced with efpeglenatide (−27%). A dose dependent effect was observed (−18%, P = NS) for 4 mg and (−35%) for 6 mg. Further reductions in risk included non-fatal myocardial infarction (−25%, P = NS), stroke (−20%, P = NS), cardiovascular mortality (−28%, P = NS), total mortality (−22%, P = NS) and heart failure (−39%, P= 0.04).67
Renal composite endpoint (Incident macroalbuminuria, increase in urinary albumin-to-creatinine ratio of ≥30% from baseline, a sustained decrease in eGFR of 40%) was significantly reduced (-32%). Efpeglenatide lowered HbA1C by 1.24%, body weight by 2.6 kg, and pulse pressure by 2.1 mm Hg, and increased heart rate by 3.9 beats per min compared with placebo.
Amplitude-O trial illustrated that the cardiovascular benefit of GLP-1 RA are not restricted to the human GLP-1 RA, and that the cardiovascular benefit of efpeglenatide may be independent of concomitant treatment with a SGLT2 inhibitor.147 The trial sponsor, Sanofi, is no longer developing the drug.
5.9 Conclusion on the CVOT
Taken together, the short acting lixisenatide had a neutral effect on cardiovascular risk, whereas liraglutide, semaglutide, albiglutide, dulaglutide and efpeglenatide were beneficial. Results are summarized in Table 2.
In recent meta-analysis including the eight CVOT and comprising 60 080 patients of whom 14 804 were without established CVD, the GLP-1 RAs reduced the relative risk of MACE by 14% with apparently greater effect in people with known CVD compared with those without CVD (HR: 0.84, CL 079–0.90) vs HR: 0.94, CL 0.83–1.06).118 When assessing the individual MACE endpoints separately, the risk of CVD death was reduced by 13%, fatal and nonfatal myocardial infarction by 10% and stroke by17%, all significant. All-cause mortality was reduced by 12%, and a composite kidney endpoint by 21% with no increased risk of hypoglycaemia, retinopathy or pancreatic adverse effects.118 The GLP-1 receptor agonists had no effect on heart failure in the individual trials except for HARMONY OUTCOMEs and AMPLITUDE-O trials, but the meta-analysis suggested a significant 11% risk reduction for hospitalisation for Heart failure.118
The benefit of using a combination of a GLP-1 RA and a SGLT-2 inhibitor in relation to cardiovascular protection is sparsely investigated. In an explorative analysis of the AMPLITUDE-O trial the effect of the GLP-1 RA efpeglenatide on MACE, renal composite endpoint and heart failure as well as adverse events appeared independent of concurrent SGLT-2 inhibitor treatment at baseline (15.2%, which increased to 15.5% and 21.2% in the efpeglenatide and placebo group).147 These data may support the combined use of GLP-1 RA and a SGLT-2 inhibitor in the treatment of T2D.
6. Adverse effects of GLP-1 RAs
The most common side effects with GLP-1 RAs are gastrointestinal, including nausea, vomiting, diarrhoea or constipation and reflect both central actions of GLP-1 and/or direct actions on the intestine and are most often transient.5,6
The introduction of the GLP-1 RAs for treatment of T2D gave rise to a discussion about their safety regarding pancreatitis, pancreatic carcinoma and medullary thyroid cancer.5,6,34,118,148–150 Pancreatitis has not been a problem in the phase 3 studies and in the LEADER trial increases in serum lipase and amylase were not predictive of an event of subsequent acute pancreatitis.151 In total, in an analysis of five of the cardiovascular endpoint studies, the number of patients with acute pancreatitis was 36 vs. 29 in the groups treated with a GLP-1 RA or placebo, respectively.134
In a post-hoc analyses of the LEADER trial any neoplasm was identified in 10.1% with liraglutide versus 9.0% with placebo (HR: 1.12, p:ns) For malignant neoplasms, HR was 1.06 and for benign neoplasms 1.16, but firm conclusions about, e.g. pancreatic cancer are difficult to reach because it occurred infrequently.151,152
The use of GLP-1 RAs has been associated with increased risk of gallbladder diseases like gallstone and cholecystitis,153 but 12 weeks treatment with liraglutide was not associated with changes in gall bladder function.154
Human C-cell seems to express a very low number of GLP-1 receptors compared to rodents, and the GLP-1RAs do not stimulate release of calcitonin.148,150 In addition, there is no evidence of a causal relationship between GLP-1RAs and thyroid tumours in humans.150 In the phase 3 trials and the CVOTs there were no cases of medullary thyroid carcinoma in the exposed patients.150 Nevertheless, it is still recommended that GLP-1 RAs should not be used in patients with a personal or familiar history of medullary thyroid carcinoma.
7. GIP and CVD
GIP is an incretin hormone, initially isolated by John Brown and Victor Mutt as reviewed in details in.26 In glucose tolerant subjects, GIP seems to be quantitively the most important incretin hormone in relation to insulin secretion, however, after observations of severely reduced GIP effect on insulin secretion in individuals with T2D the interest in GIP dwindled.26 The effect of GIP on body weight in humans is unknown, but it has never been found to affect appetite or food intake. GIP may increase triglyceride stores in adipose tissue.26
The potential effect of GIP on CVD is sparsely elucidated. Animal models have shown anti-atherosclerotic activity, reduced oxidative stress in endothelial cells and inflammatory cytokines release from adipose tissue, increase in heart rate and blood pressure-lowering effects during treatment with GIP.26 Further GIP receptors have been shown in myocytes and animal studies have suggested that attenuated GIP signals impair cardiac remodelling and function in ischaemic heart disease and hypertrophy in response to experimental myocardial infarction.26 Reduction in GIP receptor activation in GIP receptor knock out mice did not alter survival after experimentally induced myocardial infarction, but increased survival during experimentally induced ischaemia.155 GIP receptor activation induced expression of the pro-atherosclerotic factor endothelin-1 and osteopontin, but also showed anti-atherosclerotic effects through secretion of NO, and prevention of foam cell formation has also been reported.156–158 No long-term studies exist with GIP or with a GIP analogue, but in two prospective, community-based studies elevated fasting levels of GIP were associated with increased carotid intima-media thickness and greater risk of all-cause and CVD mortality.159,160 Thus, the therapeutical potential of GIP in relation to effects on CVD is poorly understood. With the development of the dual GLP-1/GIP analogue tirzepatide as discussed below, the interest in GIP from academia as well as the pharmaceutical industry has been resurrected.
8. GLP-1/GIP dual agonist—tirzepatide
Tirzepatide developed by Eli Lilly is a new dual GLP-1/GIP peptide agonist activating both the GLP-1 and GIP receptors. It has a half-life of about 5 days, making it suitable for once-weekly subcutaneous dosing.161 Efficacy and safety of tirzepatide have been studied in the SURPASS clinical trial programme evaluating weekly doses of 5, 10, and 15 mg.
In the SURPASS 2 trial tirzepatide 5 mg, 10 mg and 15 mg was compared with semaglutide 1 mg.162 After 40 weeks, the reduction in HbA1c (from a baseline of 8.3%) was −2.01%, −2.24%, and −2.30% with 5 mg, 10 mg, and 15 mg of tirzepatide, respectively, and −1.86% with semaglutide. About 50% of the patients treated with 15 mg obtained a HbA1c < 5.7%. Reductions in body weight were greater with tirzepatide than with semaglutide, with a difference of −1.9 kg, −3.6 kg, and −5.5 kg for 5 mg, 10 mg and 15 mg, respectively, corresponding to a weight loss of 13 kilo for the highest dose of tirzepatide. In addition, a greater reduction in blood pressure and an improvement in lipid profile were observed with tirzepatide OW compared with semaglutid OW. The adverse events were gastrointestinal corresponding to those observed with GLP-1 RAs. More patients stopped treatment with tirzepatide in the highest dose (7.8%) compared with semaglutide (3.8%).162 Tirzepatide has also demonstrated greater reduction in HbA1c compared with insulin glargine and insulin degludec.163,164 The trials indicate that tirzepatide currently is the most effective drug in relation to reduction of HbA1c and body weight and with an acceptable safety profile. The drug is approved for treatment of type 2 diabetes in US.
In the SURPASS-4 trial comparing tirzepatide with insulin glargine in a high risk cardiovascular population (87% with a history of cardiovascular) there was an insignificant reduction in MACE-4 events (Cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina) after 104 weeks (hazard ratio 0·74, 95% CI 0·51–1·08), suggesting that tirzepatide is safe.163 SURPASS-CVOT is a large, randomized, double-blind trial assessing tirzepatide 15 mg against dulaglutide in 12.500 type 2 diabetic people with established cardiovascular disease (NCT04255433). The study is expected to report results in 2024.
9. Conclusions
The CVOTs with GLP-1 RAs and SGLT-2 inhibitors have shifted the treatment paradigm in T2DM to focus from glucose lowering to organ protection, and now requires consideration of the occurrence of CVD, heart failure and kidney disease when selecting the optimal treatment, independently of HbA1c level3,14 although it is still believed that reduction of HbA1c is important for prevention of diabetic complications.
In general, the GLP-1 receptor agonists are very effective in reducing HbA1c and body weight, with semaglutide being the most potent. The risk of hypoglycaemia is minimal during treatment except if combined with sulfonylurea or insulin. Importantly, the long acting GLP-1 receptor agonists reduce the risk of cardiovascular events.18,118
The limitations of the use of GLP-1 RAs are the GI side effects, the need for subcutaneous injections (except for oral semaglutide) and cost. The proportion of patients withdrawing from GLP-1 RA treatment in the CVOTs range from about 15% to 25%.69,118 Discontinuations of treatment in the trials are mostly explained by GI side effects, and nausea has been reported by 20–40% of the patients and vomiting in 5–10%, but both are generally transient and the risk can be reduced by slow up-titration.5,165–167
At present, there is no clear evidence of a causal relationship between GLP-1 RAs and pancreatitis and pancreatic carcinoma.5,57,64,65,118
The head-to-head comparisons show that the GLP-1 receptor agonists differ with respect to reduction in HbA1c, body weight and GI side effects. The ranking in relation to HbA1c reduction based on the 17 head-to-head studies is subcutaneous semaglutide, oral semaglutide, dulaglutide = liraglutide and exenatide OW. In relation to weight reduction, the ranking is semaglutide subcutaneous or oral, liraglutide, and dulaglutide followed by exenatide OW.
The recent findings that long-acting GLP-1 RAs reduce the risk of cardiovascular events have spurred interest in the mechanisms explaining the CVD protection.22 One suggestion is that GLP-1 RAs have beneficial effects on the progression of atherosclerosis by reducing the plaque burden or increasing plaque stability, but the exact mechanisms of action of GLP-1 RAs on CVD have yet to be elucidated. GLP-1 RAs reduce systolic blood pressure by about 2–7 mm Hg and increase pulse rate with 2 to 4 beat per min.
It is debated how eight CVOT studies could generate so different results.118 Regarding lixisenatide, this RA has a short half-life and covers only about 8 h of the day, while liraglutide, semaglutide, exenatide, albiglutide, dulaglutide and efpeglanitide cover all 24 h.23 In addition, the duration of the trials as well as the prevalence of cardiovascular disease and glycaemic control have differed significantly (Table 2). Moreover, the molecules differ in their receptor signalling capacity.15 In all the studies, a high proportion of people with manifest CVD were included, except for the REWIND trial, where only 31.5% had pre-existing CVD.66,168 The risk reduction in REWIND was similar in the groups with and without pre-existing CVD, and neither was significant.
In a recent meta-analysis including data from AMPLITUDE-0, GLP-1 RAs had their relatively greatest risk reduction on stroke followed by CVD death and myocardial infarction.118 SGLT-2 inhibitors do not reduce the risk of stroke.168 The different profiles of CV and renal benefits support the combined use of GLP-1RA and SGLT-2 inhibitors in people with T2D.169 At present, only post-hoc analyses from CVOT support the safety of adding a GLP-1 RA to people treated with a SGLT-2 inhibitor.147 Combination therapy showed superior effects on HbA1c and body weight when compared with monotherapy.170 Future dedicated trials investigating the effect on CVD of combination therapy compared with the individual treatment with a GLP-1 RA or a SGLT-2 inhibitor are of major interest.
Metformin is considered first line therapy in the treatment of T2D, and ADA/EASD and AACE consensus reports recommend GLP-1 receptor agonists as potential add-on therapy for patients with uncontrolled T2D.3,4,14 THE ADA/EASD guidelines also suggest treatment with a GLP-1RA in patients with established CVD or without established CVD but with high risk indicators including age> 55 years, carotid and/or lower extremity or coronary artery stenosis > 50%, left ventricular hypertrophy, eGFR < 60 ml/min independently of baseline HbA1c. A GLP-1 RA can also be considered as monotherapy for patients with metformin intolerance. Furthermore, European Society of Cardiology (ESC) recommends to start treatment with a SGLT-2 inhibitor or a GLP-1 RA in drug naïve patients with T2D and atherosclerotic CVD or high CV Risk.171
The use of a GLP-1 RA instead of sulfonylurea or insulin represents an opportunity to avoid hypoglycaemia and weight gain.3,4,14
The prevalence of CVD in T2D populations is about 30% and, therefore, at least one fourth of the patients are candidates to treatment with a GLP-1 RA or a SGLT-2 inhibitor to reduce the risk of CVD.18 In the real world the proportion of patients treated with a GLP-1 RA or a SGLT-2 inhibitor is much smaller.
The GLP-1 RAs also have effect on the progression of kidney disease to macroalbuminuria. The effect on the reduction in eGFR has been less conspicuous compared with the evident effect of a SGLT-2 inhibitor, which also is first choice in T2D with heart failure.3,4,14 SGLT-2 inhibitors and GLP-1RAs differ with respect to MACE and all-cause mortality.118,168 In the US there is no contraindication for the use of GLP-1 RAs in patients with severe kidney disease (except for exenatide, eGFR < 30 ml/min). Whether the GLP-1 RAs are suitable for people with severe kidney will be clarified with the FLOW study (NCT038191539) investigating the renoprotection by semaglutide.
The phase 3 programme with dual GIP/GLP-1 RA agonist tirzepatide which now is approved to treatment of type 2 diabetes in US has shown improvements in HbA1c and body weight beyond what has previously been seen with the most potent GLP-1 RA semaglutide.162–164 In the Surpass trials about 80% of participants obtained a HbA1c below 7.0% and about 50% below 5.7%.162–164 The CVOT, where tirzepatide is compared with dulaglutide will probably by reported in 2024.
With semaglutide 2.4 mg SC and tirzepatide SC, both once weekly, the treatment goals of obese type 2 diabetic individuals will probably be revised from glycaemic control and organ protection to a focus on body weight reduction and thereby the possibility to obtain ‘remission’ of diabetes with normal blood glucose concentrations (however, while on incretin-based treatment). The weight loss has additional benefits on fatty liver disease, sleep apnoea, arthrosis and muscle pain, cardiovascular risk factors and induces improved fitness as well as quality of life. Indeed, weight loss is a principal target for the treatment of patients with T2D.172
New incretin combinations for treatment of T2D and obesity are under investigation including GLP-1/glucagon, GLP-1 RA/SGLT-2 inhibitor, GLP-1/amylin, GLP-1/GIP/glucagon,173 but clear clinical results are not yet available.
Data availability
No new data or unpublished results are included in the review.
References
Author notes
Conflict of interest: S.M.: Advisory boards: AstraZeneca; Boehringer Ingelheim; Merck Sharp & Dohme; Novo Nordisk; Sanofi. Lecture fees: AstraZeneca; Boehringer Ingelheim; Merck Sharp & Dohme; Novartis; Novo Nordisk; Sanofi. Research Grant Recipient: Novo Nordisk and Boehringer-Ingelheim. J.J.H.: Advisory boards: Novo Nordisk. Lecture fees: Novo Nordisk.



