-
PDF
- Split View
-
Views
-
Cite
Cite
Laura Calabresi, Giuseppe Danilo Norata, HDL particles and infection-related death: when size matters, Cardiovascular Research, Volume 119, Issue 4, April 2023, Pages 883–885, https://doi.org/10.1093/cvr/cvad043
Close - Share Icon Share
This editorial refers to ‘Lower levels of small HDL particles associated with increased infectious disease morbidity and mortality: a population-based cohort study of 30 195 individuals’ by M. Harsløf et al., https://doi.org/10.1093/cvr/cvac194.
HDLs are a heterogeneous class of lipoproteins defined by a density ranging from 1.063 to 1.21 g/mL (PMID: 25522985). They are characterized by the presence of apolipoprotein A–I as the key structural protein, but proteomic analysis has shown that HDLs transport several proteins, including enzymes, lipid transfer proteins, acute phase response proteins, complement proteins, and proteinase inhibitors.1 Depending on the analytical approach, HDLs can be classified into HDL2, more buoyant and larger, and into HDL3, smaller and denser, following ultracentrifugation, or into HDL2a, HDL2b, HDL3a, HDL3b, and HDL3c using gradient gel electrophoresis. Moreover, a separation based on both their charge and size (two-dimensional gel electrophoresis) allows to identify up to 12 HDL subclasses, from small discoidal pre-β to large α1–α4 HDL particles. Finally, using nuclear magnetic resonance spectroscopy, HDLs can be classified according to their size into small HDL particles (<9.8 nm), medium HDL particles (9.8–11.5 nm), large HDL particles (11.6–13.2 nm), and extra-large HDL particles (13.3–16.5 nm).2
A common misinterpretation in the field is the evaluation of HDL-cholesterol (HDL-C) content as an index of circulating HDL particle levels. While the measurement of HDL-C is a proxy of the entire spectrum of circulating HDL particles and often does not reflect HDL function, different HDL subclasses have been shown to exert different functions. This is the case for small HDLs, which have been reported to be critical for reverse cholesterol transport, endothelial protection, antioxidant activity, and immunoregulation via several mechanisms.3,4 Is this detailed clustering relevant for physiology and pathology?
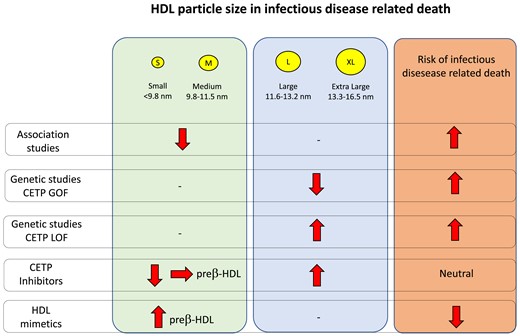
The work published by Harsløf et al.5 contributes to this topic by showing during 15 years of follow-up in more than 30 000 subjects from the general population that individuals with a lower small HDL particle count were at an increased risk of any infectious disease and of infectious disease–related death, while this was not the case when subjects were stratified according to the percentiles of medium and large HDL particle count. This work expands a previous observation from the same group showing a U-shaped relationship of HDL-C and risk of infectious disease in prospective population-based cohort studies,6 where low (<31 mg/dL) and high (>100 mg/dL) plasma HDL-C concentrations were associated with a higher risk of infectious disease. Notably, the association of increased HDL-C levels with a higher risk of infectious disease was not confirmed in the replication cohort, and elevated HDL-C levels were not associated with an increased risk of infectious disease–related death as reported by Harsløf et al.5 Several aspects might contribute to explain these differences, including that the observed association does not necessarily indicate causality and individuals with elevated HDL-C levels could present dysfunctional HDL particles as a consequence of genetic mutations in genes related to HDL pathways.
These findings set the stage for a deeper analysis of the relation between HDLs and infections. A robust increased risk of infectious disease and infectious disease–related death was observed only in individuals with very low counts of small and medium HDL particles (<1st percentile) and a modest but significant increase was also observed for individuals categorized within the 1st and the 10th percentiles. Two clear additional findings also emerged from this analysis: first, having elevated counts of small-medium size HDL particles, of large–extra-large HDLs, or presenting elevated levels of HDL-C or apolipoprotein A–I is not associated with an increased risk of infectious disease–related death; secondly, neither small-medium HDL particle count nor large–extra-large HDL particle count is associated with changes in the risk of myocardial infarction.
Could these data suggest that it is time to move from the simple HDL-C determination to a detailed analysis of HDL particle size? This question poses important issues related to the methods used to evaluate HDL subclasses. None of the techniques used so far has been standardized, and as described above, the various methods separate particles according to different HDL properties (i.e. size, density, charge). Moreover, some of the techniques do not allow a quantification of HDL subclass concentration but only give their relative plasma content.
What are the clinical and pharmacological implications of author’s observation? Currently available hypolipidaemic drugs have little effects on HDL-C levels and also, for what is known, on HDL subclass distribution. Drugs inhibiting the cholesteryl ester transfer protein (CETP), presently or under development, are known to strongly increase HDL-C levels and HDL particle size. How this increase reflects on the risk of infection is a subject of controversy. Torcetrapib, the first drug to be tested in the clinic, significantly increased the risk of infection mortality in the ILLUMINATE trial of prevention for patients with high cardiovascular risk,7 while the other CETP inhibitors, dalcetrapib, evacetrapib, and anacetrapib, did not result in an increased risk of severe infection when tested in clinical trials of cardiovascular prevention.8 This suggests (i) that the torcetrapib effect is not a class effect but rather related to off-target effects of the molecule and (ii) that increasing HDL size does not necessarily result in HDL particles less efficient in preventing infections. Indeed, despite the fact that all CETP inhibitors increase the average HDL size, the various molecules have different effects on HDL subclass distribution. Dalcetrapib and evacetrapib have been shown to preserve the formation of pre-β-HDL,9,10 while other molecules do not. Pre-β-HDL, which is very efficient in removing cholesterol from macrophages and other immune cells,11 is small and discoidal and with the various techniques used to evaluate HDL subclasses is included in the small HDL, being indeed the smallest. Notably, synthetic HDL, which mimics pre-β-HDL, prevented infections in mice12 and humans.13
Genetic studies are also controversial; CETP gain-of-function variants have been shown to associate with an increased risk of acute sepsis mortality, while a genetic score for decreased CETP function was associated with significantly decreased sepsis mortality in various cohorts.14 Although none of the genetic studies have analysed the effects of CETP variants on HDL subclass distribution, it could be expected that gain-of-function variants would lead to an increase in the prevalence of HDL with decreased size and yield the opposite result for the CETP loss-of function variants.
In summary, the results reported by Harsløf et al., together with those collected in years, suggest that the smallest pre-β-HDL particles could be those involved in the protection toward infection morbidity and mortality. Vice versa, data on larger HDL particles and infections are inconclusive, which calls for a better characterization of changes in the protein cargo in larger HDL particles rather than focusing on the size per se in the context of infections.
Funding
L.C. is supported by Italian Ministry of Education PRIN2017PFYK27 e Global NASH ASPIRE Competitive Grant no. 70215089. G.D.N. is supported by Italian Ministry of Education [PRIN 2017 K55HLC], Italian Ministry of Health [RF-2019-12370896], Next Generation EU_PNRR Missione 4 (Progetto CN3—National Center for Gene Therapy and Drugs based on RNA Technology), Next Generation EU PNRR Missione 4 (Progetto MUSA- Multilateral urban sustainability action), PNRR-MAD-2022-12375913.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
Conflict of interest: None declared.



