-
PDF
- Split View
-
Views
-
Cite
Cite
Kimberly P Keil, Sunjay Sethi, Pamela J Lein, Sex-Dependent Effects of 2,2′,3,5′,6-Pentachlorobiphenyl on Dendritic Arborization of Primary Mouse Neurons, Toxicological Sciences, Volume 168, Issue 1, March 2019, Pages 95–109, https://doi.org/10.1093/toxsci/kfy277
Close - Share Icon Share
Abstract
Early life exposures to environmental contaminants are implicated in the pathogenesis of many neurodevelopmental disorders (NDDs). These disorders often display sex biases, but whether environmental neurotoxicants act in a sex-dependent manner to modify neurodevelopment is largely unknown. Since altered dendritic morphology is associated with many NDDs, we tested the hypothesis that male and female primary mouse neurons are differentially susceptible to the dendrite-promoting activity of 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95). Hippocampal and cortical neuron-glia co-cultures were exposed to vehicle (0.1% dimethylsulfoxide) or PCB 95 (100 fM–1 μM) from day in vitro 7–9. As determined by Sholl analysis, PCB 95-enhanced dendritic growth in female but not male hippocampal and cortical neurons. In contrast, both male and female neurons responded to bicuculline with increased dendritic complexity. Detailed morphometric analyses confirmed that PCB 95 effects on the number and length of primary and nonprimary dendrites varied depending on sex, brain region and PCB concentration, and that female neurons responded more consistently with increased dendritic growth and at lower concentrations of PCB 95 than their male counterparts. Exposure to PCB 95 did not alter cell viability or the ratio of neurons to glia in cultures of either sex. These results demonstrate that cultured female mouse hippocampal and cortical neurons are more sensitive than male neurons to the dendrite-promoting activity of PCB 95, and suggest that mechanisms underlying PCB 95-induced dendritic growth are sex-dependent. These data highlight the importance of sex in neuronal responses to environmental neurotoxicants.
Polychlorinated biphenyls (PCBs) are synthetic organic compounds produced worldwide in large quantities for use in diverse industrial applications and commercial products beginning in the 1930s. PCB production was banned by the United States Congress in 1979, and by the Stockholm Convention on Persistent Organic Pollutants in 2001, because of significant human health concerns (Carpenter, 2006; White and Birnbaum, 2009). However, PCBs continue to pose a risk to human health because of not only the release of legacy congeners from old buildings, electrical transformers, and waste disposal facilities (Herkert et al., 2018; Herrick et al., 2007; Marek et al., 2017), but also the unintentional production of PCBs as byproducts of contemporary industrial processes, notably, pigment production (Guo et al., 2014; Hu and Hornbuckle, 2010). Exposure studies confirm continued widespread human exposure to both legacy and contemporary PCBs, including women of childbearing age living in the United States (Koh et al., 2015; Sethi et al., 2017b; Thompson and Boekelheide, 2013).
The primary health outcome of concern for PCBs is developmental neurotoxicity. The weight of evidence from epidemiologic studies supports a negative association between developmental PCB exposures and measures of neuropsychological function in infancy and childhood (Berghuis et al., 2015; Korrick and Sagiv, 2008; Schantz et al., 2003). Elevated prenatal levels of PCBs have more recently been associated with increased risk of neurodevelopmental disorders (NDDs), including autism spectrum disorders (ASDs) (Cheslack-Postava et al., 2013; Lyall et al., 2017) and attention deficit hyperactivity disorder (ADHD) (Eubig et al., 2010; Rosenquist et al., 2017; Sagiv et al., 2010). A PCB congener of particular interest in the context of NDDs is PCB 95. Postmortem analyses of multiple PCBs and polybrominated diphenyl ethers identified higher levels of PCB 95, but not the other analytes, in brains of children with a syndromic form of autism relative to neurotypical controls (Mitchell et al., 2012). Mechanistic studies in rodent models have revealed that developmental exposure to PCB 95 enhances dendritic arborization of central neurons both in vitro and in vivo (Wayman et al., 2012a,b; Yang et al., 2009). Because dendritic architecture is a key determinant of neuronal connectivity (Chen and Nedivi, 2010; Penzes et al., 2011), and altered patterns of dendritic arborization are associated with many NDDs (Alaerts et al., 2016; Keown et al., 2013), these preclinical observations suggest a mechanism by which PCB 95, and mechanistically similar PCBs, contribute to the pathogenesis of NDDs (Pessah et al., 2010; Stamou et al., 2013).
Sex biases in onset, severity and/or prevalence are common in NDDs, including ASD (Alaerts et al., 2016; CDC, Center for Diseasee Control and Prevention, 2014; Halladay et al., 2015; McCarthy and Wright, 2017) and ADHD (Poissant et al., 2016). The mechanism(s) underlying the sex bias in NDDs are not completely understood but are thought to include inherent sex differences in the brain established by gonadal hormones (McCarthy et al., 2015; Nugent et al., 2011), as well as sex differences in the rates of neurodevelopment (McCarthy et al., 2018). Another relatively unexplored possibility is that environmental neurotoxicants contribute to sex biases in NDDs via sex-specific effects on neurodevelopmental outcomes of relevance to NDDs. To test this hypothesis, we examined the effects of PCB 95 on dendritic arborization in primary neuron-glia cocultures derived from hippocampi and neocortices of male versus female C57BL/6J mice. Using this in vitro model of neurodevelopment, we identified sex-dependent dendritic responses to PCB 95 in primary mouse hippocampal and cortical neurons.
MATERIALS AND METHODS
Materials
PCB 95 (2,2′,3,5′,6-pentachlorobiphenyl) was purchased from AccuStandard, (Lot no. 010610KS, 99.7% pure, New Haven, Connecticut) and purity was confirmed by 1H-NMR, 13C-NMR, and GC-MS. Bicuculline was purchased from Tocris (Minneapolis, Minnesota). All stock solutions were made in dry sterile dimethylsulfoxide (DMSO, Sigma-Aldrich, Saint Louis, Missouri). Plasmid encoding microtubule-associated protein-2B (MAP2B) fused to enhanced green fluorescent protein (MAP2B-EGFP) was generously provided by Dr Gary Wayman (University of Washington, Pullman, Washington) (Wayman et al., 2006).
Animals
All procedures involving animals were approved by the University of California-Davis Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. C57BL/6J mice were purchased from Jackson Labs (Sacramento, California) and housed in clear plastic shoebox cages containing corn cob bedding. Mice were maintained on a 12-h light/dark cycle at 22°C ± 2°C. Food (Diet 5058, LabDiet, St Louis, Missouri) and water were available ad libitum.
Cell culture and morphometric analyses
Sex-specific primary neuron-glia co-cultures dissociated from the hippocampus and neocortex of postnatal day (P) 0–P1 male and female mouse pups were sexed (Keil et al., 2017) and prepared as previously described (Wayman et al., 2012a). Briefly, dissociated cells from pooled male or female hippocampi or neocortices were plated at 83,000 cells/cm2 on glass coverslips (Bellco Glass, Vineland, New Jersey) precoated with poly-L-lysine (0.5 mg/ml, Sigma-Aldrich, St Louis, Missouri) and maintained at 37°C in NeuralQ Basal Medium (GSM-9420; MTI-GlobalStem, Gaithersburg, Maryland) supplemented with 2% GS21 (GSM-3100; MTI-GlobalStem) and 1% GlutaMAX (ThermoScientific, Waltham, MA) under 5% CO2. On day in vitro (DIV) 6, cells were transfected with MAP2B-EGFP plasmid using Lipofectamine-2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. DIV 7 cultures were treated for 48 h with vehicle (DMSO; 1:1000) or PCB 95 at 100 femtomolar (fM), 1 picomolar (pM), 100 pM, 1 nanomolar (nM), 100 nM and 1 micromolar (μM) diluted from 1000× stocks directly into culture media. A subset of cultures was treated with bicuculline (20 μM).
At DIV 9, cultures were fixed with 4% paraformaldehyde (Sigma-Aldrich, diluted from an 8% stock in 10 mM sodium hydroxide, with 0.2 M phosphate buffer consisting of 0.144 M disodium phosphate and 0.056 M monosodium phosphate) for 20 min at room temperature and mounted to glass slides using ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (ThermoScientific). Images of EGFP-labeled neurons were acquired in an automated, unbiased manner using an ImageExpress Micro XL high content imaging system (Molecular Devices, Sunnyvale, California). The dendritic complexity of individual neurons was quantified using ImageJ software (Schneider et al., 2012) with the Sholl analysis plug-in v3.4.2 (http://fiji.sc/Sholl_Analysis) (Ferreira et al., 2014). Sholl rings were set at 10 pixel increments from the soma (1 pixel = 0.65 μm). The NeuronJ plug-in (Meijering et al., 2004) was used to quantify dendritic length.
Cell viability
Cell viability was quantified in DIV 9 cultures as described previously (Keil et al., 2017). Briefly, lactate dehydrogenase (LDH) release was measured using the CytoTox-ONE Homogenous Membrane Integrity Assay (Promega, Madison, Wisconsin) per the manufacturer’s instructions. Separate cultures were costained with calcein-AM (0.25 μM, ThermoFisher Scientific) and propidium iodide (1.25 μM, Sigma-Aldrich) to identify live versus dead cells, respectively. Plates were imaged and percent live cells quantified using an ImageExpress Micro XL high content imaging system (Molecular Devices). At least 5 sites were imaged per well; images that contained fewer than 250 cells were not included in the analysis.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 20 min on DIV 9 and immunostained as described (Chen et al., 2017a). Briefly, after fixation, cells were washed with phosphate buffered saline (PBS; 3.6 mM disodium phosphate, 1.4 mM monosodium phosphate, 150 mM sodium chloride, pH 7.2) and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 5 min, incubated in blocking buffer containing 10% bovine serum albumin (Sigma-Aldrich) and 5% goat serum (Vector Laboratories, Burlingame, California) in PBS for 1 h and coincubated overnight at 4°C with antibodies targeting MAP2B (1:1000, AB5622, Millipore, Burlington, Massachusetts) to label neurons and glial fibrillary acidic protein (GFAP, 1:1000, no. 3670 Cell Signaling Technology, Danvers, Massachusetts) to label astrocytes. A subset of cultures was co-incubated with antibodies targeting MAP2B and ryanodine receptor 1/2 (C34, 1:100, Developmental Studies Hybridoma Bank, Iowa City, Iowa). Cells were washed with PBS, and incubated for 1 h at room temperature with goat antirabbit Alexa Fluor 546 (1:500, A11035, Molecular Probes, Invitrogen, Waltham, Massachusetts) and goat antimouse Alexa Fluor 488 (1:500, A11029, Molecular Probes, Invitrogen). Coverslips were mounted to glass slides using ProLong Gold antifade reagent containing DAPI (ThermoScientific) and imaged using ImageExpress Micro XL high content imaging system (Molecular Devices) to acquire automated nonbiased images of MAP2B and GFAP immunopositive cells.
Statistics
Data were assessed for normality using Kolmogorov-Smirnov, D’Agostino and Pearson omnibus, and Shapiro-Wilk normality tests and were assessed for homogeneity of variance using Bartlett’s test using GraphPad Prism v 6.07 (San Diego, California). Differences between 2 groups were assessed using Student’s t test or Student’s t test with Welch’s correction for parametric data or by the Mann Whitney U test for nonparametric data. Comparisons between more than 2 groups were analyzed using a 1-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test for parametric data or Kruskal-Wallis test with Dunn’s multiple comparison test for nonparametric data. Sholl plots were analyzed with respect to the area under the curve (AUC), the number of dendritic intersections, distance from the soma of the peak (maximal) number of intersections (peak X), and the maximum number of dendritic intersections (peak Y) using built in AUC analysis in GraphPad Prism Software. Data are reported as mean fold-change from vehicle control ± SE, p values ≤ .05 were considered significant.
RESULTS
Female Neurons Are More Sensitive Than Male Neurons to the Dendrite-Promoting Effects of PCB 95
Primary sex-specific neuron-glia co-cultures isolated from P0 mouse hippocampi or neocortices were transfected with a MAP2B-EGFP cDNA construct on DIV 6 in order to visualize the complete dendritic arbor of individual neurons. Expression of MAP2B-EGFP is restricted to the somatodendritic compartment in cultured neurons and does not alter their intrinsic dendritic growth patterns (Wayman et al., 2006). Under the culture conditions used for these experiments, the dendritic arbor expands most rapidly between DIV 5 and 10 (Wayman et al., 2006); therefore, cultures were exposed to vehicle (0.1% DMSO in culture medium) or varying concentrations of PCB 95 between DIV 7 and 9. At the end of the 48 h exposure, cultures were fixed for morphometric analyses of the dendritic arbors of EGFP positive neurons.
Sholl analysis of mouse hippocampal neurons revealed sex differences in PCB 95-induced dendritic growth. In male hippocampal neurons, exposure to PCB 95 at concentrations ranging from 100 fM to 1 µM had no effect on dendritic arborization relative to sex-matched hippocampal neurons exposed to vehicle, as determined by total AUC of Sholl plots (Figs. 1A and 1B). In male cultures exposed to PCB 95 at 1 μM, but not those exposed to other concentrations of PCB 95, both the distance from the soma at which the peak dendritic intersection occurs (peak X) (Figure 1C) and the peak number of dendritic intersections (peak Y) was decreased (Figure 1D). In contrast, as determined by total AUC of the Sholl plot, PCB 95 at 100 fM significantly increased dendritic arborization in female hippocampal neurons relative to sex-matched vehicle control (Figs. 1E and 1F). PCB 95 did not alter the distance from the soma at which the peak dendritic intersection occurred (Figure 1G) but did increase the peak number of dendritic intersections in female cultures exposed to PCB 95 at 100 fM and 1 pM (Figure 1H).
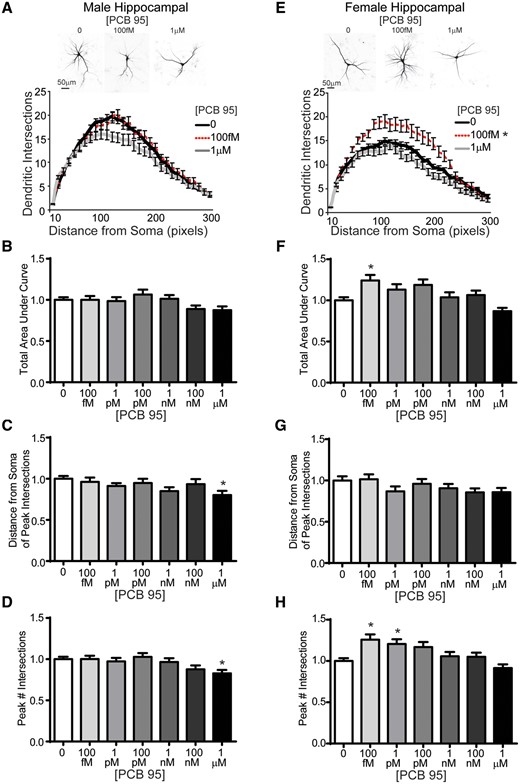
Female mouse hippocampal neurons are more sensitive than male mouse hippocampal neurons to PCB 95-induced dendritic arborization. Representative photomicrographs and Sholl plots of DIV 9 GFP+ male (A) and female (E) mouse hippocampal neurons exposed for 48 h to either vehicle (0.1% DMSO) or PCB 95 at the lowest (100 fM) or highest (1 μM) concentration examined. Fold-change relative to sex-matched vehicle control was quantified with respect to: (B, F) the total area under the Sholl curve (10–300 pixels from the soma); (C, G) the distance from the soma at which the peak (maximal number of) dendritic intersection occurs (peak X); and (D, H) the peak (maximal) number of dendritic intersections (peak Y). Data are presented as mean ± SE (n = 33–93 neurons from at least 3 independent dissections). *Significantly different from vehicle control at p < .05, as determined using 1-way ANOVA followed by Dunnett’s multiple comparison post hoc test (B) or Kruskal-Wallis test followed by Dunn’s multiple comparison post hoc test (C, D, F, G, H).
Similar to hippocampal neurons, female cortical neurons were more sensitive than male cortical neurons to the effects of PCB 95 on dendritic arborization. In male cortical neurons, exposure to PCB 95 did not alter dendritic arborization, as determined by the total AUC in Sholl plots (Figs. 2A and 2B), nor did it alter the distance from the soma at which the peak number of dendritic intersections occurred (Figure 2C), or the peak number of dendritic intersections (Figure 2D) relative to sex-matched vehicle control cortical cultures. However, in female cortical neurons, PCB 95 at 100 fM and 100 pM increased dendritic arborization relative to sex-matched vehicle control cultures, as determined by total AUC of the Sholl plot (Figs. 2E and 2F). There were no differences between groups in the distance from the soma at which the peak dendritic intersections occurred in female cortical neurons (Figure 2G); however, PCB 95 at 100 fM and 100 pM increased the peak number of dendritic intersections relative to sex-matched vehicle controls (Figure 2H).
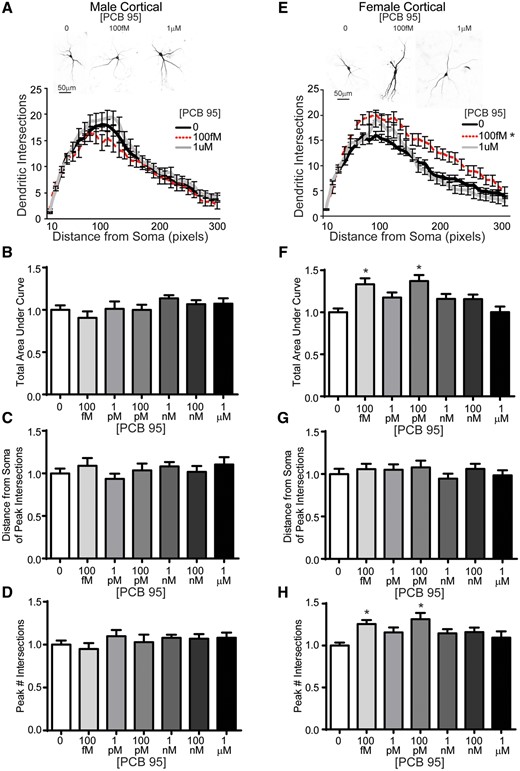
Female mouse cortical neurons are more sensitive than male mouse cortical neurons to PCB 95-induced dendritic arborization. Representative photomicrographs and Sholl plots of DIV 9 GFP+ male (A) and female (E) mouse cortical neurons exposed for 48 h to either vehicle (0.1% DMSO) or PCB 95 at the lowest (100 fM) or highest (1 μM) concentration examined. Fold-change relative to sex-matched vehicle control was quantified with respect to: (B, F) the total area under the Sholl curve (0–300 pixels from the soma); (C, G) the distance from the soma at which the peak (maximal number of) dendritic intersections occurs (peak X); and (D, H) the peak (maximal) number of dendritic intersections (peak Y). Data are presented as mean ± SE (n = 13–70 neurons from at least 3 independent dissections). *Significantly different from vehicle control at p < .05, as determined using Kruskal-Wallis test followed by Dunn’s multiple comparison post hoc test.
In order to determine the site(s) within the dendritic arbor that were altered by PCB 95, we further characterized the number and length of primary and nonprimary (defined as secondary, tertiary, etc.) dendrites. In hippocampal neurons, PCB 95 exerted concentration-dependent effects on dendritic arborization. In male hippocampal neurons PCB 95 at 1 nM increased primary dendrite number (Figure 3A) in the absence of effects on mean length of primary dendrites (Figure 3B). PCB 95 did not alter the number or mean length of nonprimary dendrites in male hippocampal neurons relative to sex-matched vehicle control cultures (Figs. 3C and 3D). In contrast, in female hippocampal neurons, PCB 95 at 100 fM decreased primary dendrite number relative to sex-matched vehicle control cultures (Figure 3E), without altering mean primary dendritic length (Figure 3F). PCB 95 at 100 fM, 100 pM, and 1 nM increased the number of nonprimary dendrites in female hippocampal neurons relative to sex-matched vehicle control cultures (Figure 3G), and at 100 nM increased the mean length of nonprimary dendrites (Figure 3H). In summary, PCB 95 influenced dendritic arborization in hippocampal neurons mainly via changes in the number rather than mean length of dendrites, with the former affecting primary dendrites in male neurons and to greater extent nonprimary dendrites in female neurons.
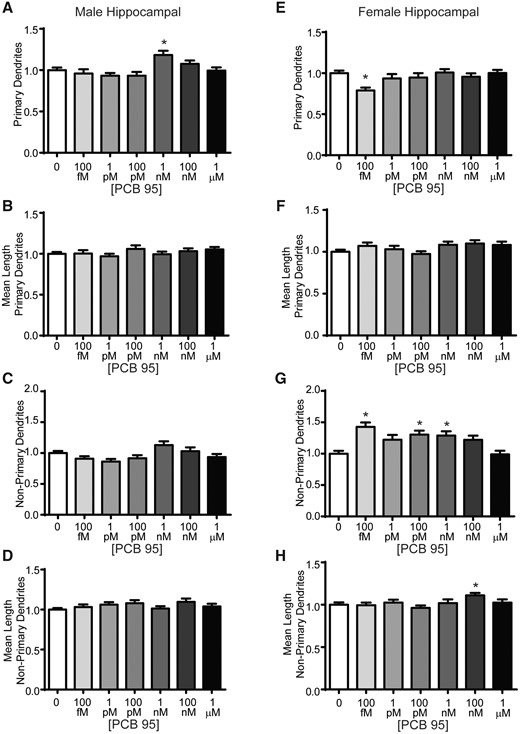
PCB 95 increases dendritic arborization of female hippocampal neurons via effects on nonprimary dendrites. Male (A–D) and female (E–H) mouse hippocampal neuron-glia cocultures were exposed for 48 h to vehicle (0.1% DMSO) or varying concentrations of PCB 95. Fold-change relative to sex-matched vehicle control was quantified with respect to: (A, E) the total number of primary dendrites; (B, F) mean length of primary dendrites (μm); (C, G) the number of nonprimary dendrites; and (D, H) the mean length of nonprimary dendrites (μm). Data are presented as mean ± SE (n = 33–93 neurons from at least 3 independent dissections). *Significantly different from vehicle control at p < .05, as determined using Kruskal-Wallis test followed by Dunn’s multiple comparison post hoc test.
In male cortical neurons, PCB 95 at 1 μM increased primary dendrite number (Figure 4A), without altering the mean length of primary dendrites (Figure 4B). In contrast, there were no significant differences in the number or mean length of nonprimary dendrites of male cortical neurons relative to sex-matched vehicle control cultures (Figs. 4C and 4D). In contrast to hippocampal neurons and male cortical neurons, in female cortical neurons, PCB 95 at 100pM, 1 nM, 100 nM, and 1 μM increased primary dendrite number (Figure 4E), without altering the mean length of primary dendrites (Figure 4F). PCB 95 at 1 and 100 nM increased the number of nonprimary dendrites in female hippocampal neurons relative to sex-matched vehicle control cultures, without altering mean length of nonprimary dendrites (Figs. 4G and 4H). In summary, PCB 95 influenced dendritic arborization in cortical neurons via changes in the number of dendrites, and these growth promoting effects were seen in both primary and nonprimary dendrites and to a greater extent in female cortical neurons.
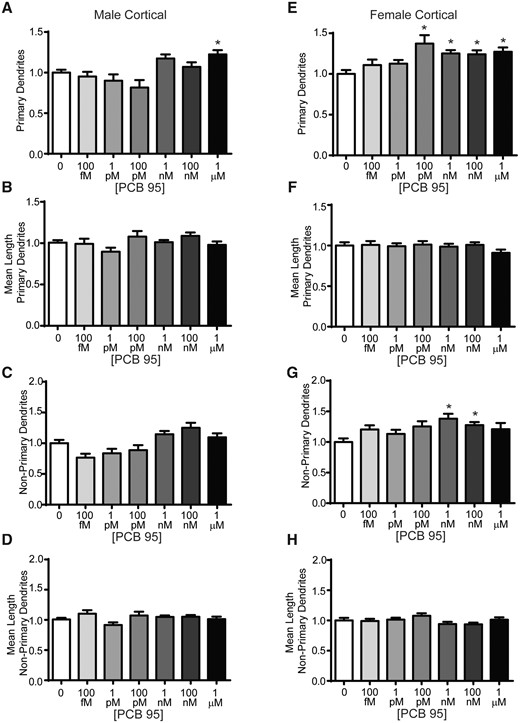
PCB 95 increases dendritic arborization of female cortical neurons via effect on primary and nonprimary dendrites. Male (A–D) and female (E–H) mouse cortical neuron-glia co-cultures were exposed for 48 h to vehicle (0.1% DMSO) or varying concentrations of PCB 95. Fold-change relative to sex-matched vehicle control was quantified for: (A, E) the total number of primary dendrites; (B, F) mean length of the primary dendrites (μm); (C, G) the number of nonprimary dendrites; and (D, H) the mean length of nonprimary dendrites (μm). Data are presented as mean ± SE (n = 13–70 neurons from at least 3 independent dissections). *Significantly different from vehicle control at p < .05, as determined using Kruskal-Wallis test followed by Dunn’s multiple comparison post hoc test.
PCB 95 Does Not Alter the Ratio of Neurons to Astrocytes or Cell Viability
Previous studies have demonstrated that neuron-glia co-cultures grown under the culture conditions used in these studies consist predominantly of neurons and astrocytes with negligible numbers of oligodendrocytes or microglia (Stamou et al., 2018). Astrocytes have been demonstrated to regulate dendritic growth in primary central neurons (Prochiantz, 1995), and in vivo studies have demonstrated that developmental PCB exposure changes glial cell populations in the brains of exposed offspring (Miller et al., 2010; Morse et al., 1996; Nguon et al., 2005). Therefore, to determine whether sex-dependent effects of PCB 95 on dendritic growth reflect differential effects of PCB 95 on the ratio of neurons to astrocytes in neuron-glia cocultures in male versus female cultures, we immunostained DIV 9 cultures for MAP2B to label neurons and GFAP to label astrocytes (Figure 5A). In male and female hippocampal cultures, relative to sex-matched vehicle control cultures, PCB 95 did not alter the percentage of MAP2B immunopositive cells (Figs. 5B and 5E), the percentage of GFAP immunopositive cells (Figs. 5C and 5F), or the ratio of MAP2B to GFAP immunopositive cells (Figs. 5D and 5G). Similarly, in male and female cortical cultures, relative to sex-matched vehicle control cultures, PCB 95 did not alter the percentage of MAP2B (Figs. 6B and 6E) or GFAP (Figs. 6C and 6F) immunopositive cells, or the ratio of MAP2B to GFAP immunopositive cells (Figs. 6D and 6G).
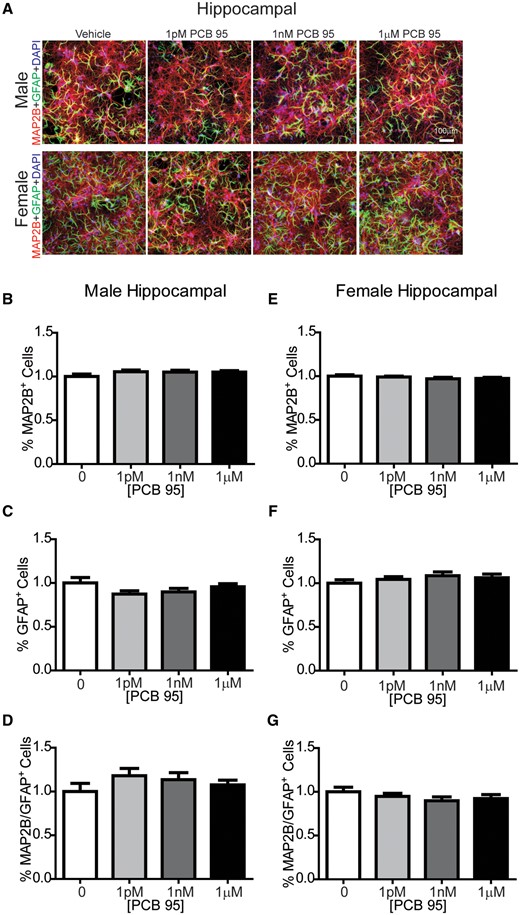
PCB 95 does not alter the number of neurons or astrocytes in hippocampal cocultures. A, Representative images of male and female mouse hippocampal neuron-glia cocultures exposed for 48 h to vehicle (0.1% DMSO) or varying concentrations of PCB 95 beginning at DIV 7. At the end of the exposure period, cultures were immunostained for MAP2B (red) to identify neurons; glial fibrillary acidic protein (GFAP, green) to identify astrocytes, and DAPI (blue) to identify nuclei. Fold-change relative to sex-matched vehicle control was determined for (B, E) the percentage of cells immunopositive for MAP2B; (C, F) the percentage of cells immunopositive for GFAP; and (D, G) the ratio of MAP2B/GFAP immunopositive cells. Data are presented as mean ± SE (up to 9 sites were averaged per well with n = 6–9 wells analyzed per group from at least 3 independent dissections). No significant differences from vehicle control were detected 1-way ANOVA (B, C, D, G) or Kruskal-Wallis test (E, F) at p < 0.05.
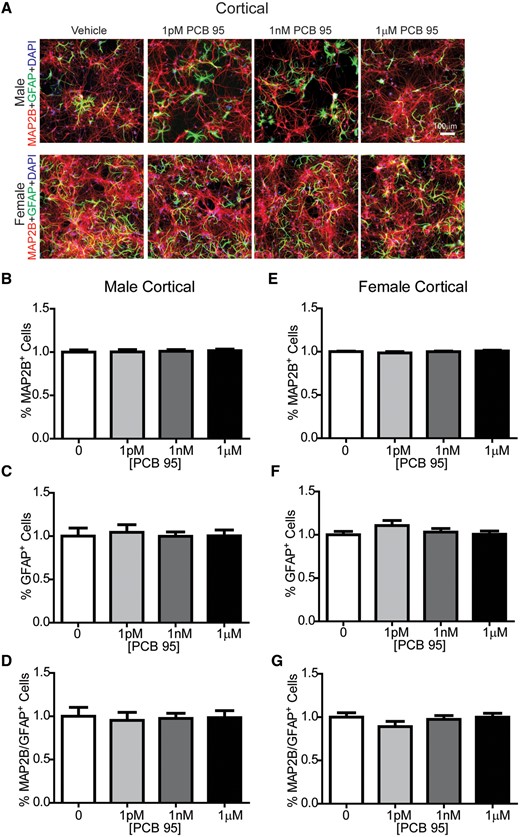
PCB 95 does not alter the number of neurons or astrocyte in cortical cocultures. A, Representative images of male and female mouse cortical neuron-glia co-cultures exposed for 48 h to vehicle (0.1% DMSO) or varying concentrations of PCB 95 beginning at DIV7. At DIV9, cultures were immunostained for MAP2B (red) to identify neurons; GFAP (green) to label astrocytes, and DAPI (blue) to label nuclei. Fold-change relative to sex-matched vehicle control was determined for: (B, E) the percentage of MAP2B immunopositive cells; (C, F) the percentage of GFAP immunopositive cells; and (D, G) the ratio of MAP2B/GFAP immunopositive cells. Data are presented as mean ± SE (up to 9 sites were averaged per well with n = 7–9 wells per group from at least 3 independent dissections). No significant differences from vehicle control were detected by 1-way ANOVA (C, D, G) or Kruskal-Wallis test (B, E, F) at p < .05.
To rule out cell viability as a factor contributing to sex-specific dendritic responses to PCB 95, we quantified PCB 95 effects on LDH release into the media and the ratio of live to dead cells as determined by calcein AM (live cells) and propidium iodide (dead cells) staining. In male and female hippocampal and cortical cell cultures, PCB 95 did not alter LDH release or the percentage of live cells relative to sex-matched vehicle control cultures (Figure 7). We also confirmed that ryanodine receptors 1 and 2, which are molecular targets of PCB 95 that mediate PCB 95 effects on dendritic growth (Wayman et al., 2012b), are expressed in male and female hippocampal neurons under our culture conditions (Supplementary Figure 1).
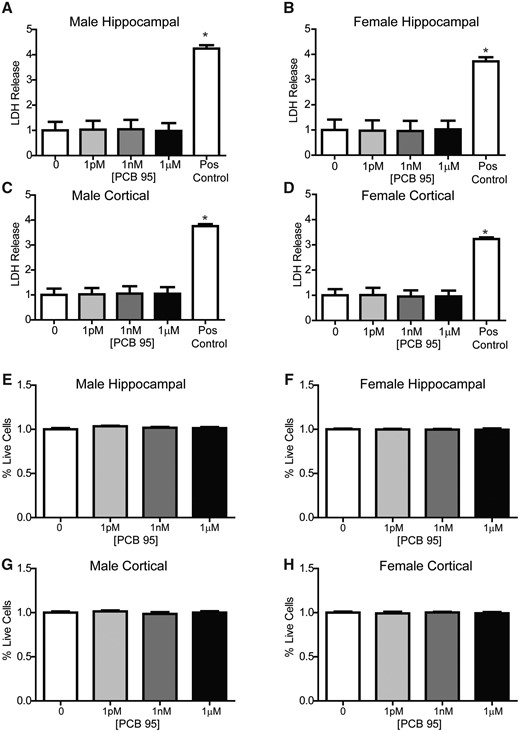
PCB 95 does not alter cell viability in male or female hippocampal and cortical cultures. Male and female mouse hippocampal (A, B, E, F) or cortical (C, D, G, H) neuron-glia cocultures were exposed to vehicle (0.1% DMSO) or varying concentrations of PCB 95 for 48 h beginning on DIV7. (A–D) On DIV 9, cell viability was determined by measuring LDH release into the medium. As a positive control, a subset of cultures were lysed with 0.2% Triton X. Data are presented as mean ± SE fold change from sex-matched vehicle control (n = 3–4 wells per group from at least 3 independent dissections). *Significantly different from vehicle control at p < 0.05, as determined using 1-way ANOVA followed by Dunnett’s multiple comparison post hoc test. (E-H) Cell viability was also analyzed by quantifying the percentage of live cells in cultures stained with calcein AM (biomarker of live cells) and propidium iodide (biomarker of dead cells) using Metamorph image analysis software. Data are presented as mean ± SE fold change from sex-matched vehicle control (n = 6–9 wells per group from 2-3 independent dissections). At least 5 sites were imaged per well; images that contained fewer than 250 cells were not included in the analysis. No significant differences from vehicle control were detected by 1-way ANOVA (F, H) or Kruskal-Wallis test (E, G) at p < .05. Raw mean percentage of live cells for vehicle groups was 95.3% ± 1.3%, 96.8% ± 0.76%, 95.7% ± 1.41%, and 96% ± 1.19% for male hippocampal, female hippocampal, male and female cortical neurons, respectively. The highest lysis control value was 0.02% ± 0.01% live cells (not shown).
Male Hippocampal and Cortical Neurons Exhibit Activity-Dependent Dendritic Growth
Data from previous studies demonstrating that the dendritic arbors of primary neurons cultured from male mouse hippocampi and neocortices are significantly larger than that of their female counterparts (Keil et al., 2017; Sethi et al., 2017c), raised the possibility that the lack of dendritic response to PCB 95 by male neurons may reflect the fact that their dendritic arbors are already at their maximal size and not capable of further expansion. To test this hypothesis, male and female hippocampal neuron-glia co-cultures were exposed to bicuculline (BIC, 20 μM), a type A γ-aminobutyric acid (GABAA) receptor antagonist, which has been shown to trigger activity-dependent dendritic growth in primary hippocampal neurons via transient activation of Ca2+-dependent signaling pathways (Wayman et al., 2006). In male hippocampal neurons, bicuculline increased the total and distal AUC of the Sholl plot relative to sex-matched vehicle control cultures (Figs. 8A–C). In female hippocampal neurons, bicuculline did not alter the total AUC of the Sholl plot relative to sex-matched vehicle control cultures (Figs. 8D and 8E); however, bicuculline increased the distal AUC of the Sholl plot relative to sex-matched vehicle control cultures (Figure 8F). Male and female cortical neurons responded similarly to bicuculline, showing an increase in the total AUC of the Sholl plot (Figs. 8G–J).
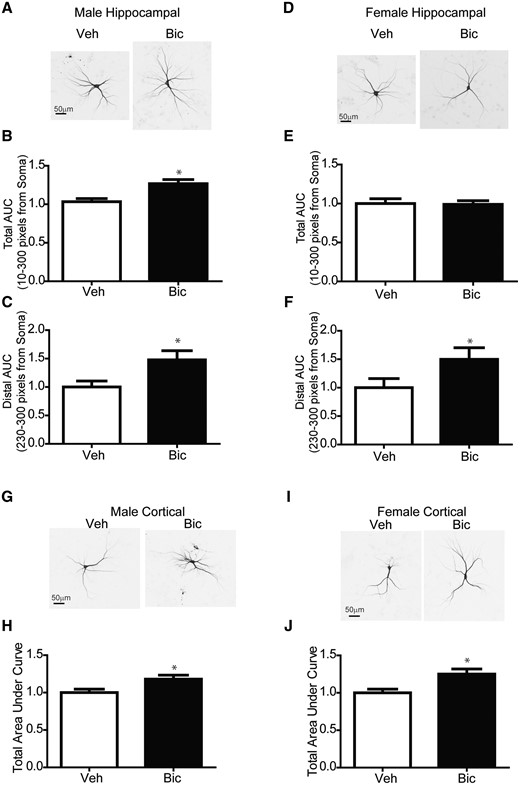
Bicuculline increases dendritic arborization in male and female hippocampal neurons. (A, D, G, I) Representative photomicrographs of DIV 9 GFP+ male and female mouse (A–F) hippocampal or (G–J) cortical neurons exposed for 48 h to either vehicle or bicuculline (20 μM) beginning on DIV7. Fold-change relative to sex-matched vehicle control was quantified for: (B, E, H, J) the total area under the Sholl curve (0–300 pixels from the soma); and (C, F) the total distal area under the Sholl curve (230–300 pixels from the soma). Data are presented as mean ± SE (hippocampal, n = 33–74 neurons from at least 3 independent dissections; cortical, n = 32–61 neurons from 2 to 3 independent dissections). *Significantly different from male at p < .05, as determined using Student’s t test (B, H, J) or Mann-Whitney test (C, E, F). Abbreviations: AUC, area under curve; Bic, Bicuculline; Veh, Vehicle.
DISCUSSION
Rodent models have revealed sex-dependent effects of developmental PCB exposure on brain neurochemistry (Dervola et al., 2015) and behavior (Bell et al., 2016; Gillette et al., 2017; Tian et al., 2011). Here, we extend these in vivo observations to demonstrate sex-dependent PCB effects on neurodevelopment in vitro. Specifically, we observed that dendritic responses to PCB 95 varied according to sex in neuron-glia cocultures derived from developing mouse hippocampi and neocortices. Female neurons from both brain regions responded more robustly to the dendrite-promoting activity of PCB 95, and at lower concentrations, than their male counterparts. The female-specific effects of PCB 95 on dendritic arborization are consistent with female-specific performance deficits in a hippocampal-dependent memory task (Tian et al., 2011), and female-specific anxiety-like behavior in adolescent mice (Bell et al., 2016) following developmental PCB exposure. The sex specificity of PCB 95-induced dendritic growth in cultured mouse neurons was not due to intrinsic differences in dendritic arborization between male and female neurons since neurons from both sexes responded to bicuculline with increased dendritic complexity. The sex-specific dendritic responses were also not due to differential cytotoxicity of PCB 95 in female versus male cultures, as evidenced by no change in cell viability or the ratio of neurons to astrocytes in primary cultures exposed to PCB 95 at the 1pM, 1 nM, and 1 μM concentrations used to assess dendritic responses. Collectively, these data suggest sex differences specifically in the mechanism(s) of PCB 95-induced dendritic growth.
These data extend our previous work demonstrating a nonmonotonic concentration dependency of PCB 95-induced dendritic arborization in mixed sex neuron-glia cocultures derived from rat hippocampi and neocortices (Wayman et al., 2012b; Yang et al., 2009). Whether the dendrite-promoting effects of PCB 95 are sex-specific in the rat has yet to be addressed. Recent studies of PCB 11 demonstrated that dendrite-promoting effects of PCB 11 were not sex-dependent in primary rat neuron-glia cocultures (Sethi et al., 2017c). However, consistent with our observations of PCB 95, in primary mouse cultures, PCB 11 promoted dendritic growth in female but not male hippocampal neurons (Sethi et al., 2017c). In contrast to our observations with PCB 95, PCB 11 promoted dendritic growth in male but not female mouse cortical neurons. These observations identify altered dendritic arborization as a convergent mechanism of PCB developmental neurotoxicity, but demonstrate that dendritic outcomes vary according to PCB congener, brain region, sex, and species.
A key question raised by these studies concerns the mechanism(s) driving sex differences in PCB-induced dendritic growth. Sex differences in liver and brain cytochrome P450s have been proposed to impact developmental neurotoxicity of PCBs by differentially altering PCB metabolism and disposition (Nagai et al., 2016; Wu et al., 2013). Although this mechanism may influence sex-dependent effects of PCBs in vivo, it is unlikely to contribute significantly to sex-dependent effects of PCBs in vitro. PCB 95 promotes dendritic growth via ryanodine receptor (RyR) sensitization (Wayman et al., 2012b), suggesting sex differences in RyR expression and/or activity as an alternate hypothesis. RyRs are differentially expressed in male versus female heart tissue (Chu et al., 2005), and they have been linked to the enhanced sensitivity of females to hyperalgesic priming (Ferrari et al., 2016). Whether RyR expression and/or activity varies significantly between male and female neurons has yet to be rigorously tested but immunocytochemical data suggest that both male and female neurons express RyR 1/2 under the culture conditions used in this study (Supplementary Figure 1). Moreover, observations that PCB 11 has negligible RyR activity (Holland et al., 2017) argue against sex differences in RyRs as a conserved mechanism of sex-specific effects of PCBs on dendritic arborization.
Sex hormones influence neuronal morphogenesis (McCarthy et al., 2018), and we recently demonstrated that tissue culture media containing phenol red, an estrogen mimetic (Liu et al., 2013), influences dendritic growth in primary mouse neurons in a sex-dependent manner (Keil et al., 2017). Further, androgen receptor, estrogen receptor (alpha and beta), and aromatase transcripts are expressed in our culture system (Keil et al., 2017). In vitro reporter assays indicate that PCB 95 has estrogenic activity (Rogers and Denison, 2000; Zou et al., 2002), but whether PCB 95 effects on dendritic growth in cultured neurons are mediated by estrogenic mechanisms remains to be tested. Arguing against hormone receptor signaling as a generalized mechanism of sex-dependent PCB effects on dendritic arborization, PCB 11 was reported to have negligible activity at estrogen receptors, although it was shown to be antiandrogenic (Takeuchi et al., 2017). Together, these data suggest that if steroid hormone signaling plays a role in PCB neurotoxicity, it is in a congener, sex and brain region specific manner.
Sex-specific effects of PCB 95 on dendritic arborization may reflect sex differences in the rate of brain maturation during development (McCarthy et al., 2018). In mice, the critical period for sexual differentiation in the brain occurs around embryonic day 16 for males (coinciding with testosterone production) through birth, while in the female brain, responsiveness to endogenous hormones extends for several days after birth (McCarthy et al., 2018). The ability of estrogen to alter the developmental trajectory of the female brain after birth may contribute to female susceptibility to the dendrite-promoting activity of PCB 95 in neurons cultured from P0 mouse brains. The hypothesis that the developmental stage of neurons influences susceptibility to the effects of PCB 95 on dendritic growth may also explain species differences in the sex-dependency of PCB effects on dendritic growth. Rat and mouse neurons mature at different stages in vivo, thus primary cultures established from a P0 rat and mouse do not begin at the same developmental stage (Bowers et al., 2010). Further, the maturation rates of rat and mouse neurons follow a different trajectory in vitro (Baj et al., 2014). DIV 7–9 is a period of dynamic growth and retraction for the formation and branching of dendrites in mouse hippocampal neurons, but not nearly as much for rat hippocampal neurons which show the most drastic changes in branching from DIV 4–6 to 10–13 (Baj et al., 2014). Collectively, these observations suggest that the maturational state of the neurons at the time of exposure is critical in determining the dendritic response to PCBs.
An interesting observation from these studies is that while male mouse hippocampal and cortical neurons did not elaborate more complex dendritic arbors in response to PCB 95, they did in response to bicuculline. Bicuculline is a GABAA receptor antagonist, previously shown to enhance activity-dependent dendritic growth in rat hippocampal neurons via sequential activation of NMDARs, CaMKK, CaMKI, Ras, MEK/ERK, and CREB-dependent transcription of Wnt-2 (Wayman et al., 2006). PCB 95 phenocopies bicuculline via RyR-dependent mechanisms, which converge on the same downstream CaMKI–CREB–Wnt2 signaling pathway (Wayman et al., 2012a). The reason(s) for the differential sex-dependency of dendritic responses to bicuculline versus PCB 95 despite their convergence on the same downstream signaling pathway are unknown, but suggest that other upstream or parallel molecular targets including NMDARs (van den Buuse et al., 2017), scaffolding proteins (Brady and Jacob, 2015) and histone deacetylases (Formisano et al., 2011) contribute to differences in sex-specificity of dendritic responses to PCB 95 versus bicuculline. One molecular target of interest in this context is the mTOR pathway. mTOR signaling has been causally linked to PCB 95-induced dendritic growth in rat neurons (Keil et al., 2018), and sex differences in protein abundance of mTOR signaling components have been reported in mouse brain (Block et al., 2015). Whether the differential effect of sex on dendritic growth induced by PCB 95 versus bicuculline is due to the fact that mTOR signaling is necessary for the dendrite-promoting activity of PCB 95, but not bicuculline, remains to be determined. An interesting observation is that the dendritic response of mouse hippocampal neurons to bicuculline included changes in the total and distal dendritic arbor of male neurons, but was restricted to the distal portion of the dendritic arbor in female neurons, which suggests that sex may exert subtle influences on bicuculline-induced dendritic growth. This is consistent with reports of sex differences in bicuculline-induced secretion of somatostatin from hypothalamic explants (Murray et al., 1999). Further characterization of sex differences in response to activity-dependent signaling in mouse neurons are needed to better understand the influence of these pathways in development and disease.
An outstanding question is whether sex differences in the response to environmental neurotoxicants contribute to NDD sex biases. Although both males and females are diagnosed with NDDs, males tend to be more frequently diagnosed with early onset disorders such as ASD and ADHD, whereas females are more frequently diagnosed with adult onset disorders like depression (McCarthy, 2016). Within disorders there are also discrepancies in the types of clinical symptoms that manifest in male versus female patients (Gaub and Carlson, 1997; Gershon, 2002; Halladay et al., 2015). Further, sex differences in functional connectivity have been reported in NDDs (Alaerts et al., 2016). Dendritic morphology is a major determinant of neural connectivity (Cline, 2001; Penzes et al., 2011), thus, environmental factors like PCB 95 that alter dendrite morphology are likely risk factors for NDDs.
The human relevance of the findings reported here is further suggested by the observation that PCB 95 significantly enhanced dendritic growth at concentrations as low as 100 fM. The concentrations of PCB 95 used here (100 fM—1 μM) equate to 0.0000163—163 ng PCB 95 per well (in 0.5 ml of culture media). If we assume 1 ml is approximately 1 g then our concentrations range from 0.0000326 to 326 ng/g. PCB 95 serum levels in pregnant mothers were found range from below the level of detection to 0.1660 ng/g serum with a mean of 0.0059 ng/g serum (Koh et al., 2016). PCB 95 levels in adult males have been reported at approximately 0.4 ng/ml plasma which equates to 1.2 nM (Lin et al., 2013). The levels of 4 congeners, including PCB 95, were also detected in infant cord blood at approximately 56 ng/g lipid (Rahbar et al., 2016). PCBs distribute to the human adult and fetal brain (Chu et al., 2003; Dewailly et al., 1999; Lanting et al., 1998), and PCB 95 levels have been reported in the adolescent brain of both children diagnosed with a NDD and neurotypical controls at levels ranging from 3.72 to 67.15 ng/g lipid. Interestingly, in this study, PCB 95 was significantly higher in children with a genetic NDD compared with neurotypical controls (Mitchell et al., 2012). PCB 95 exposure to mice results in comparable PCB 95 levels in the dam and fetus, with PCB 95 levels in the brain of resultant offspring of 50–70 ng/g tissue, which converts to approximately 28 ng of PCB 95 in a weanling mouse brain (Kania-Korwel et al., 2017). Further, PCB 95 has been detected in the serum of cattle in California at approximately 0.4 ng/ml (1.2 nM) and sheep/goats at approximately 1 ng/ml (3 nM) (Sethi et al., 2017a), as well as in commercial milk at 0.0061 ng/ml (4.26 ng/g lipid) which is approximately 18.6 pM (Chen et al., 2017b). Together, our results suggest that at concentrations that are within the range of human exposure, PCB 95 induces dendritic growth via sex-dependent mechanisms. This observation suggests a novel mechanism by which environmental exposures may contribute to sex biases of NDDs.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This work was supported by the National Institutes of Health, National Institutes of Environmental Health Sciences (R01 ES014901 to P.J.L.; P01 ES011269, P30 ES023513, and T32 ES007059; predoctoral fellowship to S.S.); the Eunice Kennedy Shriver National Institute of Child Health & Human Development (F32 HD088016 to K.P.K.); and the U.S. Environmental Protection Agency (R833292). This project used core facilities supported by the MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the USEPA. Further, the National Institutes of Health and USEPA did not endorse the purchase of any commercial products or services mentioned in the publication.
REFERENCES
CDC, Center for Diseasee Control and Prevention. (




Comments