-
PDF
- Split View
-
Views
-
Cite
Cite
Jeffrey L. Larson, Seong-Kwi Kang, Bo In Choi, Maria Hedlund, Laura M. Aschenbrenner, Beth Cecil, GloriaMay Machado, Matthew Nieder, Fang Fang, A Safety Evaluation of DAS181, a Sialidase Fusion Protein, in Rodents, Toxicological Sciences, Volume 122, Issue 2, August 2011, Pages 567–578, https://doi.org/10.1093/toxsci/kfr109
Close - Share Icon Share
Abstract
DAS181 is a novel inhaled drug candidate blocking influenza virus (IFV) and parainfluenza virus (PIV) infections through removal of sialic acid receptors from epithelial surface of the respiratory tract. To support clinical development, a 28-day Good Laboratory Practices inhalation toxicology study was conducted in Sprague-Dawley rats. In this study, achieved average daily doses based on exposure concentrations were 0.47, 0.90, 1.55, and 3.00 mg/kg/day of DAS181 in a dry powder formulation. DAS181 was well tolerated at all dose levels, and there were no significant toxicological findings. DAS181 administration did not affect animal body weight, food consumption, clinical signs, ophthalmology, respiratory parameters, or organ weight. Gross pathology evaluations were unremarkable. Histological examination of the lungs was devoid of pulmonary tissue damage, and findings were limited to mild and transient changes indicative of exposure and clearance of a foreign protein. DAS181 did not show any cytotoxic effects on human and animal primary cells, including hepatocytes, skeletal muscle cells, osteoblasts, or respiratory epithelial cells. DAS181 did not cause direct or indirect hemolysis. A laboratory abnormality observed in the 28-day toxicology study was mild and transient anemia in male rats at the 3.00 mg/kg dose, which is an expected outcome of enhanced clearance of desialylated red blood cells resulting from systemic exposure with DAS181. Another laboratory observation was a transient dose-dependent elevation in alkaline phosphatase (ALP), which can be attributed to reduced ALP clearance resulting from increased protein desialylation due to DAS181 systemic exposure. These laboratory parameters returned to normal at the end of the recovery period.
DAS181 (Fludase) is a first-in-class drug candidate currently in clinical development for the treatment of influenza virus (IFV) and parainfluenza virus (PIV) infections. DAS181 is a recombinant fusion protein, consisting of a sialidase catalytic domain (derived from Actinomyces viscosus sialidase) and an epithelial surface-anchoring heparin-binding domain derived from the human protein amphiregulin (Malakhov et al., 2006). The sialidase activity of DAS181 cleaves both α2,3- and α2,6-linked sialic acids (Nicholls et al., 2008). By removing sialic acid receptors from the host cell surface, DAS181 potentially confers broad-spectrum protection as an entry blocker against all subtypes and strains of IFV and PIV (Belser et al., 2007; Moscona et al., 2010; Triana-Baltzer et al., 2009a,b). DAS181 is designed to function topically in the upper and central respiratory tract. To achieve this, DAS181 drug substance has been formulated as dry powder microparticles in the range of 5–10 μm in size for optimal upper and central airway deposition when delivered via oral inhalation. DAS181 drug product formulation and delivery are also designed to minimize drug deposition in the deep lungs to avoid systemic drug absorption through the permeable alveoli epithelium in the lungs (Frijlink and De Boer, 2004; Labiris and Dolovich, 2003; Patton, 1996; Pauwels et al., 1997). The anticipated clinical dose range of DAS181 is 5–20 mg, daily for up to 5 days.
Normally in vivo, sialidase activity is found intracellularly (Achyuthan and Achyuthan, 2001); in the plasma, sialidase activity is kept extremely low. Significant elevation of sialidase activity in the systemic circulation is predicted to have some untoward effects, such as disturbance of hematological homeostasis due to exaggeration of the physiological mechanism that recognizes and destroys desialylated erythrocytes (red blood cell [RBC]), as desialylation is a marker of RBC senescence (Schauer, 2004). Although the normal survival time of RBC in humans is about 120 days, desialylated RBC can be cleared from the blood circulation within a few hours. Clearance of the desialylated RBC is mediated by asialoglycoprotein receptors (ASGR) expressed by macrophages (Kupffer cells) in the liver and spleen. These receptors are also involved in the clearance of desialylated glycoproteins (Aminoff et al., 1977; Bilzer et al., 2006; Stockert, 1995). Thus, a potential undesirable effect of DAS181, if absorbed at significant levels into the systemic circulation, is an increased rate of RBC elimination due to the pharmacological effect of DAS181 (desialylation), resulting in reduced RBC counts. However, such an effect is expected to be reversible when DAS181 is cleared from the systemic circulation.
Topical local desialylation of the respiratory epithelium has been known to be well tolerated. In guinea pigs and in rats, sialidase treatment does not induce airway hyperreactivity; on the contrary, sialidase treatment may significantly reduce bronchoconstriction (Jarreau et al., 1992; Kai et al., 1992). In our experiments using guinea pigs and mice, DAS181 did not cause airway hyperreactivity in normal animals; in addition, it effectively reduced airway hyperreactivity in allergen-sensitized animals as well as methacholine-challenged animals (Larson, Hedlund, and Fang, unpublished data).
To support the use of DAS181 in clinical trials, the drug candidate has been subjected to toxicology studies under Good Laboratory Practices (GLP) compliance. Here, we report the results of a 28-day inhalation toxicology study of DAS181 dry powder in Sprague-Dawley rats. The study results show no significant toxicology findings and no systemic target organ toxicity at a daily dose of up to 3.0 mg/kg, corresponding to a human equivalent dose (HED) of 28.8 mg. Laboratory findings at higher doses included mild and transient anemia, an expected effect of RBC desialylation, as well as mild and transient elevations of serum alkaline phosphatase (ALP). Additional in vitro and in vivo mechanistic studies indicated that ALP elevation was due to a reduced ALP clearance resulting from systemic exposure with DAS181. This mechanism is supported by the lack of any histopathological findings in the livers of rats exposed to DAS181 by inhalation and the lack of toxicity of DAS181 in hepatocyte cultures from multiple species.
MATERIALS AND METHODS
Study conducts.
The GLP toxicology study in rats was conducted by ITR Laboratories and GLP compliance was monitored by ITR Quality Assurance Department (Montreal, Quebec). The in vitro cytotoxicity assays and non-GLP studies in mice and rats were conducted at NexBio, Inc. (San Diego, CA). Clinical pathology analyses for non-GLP studies were conducted by BioQuant Clinical Pathology Laboratories (San Diego, CA). ALP isoenzyme analyses were conducted at Anilytics (Gaithersburg, MD).
Chemicals.
DAS181 dry powder formulation consists of approximately 65% DAS181 drug substance in a proprietary excipient blend. The control article for the inhalation study was Respitose ML001, monohydrate lactose (DMV International B.V., The Netherlands).
Internal in vivo studies in rodents used DAS181 drug substance prepared as a solution in PBS at a concentration of 20.9 mg/ml (by ultraviolet assay) and sterile filtered prior to administration. Lyophilized asialofetuin (ASF) (Sigma, St Louis, MO) or fetuin (Calbiochem, San Diego, CA) were resuspended in PBS and sterile filtered prior to injection. Sterile (PBS) was obtained from Hyclone (Logan, UT). All test articles were prepared for administration prior to initiation of each treatment period and stored in aliquots at 4°C until needed for dosing.
Growth medium for cell lines: (1) Williams E medium (Lonza, Walkersville, MD) supplemented with 0.1% Pen/Strep (Sigma), 1 μl 10mM dexamethasone (Fisher Scientific, San Diego, CA) in dimethyl sulfoxide (Sigma), 0.2% selenite solution (ITS, Gibco, Grand Island, NY) 0.2% l-glutamine (Gibco), and 0.3% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (Sigma) (primary hepatocytes); (2) InVitroGRO CP Medium (Celsis In Vitro Technologies, Baltimore, MD) (kidney cells); (3) osteoblast growth medium (Cambrex, Rockland, ME) (osteoblasts); (4) skeletal muscle growth medium supplemented with Cambrex SkGM SingleQuots (Cambrex) (skeletal muscle cells); (5) myoblast growth medium supplemented with Cambrex SkGM-2 SingleQuots (Cambrex) (smooth muscle myoblasts); (6) Dulbecco's Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS), 1× Glutamax (Invitrogen, Carlsbad, CA), and 1× antibiotic/antimycotic solution (Sigma) (A549 cells); (7) DMEM-F12 (Gibco) supplemented with 10% FBS, 1× Glutamax (Invitrogen), 1× antibiotic/antimycotic solution (Sigma), and 1× nonessential amino acids (Invitrogen) (human bronchial epithelial BEAS-2B cells); and (8) AIR-100-MM fresh maintenance medium (Mattek Corp., Ashland, MA) (human airway epithelium [HAE]). In vitro cytotoxicity assays utilized sterile-filtered PBS solutions of DAS181 to provide the requisite concentrations in cell media.
Animals.
Sprague-Dawley rats for the GLP toxicology study obtained from Charles River Canada (St-Constant, Quebec) were 8–10 weeks at treatment initiation. For the mechanistic studies, female BALB/c mice 8–11 weeks of age and Sprague-Dawley rats 6–8 weeks of age at study start were obtained from Charles River (Hollister, CA).
The protocol for the GLP toxicology study was reviewed and assessed by the Animal Care Committee of ITR. Protocols for in vivo studies conducted at NexBio were reviewed and approved by the NexBio Animal Care and Use Committee. All animals used in studies described in this article were in accordance with the principles outlined in the “Guide for the Care and Use of Laboratory Animals,” the National Institutes of Health publication.
Primary cells and cell lines.
Freshly isolated hepatocytes (three rat donors, one dog donor, three human donors) were obtained from CellzDirect (Pittsboro, NC). The following cells were obtained from single human donors: Cryopreserved human proximal renal tubule cells were obtained from InVitro Technologies (Baltimore, MD), cryopreserved human skeletal muscle myoblasts (differentiated and undifferentiated), human osteoblasts (differentiated and undifferentiated), and human skeletal muscle cells were obtained from Cambrex (Walkersville, MD). An A549 cell line and a BEAS-2B cell line, derived from human bronchial epithelial cells, were obtained from ATCC (Manassas, VA); these cell lines were expanded and individual aliquots frozen for use in the studies. Mattek’s Epi-airway well-differentiated HAE cells were obtained from Mattek Corp..
Inhalation exposures GLP study.
Animals were exposed by inhalation using nose-only exposure for 30 min daily. The aerosol generated using DAS181 dry powder formulation was produced using a piston feed/rotating brush generator. A micronizing air jet mill in addition to the piston feed/rotating brush generator was used to generate the lactose control article aerosols. The aerosols produced were diluted with air as necessary to achieve the target aerosol concentration and discharged through a 40-mm diameter tube into a flow-past nose-only exposure system. Aerosol concentration and particle size distribution were determined on samples collected from a representative port of the exposure chamber. Aerosol concentration was monitored daily for gravimetric analysis, and filter samples were collected for determination of DAS181 content using a validated high-performance liquid chromatography (HPLC) analytical method. DAS181 analyses were performed using Waters HPLC system (Waters Ltd, Ontario, Canada) with separations module (Waters 2695, VYDAC, Protein and Peptide C18 analytical columns [Grace Davidson Discovery Sciences, Grace], and a dual λ absorbance detector [Waters 2487]). Estimated achieved doses were calculated using analytical results obtained from these filters. Target dose levels were based on an estimated body weight of 0.25 kg per rat because it is not feasible to individually expose rats on the basis of body weight. The achieved dose levels were estimated by calculation of the actual concentration delivered to the animals (mg/l air), the respiratory minute volume (l/min) according to Bide et al. (2000), and the duration of daily exposure and the mean body weight during the exposure period. This estimation of total inhaled dose assumed 100% deposition within the respiratory tract. The target dose level and corresponding aerosol concentration for the lactose control group (mass of control article administered) matched the aerosol concentration for the high-dose DAS181 group by gravimetric analysis. Gravimetric and chemical determinations of particle size distributions were measured once weekly by collecting samples into a seven-stage Mercer cascade impactor and determining the mass median aeriodynamic diameter and geometeric standard deviation.
28-Day GLP inhalation toxicology study design.
For the main study, 10/sex/group were assigned to either the lactose control (group 1) or target DAS181 daily doses of 0.5, 1.0, 1.5, or 3.0 mg/kg/day (groups 2, 3, 4, and 5, respectively). An additional 5/sex/group assigned to groups 1, 4, and 5 were similarly exposed for 28 days and then held without exposure for another 28 days. Bronchoalveolar lavage (BAL) fluid was collected from an extra 3/sex/group in groups 1, 4, and 5 and from the main study animals upon necropsy for evaluation of total and white blood cell (WBC) differentials. An extra 6/sex/group in each DAS181 treatment group were designated as satellite animals used solely for toxicokinetic (TK) evaluation at predose, 1, 2, 4, 8, 12, and 24 h, with 3/sex/group/time point and evaluation after dosing on days 1 and 28 (an extra 48-h time point was included for the day 28 dose). Antidrug antibody (ADA) titers were determined in the pharmacokinetic (PK) animals at the last respective time point, 24 or 48 h, after the day 28 dose. ADA titers were also determined in the 5/sex/group recovery animals at baseline and on days 29 and 57. Standard GLP toxicology assessments were conducted including clinical signs, body weights, food consumption, ophthalmology and respiratory evaluations, clinical pathology analyses, and terminal necropsies. Organ weights were collected and the standard panel of tissues was collected from all animals. Histopathological examinations were performed on all tissues/organs from all animals in groups 1, 4, and 5 at main study and recovery terminations and from the liver, spleen, kidneys, and five sections of lungs from rats in groups 2 and 3.
Plasma DAS181 analysis.
Blood samples were collected into acid citrate dextrose tubes and processed to plasma, which was used for both DAS181 concentration and anti-DAS181 antibody analyses. Plasma concentrations of DAS181 were determined using a validated biological sialidase assay method with a lower limit of quantitation (LLOQ) of 0.23 ng/ml. In this method, standard, controls, and study plasma samples were incubated at room temperature in a microtiter plate with 100μM 2′-(4-methlyumbellyferyl)-α-d-N-acetylneuraminic acid (MuNANA) in pH 4.75, 100 mM citrate buffer (sodium), 0.5% bovine serum albumin (BSA), 0.025% Tween 20, and 0.02% thimerosal. After 20 min, the reaction is stopped with an equal volume of pH 10.2 500mM glycine (sodium), and the intensity of the fluorescence due to released N-acetyl neuraminic acid was measured (λex 365 nm, λem 455 nm). The equivalent DAS181 concentration was calculated from the relative fluorescence units (RFU) of each sample using a standard curve prepared with known concentrations of DAS181.
Anti-DAS181 antibody and neutralizing antibody analyses.
High-binding polystyrene 96-well microtiter plate wells were coated with 1 μg/ml DAS181 in pH 9.6 200mM carbonate (sodium) at 2–8°C overnight, washed with PBS + 0.1% Tween 20, and blocked with 3% BSA in PBS. Controls (affinity-purified rat anti-DAS181 polyclonal antibody in pooled normal rat plasma and a panel of 10 normal rat plasma) and study plasma samples were incubated for 1 h at room temperature. The plate was then washed and bound antibody was detected by further sequential incubations using biotin-conjugated DAS181, avidin-horseradish peroxidase (HRP), tetramethylbenzidine HRP substrate, and lastly, 2M sulfuric acid was added as a stop solution. A study sample was considered positive if the optical density (OD) was greater than or equal to the assay cutpoint OD. The assay cutpoint was defined as the mean OD of 10 individual normal rat plasma samples plus 1.645 times the SD for each assay plate. The titer of a positive ADA sample was the highest dilution factor giving an OD greater than or equal to the plate cutpoint OD. The titer of a negative ADA sample was considered less than the minimum required dilution factor (<10).
Study samples from 10 rats/group in groups 4 and 5 with positive ADA and corresponding predose samples were further characterized for DAS181 neutralizing antibody (NAb) titer to determine whether the immune response would be neutralizing of DAS181 sialidase activity. 2 ng/ml of DAS181 was incubated with controls and plasma samples in a black microtiter plate for 1 h at 37°C to achieve binding equilibrium. MuNANA sialidase substrate was then added and sialidase activity was determined as described above, except that pH 7.4 PBS was used instead of citrate buffer and the reaction period was 1 h at 30°C. Inhibition of DAS181 activity was calculated relative to the mean signal from the 10 normal individual plasmas. A study sample was considered positive if the relative inhibition was greater than or equal to the assay cut point. The NAb assay cut point was 1.645 times the SD for the normal panel relative inhibition on each plate. The NAb titer was defined as above for ADA, and the minimum required dilution factor was also 10.
Pharmacokinetic analyses.
WinNonlin version 5.2 from Pharsight Corp. (Mountain View, CA) was used to calculate systemic exposures over time area under the curve 1 (AUC) using the linear trapezoidal method. Because the sampling used different animals at the different time points, analyses were based on a single mean value at each time point. For estimation of overall systemic exposures in the 28-day GLP inhalation study, the AUC was calculated at AUC from 0 to 24 h (AUC0–24 h). Values less than the LLOQ of 0.23 ng/ml were input as zero for calculation purposes using linear interpolation. The 0–24 h cutoff for AUC determination and the use of linear interpolation including values less than the LLOQ as 0.0 was believed to provide the best estimation of systemic exposures in this highly variable data set where many values were at or below the LLOQ in this biological assay.
Mechanistic study on ALP elevation in mouse.
In the first experiment, female BALB/c mice were randomized 3/group to receive 100, 200, or 300 μl of untreated BALB/c serum proteins or 100, 200, or 300 μl of BALB/c serum proteins desialylated with mild acid hydrolysis using 0.025 N sulfuric acid for 1 h at 80°C. The desialylated serum proteins were loaded onto a PD10 column for buffer exchange, prior to injection of the specified volume. These conditions are reported to remove 98% of the sialic acid content (Spiro, 1960). Untreated or desialylated serum proteins were administered by intraperitoneal injection once daily for 2 days. Blood samples were collected 24 h after the final dose and processed to serum for determination of ALP levels. In the second experiment, female BALB/c mice were randomized 3/group to receive 1, 5, or 10 mg fetuin (sialylated protein) or 1, 5, or 10 mg ASF (desialylated protein) in saline in a dose volume of 200 μl once daily by bolus tail vein intravenous injection for 3 days. Blood samples were collected at 24 h after the final dose and processed to serum for determination of ALP levels.
Mechanistic study on ALP elevation in rats.
Rats were randomized 3/group to receive 40 or 80 mg ASF or 500 μl of untreated rat serum proteins or 500 μl of desialylated rat serum proteins. Rat serum proteins were desialylated using DAS180, which contains the identical sialidase domain as DAS181 plus a histidine (×6) tag rather than the amphiregulin anchor, for 1 h at 37°C. DAS180 protein was removed using Ni-NTA Spin kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Rats were administered fetuin, ASF, plasma, or desialylated plasma twice daily for 2 days by bolus tail vein injection (dose volume 500 μl/injection). On the third day, rats received one bolus tail vein injection and were bled 2 days later for ALP concentration analyses.
Rat ALP isoenzyme analyses.
Sprague-Dawley rats (n = 3/sex/group) were administered 0.012 or 0.12 mg/kg DAS181 in a dose volume of 1 ml/kg by bolus tail vein injection for seven consecutive days. Blood samples were collected 24 h after the final dose and processed to plasma for determination of ALP levels and ALP isoenzyme analyses. Protein was precipitated using wheat germ lectin (Schrelber and Whitta, 2011). Isoenzymes were separated by agarose gel electrophoresis using an HYDRAGEL ISOPAL kit (Sebia, Norcross, GA) according to the manufacturer’s instructions and expressed as a percentage of total ALP activity.
DAS181 in vitro cytotoxicity.
In vitro cytotoxicity experiments of the different rat, dog, and human primary cell lines were conducted in the same manner. Before DAS181 treatment, on day 0, the cell media supernatants were collected for baseline controls. DAS181 was diluted in the appropriate cell maintenance media such that 5 μl of test article or vehicle control (PBS) were added to the wells to reach the desired concentrations of 1 ng/ml to 100 μg/ml. In addition, 5 μl of 20% sodium azide was added to some of the wells to serve as a positive control for cytotoxicity. Starting from day 0, the DAS181, sodium azide, or vehicle control was incubated with the cells for 24 h. Every 24 h, on days 1–3 (24–72 h posttreatment), the cell culture supernatants were collected and fresh media was added to all the wells. In addition, every 24 h starting from day 1, two vehicle control-treated wells were lysed completely by adding 50 μl of a 9% solution of Triton X-100 (Dow Chemical) to the 500 μl of cell growth media. Both the lysed cell controls and the collected cell culture supernatant samples were stored at −80°C until further use in lactate dehydrogenase (LDH) or ALP assays. In addition to collecting cell culture supernatants and replacing the media, an MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay was performed on the final day of the experiment (day 3).
A549 and BEAS-2B cells were seeded and allowed to adhere to the plate overnight. The growth medium was exchanged with fresh growth medium, growth medium with 0.8 mg/ml DAS181, or vehicle control. At various time points over a period of 10 days, the relative number of cells per well was determined by Crystal Violet uptake. The relative number of cells was proportional to the absorbance reading at 570 nm.
Mattek’s Epi-airway well-differentiated HAE cells were grown on collagen-coated membranes and grown at the air-liquid interface, such that their apical surface was exposed to air and they obtained growth factors and nutrients from the basolateral medium. Two independent experiments were performed on two different lots of HAE. Starting from day 1, a liquid formulation of DAS181 at 64, 192, 641, or 1282 μg/cm2 or the vehicle control was applied daily for 7 days to the apical side of the tissues. Throughout the duration of the experiment, the test articles or vehicle controls were not washed away from the apical surface of the tissues. Starting from day 0 (pretreatment) through day 7, the basolateral medium was harvested once a day and was replaced with fresh maintenance medium. All the collected basolateral medium were stored at −80°C until evaluated for LDH and cytokine/chemokine levels.
Cell culture supernatants were tested for LDH activity by the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI) according to the manufacturer’s instructions. This colorimetric assay couples enzymatic activity of LDH to reduction of tetrazolium salt. As a 100% control, the relative amount of LDH activity in the Triton X-100 lysed samples was assessed. The Fluorometric Enzolyte FDP Alkaline Phosphatase Assay Kit (AnaSpec, San Jose, CA) was used to detect ALP activity in cell culture supernatants. ALP levels were detected using 3,6-fluorescein diphosphate as a fluorogenic phosphatase substrate. Fluorescence units were measured in a fluorescent microtiter plate reader at excitation at 485 nm and emission at 528 nm. Samples were also tested for cell viability using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (MTS) kit (Promega) according to the manufacturer’s instructions. MTS is converted by viable cells into a media-soluble formazan product that absorbs light at 490 nm. There is a direct correlation between the number of viable cells and the amount of formazan produced.
For cytokine/chemokine analyses, equivalent amounts of basolateral medium samples from days 1 to 7 posttreatment were pooled, such that cumulative amount of cytokines/chemokines present could be analyzed. The levels of interleukin (IL)-1α, IL-1β, IL-6, IL-10, IL-13, interferon (IFN)-α, IFN-γ, tumor necrosis factor-α (TNF-α), and RANTES in the days 1–7 pooled PBS- and DAS181-treated samples along with the baseline pretreatment samples (not pooled, only from day 0) were determined using Pierce’s SearchLight Analysis (Thermo Scientific, Rockford, IL). A media alone background control was also included. Limits of detection of the SearchLight Analysis were as follows: IL-1α < 0.4 pg/ml, IL-1β < 0.2 pg/ml, IL-6 < 0.8 pg/ml, IL-10 < 0.4 pg/ml, IL-13 < 3.9 pg/ml, IFN-α < 0.4 pg/ml, IFN-γ < 0.4 pg/ml, TNF-α < 2.3 pg/ml, and RANTES < 0.4 pg/ml. Levels of IL-8 were determined using a Pelikine Compact IL-8 Sandwich ELISA kit (Sanquin, Amsterdam, The Netherlands).
Effect of DAS181 on hemolysis.
Rat (10 male and 10 females pooled, two sets of pooled samples) and human (three normal volunteers) whole blood samples were assayed, in duplicate, within 24–48 h of collection. Aliquots of whole blood were also processed to RBC for evaluation of lysis of washed RBC. Aliquots of whole blood or washed RBC were incubated with PBS or 0.0002, 0.002, 0.02, 0.2, 2, 20, or 200 mg/ml DAS181 for 2 h at 37°C. SDS at a final concentration of 2% was used as the 100% hemolysis control. Absorbance was read at 540 nm to determine relative amount of hemolysis. To determine the percent hemolysis of the samples, the average PBS/vehicle control background sample was subtracted from the average of each DAS181- or antibody-treated sample, and the percent hemolysis was interpolated from a linear regression of the SDS-lysed RBC standard curve using Graphpad Prism version 4.02 (La Jolla, CA).
Statistical analyses.
The clinical pathology data generated in the 28-day GLP toxicology study were analyzed using the analysis of covariance/analysis of variance (ANOVA) and the significance of intergroup differences between the control and treated groups was analyzed using Dunnett’s test, two sided. A significance level of p < 0.05, 0.01, and 0.001 was reported as appropriate. For the in vitro cytotoxicity assays (LDH, ALP, and MTS) and the rodent mechanistic studies, statistical significance was evaluated using GraphPad Prism version 4.02, with statistically significant differences between the groups determined by Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001). For cytokine and chemokine levels in HAE cells, differences between baseline pretreatment samples and vehicle control-treated samples compared with the test article-treated samples were assessed by ANOVA with Dunnett’s posttest using GraphPad Prism version 4.02.
RESULTS
28-Day GLP Inhalation Toxicology Study in Rats
The overall achieved aerosol concentrations in the DAS181 exposure groups were all within ±9% of the targeted concentrations and were considered acceptable for the study. The concentration homogeneity of the aerosols within the exposure systems was acceptable as the coefficient of variation % of the mean concentration at the different stage ports was <20%. The mean estimated DAS181 achieved dose levels were 0.47, 0.90, 1.5, and 3.00 mg/kg/day for groups 2, 3, 4, and 5, respectively. Both male and female rats were exposed to the same daily aerosol exposures, and hence, the daily exposures were lower in the heavier male rats: 0.45 and 0.49, 0.87 and 0.94, 1.50 and 1.63, and 2.89 and 3.15 mg/kg/day for groups 2, 3, 4, and 5 males and females, respectively.
There were no deaths related to treatment with DAS181. The clinical signs, body weights, food consumption, ophthalmology, respiratory parameters, coagulation, and urinalysis parameters were all unaffected by treatment with DAS181. There were no changes in the bone marrows of all animals associated with treatment of DAS181. There was no effect on the total WBC counts or WBC differential data in the BAL samples collected in rats at the end of the DAS181 treatment period.
Statistically significant mild decrements in RBC, hematocrit (HCT), and hemoglobin (HGB), along with a decrease in mean corpuscular volume (MCV), were seen in group 5 male rats on day 14, indicating mild anemia (Table 1). These decrements seen at day 14 returned to baseline at day 29, and a statistically significant increase in reticulocytes was observed in group 5 males on day 29 (Table 1). Together, these findings indicate a mild and transient anemia accompanied by a regenerative response of the erythrocyte lineage. There were no statistically significant differences in any hematological parameters at the end of the recovery period. Statistically significant changes in the lower dose groups were considered incidental as they were not observed with a relationship to dose. There were no consistent treatment-related hematological findings indicating anemia in female rats. No other treatment-related findings on hematology parameters were observed.
| Group | Day 14 | Day 29 | |||
| RBC (1012/l) | HGB (g/l) | HCT (l/l) | MCV (fl) | Retic (1012/l) | |
| 1 | 8.65 ± 0.55 | 169 ± 9 | 0.50 ± 0.03 | 57.3 ± 1.2 | 0.200 ± 0.021 |
| 2 | 8.61 ± 0.48 | 167 ± 7 | 0.49 ± 0.02 | 56.6 ± 1.5 | 0.213 ± 0.037 |
| 3 | 8.41 ± 0.41 | 160 ± 6* | 0.47 ± 0.02* | 55.7 ± 1.7* | 0.241 ± 0.055* |
| 4 | 8.65 ± 0.45 | 168 ± 6 | 0.49 ± 0.02 | 56.2 ± 1.7 | 0.233 ± 0.026 |
| 5 | 8.20 ± 0.26** | 155 ± 8*** | 0.45 ± 0.02*** | 55.1 ± 1.4*** | 0.272 ± 0.042*** |
| Group | Day 14 | Day 29 | |||
| RBC (1012/l) | HGB (g/l) | HCT (l/l) | MCV (fl) | Retic (1012/l) | |
| 1 | 8.65 ± 0.55 | 169 ± 9 | 0.50 ± 0.03 | 57.3 ± 1.2 | 0.200 ± 0.021 |
| 2 | 8.61 ± 0.48 | 167 ± 7 | 0.49 ± 0.02 | 56.6 ± 1.5 | 0.213 ± 0.037 |
| 3 | 8.41 ± 0.41 | 160 ± 6* | 0.47 ± 0.02* | 55.7 ± 1.7* | 0.241 ± 0.055* |
| 4 | 8.65 ± 0.45 | 168 ± 6 | 0.49 ± 0.02 | 56.2 ± 1.7 | 0.233 ± 0.026 |
| 5 | 8.20 ± 0.26** | 155 ± 8*** | 0.45 ± 0.02*** | 55.1 ± 1.4*** | 0.272 ± 0.042*** |
Note. Mean ± SD; n = 15/group, groups 1, 4, and 5 and n = 10/group, groups 2 and 3. Statistical test: Dunnett’s two-sided: *p < 0.05, **p < 0.01, ***p < 0.001.
| Group | Day 14 | Day 29 | |||
| RBC (1012/l) | HGB (g/l) | HCT (l/l) | MCV (fl) | Retic (1012/l) | |
| 1 | 8.65 ± 0.55 | 169 ± 9 | 0.50 ± 0.03 | 57.3 ± 1.2 | 0.200 ± 0.021 |
| 2 | 8.61 ± 0.48 | 167 ± 7 | 0.49 ± 0.02 | 56.6 ± 1.5 | 0.213 ± 0.037 |
| 3 | 8.41 ± 0.41 | 160 ± 6* | 0.47 ± 0.02* | 55.7 ± 1.7* | 0.241 ± 0.055* |
| 4 | 8.65 ± 0.45 | 168 ± 6 | 0.49 ± 0.02 | 56.2 ± 1.7 | 0.233 ± 0.026 |
| 5 | 8.20 ± 0.26** | 155 ± 8*** | 0.45 ± 0.02*** | 55.1 ± 1.4*** | 0.272 ± 0.042*** |
| Group | Day 14 | Day 29 | |||
| RBC (1012/l) | HGB (g/l) | HCT (l/l) | MCV (fl) | Retic (1012/l) | |
| 1 | 8.65 ± 0.55 | 169 ± 9 | 0.50 ± 0.03 | 57.3 ± 1.2 | 0.200 ± 0.021 |
| 2 | 8.61 ± 0.48 | 167 ± 7 | 0.49 ± 0.02 | 56.6 ± 1.5 | 0.213 ± 0.037 |
| 3 | 8.41 ± 0.41 | 160 ± 6* | 0.47 ± 0.02* | 55.7 ± 1.7* | 0.241 ± 0.055* |
| 4 | 8.65 ± 0.45 | 168 ± 6 | 0.49 ± 0.02 | 56.2 ± 1.7 | 0.233 ± 0.026 |
| 5 | 8.20 ± 0.26** | 155 ± 8*** | 0.45 ± 0.02*** | 55.1 ± 1.4*** | 0.272 ± 0.042*** |
Note. Mean ± SD; n = 15/group, groups 1, 4, and 5 and n = 10/group, groups 2 and 3. Statistical test: Dunnett’s two-sided: *p < 0.05, **p < 0.01, ***p < 0.001.
Test article-related increases in ALP level were observed in all DAS181-treated groups of male rats and in females in group 5 on day 14 as illustrated in Figure 1. Interestingly, on day 29, the ALP levels were back to baseline levels in all groups except group 5 male rats. The potential reason for this observation will be discussed in the next section. Although in group 5 males the group mean ALP on day 29 was still elevated, the values had declined by more than 50% of the day 14 values, and individually, each male rat in group 5 also had a reduced ALP value on day 29 relative to their day 14 value.
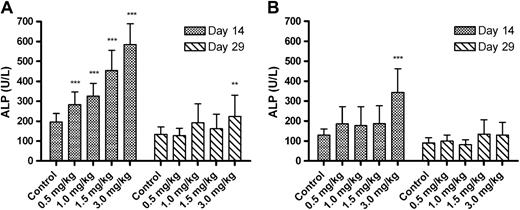
Group mean ALP levels. (A) Mean (±SD) ALP values in male rats; (B) mean ALP values in female rats. For each sex, 15/group for the control, 1.5 and 3.0 mg/kg dose groups and 10/group for the 0.5 and 1.0 mg/kg dose groups at both time points (except where n = 14 control females on day 29). Asterisks indicate significant difference between the control and treated groups, *p < 0.05, **p < 0.01, ***p < 0.001.
In group 3, 4, and 5 males, the group mean alanine aminotransferase (ALT) levels were higher than the lactose control animals in group 1 according to statistical analysis (Table 2). However, the increases in mean values, although statistically significant, were marginal, 45, 45, and 48 U/l for groups 3, 4, and 5, respectively, versus a mean of 36 U/l in group 1 controls. Likewise, an increase in group mean ALT group 5 males on day 29 was marginal, 43 U/l versus a mean of 33 U/l in group 1 controls. In addition, the individual ALT levels in each DAS181-treated groups were still within the historical range of normal rats at the conducting contract research organization (unpublished data from ITR). In group 5 males on day 14, the mean value of aspartate aminotransferase (AST), 202 U/l, was statistically different from the value of 171 U/l in group 1 controls (Table 2), but in the same group of male rats on day 29, the mean AST values were normal. There was no increase in ALT or AST among DAS181-treated female rats at either time point. There were no histological findings in the livers of any DAS181-treated animals. Additionally, other laboratory parameters of liver function, such as gamma glutamyl transpeptidase and bilirubin, were normal in all animals at all time points. As a result, it was concluded that the observed ALT and AST differences were of no biological or toxicological significance.
| Group | Day 14 | Day 29 | ||
| ALT (U/l) | AST (U/l) | ALT (U/l) | AST (U/l) | |
| 1 | 36 ± 5 | 171 ± 30 | 33 ± 5 | 181 ± 34 |
| 2 | 41 ± 6 | 153 ± 18 | 37 ± 8 | 219 ± 70 |
| 3 | 45 ± 7** | 168 ± 10 | 40 ± 11 | 220 ± 38 |
| 4 | 45 ± 4** | 198 ± 39 | 37 ± 9 | 197 ± 82 |
| 5 | 48 ± 9*** | 202 ± 32* | 43 ± 9** | 207 ± 55 |
| Group | Day 14 | Day 29 | ||
| ALT (U/l) | AST (U/l) | ALT (U/l) | AST (U/l) | |
| 1 | 36 ± 5 | 171 ± 30 | 33 ± 5 | 181 ± 34 |
| 2 | 41 ± 6 | 153 ± 18 | 37 ± 8 | 219 ± 70 |
| 3 | 45 ± 7** | 168 ± 10 | 40 ± 11 | 220 ± 38 |
| 4 | 45 ± 4** | 198 ± 39 | 37 ± 9 | 197 ± 82 |
| 5 | 48 ± 9*** | 202 ± 32* | 43 ± 9** | 207 ± 55 |
Note. Mean ± SD; n = 15/group, groups 1, 4, and 5 and n = 10/group, groups 2 and 3. ITR historical normal range for male rats of this age: ALT is 23–65 U/l and AST is 79–187 U/l. Statistical test: Dunnett’s two-sided: *p < 0.05, **p < 0.01, ***p < 0.001.
| Group | Day 14 | Day 29 | ||
| ALT (U/l) | AST (U/l) | ALT (U/l) | AST (U/l) | |
| 1 | 36 ± 5 | 171 ± 30 | 33 ± 5 | 181 ± 34 |
| 2 | 41 ± 6 | 153 ± 18 | 37 ± 8 | 219 ± 70 |
| 3 | 45 ± 7** | 168 ± 10 | 40 ± 11 | 220 ± 38 |
| 4 | 45 ± 4** | 198 ± 39 | 37 ± 9 | 197 ± 82 |
| 5 | 48 ± 9*** | 202 ± 32* | 43 ± 9** | 207 ± 55 |
| Group | Day 14 | Day 29 | ||
| ALT (U/l) | AST (U/l) | ALT (U/l) | AST (U/l) | |
| 1 | 36 ± 5 | 171 ± 30 | 33 ± 5 | 181 ± 34 |
| 2 | 41 ± 6 | 153 ± 18 | 37 ± 8 | 219 ± 70 |
| 3 | 45 ± 7** | 168 ± 10 | 40 ± 11 | 220 ± 38 |
| 4 | 45 ± 4** | 198 ± 39 | 37 ± 9 | 197 ± 82 |
| 5 | 48 ± 9*** | 202 ± 32* | 43 ± 9** | 207 ± 55 |
Note. Mean ± SD; n = 15/group, groups 1, 4, and 5 and n = 10/group, groups 2 and 3. ITR historical normal range for male rats of this age: ALT is 23–65 U/l and AST is 79–187 U/l. Statistical test: Dunnett’s two-sided: *p < 0.05, **p < 0.01, ***p < 0.001.
There were no macroscopic or microscopic changes that were considered to be of toxicological significance for DAS181 treatment. In males, a transient increase in severity and incidence of accumulation of foamy macrophages and increase in cellularity of bronchus-associated lymphoid tissue (BALT) in the lungs was observed in all treated groups when compared with the control group (Figs. 2A and 2B). These findings were not observed in conjunction with any evidence of local tissue damage, thus indicating a lack of direct toxicological effect of DAS181 on lung tissue. In the females, relative to the lactose control group, a transient increase in severity and incidence of accumulation of foamy macrophages in the lungs was observed in group 5 and increase in cellularity of BALT in the lungs was noted in rats in groups 3, 4, and 5 (Fig. 2C). After a 28-day recovery period, these findings were no longer present in both males and females, indicating complete reversal of this finding. The observed changes are consistent with common and nonspecific responses to an inhaled protein in the lungs. The dose-related severity of the response reflects the increased amount of foreign protein to which the animals are exposed with increasing dose. These lesions were totally reversed in the recovery phase.
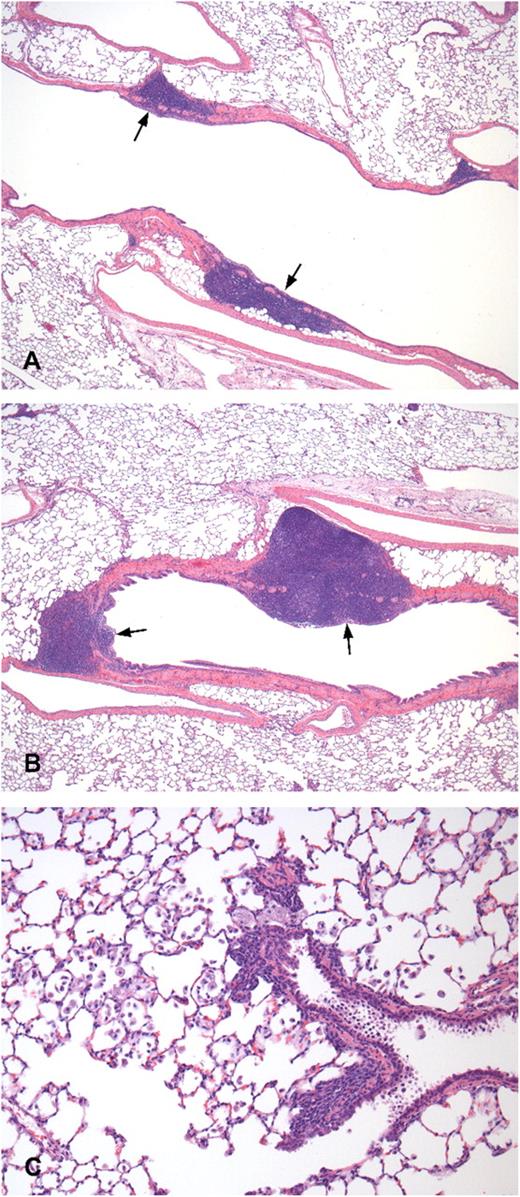
Lung histology of control- and DAS181-treated rats. Histological examination of the lungs of rats treated with lactose (A) and 3 mg/kg/day of DAS 181 (B and C) on day 29, hematoxylin and eosin stain. (A) BALT (arrow) of lactose control, ×40. (B) Increased size/cellularity of the BALT (arrow) is present compared with lactose control (A), ×40. (C) Accumulation of foamy macrophages in the bronchoalveolar junction and alveoli, ×200.
TK and Immunogenicity in 28-Day GLP Inhalation Toxicology Study in Rats
In the toxicology study, a low level of systemic exposures to DAS181 (in the low ng/ml range) was observed upon 30-min inhalation exposures to DAS181 (Table 3). Curves of the exposures in males, females, and both sexes combined for day 1 are provided in Figure 3. The considerable variability in plasma concentrations among the individual rats across the treatment groups precluded making any definitive statements regarding dose-response or gender dependencies; in fact, the greatest DAS181 concentration of 24 ng/ml was observed in a group 2 rat. The Tmax ranged from 1 to 24 h, reflecting the small differences between plasma concentrations determined at multiple time points and real lack of any substantial peak at any one time after exposure. According to a preliminary intravenous PK study of DAS181 (0.025 mg/kg) in rats, the intravenously injected DAS181 has an elimination half-life of approximately 1.5 h and an AUC of approximately 40 ng · h/ml (data not shown). By comparing the TK result of the current inhalation study with the preliminary intravenous PK study results, it can be concluded that the variable and low systemic exposure of DAS181 reflects the nature of DAS181 absorption from the respiratory tract; the systemic availability of DAS181 inhalant is very low and estimated to be < 2%.
| Group | Gender | Day 1 | Day 28 | ||||
| Cmax (ng/ml) | Tmax (h) | AUC0–24 h (h × ng/ml) | Cmax (ng/ml) | Tmax (h) | AUC0–24 h (h × ng/ml) | ||
| 2 | M | 1.52 | 4 | 13.93 | 1.54 | 1 | 8.74 |
| 2 | F | 2.57 | 8 | 18.60 | 0.34 | 24 | 4.60 |
| 3 | M | 14.98 | 1 | 28.64 | 0.31 | 2 | 1.55 |
| 3 | F | 2.58 | 24 | 20.38 | 0.44 | 24 | 5.43 |
| 4 | M | 1.48 | 1 | 17.36 | 0.78 | 2 | 4.71 |
| 4 | F | 1.49 | 8 | 18.22 | 1.73 | 8 | 10.55 |
| 5 | M | 4.78 | 4 | 60.21 | 1.04 | 2 | 4.33 |
| 5 | F | 2.20 | 4 | 31.64 | 0.18 | 1 | 0.18 |
| Group | Gender | Day 1 | Day 28 | ||||
| Cmax (ng/ml) | Tmax (h) | AUC0–24 h (h × ng/ml) | Cmax (ng/ml) | Tmax (h) | AUC0–24 h (h × ng/ml) | ||
| 2 | M | 1.52 | 4 | 13.93 | 1.54 | 1 | 8.74 |
| 2 | F | 2.57 | 8 | 18.60 | 0.34 | 24 | 4.60 |
| 3 | M | 14.98 | 1 | 28.64 | 0.31 | 2 | 1.55 |
| 3 | F | 2.58 | 24 | 20.38 | 0.44 | 24 | 5.43 |
| 4 | M | 1.48 | 1 | 17.36 | 0.78 | 2 | 4.71 |
| 4 | F | 1.49 | 8 | 18.22 | 1.73 | 8 | 10.55 |
| 5 | M | 4.78 | 4 | 60.21 | 1.04 | 2 | 4.33 |
| 5 | F | 2.20 | 4 | 31.64 | 0.18 | 1 | 0.18 |
Note. Systemic exposures are derived from the mean values for three rats/gender/time point. F, female; M, male.
| Group | Gender | Day 1 | Day 28 | ||||
| Cmax (ng/ml) | Tmax (h) | AUC0–24 h (h × ng/ml) | Cmax (ng/ml) | Tmax (h) | AUC0–24 h (h × ng/ml) | ||
| 2 | M | 1.52 | 4 | 13.93 | 1.54 | 1 | 8.74 |
| 2 | F | 2.57 | 8 | 18.60 | 0.34 | 24 | 4.60 |
| 3 | M | 14.98 | 1 | 28.64 | 0.31 | 2 | 1.55 |
| 3 | F | 2.58 | 24 | 20.38 | 0.44 | 24 | 5.43 |
| 4 | M | 1.48 | 1 | 17.36 | 0.78 | 2 | 4.71 |
| 4 | F | 1.49 | 8 | 18.22 | 1.73 | 8 | 10.55 |
| 5 | M | 4.78 | 4 | 60.21 | 1.04 | 2 | 4.33 |
| 5 | F | 2.20 | 4 | 31.64 | 0.18 | 1 | 0.18 |
| Group | Gender | Day 1 | Day 28 | ||||
| Cmax (ng/ml) | Tmax (h) | AUC0–24 h (h × ng/ml) | Cmax (ng/ml) | Tmax (h) | AUC0–24 h (h × ng/ml) | ||
| 2 | M | 1.52 | 4 | 13.93 | 1.54 | 1 | 8.74 |
| 2 | F | 2.57 | 8 | 18.60 | 0.34 | 24 | 4.60 |
| 3 | M | 14.98 | 1 | 28.64 | 0.31 | 2 | 1.55 |
| 3 | F | 2.58 | 24 | 20.38 | 0.44 | 24 | 5.43 |
| 4 | M | 1.48 | 1 | 17.36 | 0.78 | 2 | 4.71 |
| 4 | F | 1.49 | 8 | 18.22 | 1.73 | 8 | 10.55 |
| 5 | M | 4.78 | 4 | 60.21 | 1.04 | 2 | 4.33 |
| 5 | F | 2.20 | 4 | 31.64 | 0.18 | 1 | 0.18 |
Note. Systemic exposures are derived from the mean values for three rats/gender/time point. F, female; M, male.
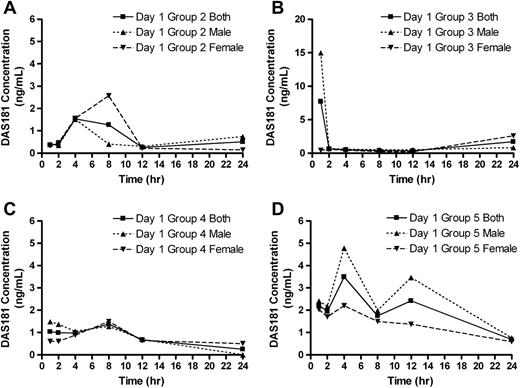
DAS181 plasma concentrations. Group mean DAS181 plasma concentrations for males, females, and both sexes combined are shown. (A) Mean DAS181 plasma concentrations in group 2 rats; (B) mean DAS181 plasma concentrations in group 3 rats; (C) mean DAS181 plasma concentrations in group 4 rats; and (D) mean DAS181 plasma concentrations in group 5 rats, n = 3 rats/sex/time point.
Immunogenicity analysis results showed that on day 29/30 and day 57 postdosing, anti-DAS181 antibody (ADA) was detectable in all DAS181-treated groups but not the lactose control group. The incidence of positive ADA titers was 11/12, 11/12, 21/22, and 22/22 rats in groups 2, 3, 4, and 5, respectively. Despite the occurrence of ADA in all animals, the ADA response was highly variable, ranging from 50 to 1.9 × 107 in titer. The ADA titers in individual animals did not correlate with DAS181 dose or blood collection time (Fig. 4A). The levels of neutralizing anti-DAS181 antibody (NAb) were measured in 10 rats/group in groups 4 and 5. A positive NAb titer was observed in 8/10 and 10/10 rats in groups 4 and 5, respectively. NAb titers were low, relative to ADA titers, with the maximum titer being 2560 in group 4 and 10,240 in group 5. Because most NAb titers were 640 or 2560, there were no correlations between ADA and NAb titers, and high ADA titers were not predictive of a high NAb response. As with the ADA titers, the NAb titer did not seem to correlate with DAS181 dose or blood collection time (Fig. 4B).
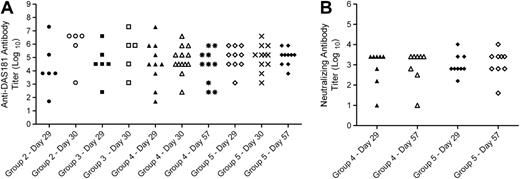
Immunogenicity analysis results. (A) ADA titer in dose groups 2–5 at days 28–57. (B) NAb titer in dose groups 4 and 5 at days 29 and 57.
The overall systemic exposures to DAS181 on day 28 were considerably less than that on day 1 (Table 3). Plasma concentrations were quantifiable in all rats on day 1 but were below the lower limit of quantitation (LLOQ) of 0.23 ng/ml in 4/12 group 2, 8/12 group 3, 3/12 group 4, and 7/12 group 5 rats at day 28. This phenomenon correlated with the occurrence of ADA response at day 28. Most rats that developed high ADA titer had reduced or undetectable levels of DAS181 in the plasma. There were two rats that did not develop anti-DAS181 antibodies, and these rats had similar plasma concentrations of DAS181 on days 1 and 28. The decreased systemic levels of DAS181 during the latter part of the 28-day DAS181 exposure, due to appearance of ADA, is also the most likely explanation for the return of serum ALP values to the baseline levels noted above.
Mechanistic Studies of Elevated ALP in Rodents
ALP is a glycoprotein catalyzing the hydrolysis of phosphomonoesters at alkaline pH. It has been demonstrated that ALP level can be determined by the ALP elimination rate from the circulation (Blom et al., 1998). Furthermore, ALP is eliminated through ASGR and its serum half-life can be markedly prolonged by infusion of a desialylated glycoprotein, ASF, in the blood (Blom et al., 1998). To verify if ALP elevation can be induced by increased level of desialylated proteins in the systemic circulation, independent of DAS181, we treated mice with intraperitoneal injections of serum samples that were desialylated by mild acid treatment or ASF. As expected, desialylated serum at 300 μl dose and ASF at 10 mg dose were both able to cause significant elevation of ALP level in mice (Fig. 5A). Interestingly, the daily dose of 300 μl of serum that caused significant ALP elevation only contained 8.7 mg glycoprotein, which is a small fraction (4–12%) of the total mice serum glycoprotein level that ranges between 72 and 216 mg (Park et al., 2005). Similarly, administration of 10 mg of ASF also caused equivalent increase of ALP level (Fig. 5B).
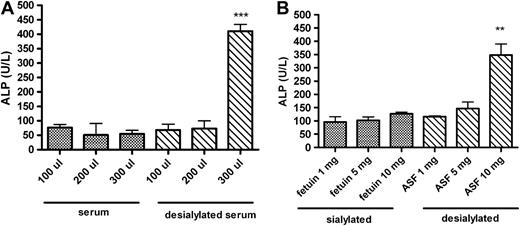
Induction of ALP elevation by desialylated glycoproteins. (A) Mean (±SEM) ALP values in female mice receiving sialylated (normal) serum or desialylated serum once daily for 2 days; (B) mean ALP values in female mice administered fetuin (sialylated protein) or ASF (desialylated protein) once daily for 3 days, n = 3 female mice/group. Asterisks indicate significant difference between the sialylated and desialylated treatment groups at the respective dose amounts, *p < 0.05, **p < 0.01, ***p < 0.001.
To confirm these findings in a different animal species, we also treated male rats with desialylated plasma proteins via intravenous injections. To this end, rat plasma samples were desialylated in vitro using DAS180, which is a DAS181 analog that carries an Hisx6 tag but lacks the amphiregulin-anchoring domain. Before injection, DAS180 was removed by passing the plasma samples through a Ni column. Twice daily administration of 500 μl of desialylated plasma for 3 days significantly increased the plasma ALP level, whereas the same amount of normal plasma did not have this effect (data not shown).
Isoenzyme Analyses of Elevated ALP in Rats
There are three isoforms of cellular ALP, which are richly expressed in the liver, bone, and kidney, respectively. These ALP isoenzymes differ in their glycosylation patterns, although they have identical polypeptide sequence. It has been shown that different ALP isoenzymes are cleared from circulation at different rate (Blom et al., 1998). To assess how ALP isoenzyme levels are affected by DAS181, a liquid formulation of DAS181 was administered by bolus tail vein injection to rats for 7 days at dose levels of 0.012 or 0.12 mg/kg/day. Plasma concentrations of ALP were elevated to 491 ± 32 and 600 ± 48 U/l for the 0.012 and 0.12 mg/kg dose groups, respectively, whereas the normal range for ALP in rats is 16–302 U/l. Isoenzyme analyses showed that the elevated ALP was predominantly the isoenzyme from the liver (Fig. 6). The percentage of total ALP isolated in the liver fraction was consistent between the two dose groups of DAS181. Isoenzymes from the bone and gastrointestinal tract accounted for < 10% of the induced level of ALP. This result indicates that the ALP isoenzyme is the one for which the elimination rate is most sensitive to desialylation.
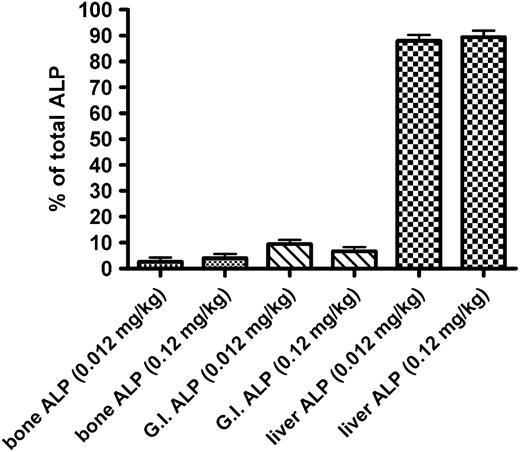
Isoenzyme analysis of elevated ALP. Mean (±SD) ALP activity for bone, gastrointestinal, and liver isoenzymes expressed as a percentage of the total ALP activity. Rats (3/sex/group) were administered 0.012 or 0.12 mg/kg of DAS181 for seven consecutive days by intravenous injection. Blood samples were taken at 24 h after the final dose and processed for ALP isoenzyme analysis.
In Vitro DAS181 Cytotoxicity
Potential cytotoxic effects of DAS181 on hepatocytes from rats, dogs, and human donors were evaluated. Multiple cell donors (3/species) were used in the study. Since the data were consistent between species, the results of one donor from each species are presented in Figure 7. DAS181 was not cytotoxic to hepatocytes from the rat, dog, or human donors. Concentrations of up to 1 μg/ml were used in these assays, which are far in excess of the highest concentration of 24 ng/ml observed in the 28-day rat GLP toxicology study. During the cytotoxicity assays, the level of ALP in the culture media was also measured, and there was no significant difference between the ALP levels in the vehicle or DAS181-treated hepatocytes (data not shown). Additionally, DAS181 did not affect cell viability in differentiated human skeletal muscle cells, differentiated or undifferentiated human osteoblasts, differentiated or undifferentiated human smooth muscle myoblasts, or human renal proximal tubule cells or cortical epithelial cells (data not shown).
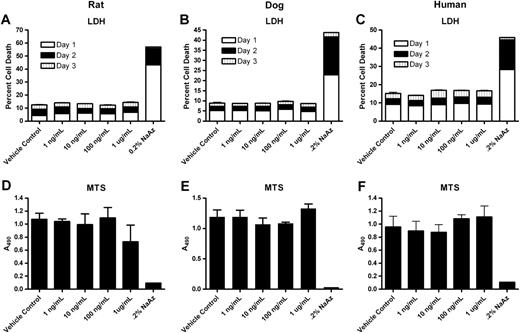
Cytotoxic effects of DAS181 on hepatocytes. Hepatocytes from a Sprague-Dawley rat (A, D), beagle dog (B, E), and human donor (C, F) were used in the experiment. The LDH assay (A, B, and C) was performed on samples collected from day 1 (white), day 2 (black), and day 3 (vertical stripe). The MTS assay (D, E, and F) was performed only on day 3 samples. No significant difference between vehicle control and DAS181 treatment groups. Cells treated with 0.2% sodium azide were positive controls. Error bars are SEM of triplicate samples with the exception of the 0.2% sodium azide control, which were single replicates.
DAS181 at up to 0.8 mg/ml did not affect the growth of the A549 or BEAS-2B cell lines, two cell lines derived from human bronchial epithelial cells, over a period of 10 days (data not shown). In the cultures of well-differentiated HAE, a culture system that mimics the native HAE, DAS181 did not produce any evidence of cytotoxicity at up to 1282 μg/cm2/day, nor did DAS181 cause aberrant production of cytokines, including IL-1α, IL-1β, IL-6, IL-10, IL-13, IFN-α, IFN-γ, TNF-α, and RANTES (data not shown). Notably, the concentration of DAS181 used in the HAE system was > 100-fold higher than the projected DAS181 local concentration of 5–10 μg/cm2 on the airway surface based on the projected therapeutic dose range (Triana-Baltzer et al., 2010).
To evaluate the potential cytotoxic effect of DAS181 on RBC, tests were performed to assess if DAS181 would indirectly cause hemolysis via activation of complements, or if DAS181 would directly cause hemolysis. As a positive control for the complement-mediated hemolysis, species-specific antibodies against RBC was used to activate the classical complement pathway and initiate the signaling cascade leading to the lysis of the RBC. For evaluating direct hemolytic activity of DAS181, the RBC were washed to remove any complement proteins and then resuspended with heat-inactivated plasma or serum. In both the rat and the human blood, DAS181 did not cause any hemolytic reactions, neither direct hemolysis nor complement-mediated hemolysis, at concentrations up to 15 mg/ml (data not shown).
DISCUSSION
Repeated daily exposures of inhaled DAS181 for 28 days at achieved doses of up to 3.00 mg/kg/day were well tolerated by rats. This dose level corresponds to a daily HED of 28.8 mg for a 60-kg person based on conversion of the rat dose to the human equivalent using body surface area. This is above the predicted therapeutic dose range of 5–20 mg/day for 5 days in humans. Additionally, the 28-day treatment duration in the toxicology study greatly exceeds the expected duration of a therapeutic course in humans (≤5 days). Clinical signs, body weights, food consumption, ophthalmology, respiratory parameters, coagulation, and urinalysis parameters were unaffected by treatment with DAS181. Histopathological findings in the standard panel of tissues were limited to findings indicative of nonspecific response to an inhaled foreign protein in the lungs that were totally reversed in the recovery phase. Similar findings have been reported with other inhaled proteins (Guimond et al., 2008). Importantly, DAS181 treatment did not cause lung parenchymal damage in this inhalation toxicology study.
The dry powder formulation of DAS181 has been engineered to provide relatively larger particles than the particles intended for deep lung deposition. Although the DAS181 dry powder mainly targets the upper and central respiratory tract for treatment of IFV and PIV infection, a very small fraction of the inhaled DAS181 dry powder consists of smaller particles that may deposit in the deep lungs and be systemically bioavailable (Folkesson et al., 1990; Patton, 1996; Schanker, 1978). It is possible that the variable and low-level systemic exposure to DAS181 observed in the GLP toxicology study represented the worst case scenario in terms of DAS181 systemic exposure because animals were exposed to DAS181 aerosols for 30 min daily during the toxicology studies. Such an exposure mode, consisting of slow inhalation over a long period of time, favors drug deposition in the deep lungs where alveolar epithelium is permeable to proteins like DAS181. In contrast, the bolus dose inhalation method intended for human use favors drug deposition in the upper and central airways where the epithelium is relatively impermeable to DAS181. Importantly, even at the levels of systemic exposure observed in the toxicology study, DAS181 is well tolerated without target organ toxicity.
DAS181 is designed to be of low immunogenicity in humans. Indeed, very low levels of ADA and NAb titers have been observed in human clinical trials so far (data not shown). Not surprisingly, high levels of ADA, up to 1.9 × 107 in titer, were observed in rats after 28-day exposure to DAS181. It is notable that ADA at such high levels did not cause any adverse effects in the animals.
In contrast to the high ADA responses, the NAb plasma levels observed in the rats were much lower, to a maximum of 104 in titer. Because antibody titer in the airway is 10–250 times lower than that in the plasma in general (Biberfeld and Sterner, 1971; Waldman et al., 1970), the level of NAb in the rat respiratory tract is expected to be no more than 1000 in titer. NAb at such titer would inhibit 30% of DAS181 at the concentration of 2 μg/ml (calculated based on NAb assay condition, data not shown). However, at the projected DAS181 therapeutic dose, the local concentration of inhaled DAS181 in human airway is estimated to be at least 250 μg/ml. Therefore, even the highest level of plasma NAb observed in the toxicology study is not expected to significantly affect DAS181 therapeutic function in humans.
There were notable findings in hematology and in clinical chemistry parameters in the rats. These findings are the results of DAS181 pharmacological activity in the systemic circulation; although given the variability in the low-level systemic exposures in satellite toxicokinetics animals, it was not possible to draw correlations to the clinical pathology findings in the main study animals. It is unclear why male rats showed greater effects on clinical pathology parameters compared with female rats because systemic exposures were similar, and the exposures on a body weight basis were actually lower in male rats. Minimal, albeit statistically significant, decrements in RBC, HCT, and HGB along with a decrease in MCV were seen indicating mild anemia. This finding was seen only at day 14 in male rats at the highest dose level of 3.00 mg/kg/day. The statistically significant increase in reticulocytes in these males on day 29 was indicative of an erythrogenic regenerative response. As sialic acids are involved in the designation of senescent erythrocytes for elimination, mild anemia is an expected outcome on an enhanced clearance of desialylated erythrocytes (Aminoff et al., 1977; Schauer, 2004). As this finding is to be expected from DAS181 pharmacological activity in the systemic circulation, it was seen only at the highest exposure in male rats and was entirely reversible, and it is not expected to have significant toxicological implication.
A prominent finding in the GLP toxicology study was the dose-dependent increase in ALP in the absence of any treatment-related histopathological changes in the DAS181-treated rats of both sexes. Neither did DAS181 induce toxicity in hepatocytes in multiple species further, confirming the lack of toxic effect on the liver. Our mechanistic research on this finding is in line with others who have demonstrated that desialylation of glycoproteins notably alters the elimination rate of ALP. Plasma glycoproteins in the blood, including some of the highly abundant sialoglycoproteins such as immunoglobulin (Ig)G, IgA, α2-macroglobulin, transferrin, haptoglobin, α1-antitrypsin, all compete for access to the ASGR system with desialylated molecules being cleared more rapidly relative to sialylated counterparts (Fukuda et al., 1989; Hagglund et al., 2007; Morell et al., 1971). This differential clearance has been shown to lead to an impaired elimination of the less abundant sialylated glycoproteins in situations with an increased desialylated glycoprotein levels (Stockert, 1995). Because ALP is a sialylated glycoprotein of low abundance, it is expected that ALP elimination rate is susceptible to increased level of desialylated glycoproteins in the systemic circulation. Indeed, this has been previously demonstrated in rats where administration of ASF prolonged the elimination of ALP from the circulation by roughly twofold (Blom et al., 1998; Young et al., 1984). In our studies with desialylated protein administered to rats and mice, increased concentrations of ALP were also observed, consistent with the literature. Administration of ASF was likewise able to cause elevated ALP levels in mice. Together, our studies results and the literature have revealed that ALP elevation is the result of DAS181 systemic pharmacological effect, rather than a sign of liver toxicity.
In summary, we have shown that daily inhalation exposures to rats of up to 3.00 mg/kg/day DAS181 were well tolerated with no direct test article toxicities. Observed effects in the lungs were consistent with inhalation of a foreign protein and the clinical pathology findings were attributed to systemic pharmacological action of DAS181. The safety and efficacy of this novel drug candidate in humans is currently being evaluated in clinical trials.
FUNDING
National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services (contract no. HHSN266200600015C).
We thank Dr David Wurtman for critical reading and suggestions. Conflicts of interest J.L.L., G.M., M.N., and F.F. are shareholders in NexBio, Inc. (San Diego, CA).




Comments