-
PDF
- Split View
-
Views
-
Cite
Cite
Anne-Catherine Bachoud-Lévi, Renaud Massart, Anne Rosser, Cell therapy in Huntington's disease: Taking stock of past studies to move the field forward, Stem Cells, Volume 39, Issue 2, February 2021, Pages 144–155, https://doi.org/10.1002/stem.3300
Close - Share Icon Share
Abstract
Huntington's disease (HD) is a rare inherited neurodegenerative disease that manifests mostly in adulthood with progressive cognitive, behavioral, and motor dysfunction. Neuronal loss occurs predominantly in the striatum but also extends to other brain regions, notably the cortex. Most patients die around 20 years after motor onset, although there is variability in the rate of progression and some phenotypic heterogeneity. The most advanced experimental therapies currently are huntingtin-lowering strategies, some of which are in stage 3 clinical trials. However, even if these approaches are successful, it is unlikely that they will be applicable to all patients or will completely halt continued loss of neural cells in all cases. On the other hand, cellular therapies have the potential to restore atrophied tissues and may therefore provide an important complementary therapeutic avenue. Pilot studies of fetal cell grafts in the 2000s reported the most dramatic clinical improvements yet achieved for this disease, but subsequent studies have so far failed to identify methodology to reliably reproduce these results. Moving forward, a major challenge will be to generate suitable donor cells from (nonfetal) cell sources, but in parallel there are a host of procedural and trial design issues that will be important for improving reliability of transplants and so urgently need attention. Here, we consider findings that have emerged from clinical transplant studies in HD to date, in particular new findings emerging from the recent multicenter intracerebral transplant HD study, and consider how these data may be used to inform future cell therapy trials.
Cell therapy is the only approach currently focused on structural and functional restoration in Huntington's disease. The lack of benefit shown in the largest fetal cell transplant trial to date, the multicenter intracerebral transplant in Huntington's disease trial (MIG-HD), cautions against uncontrolled cell therapy treatments. However, MIG-HD resulted in new procedures that significantly improved patient safety and enlightened upcoming cell transplant protocols. Stem cells should dramatically change the landscape by permitting better control of the quantity and homogeneity of the donor cell product. This study proposes ways to improve future trials, thereby increasing their chance for success.
Huntington's disease (HD) is a rare inherited neurodegenerative disease associated with progressive neuronal degeneration, with a marked and early striatal degeneration. Onset is usually in adulthood and is associated with progressive cognitive, motor, and behavioral deterioration. Death occurs after a median of 18 years.1 The diagnosis is proven genetically with full penetrance above 39 CAG repeats in the huntingtin gene.1 There are no disease-modifying treatments yet available, and among symptomatic therapies, only increased activity and a quality health care environment have proven capacity to improve outcomes.2 Therapies aimed at slowing or stopping the disease process are the subject of intense research efforts, with several huntingtin lowering trials in, or approaching, phase III.3 Cell transplants offer a different strategy; being the only means of restoring the atrophied tissue by implanting cells with the capacity to take over at least some functions of the degenerated cells. Based on some successes in open label studies,4-6 the largest randomized study of intracerebral human fetal cell transplantation to date (the multicentric intracerebral grafting trial in HD; MIG-HD NCT00190450) was established in 2001. MIG-HD did not demonstrate benefit of transplants in HD, but solved some major safety issues and has illuminated the way forward.7 Beyond the choice of cells to be injected, patient selection, transplant procedures, immunosuppression, and measure outcomes urgently require optimization and standardization for future trials. Each of these aspects is discussed here in light of these recent results (Figure 1).
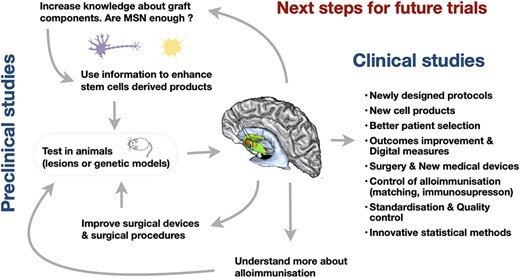
An overview of the iterative process needed to address current biological and clinical challenges in order to improve reliability of future cell therapy trials in Huntington's disease
RATIONAL FOR INTRACEREBRAL TRANSPLANT IN HD
Striatal medium spiny neurons (MSNs) are the major cell type loss in HD. This loss starts well before symptom onset, correlates best with clinical evolution, and is the best marker of disease progression.8 In common with other neurodegenerative conditions, HD progresses atrophy spreads to other brain regions, in particular the cortex,9 thus leading to debate about the usefulness of a graft limited to the striatum.10 Yet, no other approach to date has the potential to restore cells lost to the disease process. Moreover, degeneration of extrastriatal regions may be partly due to retrograde degeneration after the disappearance of their striatal targets, in accordance with the hierarchy of neuronal network disturbance in HD.11 Thus, restoration of cortico-striatal afferent and efferent connections could even reduce retrograde degeneration.12 Although we anticipate cell therapy being a stand-alone treatment for some patients, it is fully compatible with most potential disease-modifying treatments on the horizon13 (Figure 2), which could enable us to address both existing and ongoing cell loss. Furthermore, in the future, engineering stem cells to deliver disease-modifying molecules could allow grafts to directly contribute to disease-modification as well as repairing existing damage.14-16
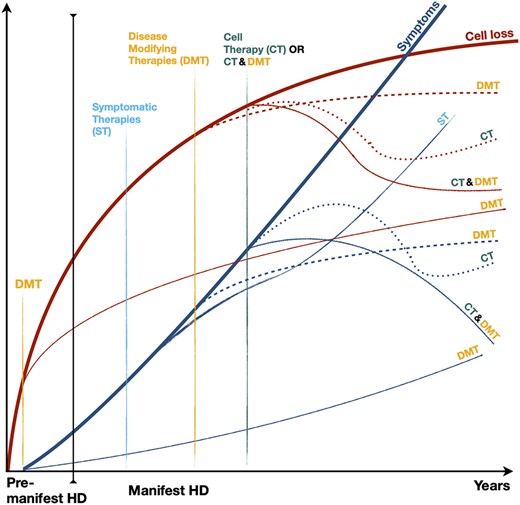
Illustration of the theoretical impacts of various classes of therapies on the course of brain cell loss (red lines) and clinical symptoms (blue lines) in Huntington's disease. Symptomatic therapies (ST), the only ones currently available, may modify the progress of symptoms, but do not modify the course of the underlying condition. Current research is aimed at generating disease-modifying therapies (DMT) to slow or halt the disease process, and could theoretically prevent disease onset if given in the premanifest period (shown as separate lines on the schematic). Cell therapy (CT) is the only means envisaged to date to reverse the process of cell death and recover lost functions. The combination of CT and DMT is feasible and could amplify the beneficial effect of both strategies
OVERVIEW OF CELL TRANSPLANT TRIALS IN HD
Pilot studies
Since the 1990s, 70 HD patients worldwide have been enrolled in open-label, nonrandomized, monocentric cell transplant trials (1-16 participants) using human fetal donor cells derived from the fetal ganglionic eminence (GE) (Table 1).6 Of these participants, and where reliable data are available, four show dramatic and long-lasting improvements over more than 6 years. First, in the Créteil pilot trial, three out of five participants benefited from transplantation, with far superior results to previous studies. The correlation between different blinded evaluations (clinical outcomes, positron emission tomography [PET], electrophysiology, digitalized movement analysis and video records of the total motor score [TMS]) highlighted the robustness of those results.4,5,22 The two remaining participants followed the usual deterioration expected in HD. It was postulated that in one of them, the disease was too advanced to permit efficient graft vascularisation,21 an idea supported by subsequent postmortem findings.25 The graft failure in the other, a multiparous women, might now be interpreted as graft rejection considering the alloimmunization subsequently reported in MIG-HD.43 In a separate study in London, two individuals showed increased raclopride PET signal, confirming graft survival.32 All parameters remained relatively stable in one participant, but the second, who was unable to cope with daily life activities presurgically, was able to return to normal life after 5 years and registered an improved Total Functional Capacity (TFC) score44 from 4 to 13 (range from 0 to 13). It is worth noting, however, that this latter participant suffered from significant depression at the time of surgery, and although the subsequently improvement in depressive symptoms may be related to striatal repair, it cannot be excluded that incidental treatment of depression contributed to clinical improvement.
List of Huntington's disease cell transplantation trials using human fetal cells performed worldwide
| . | Los Angeles . | Créteil . | Tampa . | Nest, UK . | London . | Florence . | MIG-HD . | MIG-HD German extension . |
|---|---|---|---|---|---|---|---|---|
| References | 17-20,46 | 4,5,9,21-24 | 25-28 | 29-31 | 32 | 33-38 | 7,43 | 40-42 |
| Injectate | Micropieces | Micropieces | Micropieces | Suspension | Suspension | Suspension | Micropieces | Micropieces |
| GE part | LGE; 1 sural nerve | WGE | LVE | WGE | WGE | WGE | WGE | WGE |
| Hibernation (N hours) | <48 | <48 | — | 24-108 | <24 | 4-6 | <48 | <12 |
| N fetuses / age | 5-8/~9-10 W | 1-2/7-9 W | 1-8/8-9 W | 1/8.5-12 W | 2-3/9-10 W | 1/9-12.4 W | 8.5-12 W | 7-12 W |
| N and tracts location | 1-2 C; 3-4 P | 2 C; 2 ant-P; 1 post-P | 0-2 C; 3-6 P (max post) | 4-6:2 C; 2 ant-P; 2 post-P | 1 C; 3 P ant, mid, post | Mean 6.6 (3-9 C and P) | 2C, 2 ant-P, 2 post-P | 2 C; 2 ant-P; 2 post-P |
| Volume/tract | 80-120 μL | 40 μL | 16-20 μL | 12 μL | NA | 50 μL | 50 μL | 40 μL |
| Delay between sides | 0 | 1 Y | 4.5 W | >1 Y except Patient 5 = 0 | 2 M | 2-7 M | 2.45 ± 3.03 M | 5.5 W ± 3.3 (range 1-14) |
| Immunosuppression | Cyclo M18-35 | Cyclo, Pred, Aziat M18 | Cyclo M6 | Cyclo, Pred, Aziat>M6 | Pred, Cyclo1 Y | Cyclo, Pred, Aziat 1Y | Cyclo, Pred, Aziat 1Y then 18 M | Cyclo, Pred, Aziat1Y then M18 |
| Grafted patients (N) | 14 | 5 | 7 | 5 | 2 | 16 | 45 | 22 |
| Autopsied cases (N) | 3 | — | 4 | 1 | — | 1 | — | 1 |
| HLA antibodies | — | — | — | — | — | 9/16 | 18/43 | 5/10 |
| . | Los Angeles . | Créteil . | Tampa . | Nest, UK . | London . | Florence . | MIG-HD . | MIG-HD German extension . |
|---|---|---|---|---|---|---|---|---|
| References | 17-20,46 | 4,5,9,21-24 | 25-28 | 29-31 | 32 | 33-38 | 7,43 | 40-42 |
| Injectate | Micropieces | Micropieces | Micropieces | Suspension | Suspension | Suspension | Micropieces | Micropieces |
| GE part | LGE; 1 sural nerve | WGE | LVE | WGE | WGE | WGE | WGE | WGE |
| Hibernation (N hours) | <48 | <48 | — | 24-108 | <24 | 4-6 | <48 | <12 |
| N fetuses / age | 5-8/~9-10 W | 1-2/7-9 W | 1-8/8-9 W | 1/8.5-12 W | 2-3/9-10 W | 1/9-12.4 W | 8.5-12 W | 7-12 W |
| N and tracts location | 1-2 C; 3-4 P | 2 C; 2 ant-P; 1 post-P | 0-2 C; 3-6 P (max post) | 4-6:2 C; 2 ant-P; 2 post-P | 1 C; 3 P ant, mid, post | Mean 6.6 (3-9 C and P) | 2C, 2 ant-P, 2 post-P | 2 C; 2 ant-P; 2 post-P |
| Volume/tract | 80-120 μL | 40 μL | 16-20 μL | 12 μL | NA | 50 μL | 50 μL | 40 μL |
| Delay between sides | 0 | 1 Y | 4.5 W | >1 Y except Patient 5 = 0 | 2 M | 2-7 M | 2.45 ± 3.03 M | 5.5 W ± 3.3 (range 1-14) |
| Immunosuppression | Cyclo M18-35 | Cyclo, Pred, Aziat M18 | Cyclo M6 | Cyclo, Pred, Aziat>M6 | Pred, Cyclo1 Y | Cyclo, Pred, Aziat 1Y | Cyclo, Pred, Aziat 1Y then 18 M | Cyclo, Pred, Aziat1Y then M18 |
| Grafted patients (N) | 14 | 5 | 7 | 5 | 2 | 16 | 45 | 22 |
| Autopsied cases (N) | 3 | — | 4 | 1 | — | 1 | — | 1 |
| HLA antibodies | — | — | — | — | — | 9/16 | 18/43 | 5/10 |
Abbreviations: ant, anterior; Aziat, azathioprine; C, caudate; Cyclo, cyclosporine; GE, ganglionic eminence; LGE, lateral ganglionic eminence; LVE, lateral ventricular eminence; M, months; mid, median; P, putamen; post, posterior; Pred, prednisolone; W, weeks; WGE, whole ganglionic eminence; Y, years.
List of Huntington's disease cell transplantation trials using human fetal cells performed worldwide
| . | Los Angeles . | Créteil . | Tampa . | Nest, UK . | London . | Florence . | MIG-HD . | MIG-HD German extension . |
|---|---|---|---|---|---|---|---|---|
| References | 17-20,46 | 4,5,9,21-24 | 25-28 | 29-31 | 32 | 33-38 | 7,43 | 40-42 |
| Injectate | Micropieces | Micropieces | Micropieces | Suspension | Suspension | Suspension | Micropieces | Micropieces |
| GE part | LGE; 1 sural nerve | WGE | LVE | WGE | WGE | WGE | WGE | WGE |
| Hibernation (N hours) | <48 | <48 | — | 24-108 | <24 | 4-6 | <48 | <12 |
| N fetuses / age | 5-8/~9-10 W | 1-2/7-9 W | 1-8/8-9 W | 1/8.5-12 W | 2-3/9-10 W | 1/9-12.4 W | 8.5-12 W | 7-12 W |
| N and tracts location | 1-2 C; 3-4 P | 2 C; 2 ant-P; 1 post-P | 0-2 C; 3-6 P (max post) | 4-6:2 C; 2 ant-P; 2 post-P | 1 C; 3 P ant, mid, post | Mean 6.6 (3-9 C and P) | 2C, 2 ant-P, 2 post-P | 2 C; 2 ant-P; 2 post-P |
| Volume/tract | 80-120 μL | 40 μL | 16-20 μL | 12 μL | NA | 50 μL | 50 μL | 40 μL |
| Delay between sides | 0 | 1 Y | 4.5 W | >1 Y except Patient 5 = 0 | 2 M | 2-7 M | 2.45 ± 3.03 M | 5.5 W ± 3.3 (range 1-14) |
| Immunosuppression | Cyclo M18-35 | Cyclo, Pred, Aziat M18 | Cyclo M6 | Cyclo, Pred, Aziat>M6 | Pred, Cyclo1 Y | Cyclo, Pred, Aziat 1Y | Cyclo, Pred, Aziat 1Y then 18 M | Cyclo, Pred, Aziat1Y then M18 |
| Grafted patients (N) | 14 | 5 | 7 | 5 | 2 | 16 | 45 | 22 |
| Autopsied cases (N) | 3 | — | 4 | 1 | — | 1 | — | 1 |
| HLA antibodies | — | — | — | — | — | 9/16 | 18/43 | 5/10 |
| . | Los Angeles . | Créteil . | Tampa . | Nest, UK . | London . | Florence . | MIG-HD . | MIG-HD German extension . |
|---|---|---|---|---|---|---|---|---|
| References | 17-20,46 | 4,5,9,21-24 | 25-28 | 29-31 | 32 | 33-38 | 7,43 | 40-42 |
| Injectate | Micropieces | Micropieces | Micropieces | Suspension | Suspension | Suspension | Micropieces | Micropieces |
| GE part | LGE; 1 sural nerve | WGE | LVE | WGE | WGE | WGE | WGE | WGE |
| Hibernation (N hours) | <48 | <48 | — | 24-108 | <24 | 4-6 | <48 | <12 |
| N fetuses / age | 5-8/~9-10 W | 1-2/7-9 W | 1-8/8-9 W | 1/8.5-12 W | 2-3/9-10 W | 1/9-12.4 W | 8.5-12 W | 7-12 W |
| N and tracts location | 1-2 C; 3-4 P | 2 C; 2 ant-P; 1 post-P | 0-2 C; 3-6 P (max post) | 4-6:2 C; 2 ant-P; 2 post-P | 1 C; 3 P ant, mid, post | Mean 6.6 (3-9 C and P) | 2C, 2 ant-P, 2 post-P | 2 C; 2 ant-P; 2 post-P |
| Volume/tract | 80-120 μL | 40 μL | 16-20 μL | 12 μL | NA | 50 μL | 50 μL | 40 μL |
| Delay between sides | 0 | 1 Y | 4.5 W | >1 Y except Patient 5 = 0 | 2 M | 2-7 M | 2.45 ± 3.03 M | 5.5 W ± 3.3 (range 1-14) |
| Immunosuppression | Cyclo M18-35 | Cyclo, Pred, Aziat M18 | Cyclo M6 | Cyclo, Pred, Aziat>M6 | Pred, Cyclo1 Y | Cyclo, Pred, Aziat 1Y | Cyclo, Pred, Aziat 1Y then 18 M | Cyclo, Pred, Aziat1Y then M18 |
| Grafted patients (N) | 14 | 5 | 7 | 5 | 2 | 16 | 45 | 22 |
| Autopsied cases (N) | 3 | — | 4 | 1 | — | 1 | — | 1 |
| HLA antibodies | — | — | — | — | — | 9/16 | 18/43 | 5/10 |
Abbreviations: ant, anterior; Aziat, azathioprine; C, caudate; Cyclo, cyclosporine; GE, ganglionic eminence; LGE, lateral ganglionic eminence; LVE, lateral ventricular eminence; M, months; mid, median; P, putamen; post, posterior; Pred, prednisolone; W, weeks; WGE, whole ganglionic eminence; Y, years.
MIG-HD: A randomized, multicenter, phase II trial
Despite improvements observed up to 18 months postoperatively in other studies,33,40,41 most were too heterogeneous (different participant characteristics, cell sources, surgical protocols, immunosuppression) and too underpowered to be conclusive or to identify areas for procedural improvement. For these reasons, MIG-HD was designed as a multicenter randomized delayed-start trial of 45 HD participants. MIG-HD included a run-in period between Month (M)0 and M12, followed by randomization to either the treatment group (transplanted at M13-M14 with 1 month between sides), or to the control group who received delayed transplants (transplanted at M33-M34). Group efficacy (treatment vs control) was assessed at M32 and pre- and postgraft TMS slopes were compared at M52 in all patients. Participants were transplanted between 2001 and 2013 within six sites in France and Belgium. A relatively long follow-up was planned, initially up to 2008, anticipating that implanted human cells required around 18 months to develop, integrate and become functional based on previous trajectories of clinical effects,5 the appearance of tyrosine hydroxylase fibers from the graft to the brain host,42,45-47 and the activation of the frontal cortex measured by [18F]-fluorodeoxyglucose (FDG)-PET scans 24-months after transplant.22
Complications of MIG-HD
The actual study timelines in MIG-HD were more protracted than planned, and illustrate the difficulty of undertaking a multicenter transplant study with fetal cells, as well as the detrimental effect of a poorly established regulatory framework (Figure 3). Fetal cells were transplanted within 2 days of abortion, yielding 86 actual grafts but with 163 occasions on which surgery was cancelled due to inadequate/insufficient donor tissue, thereby emphasizing the need to identify nonfetal donor cells. Institutional and regulatory constraints contributed to the delay, first by prolonged suspension of the trial after the occurrence of a postoperative putaminal hematoma (approximately 1 year), resulting in three of the six centers being unable to continue transplants. Obtaining authorization for patients to cross the frontiers for surgery took years. Furthermore, regulation of donor serology changed in France rendering grafting of the final participants impossible. Changes in the regulation of human fetal tissue collection also truncated the NEST-UK trial.48 One cannot easily measure the impact of such delays on participants nor the impact of associated stress on their performance. Future transplant trials require a more integrated and consistent regulatory framework.
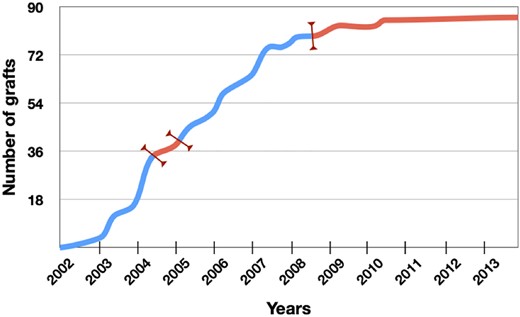
Detrimental impact of regulatory uncertainty on inclusion of participants in the surgical arm of multicenter intracerebral transplant in Huntington's disease (MIG-HD). Red portions of the curve indicate the periods where regulatory and institutional issues impacted on the trial (trial suspension, delays in obtaining authorizations for adapting to regulatory changes, changes in French law with respect to serological testing)
Factors influencing the graft in MIG-HD
Graft function is reliant on survival of sufficient donor cells of the target phenotype (in this case MSNs) that can innervate the host tissue, make synaptic connection and restore neural circuitry. Clearly, a graft cannot be expected to improve function if it is too small or unhealthy. In 82% of the grafted patients in MIG-HD, FDG binding (indicating metabolic activity of the tissue) did not increase, suggesting that in these grafted individuals, graft survival and/or function was suboptimal. Raclopride binding (measuring D2 signaling, consistent with functional MSNs) was compromised by neuroleptics intake which rendered the data unusable; only 15% of patients were free from neuroleptics by the end of the trial. MRI data were available for a subset of “first treated” individuals (N = 19) and showed striatal volume increase of 27% by M32, and for a smaller subset (N = 12) from both groups (first-treated patients and controls who received a delayed transplant) in which data were available at each time point and in which a 14% increase was seen by M52. These data should be treated with caution, but they might indicate progressive atrophy of the graft due to its lack of integration in the host tissue. In contrast, in the small number of previous transplant recipients who showed unequivocal evidence of functional improvement, there was clear evidence of increased FDG or raclopride binding.4,32 There are a number of potential reasons for suboptimal grafts, which may include poor viability of transplanted cells, donor tissue being outside of the optimal gestational window (which would reduce the proportion of MSNs10), poor vascularization of the graft, immune rejection and a nonpermissive host environment. An in-depth discussion of all graft survival factors is outside the scope of this article, but some of the key factors are briefly discussed below and graft rejection is considered in more detail.
In MIG-HD, the average fetal gestation age and age range were higher than in the Creteil pilot trial (8.5-12 weeks compared to 7.5-9 weeks) with an average delay 9 hours 55 minutes (range 1 hour 20 minutes to 23 hours 50 minutes) between collection and tissue dissection, which was longer than that in the pilot study. Unfortunately, the impact of these factors is unquantifiable as cell viability was not assessed prior to implantation. This may be a critical issue and it will be essential for future trials to document donor cell viability and define viability thresholds. Likewise, fetal brain dissection is a specialist undertaking and can be highly challenging as fetal brain is rarely fully intact post termination, but in MIG-HD local investigators were trained by the coordination to undertake the dissections but the level of expertise reached was not uniform. Photographs of dissected ganglionic eminence were checked remotely pretransplantation by coordination center experts, but the efficacy of this strategy has not been validated. Errors in dissection could result in insufficient target tissue being transplanted and/or inclusion of nontarget tissue. We reported one patient in whom cells from choroid plexus were transplanted. This did not result in long term damage thanks to effective surgical cauterization of the secreting zone in the graft. Future trials of fetal tissue transplantation need to pay careful attention to training of dissectors and should document the dissection (eg, by video recording of the process through the dissecting microscope rather than still images).
The host environment is also important, as the HD brain may not provide the same level of support as a healthy striatum. There is abundant evidence that immune dysregulation is important in many neurodegenerative conditions, including HD49 and although this is difficult to image satisfactorily in life, postmortem analysis of nine transplant patients suggested that long-term grafts could be subject to chronic damage, possibly due to an inhospitable host milieu.50 There has been little direct preclinical exploration of this question, which is a gap in our knowledge, as it may lead to strategies to improve survival. Another host environmental consideration in HD is “prion-like” transfer of mutant HTT aggregates from diseased to healthy cells. Animal studies have supported the notion that this can occur in HD in an analogous way to grafts in patients with Parkinson's disease (PD).51 There is a paucity of postmortem evidence although Maxan et al29 reported mHTT aggregates in the graft region of an individual who received fetal grafts 12 years earlier. This is consistent with aggregate transfer although, unfortunately, the identity of the mHTT-containing cells was not proven to be of donor, rather than host, origin (grafted cells integrate with the host tissue over time, so not every cell in the graft region will be of donor origin). Confirmation in human specimens is awaited, although even if confirmed, the relevance of small percentages of cells containing aggregates after many years is yet to be determined.
An important issue uncovered in MIG-HD was evidence of alloimmunization despite participants undergoing a period of triple immunosuppression.43 When cell therapy trials in HD started (Table 1), the brain was considered to be an immune privileged site, a concept since revised, notably with the discovery of a lymphatic system in the central nervous system (CNS)52 which may play a role in the pathophysiology of neurodegenerative diseases.53,54 Moreover, cerebrovascular and blood-brain barrier impairments have been reported in HD patients, which might facilitate the transmigration of immune cells to the brain.55 The first case of graft rejection in the brain was reported in MIG-HD43 where immunosuppression included cyclosporine A, started 3 days preoperatively until 6 months after the second transplantation, with prednisolone and azathioprine administered from the day of surgery to 6 months after the second graft. Graft rejection was diagnosed in the only participant not receiving immunosuppressant as per protocol; this participant lost weight, collapsed, and a hyposignal was found in the graft location on T1 magnetic resonance imaging (MRI). Class 1 human histocompatibility antigen (HLA) antibodies specific to the fetal graft were detected both in blood and cerebrospinal fluid. High doses of intravenous prednisolone resulted in regression of clinical and imaging signs with an almost complete return to the presurgery state within 6 months. Following this event, triple immunosuppression was maintained in the remaining (20) participants for up to 1 year after the second graft. No further graft rejection was observed, but in the 39 participants assessed during the trial, HLA class 1 and 2 antibodies appeared in 34% of posttransplant participants, being present in 9% of pretransplant women (presumably due to pregnancy).7 HLA antibodies against transplant were also detected by the Firenze group (in 37.5% of patients) with a year immunosuppression34,35 and in the German branch of MIG-HD (in 50% of patients) with 18 months immunosuppression,40 and different immunosuppressive protocols (Table 1). Inflammation has been reported in postmortems of both grafted HD and PD patients having had immunosuppression, despite surviving grafts identified on MRI. Within grafts, healthy regions (with internal organization similar to normal striatum, expressing markers of MSNs and interneurons and receiving cortical afferents) often coexisted with areas of apoptosis,47 astrocytic activation,25 and inflammation (cluster of differentiation [CD]45) around or within grafts tissue and vasculature (CD3, CD68 and T-cell CD68 microglia, natural killers cells, CD8 T cytotoxic cells, and CD4 T helper cells).29,40,42,46,47,56 Grafts generally lack vascularization compared to host tissue, and mutant huntingtin has been reported in grafts, although evidence that it was located in donor, rather than host, cells is still awaited.29,56
Additional parameters, that were not part of the MIG-HD initial analysis plan, were sought to explain the efficacy failure and to inform future trials. These included host characteristics, procedural data, volume injected, graft size after 20 months and presence of HLA antibodies. Counterintuitively, a high number of tracks (>5 per side) was associated with a poorer outcome. Although Paganini et al36 concluded that the grafts slowed motor and cognitive decline, even in HD patients with large ectopic tissue nodules in the frontal lobe, MIG-HD showed that ectopic graft tissue was detrimental, calling for caution in future trials.
It is unclear whether the methodological differences between the pilot study and MIG-HD turned success into failure.4,5 For example, reducing the delay between ipsilateral and contralateral transplant surgeries to 1 month, compared to 1 year in the pilot, might have constituted a more severe immunogenic challenge. HLA antibodies searched 10 years after transplantation were not found in the pilot study but were present 3 years after transplants in MIG-HD. Although knowledge is lacking in the field of intracerebral transplantation, in that of solid organ transplants, the presence of HLA antibodies against donor tissues is considered to indicate alloreactivity.57 Likewise, the increased number of fetal samples per host and older gestational window from 7-9 to 8.5-12 weeks postconception may also have increased the overall immunogenicity burden. Finally, moving from a monocentric pilot study to a multicentric one in MIG-HD might have compounded procedural variability. Notwithstanding intensive training, full standardization of surgical procedures was not achieved; the surgeon adapting the intervention for each participant. We strongly recommend full standardization and documentation of all procedures in future studies and highlight suggested improvements in the next sections.
FUTURE CHALLENGES
Donor cell product
The rationale for using fetal GE as donor tissue is the replacement of MSNs, with the idea that these cells will make synaptic connections with appropriate striatal targets and provide some reconstruction of the neural circuitry. These cells have developed “naturally” and so are the donor cell source most likely to be properly specified. There is evidence of circuit reconstruction by fetal-derived cells in animal studies,58 and also in humans where [18F]-FDG-PET signal increase in the frontal lobes suggested that circuit reconstruction was the mechanism underlying successful grafts.22
However, ultimately, the generation of donor cells from renewable sources, such as human pluripotent stem cells (hPSCs), will be essential for transplantation to be an efficient therapy; fetal-derived donor cells are a scarce resource and cannot be stored beyond a few days, making quality control very difficult and presenting a major challenge in terms of surgical scheduling. Indeed, even if no difference in cell processing was introduced between the successful pilot and MIG-HD, in both trials viability assessment was not possible and may have proved critical in ensuring graft survival in MIG-HD as discussed above. Nevertheless, a nuanced understanding of the factors determining a successful fetal GE graft underpins our ability to produce suitable hPSC-derived donor cells. For example, fetal grafts have taught us that the stage of precursor development at the time of transplantation is critical (precursors need to be sufficiently committed to an MSN phenotype but sufficiently immature to integrate well in the host tissue12). The questions of whether a fetal graft should be restricted to the lateral GE, in which the majority of MSNs develop, is unresolved and related to consideration of optimal graft cell content; human fetal GE contains not only MSN precursors, but a range of neural and glial precursors, microglia and vascular cells and the contribution of these none-MSN elements to fetal graft function have not yet been determined.59 The question of whether fetal tissue should be prepared as tissue pieces or cell suspension also remains unresolved; theoretically, a suspension graft may integrate better and may be less immunogenic, as it probably contained fewer donor vascular elements although the most promising clinical study to date employed tissue pieces.4,5 However, this question may be less relevant for stem cell-derived cells, which are likely to be suspension grafts and so attract vascular elements from the host rather than donor (although this will depend to some extent on the precise differentiation protocol). It is also possible that cell clusters or stem cell derived organoids (delivered alone or associated with various biomaterials60) may prove to be a useful delivery mechanism.
A variety of renewable cell sources are actively being explored as donor cells for cell therapy. These include human embryonic stem cells (hESCs), induced pluripotent stem cells (hiPSCs), fetal derived neural stem cells, and directly induced stem cells (hiNSCs). As well as addressing the critical issues of tissue availability and quality control, these sources have other potential advantages over fetal cells and also a number of drawbacks. These are outlined briefly below and a detailed discussion of the pros and cons of specific cell types can be found in Reference 61. There has also been considerable interest in deriving donor cells from nonneural stem cells, such as mesenchymal stem cells, for HD. Such cells have the capacity to secrete neuroprotective, anti-apoptotic, immunomodulatory, and/or anti-inflammatory molecules and may be engineered to produce and release specific therapeutic molecules such as brain-derived neurotrophic factor (BDNF). These cells, and others also engineered to produce therapeutic molecules, are being explored for their neuroprotective potential.14,15 However, there is little convincing evidence that such cells differentiate into neuronal phenotypes suitable for circuit reconstruction and so they will not be considered further here.
hESCs are arguably the closest to clinical trial at the current time, largely because they have been the subject of research for the longest time and are thus underpinned by a large body of knowledge, added to which good manufacturing practices (GMP)-grade cell lines are already in existence, some of which have already been used in clinical studies for other conditions. However, iPSC, and to some extent iNSC, technology has been accelerating quickly and some iPSC lines have entered clinical trials. iPSCs can be generated from adult somatic tissues and so have the potential advantage of circumventing some ethical issues associated with hESCs (namely the use of human blastocysts). iNSCs are produced by directly trans-differentiating somatic cells to a neural stem cell, rather than pluripotent, phenotype, with the potential advantage of a lower risk of tumor formation,62 although there have been fewer preclinical studies to date than for hESC or hiPSCs exploring these cells for their cell therapy potential. There is also the possibility of directly transdifferentiating host cells to another phenotype, such as has been achieved for dopaminergic cells from astrocytes, although this exciting technology is currently probably a long way from clinical application.63,64
Stem cells and fetal cells have some challenges in common, including cell viability on implantation, graft vascularization, graft placement and distribution, the permissiveness of the host environment, and graft rejection (the latter is discussed further below). There are also some challenges that apply to stem cell sources, but not to fetal cells; in particular, the development of protocols to ensure the presence of correctly specified target neural cells and the absence of unwanted cell types. Fetal cells have the advantage of being programmed through normal development whereas stem cell-derived neural cells have to be artificially programmed.65,66 Indeed, preclinical research using fetal striatal cells (which contain precursors for the full complement of neuronal and glial components of the adult striatum) underpins the use of stem cells by helping to define which cells are critical for a fully functional graft.59 The elimination of cells with tumerogenic potential is a much greater problems for stem cells compared to fetal cells and can potentially be achieved through a range of means, including adjusting the protocol and timing of transplantation to ensuring complete differentiation and elimination of proliferating cells,66 and cell sorting strategies.67 Through in vitro proliferation, stem cells are also subject to quality control issues, in particular, the acquisition of karyotype and genetic abnormalities, batch to batch variation and between line differences in response to differentiation factors. These challenges will need to be addressed for safe and optimum clinical application of stem cell derived products.
iPSCs and iNSCs also have the potential advantage of providing a source of autologous donor cells (with or without correction of any genetic abnormality),68 which could circumvent the need for immunosuppression. However, generating GMP-grade cells on an individual basis is currently extremely costly and thus not currently suitable for widespread application, and the immune tolerance to autologous iPSC grafts may not be as straightforward as originally perceived, as it has been demonstrated that autologous primate iPSCs still induce an immune reaction after grafting into the CNS, albeit at a lower level than an allograft.69 A challenge for both iPSCs and iNSCs is that they are generated through genetic manipulations, which add an additional layer of regulatory scrutiny in terms of clinical use and additional cost. In addition, their use in autologous grafting may require correction of a host mutation prior to use, as is the case for HD. The extent to which this would be necessary is currently uncertain, but it would clearly incur additional steps that would further increase the regulatory burden and cost. However, putting the regulatory burden to one side, the relative ease with which stem cells can be genetically manipulated opens up possibilities that are much more difficult to achieve with primary fetal cells; for example, the vision of producing “universal cells” that are invisible to the immune system through CRISPR/Cas9-mediated deletion of major histocompatibility genes.70 However, the CRISPR/Cas9 system is not without its dangers, such as its autonomous evolution into the brain which has led to the KamiCas9 construct designed by Merienne et al71 to avoid section of additional genes beyond the targeted one. This construct is a self-inactivating editing system that achieved transient expression of the Cas9 protein in mice models. It might take years to achieve safety in patients.
Several recent studies have reported some functional improvements in HD animals transplanted with hPSC-derived striatal-like precursors,61,72-76 although we believe it is important to demonstrate in animals that these approaches are at least as effective as implantation of fetal-derived cells before progressing to clinical trials. This is highly likely to necessitate implantation in animal models of HD.
Animal models
Animal models of HD remain an important experimental tool, in particular for assessing donor sources that may replace fetal-derived cells, as outlined above, but it is important to understand their limitations and also to continue the quest for models that better represent the relevant elements of the human situation. Lesion models can reproduce the striatal, and sometimes even the cortical, cell loss seen in HD patients, as well as mimicking the behavioral and metabolic changes.77 Such models in rodents have been essential for understanding the biology of grafts, but they do not replicate the progressive cell loss seen in HD, nor reliably replicate the extrastriatal damage.78 A variety of genetic models exist in mice, but these have so far proven problematic for assessing striatal grafts; they have substantially longer CAG repeat expansions than those seen in adult onset HD and do not accurately reflect the anatomy of changes of the CNS.39 Rat models with CAG repeats much closer to that of adult onset HD are being optimized and may provide more relevant models with which to work.79 It is likely that these relatively cheap and accessible rodent models will continue to provide the cornerstone for experimental work addressing both mechanistic and procedural questions, and perhaps even alloimmunization issues through the use of humanized mice.80
Large animal models are significantly more costly and complex, but some translationally important issues would be better addressed in such models.81 For example, refining cell distribution, assessing the capacity of cells to extend processes in a brain with anatomical distances closer to a human brain, testing the efficacy of grafts in animals with more human-like behavioral repertoires, and working out the details of assessment tools such as in vivo imaging to track graft size, all fall into this category. Large animal models also facilitate long-term studies, potentially over several years, to more comprehensively assess safety and efficacy of treatments. Both lesion and genetic models in larger animals are becoming increasingly available.81 For example, some transgenic minipigs show progressive neurodegeneration as well as motor, cognitive and behavioral decline and nonhuman primate lesion models, although limited by ethical concerns and legal regulations, have been used to assess imaging strategies and address issues of alloimmunization.69
Protocol design
Heterogeneity is one of the hallmarks of HD, both in its clinical manifestations and rates of progression. Specific phenotypes are linked to patterns of brain alterations,82,83 gender,84 and genetic factors.85-87 Variability, which results in the need to recruit large numbers of patients and for prolonged periods of follow-up, is at the forefront of obstacles to be lifted for future trials.8,88 The problem is heightened in trials aimed at restoring atrophied tissues where the response to the transplant cannot be readily predicted and might encompass improvement in a wide range of symptoms, thus necessitating a broad-spectrum evaluation of patients to meet safety and efficacy requirements.
One way to address these challenges is the refinement and automation (and/or video recording) of outcome measures to reduce rater bias. Participants in MIG-HD were assessed using an adapted version of the CAPIT-HD (Core Assessment Protocol for Intracerebral Transplantation in HD) protocol89; which was heavily reliant on the Unified Huntington's disease Rating Scale (UHDRS). In retrospect, these assessments are unwieldy and lack sensitivity. Likewise, the use of the TMS as primary endpoint was also suboptimal as the cognitive and behavioral disturbances are now recognized as the major drivers of poor function in HD.90 When this study commenced, no cognitive assessments were universally accepted except the UHDRS cognitive score, which also lacks sensitivity, and TFC was discounted considering the large numbers of participants required to detect change. The global composite score cUHDRS, combining the TFC, TMS, Stroop and Symbol Digit Modalities Test, is currently used in trials of antisense oligonucleotides and constitutes a first step toward improvement.88 Yet, objective digitized tasks, such as QMotor which has already been used in clinical studies,91 might allow evaluation and comparison of many functions within in a short time and eliminate rater bias. Sensitive digitalized measures of cognition, suitable for intracranial intervention studies including cell therapy, are under validation in French, English and German thanks to the Repair-HD program (eg, Selfcog).
Although electrophysiological assessments were optional in the CAPIT-HD protocol, their inclusion in our pilot study4 allowed us, in conjunction with clinical and PET assessments, to identify transplants benefits; similar improvements never having been previously reported in longitudinal assessment of either observational cohorts92 or pharmacological trials.21 However, duration, availability, and lack of full standardization of electrophysiology assessment might hamper its use in multicenter trials. We would recommend using it only in the future open label trial, in single center or after full homogenization of the procedure by expert investigators. It will complete the panel of arguments before an eventual dissemination of the transplant procedure.
So far, brain imaging has involved the use of structural MRI and PET. [18F]-FDG-PET was used in the early days of cell transplants trials22,33,40,41 but could not disentangle edema or graft rejection from functional grafts. [11C]raclopride PET is specific for dopamine D2 receptors, which are expressed by MSNs, but is affected by neuroleptics which are frequently prescribed in HD for involuntary movements and behavioral disorders. Thus, it is less suitable for participants with moderate/advanced disease. New tracers, such as ligands to phosphodiesterase-10A, an enzyme expression by striatal MSN whose reduction is one of the earliest biochemical changes in HD93 may prove to be a reliable indicator of disease progression.94 Likewise future trials should capitalize on volumetric MRI and connectivity measures.95,96
Additional trial design measures might include enrichment strategies to recruit patients predicted to have faster rates of progression based on neuroimaging analyses, genetic polymorphisms, clinical profiles, or retest effects85,97-100 to increase the likelihood of demonstrating treatment efficacy with small cohorts over shorter durations. Identification of markers to select patients better able to tolerate grafts (eg, with a low risk of graft rejection) or to ensure compatibility between the host and donor cells are needed.6,101 The inclusion of sham surgery is another important consideration, but may not be deemed ethical in early stage trials. Moreover, failed grafts (rejected/too small) may be more informative comparators than partial burr holes sham cases, in which associated experimental procedures are not undertaken.
Stage of disease is important; advanced HD patients were more likely to develop subdural hematomas45 and to have defects of vascularization,25 compromising transplant development.21 Improvements in the surgical procedures in MIG-HD might have solved some of these issues7 but the question of efficacy in more advanced patients remains. The complexity of patient profiles makes the interpretation of transplant response extremely complex. The use of new outcome measures and biomarkers combined with innovative statistical methods, including machine learning approaches able to analyze high-dimensional sources of data and enable inferences from fewer samples, all have the potential to improve patient stratification for inclusion in trials and/or to identify subsets of patients with differential response to treatment.102,103 They will improve the design and evaluation of cell therapy strategies in a cost-effective and efficient manner.
Surgical procedures
Adverse events in transplant surgery are relatively common across all disciplines. A key adverse event for neural transplant surgery is intracranial bleeding. For example, Freeman et al45 reported 26% subdural hematomas following cell transplants in HD; subdural hematomas being anyway more frequent in HD than in control populations. However, reducing the number of tracts in participants with marked atrophy and providing hyperhydration and 48 hours bed confinement abolished further subdural hematomas in MIG-HD.7 Furthermore, endoscopic surgery to cauterize a choroid cyst successfully rescued one participant, who continued to enjoy improvement associated with a surviving graft. Nevertheless, better control of surgical and perioperative procedures and the development of improved cell-delivery devices will be critical for future transplant studies. Systematic video records of surgery should improve homogenization of procedures.
Alloimmunization
The first case of graft rejection43 and the identification of HLA antibodies against transplants in trials using various immunosuppressive protocols34,35,40 suggest that such responses to the graft are not linked to inadequacy of a specific immunosuppressive protocol, but rather related to poor understanding of graft-host responses in the CNS. As outlined above, stem cell-derived products may provide better methods for circumventing immune rejection, although they are not without their own practical and regulatory problems. In MIG-HD there was no clear association between the presence of functional grafts and graft-directed HLA antibodies. However, since alloimmunization was not anticipated in MIG-HD, HLA antibodies were not systematically measured and techniques were not uniform across centers. Thus, conclusions cannot yet be drawn as to the importance of graft-derived antibodies nor how this should affect future trials. Coordinated analysis of immune status will be essential in future trials, but for now, donor/host HLA matching may provide a partial solution in future stem cell trials.104 If fetal cells are still to be used, crossmatching of alloantibodies and antigens could be attempted prior to transplantation.
CELL TRANSPLANTS IN THE FUTURE HD THERAPEUTIC LANDSCAPE
The results of MIG-HD, even if they shed light on the way forward, undoubtedly constitute a disappointment for the field, and probably underlie the extreme points of view of some researchers who condemn all further research in the field.105 Yet these same researchers should remember the history of transplantation in PD, in which they participated, and in which condition (in contrast to HD) some meaningful treatments have been available for many years.
The rationale for transplantation in PD is to restore dopamine levels in the striatum through implantation of dopamine-releasing cells. Between the 1970s and the 2000s, open pilot studies reported variables results with successful clinical outcome of ventral mesencephalic grafts in some patients that correlated with [18F]-FDG-PET increases and postmortem evidence of long-term cell survival,106-108 whereas other studies showed small/no benefit (reviewed in Reference 109). The phase II randomized trials that followed56,110 were negative and resulted in this area of research shutting down for around 15 years. The reason for these trials failing was likely related to poor cell viability linked to their storage, and alloimmunization of transplants; the first trial did not include immunosuppressants and the second only 6 months, and autopsies revealed inflammation around and within the grafts.110 Endpoints were also regarded as subjective,56,110 thereby justifying the use of shams and, as an example, nourished a discussion on the choice of clinical vs imaging endpoints. However, reanalysis of surviving transplants in these and other previous studies demonstrated that the transplant efficacy was often delayed beyond the end of clinical study and thus not recognized in the original report.109 Variability in procedures and patient selection also contributed to the disparity of the results. Following these observations, a European fetal cell trial (TRANSEURO - NCT01898390) was established in 2013, attempting to respond to the deficiencies recognized in previous trials. Transplants in HD (albeit only in four patients) have so far produced clinical improvement beyond any other therapeutic approach. Thus, to avoid repetition of the PD scenario, with a long and damaging interruption of research activities, this review attempts to identify the critical trial design and technical factors responsible for graft failure in order to facilitate progress in this area. As outlined above, it is essential to note that many of these factors are pertinent to stem cell-derived, as well as fetal, donor cell products.
CONCLUSIONS
MIG-HD failed to demonstrate a benefit associated with transplantation. Nevertheless, the benefits reported in hundreds of animal studies and in human pilot trials4,32 in addition to areas for improvement identified in MIG-HD encourage further research into cell therapy for HD. MIG-HD should be understood in the context of having started in 2001. Since then, there have been considerable advances in knowledge and technical development, including transplant and immunosupression regimens, stem cell differentiation protocols, and development of more sensitive objective outcome measures. Disease-modifying treatments, such as huntingtin lowering therapies, could slow or even prevent the disease from developing, although their degree of efficacy and long term adverse impacts are yet to be determined. However, cell therapy, which could be given alongside a disease-slowing therapy (Figure 2), is the only approach currently focused on structural and (hopefully) functional restoration. Until we have a cure applicable to the whole HD community, it seems reasonable to continue exploring a broad range of therapeutic options. We therefore propose that the door is left open for future cell therapy research.
We anticipate progress being an iterative process requiring both preclinical and clinical work. The availability of stem cell-derived donor cells has the potential to dramatically change the landscape by permitting better control of the quantity and homogeneity of the donor cell product, but it is essential that their clinical translation is based on sound evidence. Indeed, we and others have established an international platform (Stem Cells for HD, www.sc4hd.org) to review such evidence and produce guidance, and we encourage great caution around the use of private transplant clinics worldwide which offer cell therapy treatments without clear evidence of efficacy or guarantees for patients safety.
ACKNOWLEDGMENTS
Anne-Catherine Bachoud-Lévi's team is supported by NeurATRIS ANR-11-INBS-0011/Agence Nationale de la Recherche (French National Research Agency), ANR-17-EURE-0017, and the Henri-Mondor Hospital National Reference Centre for Huntington's disease (Ministry of Health). Anne-Catherine Bachoud-Lévi: Consulting and Advisory Board Membership with honoraria: Roche; Grants and Research: investment for the future ANR grant (Neuratris, Front EUR), National Center of Reference for Huntington's disease (DGOS, Ministry of Health), PHRCs (DRCI grants); Intellectual Property Rights: Cognitive assessments (SelfCog, CATEX, CALAP). Anne Rosser: Employment with Cardiff University; Consultant/Advisory role for Roche; research funding from EHDN/MRC UK/HCRW/EC/CARE/Jacques & Gloria Gossweiler Foundation; and other from EHDN/Triplet. Renaud Massart declared no potential conflicts of interest.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
A.-C.B.-L., R.M., A.R.: wrote the manuscript.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
Author notes
Funding information EHDN/Triplet; EHDN/MRC UK/HCRW/EC/CARE/Jacques & Gloria Gossweiler Foundation; Henri-Mondor Hospital National Reference Centre for Huntington's disease (Ministry of Health); ANR-17-EURE-0017; NeurATRIS ANR-11-INBS-0011/Agence Nationale de la Recherche (French National Research Agency)



