-
Views
-
Cite
Cite
Xuchi Pan, Chie Naruse, Tomoko Matsuzaki, Ojiro Ishibashi, Kazushi Sugihara, Hidetsugu Asada, Masahide Asano, Critical role of the potential O-linked glycosylation sites of CXCR4 in cell migration and bone marrow homing of hematopoietic stem progenitor cells, Stem Cells, 2025;, sxaf025, https://doi.org/10.1093/stmcls/sxaf025
Close - Share Icon Share
Abstract
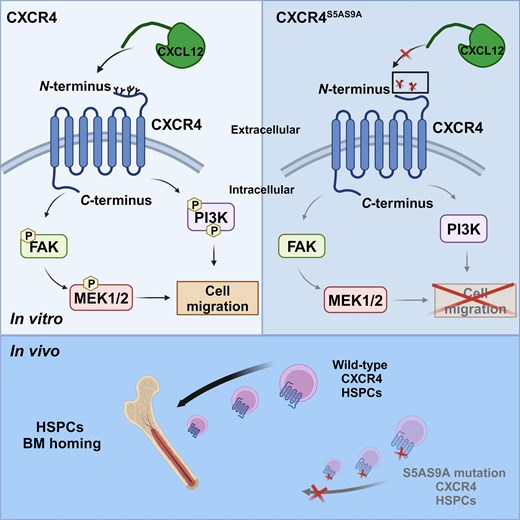
The C-X-C chemokine receptor type 4 (CXCR4) and its ligand, C-X-C motif chemokine ligand 12 (CXCL12), are critical for the homing of hematopoietic stem progenitor cells (HSPCs) to bone marrow (BM). Our previous study revealed that carbohydrate chains on HSPCs are vital in the homing and engraftment of HSPCs. However, the relationship between the glycosylation of CXCR4 and HSPCs homing remains unclear. In this study, we analyzed the glycosylation sites of the N-terminal 38 amino acids of mouse CXCR4, which is indispensable for CXCL12 binding. Among these, simultaneous mutations of possible glycosylation sites, Serine-5 and Serine-9 of mouse CXCR4 lost cell migration activity through CXCL12 in cultured cells and mouse HSPCs. Furthermore, Serine-5 and Serine-9 mutations in HSPCs caused a deficiency in the homing to the BM. Our findings suggest that the glycosylation of mouse CXCR4 is essential for homing HSPCs to the BM, which can be used to screen cord blood HSPCs suitable for transplantation.