-
PDF
- Split View
-
Views
-
Cite
Cite
Samirah A. Gomes, Joshua M. Hare, Erika B. Rangel, Kidney-Derived c-Kit+ Cells Possess Regenerative Potential, Stem Cells Translational Medicine, Volume 7, Issue 4, April 2018, Pages 317–324, https://doi.org/10.1002/sctm.17-0232
Close - Share Icon Share
Summary
Kidney-derived c-Kit+ cells exhibit progenitor/stem cell properties in vitro (self-renewal capacity, clonogenicity, and multipotentiality). These cells can regenerate epithelial tubular cells following ischemia-reperfusion injury and accelerate foot processes effacement reversal in a model of acute proteinuria in rats. Several mechanisms are involved in kidney regeneration by kidney-derived c-Kit+ cells, including cell engraftment and differentiation into renal-like structures, such as tubules, vessels, and podocytes. Moreover, paracrine mechanisms could also account for kidney regeneration, either by stimulating proliferation of surviving cells or modulating autophagy and podocyte cytoskeleton rearrangement through mTOR-Raptor and -Rictor signaling, which ultimately lead to morphological and functional improvement. To gain insights into the functional properties of c-Kit+ cells during kidney development, homeostasis, and disease, studies on lineage tracing using transgenic mice will unveil their fate. The results obtained from these studies will set the basis for establishing further investigation on the therapeutic potential of c-Kit+ cells for treatment of kidney disease in preclinical and clinical studies.
The search for putative stem cells or precursors within the kidney has been the focus of extensive research. The identification of a kidney progenitor/stem cell population would provide important biological insights and could therapeutically be used to generate new tubular, glomerular, and vascular cells for treatment of kidney disease. Recent findings support that c-Kit+ cells isolated from neonatal rat kidney represent a novel population of progenitor/stem cells. Therefore, c-Kit+ cells fulfill all of the criteria as a true kidney progenitor/stem cell, including self-renewal capacity, clonogenicity, and multipotentiality. c-Kit+ cells formed nephrospheres when plated at clonal density, and were positive for epithelial, endothelial, and neuronal markers. Furthermore, c-Kit+ cells exhibit the potential to treat renal failure by multi-compartment engraftment (tubular, vascular, and glomerular) following acute ischemia-reperfusion injury. In acute proteinuria induced by puromycin aminonucleoside, c-Kit+ cell treatment rescued podocyte disarrangement and accelerated foot process effacement reversal by modulating autophagy and mTOR pathway. Taken together, these findings unravel important aspects of stem cell therapy.
Introduction
Kidney-derived stem cell populations detected during embryonic and early post-natal periods are identified by the markers Six2 [1] and Lgr5 [2]. However, these stem cell populations are not found when kidney is fully developed and are not maintained throughout adult life. Conversely, in other organs, such as gut and stomach, adult stem cells are implicated in both homeostatic tissue maintenance and functional restoration after injury [3, 4]. The existence of putative progenitor/stem cell populations in the adult kidney is, therefore, a subject of debate, and multiple hypotheses have been described in the literature.
Accordingly, several studies analyzed the effect of cell labeling by thymidine analogs to trace the fate of proliferating cells during kidney homeostasis and injury. By definition, stem cells are undifferentiated and slow cycling cells, as opposed to the bulk of cycling cells that are transit amplifying and rapidly cycling cells. For example, when kidney epithelial cells in the S3 segment of proximal tubules were chased for 14 days after BrdU administration (a marker of DNA duplication), 40% of these cells were in cell cycle arrest in G1 phase and were cyclin D1 positive, although they were rapidly recruited after injury [5]. Sequential labeling with thymidine analogs after acute ischemia-reperfusion injury, revealed that intrinsic repair occurred mainly by self-duplication of surviving epithelial cells, and not by a population of slow cycling cells, for example, resident progenitor/stem cells [6]. Additionally, when parietal epithelial cells (PECs) were irreversibly labeled before ischemia-reperfusion injury, the number of labeled cells remained constant, but when PECs were labeled during injury, that number increased, suggesting that any surviving tubular cell contributed to kidney regeneration [7].
Regeneration of other adult cells, such as hepatocytes and pancreatic islet cells, may also involve self-duplication of differentiated cells instead of stem cell differentiation [8, 9].
However, cell labeling by thymidine analogs may have a time-dependent effect. Thus, during 2-week chase period after BrdU labeling, a population of label-retaining cells (LRC; slow-cycling cells), defined as possible candidates of kidney-specific stem cells, can undergo cell division during renal ischemia recovery and replace injured cells [10]. Furthermore, 2-month chase period after BrdU labeling disclosed LRCs in the interstitium and papilla [11, 12]. Notably, kidney may harbor distinct populations of LRCs that are regulated according to age, for example, in early and late neonatal periods, LRCs are found predominantly in the papilla and inner medulla, and during adult life, they are detected in the cortex, although the outer medulla seems to harbor LRCs during all stages [13]. Additionally, LRCs of the kidney papilla can migrate to the upper papilla and form a compartment of rapidly proliferating cells after ischemic injury in GFP-histone2B transgenic mice [14], yet activating SDF-1/CXCR4 signaling [15].
Similar controversies are also present in glomerular compartment regeneration. In podocyte injury models, extracted human CD24+CD133+PDX- progenitors contributed to kidney regeneration following adriamycin injection [16]. However, genetic labeling of these cells unraveled that their recruitment occurs mainly in juvenile mice [17] and only a small fraction of these cells is recruited to glomeruli in adult mice, even when glomerular hypertrophy is induced [18].
More recently, other lines of evidence support the notion that renin lineage cells may enhance glomerular regeneration by functioning as progenitors of podocytes in a model of podocyte depletion using anti-podocyte antibody [19], although these cells also exhibit limited regenerative potential in aging mice [20]. To provide further evidence that remission of glomerular disease in mice with adriamycin nephropathy is associated with the generation of novel podocytes, pharmacological approach using glycogen synthase kinases 3-α and 3-β inhibitor increases podocyte regeneration and promotes glomerular disease remission [21].
In line with those studies that investigated putative candidates of kidney-derived progenitor/stem cell population, our group set out experiments to identify that population in kidney during development, and document their cardinal properties of progenitor/stem cell population.
c-Kit Cell Population in the Kidneys: A Novel Population of Tissue Specific Progenitor/Stem Cells
The proto-oncogene c-Kit (CD117), encoded by a gene on human chromosome 4, is a 145-kD type III tyrosine kinase transmembrane receptor that is allelic with the white spotting locus (W) on mouse chromosome 5. c-Kit receptor belongs to the platelet-derived growth factor and macrophage-colony stimulating factor receptor subfamily [22]. The extracellular portion of c-Kit binds to its ligand, known as stem cell factor (SCF) located on human chromosome 12, and the intracellular domain contains the enzymatic kinase domain. c-Kit/SCF pathway is involved in transducing important signals in a variety of normal physiological and pathogenic processes related to cell survival and proliferation, migration, and differentiation [23]. That pathway is also crucial for the development of hematopoiesis, melanocytes, and primordial germ cells, yet is important in the development of many mammalian cells and organs [24].
Studies in Rodents
Embryonic and Neonatal Data
During embryonic mouse kidney development, c-Kit+ cells are detected in primordial structures, known as S-shaped bodies, a structure that gives rise to glomeruli, proximal tubules, Henle's loop, and distal tubules, and play also a critical role in nephron number and ureteric bud (UB) branching [25, 26]. Likewise, c-Kit expression was observed not only in hemangioblasts and mesenchyma metanephric, but also in UB-derived structures, such as epithelial cells of distal tubules, collecting ducts, ureter and bladder of mice [24]. These findings suggest that c-Kit can be a marker of a population with multipotentiality capacity, at least within the kidneys.
Recently, our group reported that c-Kit+ cells isolated from neonatal rat kidneys may represent a novel resident progenitor/stem cell population [27]. When c-Kit+ progenitor/stem cells were sorted out from rat neonatal kidneys, we found that these cells exhibited self-renewal capacity, clonogenicity, and multipotentiality in vitro, as shown in Figure 1. c-Kit+ progenitor/stem cells were expanded over 100 passages, formed nephrospheres when plated at clonal density in non-adherent conditions, differentiated into mesodermic (adipogenic, osteogenic, epithelial, and endothelial) and ectodermic (neuronal) progeny, and were maintained throughout adult life. Neonatal c-Kit+ nephrospheres were observed at higher frequency when compared to adult c-Kit+ nephrospheres (e.g., 2.5% vs. 0.4%). To note, those cells started to express angiotensin II type 1a receptor upon endothelial differentiation and released calcium from endoplasmic reticulum via IP3 receptors. They also exhibited distinct responses to endothelin, prostaglandin, and bradykinin when grew in endothelial medium. Furthermore, c-Kit+ stem cells were able to treat renal failure by multi-compartment engraftment, for example, tubular, vascular, and glomerular following acute ischemia-reperfusion injury [27].
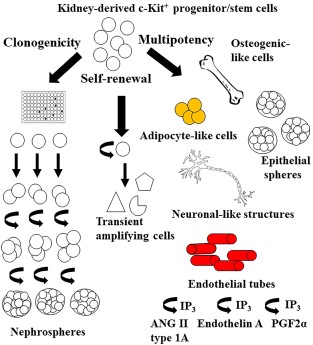
c-Kit+ cells exhibit progenitor/stem cell cardinal properties. c-Kit+ progenitor/stem cells are self-renewing, clonogenic, and multipotent. These cells can be expanded over 100 passages, form nephrospheres when plated at clonal density in non-adherent conditions, and differentiate into mesodermic (adipocytic, osteogenic, epithelial, and endothelial-like cells) and ectodermic (neuronal-like cells) progeny. c-Kit+ progenitor/stem cells started to express angiotensin II type 1a receptor upon endothelial differentiation and released calcium from endoplasmic reticulum via IP3 receptors. They also exhibited distinct responses to endothelin, prostaglandin, and bradykinin when grew in endothelial medium.
In a model of acute proteinuria induced by puromycin aminonucleoside (PAN), c-Kit+ progenitor/stem cells contributed to improve foot process effacement, not only by paracrine mechanisms, such as growth factors production and modulation of autophagy and mTOR pathway, but also by engrafting into tubule-interstitial compartment and differentiating into podocyte-like cells [28]. We observed that c-Kit+ progenitor/stem cell treatment activated autophagy, an important intracellular degradation system that maintains homeostasis by removing damaged protein and organelles. The number of autophagosomes and autophagolysosomes increased in a time-dependent manner in the c-Kit treated animals, as well as the expression of autophagy marker microtubule-associated protein light chain 3-II. Therefore, autophagy activation contributed to foot processes reorganization. We also found that autophagy activation was associated with mammalian target of rapamycin complex 1 (mTORC1) downregulation on Ser 2448, as demonstrated by others in the kidney [29, 30]. In addition, c-Kit treatment up regulated mTORC2-Rictor on Ser 2481, a rapamycin insensitive regulator of actin cytoskeleton [31], which potentially targeted ɑ-Actinin-4 in podocytes. Our results suggest that c-Kit+ progenitor/stem cell treatment can ultimately rescue podocyte disarrangement and accelerate foot processes effacement reversal [28].
Moreover, further studies are warranted to evaluate the underlying pathophysiological mechanisms of c-Kit+ progenitor/stem cell treatment on chronic kidney diseases (CKD).
Although less than 10% of the injected cells exhibited tissue engraftment in acute ischemia-reperfusion and PAN-induced proteinuria, these findings provide initial evidence that c-Kit based cell therapy is promising due to its differentiation capacity [27, 28]. Figure 2 is a representative cartoon of c-Kit progenitor/stem cell treatment and regenerative capacity in different models of acute kidney injury.
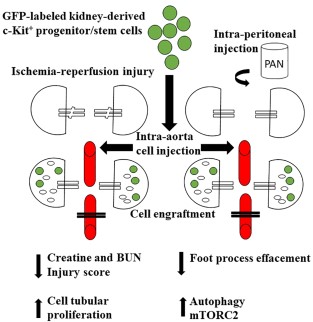
c-Kit+ progenitor/stem cells contribute to kidney regeneration following acute injury. c-Kit+ progenitor/stem cells can regenerate epithelial tubular cells following ischemia-reperfusion injury and accelerate foot processes effacement reversal in a model of acute proteinuria in rats. These cells engraft and differentiate into renal structures, such as tubules, vessels, and podocyte-like cells. Paracrine mechanisms could also account for kidney regeneration, either by stimulating proliferation of surviving cells or modulating autophagy and podocyte cytoskeleton rearrangement through mTOR-Raptor and- Rictor signaling, which ultimately lead to morphological and functional improvement. Abbreviations: BUN, blood urea nitrogen; mTORC2, mammalian target of rapamycin complex 2; PAN, puromycin aminonucleoside.
To investigate further KIT function, a mutation introduced by gene targeting at the W/Kit locus in mouse embryonic stem cells, specifically marked c-Kit expressing cells and their fate was followed during embryogenesis [24]. Of importance, melanoblasts, primordial germ cells and hematopoietic progenitor cells failed to survive in the absence of KIT, while endothelial, epithelial, and endocrine cells did not depend on KIT expression for their migration, proliferation or survival during embryogenesis. Therefore, we anticipate that KIT gene expression is only required for the normal postnatal development of kidney-derived c-Kit progenitor/stem cells described by our group.
Adult Data
The involvement of c-Kit+ progenitor/stem cells in adult kidney homeostasis, disease, and regeneration was also observed by others in rodent models, such as the juxta-glomerular c-Kit+ cell recruitment after 10 day-period treatment with low-salt and captopril (angiotensin-converting enzyme inhibitor) [32], and the shift of c-Kit+ cells from the papilla and medullary rays to the corticomedullar area following acute ischemia-reperfusion injury [33].
In line with these findings, comprehensive lineage tracing is essential to understand c-Kit cell fate during kidney development, homeostasis, and disease. Stem cell dynamics can be studied with single-clone resolution and the advent of multicolor reporter alleles [34]. This approach enables examination of the individual behavior of multiple stem cells in a single niche. To gain insights into the division dynamics of resident stem cells of intestinal crypts, studies in multicolor Cre-reporter mice unveiled that stem cells double their numbers each day and stochastically adopt stem or transient amplifying fates, and do not exhibit hierarchical or asymmetric division [3].
More recently, continuous tubulogenesis in kidney adult mice was documented to occur by fate-restricted precursors functioning as unipotent progenitors when single clones were analyzed in multicolor mice [35]. Conversely, multiple adult nephron progenitor/stem cells that reside throughout the zebrafish kidney contribute to a single nephron [36]. Likewise, a subpopulation of label retaining cells localized in the upper papilla within the kidneys possesses the capacity to proliferate and differentiate into distinct tubular segments (proximal and distal tubules and Henle's loop) when a severe injury was established [37]. These results suggest that different compartments of the kidney may have different progenitor cell pools. Other organs, such as pancreas, may also harbor multipotent precursors [38]. To determine whether kidney c-Kit+ cells represent a unipotent progenitor population or a multipotent progenitor population in vivo, further lineage tracing analyses are required to clarify that question.
Studies in Humans
Embryonic and Fetal Data
In humans, embryonic stem cells also express c-Kit receptor [39] and in fetal kidney, c-Kit+ cells are found at moderate levels in epithelial cells of the proximal tubule and Henle's loop, at weak and focal levels in distal tubules, and were negative in renal corpuscules and collecting tubules [40]. Conversely, other authors reported the absence of c-Kit cells in human fetal kidneys [41]. c-Kit+ cells represent 1% of human amniotic fluid cells and exhibit stem cells properties, such as clonogenicity and multipotentiality [42]. Of importance, considering that human amniotic fluid harbor kidney epithelial tubular cells that were detached from embryonic and fetal kidneys and that these cells repair mice kidney after ischemia-reperfusion injury [43], we believe that these results should be considered as supporting information of our results.
Therefore, the evaluation of c-Kit expression at different time points of human embryonic and fetal nephrogenesis is lacking in the literature and the role of c-Kit cells during kidney development requires further investigation.
Adult Data
c-Kit expression is also maintained in human adult kidney and is expressed at moderate levels in the cytoplasm of proximal and distal tubules [40]. However, others reported absence of c-Kit+ cells in adult kidneys [44] kidneys. This controversial data can be attributed to different techniques of c-Kit detection using paraffin-embedded samples.
c-Kit label using rabbit polyclonal antibody (DAKO, Glostrup, Denmark) was verified in both cortex and medulla tubules in healthy individuals and deceased donors with acute tubular necrosis (Fig. 3).
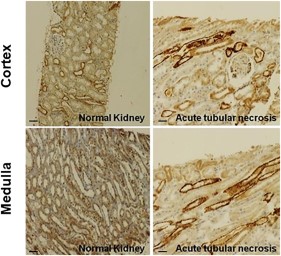
c-Kit cell immunochemistry in human kidneys. c-Kit cells are found in both cortex and medulla epithelial tubular cells from kidney biopsies of healthy individuals and deceased donors with acute tubular necrosis. Scale bars represent 50 μm for immunohistochemistry images.
Based on cross-species conservation, we speculate if human adult and fetal kidneys harbor a population of c-Kit+ progenitor/stem cells that exhibit clonogenicity, self-renewal capacity, multipotentiality, and regenerative potential. The results obtained from this approach will set the basis for establishing further investigation involving the therapeutic potential of c-Kit+ cells for treatment of kidney disease in preclinical and clinical studies.
Furthermore, we are currently working on c-Kit cell isolation from human kidney biopsies. We have already performed 12 flow cytometry analyses of eight kidney deceased donors (all male, mean age of 20 ± 15.6 years-old, ranging from 2 to 46 years-old, and non-cardiovascular cause of brain death in 70% of the individuals). In Figure 4A, a human kidney biopsy was obtained from a deceased donor, 36-year, with brain trauma. c-Kit cells were sorted out by flow cytometry and successfully expanded. c-Kit cells were found to be positive in 3.6% ± 3% of the kidney cells before sorting in human kidney biopsies obtained from deceased donors (Fig. 4B). The positive fraction for c-Kit cells that were sorted out exhibited 92% ± 5.8% positivity for c-Kit while the negative fraction exhibited negativity for c-Kit in 98.3% ± 1.6% of the cells (Fig. 4C). By qPCR, positivity for c-Kit was also higher in the c-Kit positive fraction (Fig. 4D).
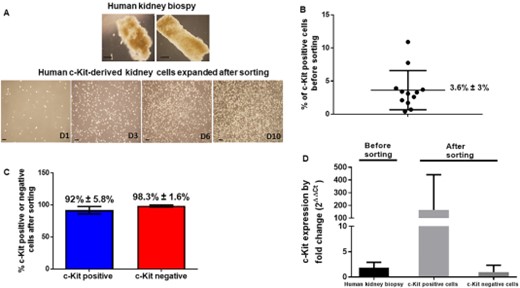
c-Kit cells are found in human kidneys. (A): A human kidney biopsy was obtained from a male deceased donor, 36-year, with brain trauma. c-Kit cells were sorted out by flow cytometry and successfully expanded for several days (D). (B): c-Kit cells were found to be positive in 3.6% ± 3% of the kidney cells before sorting in human kidney biopsies obtained from deceased donors (n = 12 analyses in eight human kidney donors). (C): The positive fraction for c-Kit cells that were sorted out exhibited 92% ± 5.8% positivity for c-Kit while the negative fraction exhibited negativity for c-Kit in 98.3% ± 1.6% of the cells. (D) By qPCR, positivity for c-Kit was also higher in the c-Kit positive fraction. Scale bars represent 20 μm for (A) images.
Of importance, understanding KIT gene regulation will provide insightful evidence of progenitor/stem c-Kit population within the kidneys. KIT mutation or activation is a major pathogenic event in certain tumors. Pathological activation of KIT through gain-function mutations leads to neoplasia in different systems: mast cells/myeloid cells (mastocytosis/acute myeloid leukaemia), germ cells (seminoma), and Cajal cells (gastrointestinal stromal tumors) [45]. In renal tumors, most conventional renal cell carcinomas (RCC) are c-Kit negative, although a subset of clear cell, papillary type and chromophobe RCCs, all oncocytomas, and most mesoblastic nephromas are c-Kit positive [40]. Notably, c-Kit localization in chromophobe RCC is not homogenous and can be found in cytoplasmic, membranous and nuclear compartments [46].
In the paediatric Wilms tumor (nephroblastoma), c-Kit expression is very rare (0%–4%), but when that mutation is detected, relapse can occur at a shorter time [40, 47].
Of importance, KIT mutations do not correlated to KIT copy number or CD117 expression in different neoplasias [48, 49].
Dysregulation of c-Kit expression may be attributed to DAB2IP, a novel Ras-GTPase activating protein frequently found in many cancer types and associated with cancer stem cells properties [50]. SCF induces activation of phosphatidylinositol (PI) 3-kinase-Akt and subsequent phosphorylation of Bad, a pro-apoptotic molecule, on Ser112 and Ser136 [51]. DAB2IP can suppress c-Kit gene expression and c-Kit-PI3K-Akt-mTOR signaling pathway that increases c-myc protein, which leads to activate ZEB1 gene expression and therefore to cancer stem cell phenotypes [50]. Likewise, DAB2IP knockout (KO) mice exhibit elevated expression of ZEB1 and CD117 in the prostate basal cell population.
Whether those signaling pathways are involved during development or adult kidneys or during homeostasis or disease, critical analyses are further required.
In benign conditions, such as piebaldism, an autosomal dominant disorder characterized by congenital patches of white skin and hair that lack melanocytes, the phenotypic severity of the disease correlates with the site and the type on the KIT gene [52]. A similar disorder of mouse, dominant white spotting (W) results from mutations of the c-Kit proto-oncogene and may serve as a disease model of human piebaldism.
c-Kit+ Cell Population in Other Organs
c-Kit receptor expression is also constitutionally expressed in differentiated cells that do not exhibit stem cell properties, such as mast cells, germ cells, melanocytes, gastrointestinal Cajal cells, fetal endothelial cells, and epithelial cells, including breast ductal cells, oesophageal and sweat glands, parotid, some cells of skin adnexa, and neurons of cerebellum, hippocampus, and spinal dorsal horn [44, 45].
However, c-Kit+ cells are reported as a population of progenitor/stem cells in many organs and tissues, as documented in bone-fide studies. In these studies, similarly to our findings, c-Kit+ cells differentiated into endothelial, epithelial, and neuronal-like cells.
In bone marrow, c-Kit signaling is involved in cellular adhesion and is essential for long-term maintenance and expansion of hematopoietic progenitor/stem cells [53]. In liver, human c-Kit+ cells are occasionally found in the biliary duct and in close proximity to biliary epithelial cells and, when sorted out, these cells can form epithelial and endothelial colonies in vitro [54]. In heart, c-Kit+ cells exhibit stem cells properties in vitro [55] and promote cardiac repair after myocardium infarction [56]. To note, c-Kit+ cells generate cardiac endothelial cells in vivo as demonstrated by lineage tracing in mice [57]. In lungs, human c-Kit+ cells participate in tissue homeostasis and regeneration, giving rise to bronchioles, alveoli, and pulmonary vessels [58]. Likewise, c-Kit+ cells are detected in murine epithelia of sub-mandibular gland during embryonic and post-natal periods, and are involved in the growth of ductal and acinar cells during development [59]. In olfactory neuroepithelial injury, c-Kit+ progenitor cell population, that resides among the globose basal cell populations, is activated to reconstitute the neuronal population [60].
Although kidney-derived c-Kit+ cells share some similarities with c-Kit+ cells from other organs, for example, the capacity to differentiate into endothelial and epithelial progeny, it has not been elucidated whether these cells come from the same unipotent precursor or from multipotent precursors, yet c-Kit+ cells are detected in tissues originated from all three germ layers.
In all these studies, staining for mast cells markers, such as CD14 and mast cell tryptase, are crucial for differentiation from mast cells and for understanding the biological properties of c-Kit+ cells [61].
Future Directions in Kidney Regeneration: Novel Therapies and Translational Aspects of c-Kit+ Progenitor/Stem Cells
CKD is a worldwide public health problem that affects millions of people from all age, racial, and ethnic groups. CKD is incurable, requiring renal replacement therapy, that is, dialysis or preferably renal transplantation. However, the critical shortage of available organs for transplantation continues to severely limit this option, underlying the importance of novel therapeutic regimens, such as stem cell therapy [62].
Current approaches include the understanding of kidney disease and development using genome and tissue engineering in human pluripotent stem cells (hPSCs) [63, 64]. hPSCs, including human embryonic stem cells and human induced pluripotent stem cells, can be differentiated toward kidney organoids using small molecules and soluble factors mimicking the signals triggering kidney development in vivo. These hPSCs can be directed to intermediate mesoderm, the embryonic lineage giving rise to both UB and metanephric mesenchyme progenitors.
In addition, genome engineering can be efficiently achieved in hPSCs using CRISPR/Cas9, which is composed by a nuclease Cas9 and a small guide (sg) RNA determining binding specificity trough base pairing to its genomic target [63]. This approach allows introducing any modification useful for disease modeling and developmental studies in hPSCs including, single and multiple KO or knockin mutations; creation of doxycycline-, CRE- or FLP inducible KO lines; reporters and lineage tracer lines; and performing genetic screenings using Cas9 and pooled sgRNA libraries.
Kidney organoids-derived form nephron progenitors and hPSCs can not only be tested for disease modeling and subsequent drug screening and toxicity testing, but also contribute to bioengineered kidney design and kidney regeneration [64–66]. In line with these perspectives, developing strategies to recreate the niche of nephron progenitor cells, such as 3D culture, and understating the signaling pathways will allow those cells to maintain genomic stability, molecular homogeneity, clonogenicity, differentiation potential, self-renewal capacity, and nephrogenic potential [66, 67].
To note, identification of progenitor/stem cell populations in mammalian tissues is also important for therapeutic applications, and for understanding developmental processes and tissue homeostasis. Furthermore, pursuing progenitor/stem cell-based kidney regenerative strategies is also a key step toward the development of a bioengineered transplantable kidney [68].
Sorting out c-Kit+ progenitor/stem cells from human adult kidneys and growing these cells as adherent cells or nephrospheres are promising therapeutic strategies. Although human fetal kidneys may harbor a c-Kit+ progenitor/stem cell population, ethical aspects require further discussion. Of importance, some considerations of preclinical studies using kidney-derived c-Kit+ stem/progenitor cells need to be highlighted, as proposed by others [69]:
Selection of the animal model (species, immunocompetent or immunocompromised, diseased or healthy animals, acute and chronic models, and feasibility of cell injection);
Design considerations (the model should mimic the intended clinical application as closely as possible and cell product manufacturing should be comparable to the intended clinical methods);
Proof-of-concept studies (endpoints should include survival, functional and morphological parameters, cell engraftment, durability of the response);
Biodistribution studies (analyses of chronic and acute time points and dose/route of administration, number of injections, which should mimic intended clinical use) and
Safety studies (dose raging, short- and long-term toxicity, and tumorogenicity).
Conclusion
Current kidney regeneration approaches include the understanding of kidney disease and development using genome and tissue engineering in hPSCs. These cells can be tested for disease modeling and ultimately be differentiated toward kidney organoids mimicking the signals triggering kidney development in vivo, and contribute to bioengineered kidney design.
c-Kit+ cells may represent a novel population of kidney-derived progenitor/stem cells found during kidney development that are also maintained throughout adult life. These cells contribute to kidney regeneration following injury. Of importance, the identification of kidney-specific population of progenitor/stem cells will abbreviate the need of direct differentiation into renal structures.
However, studies with multicolor mice and lineage tracing will unveil c-Kit+ cells dynamics during kidney development, homeostasis, and disease. Therefore, these studies possess important biological and therapeutic applications. Based on cross-species conservation, further studies in development and adult human kidneys will also establish the basis for treatment of kidney disease in preclinical and clinical studies.
Acknowledgments
E.B.R. is supported by Brazilian grants from Conselho Nacional em Pesquisa e Desenvolvimento (CNPq, 456959/2013-0), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 13/19560-6), and European Foundation for the Study of Diabetes (EFSD).
Author Contributions
S.A.G. gave substantial contributions to the conception, acquisition, analysis and interpretation of data for the work; J.M.H. reviewed the draft critically for important intellectual content; E.B.R. gave substantial contributions to the conception, acquisition, analysis and interpretation of data for the work, drafted the work, and gave final approval of the version to be published.
Disclosure of Potential Conflicts of Interest
J.H. declared advisory role with Vestion, Inc., ownership interest with Vestion, Inc., Biscayne Pharmaceuticals, Heart Genomics, Longeveron LLC and research funding from NHLBI. The other authors indicated no potential conflicts of interest.


