-
PDF
- Split View
-
Views
-
Cite
Cite
Sishi Lin, Ji Zhang, Xiaohan You, Bo Chen, Yan Liang, Yin Zhou, Xiaokai Ding, Yinqiu Lv, Huidi Zhang, Bofeng Su, Yongheng Bai, Chaosheng Chen, Efficacy and safety of belimumab in patients with lupus nephritis: a real-world retrospective observational study, Rheumatology, Volume 64, Issue 2, February 2025, Pages 614–622, https://doi.org/10.1093/rheumatology/kead707
Close - Share Icon Share
Abstract
To evaluate the differences in efficacy and safety between lupus nephritis (LN) patients who received belimumab plus standard therapy and those who received only standard therapy in real world practice.
Patients diagnosed with LN at the First Affiliated Hospital of Wenzhou Medical University from November 2012 to July 2023 were identified, and eligible cases were divided into two groups according to whether they received additional treatment with belimumab during the course of the disease.
A total of 1169 LN patients were identified from our follow-up database. In total, 112 patients receiving add-on treatment with belimumab (BLM group) and 112 control patients matched for relevant baseline characteristics were enrolled in this study. The median duration of treatment with belimumab was 13.82 [7.24, 20.29] months. Compared with the control group, the BLM group had more significant improvement in disease activity indicators such as serum albumin and complement levels, significantly lower B-cell count, immunoglobulin, and earlier first attainment of renal remission, but there was no significant improvement in renal function and kidney-related events or death during the 2-year follow-up period. In the BLM group, the treatment effect of belimumab was more prominent in patients with lower levels of proteinuria. The safety profile of belimumab treatment was favorable, with a lower incidence of respiratory tract infection in the BLM group than in the control group during the follow-up period (P = 0.015).
This real-world study revealed that add-on treatment with belimumab provided better disease remission, and the therapeutic effect was more significant in patients with lower proteinuria levels. In addition, it had a favorable safety profile and reduced the risk of respiratory tract infection.
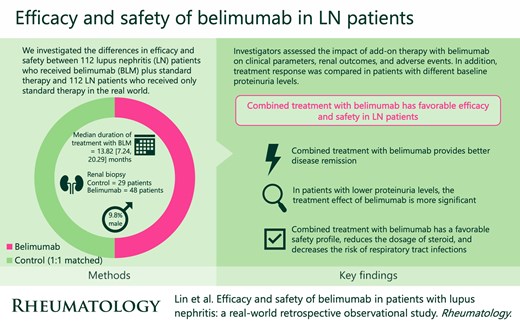
Combined treatment with belimumab provides better disease remission.
In patients with lower proteinuria levels, the treatment effect of belimumab is more significant.
Combined treatment with belimumab has a favorable safety profile, reduces the dosage of steroid, and decreases the risk of respiratory tract infections.
Introduction
Lupus nephritis (LN) is one of the most common and severe organ damage in systemic lupus erythematosus (SLE) and is also an important cause of disease progression and death in SLE patients. At present, the standard therapy for LN is induction therapy with steroid combined with immunosuppressive agents such as cyclophosphamide (CTX) or mycophenolate mofetil (MMF), as well as maintenance immunosuppressive therapy after clinical remission is achieved [1–5]. Studies have shown that despite receiving standard treatment, 10–20% of LN patients progress to end-stage renal disease (ESRD) within 5 years of diagnosis [6].
Belimumab is a recombinant human IgG1 λ monoclonal antibody that specifically inhibits B-lymphocyte stimulator (BLys), blocking the binding of BLys to its receptor on B cells, inhibiting the growth of B cells and decreasing the differentiation of B cells to plasma cells that produce immunoglobulin. The BLISS-LN phase 3 clinical trial demonstrated that the addition of belimumab to standard therapy improved the renal response of patients, with no significant difference in the incidence of adverse events [7]. A multicentre, real-world study, BeRLiSS-LN, showed that LN patients treated with belimumab add-on therapy led to durable renal response [8]. However, in the real world, the superiority and safety of add-on therapy with belimumab on the prognosis of patients compared with standard therapy has not been clearly confirmed.
The purpose of this study is to evaluate the differences in efficacy and safety between LN patients who received belimumab plus standard therapy and LN patients who received only standard therapy in the real world.
Materials and methods
Study population
A total of 1169 SLE patients were clinically diagnosed as LN in the First Affiliated Hospital of Wenzhou Medical University from November 2012 to July 2023, of which 267 patients underwent renal biopsy. The diagnostic of SLE was based on EULAR/ACR 2019 classification criteria [9], and the histological classification of LN was based on the ISN/RPS 2018 classification criteria [10]. Exclusion criteria: (i) missing clinical data or follow-up time <3 months; (ii) age <18 years at baseline; (iii) received renal replacement therapy or kidney transplant before baseline; and (iv) pregnancy or anti-malignant therapy at baseline and during follow-up. Finally, 917 patients were included, all of whom received standard therapy after being diagnosed with LN. Patients were divided into the belimumab-treated group (BLM group) and the control group according to whether they received additional treatment with belimumab during the course of the disease. All patients receiving add-on therapy with belimumab at our hospital were administered by intravenous infusion (ivgtt) with a standard dosing regimen of 10 mg/kg every 2 weeks for the first three doses and every 4 weeks thereafter. The baseline time for the BLM group was defined as the time of first belimumab treatment. Define the median time from first diagnosis of LN to initial treatment with belimumab for all patients in the BLM group as ‘A’ months, and then use the time point at ‘A’ months after the first diagnosis as the baseline time for the control group. According to the baseline age, gender, 24 h proteinuria, estimated glomerular filtration rate (eGFR), anti-dsDNA, complement 3 (C3), complement 4 (C4), daily dose of steroid and induction therapy regimen (CTX/MMF), 1:1 matched to form the control group (Fig. 1). In addition, stratified by baseline proteinuria levels, the response of patients with different proteinuria levels to add-on therapy with BLM was compared.
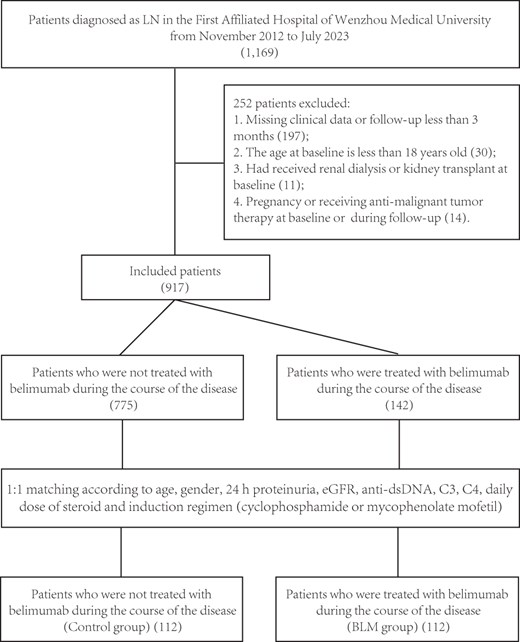
The selection process for eligible patients. BLM: belimumab; C3: complement factor 3; C4: complement factor 4; dsDNA: double-stranded DNA; eGFR: estimated glomerular filtration rate; LN: lupus nephritis
Data collection
All data were collected from the scientific research data platform of the First Affiliated Hospital of Wenzhou Medical University, and patient-identifiable information was masked. The Medical Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University approved the study. Data were collected from patients at the first diagnosis of LN, including: age, gender, body mass index (BMI) [weight (kg)/height (kg)2], blood pressure, mean arterial pressure (MBP) [diastolic blood pressure + 1/3 (systolic blood pressure—diastolic blood pressure)] and SLE disease activity index (SLEDAI) [11]. Baseline and follow-up data were collected, including: age, gender, laboratory tests including serum creatinine, estimated glomerular filtration rate (eGFR) (calculated by the 2021 CKD–EPI formula [12]), serum albumin, white blood cells, haemoglobin, platelets, lymphocytes count, T-cell count, B-cell count, NK-cell count, immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), C3, C4, serum antinuclear antibody (ANA), anti-dsDNA, anti-Sm, anti-u1RNP, anti-SSA, anti-SSB antibody, lupus anticoagulant ratio, 24 h proteinuria, urine white blood cells (WBC), urine red blood cells (RBC), urinary protein/creatinine ratio (uPCR), and therapeutic agents received at each hospitalization or follow-up visit, including hydroxychloroquine, renin-angiotensin-aldosterone system inhibitors (RAASI), steroid, CTX, MMF, azathioprine (AZA), calcineurin inhibitors (CNI) and leflunomide (LEF).
Biopsy specimens were embedded in paraffin and sectioned, and stained with HE, PAS, Masson and direct immunofluorescence. All the biopsy specimens were processed by standard light and immunofluorescence, and 80–90% were examined by electron microscopy. Two pathologists each reviewed and reclassified the renal pathology data according to the ISN/RPS 2018 classification criteria without knowing of the patient’s clinical data, and if there was a discrepancy between the two pathologists, biopsies were reviewed until consensus was reached.
End points and assessments
The complete renal response (CRR) was defined as a ratio of urinary protein to creatinine of 0.5 or less, an eGFR that was no worse than 10% below the baseline value or at least 90 mL/min per 1.73 m2. The partial renal response (PRR) was defined as a ratio of urinary protein to creatinine of 0.7 or less, an eGFR that was no worse than 20% below the baseline value or at least 60 mL/min per 1.73 m2 [7]. Renal relapse was defined as follows: (i) if baseline proteinuria <0.5 g/24 h, increase to ≥1 g/24 h; if baseline proteinuria was 0.5–1.0 g/24 h, increase to ≥2 g/24 h; and if the baseline proteinuria >1.0 g/24 h, double; (ii) if baseline serum creatinine <2.0 mg/dl, increase ≥0.2 mg/dl; and if baseline serum creatinine ≥2.0 mg/dl, increase ≥0.4 mg/dl [13]. Renal end point was defined as kidney-related events [doubling of serum creatinine, decrease in eGFR of >30%, increased proteinuria, end-stage renal disease (ESRD) or need for dialysis and/or kidney transplantation, or kidney disease-related treatment failure] or death [7]. Adverse events at each follow-up visit were assessed, including: respiratory tract infection, urinary tract infection, digestive tract infection, leukopenia, as well as mental and neurological adverse events (such as depression, suicidality, headache, insomnia, and sleep disorders).
Statistical analysis
Continuous variables with normal distribution were described by means ± standard deviation, and t test was used for comparison between groups. The continuous variables with non-normal distribution were described by median [quartile], and Wilcoxon rank sum test was used for comparison between groups. The classified variables were described by the number of cases (%), and the comparison between groups was described by χ2 or Fisher exact test. Multiple comparisons were corrected using the BH method. Kaplan–Meier curve (log-ranch test) was performed to demonstrate the disease end point. Linear mixed models were built to display the different change of variables over time. A two-sided P-value of < 0.05 was considered statistically significant. The R software (version 4.1.2) [14] and R packages (such as ggplot2 [15], Tableone [16]) were used to perform the analyses and plots.
Results
Baseline characteristics
A total of 112 patients receiving add-on treatment with belimumab and 112 control group patients matched for relevant baseline characteristics were enrolled in this study. Patients treated with belimumab in this study included 14 (12.5%) patients who were first diagnosed with lupus nephritis and treated with belimumab, 52 (46.4%) patients who had been diagnosed with lupus nephritis and were treated with belimumab because of relapse of the disease, 19 (17.0%) patients with refractory lupus nephritis and an additional 27 (24.1%) patients who were on maintenance therapy for lupus nephritis. The main reason for receiving belimumab was active SLE/LN with/without previous treatment (84.8%), followed by prevention of relapse (8.9%), and some patients received belimumab aimed to decrease the use of steroids or because of adverse events of other medications (6.3%). The median course of LN at the beginning of belimumab treatment was 9.41 [0.00, 55.74] months in the BLM group, and the median duration of treatment with belimumab was 13.82 [7.24, 20.29] months, with 12 of the patients receiving add-on treatment with belimumab having a follow-up of >24 months (Supplementary Table 1, Supplementary Fig. 1, available at Rheumatology online). Twenty-nine patients in the control group and forty-eight patients in the BLM group underwent renal biopsy. The most common histological classification in the BLM group was class III/IV+V (52.1%), whereas class IV predominated in the control group (51.7%) (Table 1). Demographic characteristics such as age and gender, disease activity such as C3, C4, renal function such as eGFR, as well as baseline treatment regimen such as daily dose of steroid, induction therapy regimen, and other baseline characteristics were similar between BLM group and control group. Compared with control group, patients in BLM group at baseline had relatively more severe disease, as evidenced by higher levels of proteinuria (P = 0.059) and lower levels of serum albumin (P < 0.001). At baseline, the proportion of CNI used in BLM group was higher (P < 0.001), and there were no significant differences in other major therapeutic agents. (Table 1, Supplementary Table 2, available at Rheumatology online).
Comparison of clinical parameters and drug between patients treated with or without belimumab at baseline
| Factor . | Standard therapy . | Belimumab . | P value . |
|---|---|---|---|
| Number of patients | 112 | 112 | |
| Laboratory examination | |||
| Serum creatinine, umol/L | 64.50 [56.38, 79.19] | 66.50 [55.00, 89.50] | 0.694 |
| eGFR | 107.64 [82.94, 120.57] | 108.58 [79.63, 120.32] | 0.700 |
| ≥90 (%) | 80 (71.4) | 76 (67.9) | 0.663 |
| ≥60 (%) | 94 (83.9) | 88 (78.6) | 0.392 |
| Serum albumin, g/L | 38.83 [34.77, 41.42] | 32.50 [28.70, 36.54] | <0.001 |
| C3, g/L | 0.93 (0.23) | 0.91 (0.25) | 0.590 |
| C4, g/L | 0.16 [0.13, 0.23] | 0.18 [0.11, 0.25] | 0.681 |
| IgA, g/L | 2.59 [1.96, 3.25] | 2.32 [1.75, 3.08] | 0.147 |
| IgG, g/L | 10.95 [8.17, 13.70] | 10.60 [7.68, 12.98] | 0.467 |
| IgM, g/L | 0.70 [0.51, 1.07] | 0.68 [0.45, 1.06] | 0.404 |
| Lupus anticoagulant | 1.58 [1.11, 2.40] | 1.08 [0.76, 1.68] | <0.001 |
| Proteinuria, g/24h | 0.78 [0.26, 2.45] | 1.17 [0.41, 2.58] | 0.059 |
| Urine WBC, WBC/ul | 12.00 [6.22, 33.11] | 13.25 [6.00, 42.25] | 0.906 |
| Urine RBC, RBC/ul | 14.75 [5.00, 52.50] | 12.00 [4.25, 53.75] | 0.443 |
| Positive ANA | 111 (99.1) | 107 (95.5) | 0.212 |
| Positive anti-dsDNA | 65 (58.0) | 58 (51.8) | 0.421 |
| Positive anti-Sm | 26 (23.2) | 31 (27.7) | 0.540 |
| Positive anti-ssA | 63 (56.2) | 62 (55.4) | 1.000 |
| Positive anti-ssB | 13 (11.6) | 18 (16.1) | 0.439 |
| Pathology | |||
| ISN/RPS class | |||
| III | 3 (10.3) | 5 (10.4) | 1 |
| IV | 15 (51.7) | 11 (22.9) | 0.013 |
| III/IV+V | 11 (37.9) | 25 (52.1) | 0.249 |
| V | 0 (0.0) | 7 (14.6) | 0.041 |
| Crescent (%) | 13.33 [0.00, 28.57] | 5.50 [0.00, 14.56] | 0.126 |
| AI | 7.00 [4.00, 9.00] | 5.00 [2.00, 10.00] | 0.150 |
| CI | 2.00 [1.00, 5.00] | 2.00 [1.00, 4.00] | 0.923 |
| Therapy | |||
| ATM (%) | 75 (67.0) | 85 (75.9) | 0.183 |
| AZA (%) | 1 (0.9) | 1 (0.9) | 1.000 |
| CNI (%) | 16 (14.3) | 54 (48.2) | <0.001 |
| CTX (%) | 6 (5.4) | 13 (11.6) | 0.149 |
| LEF (%) | 10 (8.9) | 8 (7.1) | 0.807 |
| MMF (%) | 54 (48.2) | 51 (45.5) | 0.789 |
| RAASi (%) | 43 (38.4) | 57 (50.9) | 0.080 |
| Steroid (%) | 107 (95.5) | 108 (96.4) | 1.000 |
| Dosage of steroid, mg/day | 15.00 [10.00, 20.00] | 15.00 [7.50, 25.62] | 0.726 |
| Major treatment regimen | |||
| Steroid only (%) | 32 (28.6) | 11 (9.8) | <0.001 |
| Steroid + CTX (%) | 6 (5.4) | 13 (11.6) | 0.149 |
| Steroid + MMF (%) | 50 (44.6) | 45 (40.2) | 0.589 |
| Factor . | Standard therapy . | Belimumab . | P value . |
|---|---|---|---|
| Number of patients | 112 | 112 | |
| Laboratory examination | |||
| Serum creatinine, umol/L | 64.50 [56.38, 79.19] | 66.50 [55.00, 89.50] | 0.694 |
| eGFR | 107.64 [82.94, 120.57] | 108.58 [79.63, 120.32] | 0.700 |
| ≥90 (%) | 80 (71.4) | 76 (67.9) | 0.663 |
| ≥60 (%) | 94 (83.9) | 88 (78.6) | 0.392 |
| Serum albumin, g/L | 38.83 [34.77, 41.42] | 32.50 [28.70, 36.54] | <0.001 |
| C3, g/L | 0.93 (0.23) | 0.91 (0.25) | 0.590 |
| C4, g/L | 0.16 [0.13, 0.23] | 0.18 [0.11, 0.25] | 0.681 |
| IgA, g/L | 2.59 [1.96, 3.25] | 2.32 [1.75, 3.08] | 0.147 |
| IgG, g/L | 10.95 [8.17, 13.70] | 10.60 [7.68, 12.98] | 0.467 |
| IgM, g/L | 0.70 [0.51, 1.07] | 0.68 [0.45, 1.06] | 0.404 |
| Lupus anticoagulant | 1.58 [1.11, 2.40] | 1.08 [0.76, 1.68] | <0.001 |
| Proteinuria, g/24h | 0.78 [0.26, 2.45] | 1.17 [0.41, 2.58] | 0.059 |
| Urine WBC, WBC/ul | 12.00 [6.22, 33.11] | 13.25 [6.00, 42.25] | 0.906 |
| Urine RBC, RBC/ul | 14.75 [5.00, 52.50] | 12.00 [4.25, 53.75] | 0.443 |
| Positive ANA | 111 (99.1) | 107 (95.5) | 0.212 |
| Positive anti-dsDNA | 65 (58.0) | 58 (51.8) | 0.421 |
| Positive anti-Sm | 26 (23.2) | 31 (27.7) | 0.540 |
| Positive anti-ssA | 63 (56.2) | 62 (55.4) | 1.000 |
| Positive anti-ssB | 13 (11.6) | 18 (16.1) | 0.439 |
| Pathology | |||
| ISN/RPS class | |||
| III | 3 (10.3) | 5 (10.4) | 1 |
| IV | 15 (51.7) | 11 (22.9) | 0.013 |
| III/IV+V | 11 (37.9) | 25 (52.1) | 0.249 |
| V | 0 (0.0) | 7 (14.6) | 0.041 |
| Crescent (%) | 13.33 [0.00, 28.57] | 5.50 [0.00, 14.56] | 0.126 |
| AI | 7.00 [4.00, 9.00] | 5.00 [2.00, 10.00] | 0.150 |
| CI | 2.00 [1.00, 5.00] | 2.00 [1.00, 4.00] | 0.923 |
| Therapy | |||
| ATM (%) | 75 (67.0) | 85 (75.9) | 0.183 |
| AZA (%) | 1 (0.9) | 1 (0.9) | 1.000 |
| CNI (%) | 16 (14.3) | 54 (48.2) | <0.001 |
| CTX (%) | 6 (5.4) | 13 (11.6) | 0.149 |
| LEF (%) | 10 (8.9) | 8 (7.1) | 0.807 |
| MMF (%) | 54 (48.2) | 51 (45.5) | 0.789 |
| RAASi (%) | 43 (38.4) | 57 (50.9) | 0.080 |
| Steroid (%) | 107 (95.5) | 108 (96.4) | 1.000 |
| Dosage of steroid, mg/day | 15.00 [10.00, 20.00] | 15.00 [7.50, 25.62] | 0.726 |
| Major treatment regimen | |||
| Steroid only (%) | 32 (28.6) | 11 (9.8) | <0.001 |
| Steroid + CTX (%) | 6 (5.4) | 13 (11.6) | 0.149 |
| Steroid + MMF (%) | 50 (44.6) | 45 (40.2) | 0.589 |
Values for numeric data are presented as mean (standard deviation) or median [interquartile range]; for categorical data, as count (percent).
AI: activity NIH index; ANA: antinuclear antibodies; ATM: hydroxychloroquine; AZA: azathioprine; C3: complement factor 3; C4: complement factor 4; CI: chronicity NIH index; CNI: calcineurin inhibitor; CTX: cyclophosphamide; dsDNA: double-stranded DNA; eGFR: estimated glomerular filtration rate; IgA: Immunoglobulin A; IgG: Immunoglobulin G; IgM: Immunoglobulin M; ISN/RPS international society of nephrology/renal pathology society; LEF: leflunomide; MMF: mycophenolate mofetil; RAASi: renin-angiotensin-aldosterone system inhibitors; RBC: red blood cells; ssA: Sjögren's-syndrome-related antigen A; ssB: Sjögren's-syndrome-related antigen B; WBC: white blood cells.
Comparison of clinical parameters and drug between patients treated with or without belimumab at baseline
| Factor . | Standard therapy . | Belimumab . | P value . |
|---|---|---|---|
| Number of patients | 112 | 112 | |
| Laboratory examination | |||
| Serum creatinine, umol/L | 64.50 [56.38, 79.19] | 66.50 [55.00, 89.50] | 0.694 |
| eGFR | 107.64 [82.94, 120.57] | 108.58 [79.63, 120.32] | 0.700 |
| ≥90 (%) | 80 (71.4) | 76 (67.9) | 0.663 |
| ≥60 (%) | 94 (83.9) | 88 (78.6) | 0.392 |
| Serum albumin, g/L | 38.83 [34.77, 41.42] | 32.50 [28.70, 36.54] | <0.001 |
| C3, g/L | 0.93 (0.23) | 0.91 (0.25) | 0.590 |
| C4, g/L | 0.16 [0.13, 0.23] | 0.18 [0.11, 0.25] | 0.681 |
| IgA, g/L | 2.59 [1.96, 3.25] | 2.32 [1.75, 3.08] | 0.147 |
| IgG, g/L | 10.95 [8.17, 13.70] | 10.60 [7.68, 12.98] | 0.467 |
| IgM, g/L | 0.70 [0.51, 1.07] | 0.68 [0.45, 1.06] | 0.404 |
| Lupus anticoagulant | 1.58 [1.11, 2.40] | 1.08 [0.76, 1.68] | <0.001 |
| Proteinuria, g/24h | 0.78 [0.26, 2.45] | 1.17 [0.41, 2.58] | 0.059 |
| Urine WBC, WBC/ul | 12.00 [6.22, 33.11] | 13.25 [6.00, 42.25] | 0.906 |
| Urine RBC, RBC/ul | 14.75 [5.00, 52.50] | 12.00 [4.25, 53.75] | 0.443 |
| Positive ANA | 111 (99.1) | 107 (95.5) | 0.212 |
| Positive anti-dsDNA | 65 (58.0) | 58 (51.8) | 0.421 |
| Positive anti-Sm | 26 (23.2) | 31 (27.7) | 0.540 |
| Positive anti-ssA | 63 (56.2) | 62 (55.4) | 1.000 |
| Positive anti-ssB | 13 (11.6) | 18 (16.1) | 0.439 |
| Pathology | |||
| ISN/RPS class | |||
| III | 3 (10.3) | 5 (10.4) | 1 |
| IV | 15 (51.7) | 11 (22.9) | 0.013 |
| III/IV+V | 11 (37.9) | 25 (52.1) | 0.249 |
| V | 0 (0.0) | 7 (14.6) | 0.041 |
| Crescent (%) | 13.33 [0.00, 28.57] | 5.50 [0.00, 14.56] | 0.126 |
| AI | 7.00 [4.00, 9.00] | 5.00 [2.00, 10.00] | 0.150 |
| CI | 2.00 [1.00, 5.00] | 2.00 [1.00, 4.00] | 0.923 |
| Therapy | |||
| ATM (%) | 75 (67.0) | 85 (75.9) | 0.183 |
| AZA (%) | 1 (0.9) | 1 (0.9) | 1.000 |
| CNI (%) | 16 (14.3) | 54 (48.2) | <0.001 |
| CTX (%) | 6 (5.4) | 13 (11.6) | 0.149 |
| LEF (%) | 10 (8.9) | 8 (7.1) | 0.807 |
| MMF (%) | 54 (48.2) | 51 (45.5) | 0.789 |
| RAASi (%) | 43 (38.4) | 57 (50.9) | 0.080 |
| Steroid (%) | 107 (95.5) | 108 (96.4) | 1.000 |
| Dosage of steroid, mg/day | 15.00 [10.00, 20.00] | 15.00 [7.50, 25.62] | 0.726 |
| Major treatment regimen | |||
| Steroid only (%) | 32 (28.6) | 11 (9.8) | <0.001 |
| Steroid + CTX (%) | 6 (5.4) | 13 (11.6) | 0.149 |
| Steroid + MMF (%) | 50 (44.6) | 45 (40.2) | 0.589 |
| Factor . | Standard therapy . | Belimumab . | P value . |
|---|---|---|---|
| Number of patients | 112 | 112 | |
| Laboratory examination | |||
| Serum creatinine, umol/L | 64.50 [56.38, 79.19] | 66.50 [55.00, 89.50] | 0.694 |
| eGFR | 107.64 [82.94, 120.57] | 108.58 [79.63, 120.32] | 0.700 |
| ≥90 (%) | 80 (71.4) | 76 (67.9) | 0.663 |
| ≥60 (%) | 94 (83.9) | 88 (78.6) | 0.392 |
| Serum albumin, g/L | 38.83 [34.77, 41.42] | 32.50 [28.70, 36.54] | <0.001 |
| C3, g/L | 0.93 (0.23) | 0.91 (0.25) | 0.590 |
| C4, g/L | 0.16 [0.13, 0.23] | 0.18 [0.11, 0.25] | 0.681 |
| IgA, g/L | 2.59 [1.96, 3.25] | 2.32 [1.75, 3.08] | 0.147 |
| IgG, g/L | 10.95 [8.17, 13.70] | 10.60 [7.68, 12.98] | 0.467 |
| IgM, g/L | 0.70 [0.51, 1.07] | 0.68 [0.45, 1.06] | 0.404 |
| Lupus anticoagulant | 1.58 [1.11, 2.40] | 1.08 [0.76, 1.68] | <0.001 |
| Proteinuria, g/24h | 0.78 [0.26, 2.45] | 1.17 [0.41, 2.58] | 0.059 |
| Urine WBC, WBC/ul | 12.00 [6.22, 33.11] | 13.25 [6.00, 42.25] | 0.906 |
| Urine RBC, RBC/ul | 14.75 [5.00, 52.50] | 12.00 [4.25, 53.75] | 0.443 |
| Positive ANA | 111 (99.1) | 107 (95.5) | 0.212 |
| Positive anti-dsDNA | 65 (58.0) | 58 (51.8) | 0.421 |
| Positive anti-Sm | 26 (23.2) | 31 (27.7) | 0.540 |
| Positive anti-ssA | 63 (56.2) | 62 (55.4) | 1.000 |
| Positive anti-ssB | 13 (11.6) | 18 (16.1) | 0.439 |
| Pathology | |||
| ISN/RPS class | |||
| III | 3 (10.3) | 5 (10.4) | 1 |
| IV | 15 (51.7) | 11 (22.9) | 0.013 |
| III/IV+V | 11 (37.9) | 25 (52.1) | 0.249 |
| V | 0 (0.0) | 7 (14.6) | 0.041 |
| Crescent (%) | 13.33 [0.00, 28.57] | 5.50 [0.00, 14.56] | 0.126 |
| AI | 7.00 [4.00, 9.00] | 5.00 [2.00, 10.00] | 0.150 |
| CI | 2.00 [1.00, 5.00] | 2.00 [1.00, 4.00] | 0.923 |
| Therapy | |||
| ATM (%) | 75 (67.0) | 85 (75.9) | 0.183 |
| AZA (%) | 1 (0.9) | 1 (0.9) | 1.000 |
| CNI (%) | 16 (14.3) | 54 (48.2) | <0.001 |
| CTX (%) | 6 (5.4) | 13 (11.6) | 0.149 |
| LEF (%) | 10 (8.9) | 8 (7.1) | 0.807 |
| MMF (%) | 54 (48.2) | 51 (45.5) | 0.789 |
| RAASi (%) | 43 (38.4) | 57 (50.9) | 0.080 |
| Steroid (%) | 107 (95.5) | 108 (96.4) | 1.000 |
| Dosage of steroid, mg/day | 15.00 [10.00, 20.00] | 15.00 [7.50, 25.62] | 0.726 |
| Major treatment regimen | |||
| Steroid only (%) | 32 (28.6) | 11 (9.8) | <0.001 |
| Steroid + CTX (%) | 6 (5.4) | 13 (11.6) | 0.149 |
| Steroid + MMF (%) | 50 (44.6) | 45 (40.2) | 0.589 |
Values for numeric data are presented as mean (standard deviation) or median [interquartile range]; for categorical data, as count (percent).
AI: activity NIH index; ANA: antinuclear antibodies; ATM: hydroxychloroquine; AZA: azathioprine; C3: complement factor 3; C4: complement factor 4; CI: chronicity NIH index; CNI: calcineurin inhibitor; CTX: cyclophosphamide; dsDNA: double-stranded DNA; eGFR: estimated glomerular filtration rate; IgA: Immunoglobulin A; IgG: Immunoglobulin G; IgM: Immunoglobulin M; ISN/RPS international society of nephrology/renal pathology society; LEF: leflunomide; MMF: mycophenolate mofetil; RAASi: renin-angiotensin-aldosterone system inhibitors; RBC: red blood cells; ssA: Sjögren's-syndrome-related antigen A; ssB: Sjögren's-syndrome-related antigen B; WBC: white blood cells.
Efficacy
Effect on clinical parameters
GLMM analysis showed that serum albumin (Fig. 2A), C3 (Fig. 2B) and C4 (Fig. 2C) increased significantly in both groups of LN patients during the first two years of follow-up (P < 0.001; P < 0.001; P < 0.001), and the increase was more significantly observed in the BLM group compared with the control group (P < 0.001; P < 0.001; P < 0.001) (Supplementary Table 2, available at Rheumatology online). The decrease in proteinuria (Fig. 2E) and daily dose of steroids (Fig. 2F) were more pronounced in the BLM group than in the control group during the first 2 years of follow-up, although there was no statistically significant difference. Other clinical parameters such as serum creatinine (Fig. 2D) increased gradually with time during the first two years of follow-up, but there was no significant difference between the two groups (Supplementary Table 3, available at Rheumatology online).
![Comparison of efficacy between standard and belimumab groups. A–F. Estimated marginal means and corresponding standard errors of serum albumin (A), C3 (B), C4 (C), serum creatinine (D), 24 h proteinuria (E) and daily steroid dosage (F) in standard therapy group and belimumab group using GLMM. The ANOVA of the GLMM was reported in Supplementary Table 2 (available at Rheumatology online). G–J. Comparison of efficacy endpoints between standard group and belimumab group. (G) Time to first PERR. (H) Time to first CRR. (I) Kaplan–Meier curve (P = 0.170 [log-rank test]). The end point is renal flare. (J) Kaplan–Meier curve (P = 0.064 [log-rank test]). The renal endpoint is doubling of serum creatinine, eGFR 30% decrease of the baseline or reaching end-stage renal disease or need for dialysis or renal transplantation or death. Statistical significance levels are indicated in the plot as ‘*’, which represents P < 0.05, and ‘**’, representing P < 0.01. C3: complement factor 3; C4: complement factor 4; CRR: complete renal response; PERR: primary efficacy renal response](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/rheumatology/64/2/10.1093_rheumatology_kead707/2/m_kead707f2.jpeg?Expires=1748458003&Signature=iRjEDIamWsSIEwPOzfwmHa~t0nhNYtx6idouBhZdQxoUHaKhhGDr635WJxID65VDuEOiNfYGhqHdmZ0SDBokY3Ze3XRYjOaHeL6dXshPe~25ww6GhBEj93gPG7tMVaN1R4QsduzcLXc6U-kw5Y6meveafWMFr5oswynVUwVZvLi3Cjdlo-zZT3mgP12UtG7nj9lcZnwHALFQ929SRSOat5s2xImINLZ7R4yACPIFXelIZEJN-Q~Tr~stFAGvNok80oSa7ZkUZKQjQh~3Eg8VzfkOvNfS-FB5cOptvV8gkOxgX-nLy7Paq-U5bcOm9MDIe2JkEmIgMj8qWYK~DT4Uog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Comparison of efficacy between standard and belimumab groups. A–F. Estimated marginal means and corresponding standard errors of serum albumin (A), C3 (B), C4 (C), serum creatinine (D), 24 h proteinuria (E) and daily steroid dosage (F) in standard therapy group and belimumab group using GLMM. The ANOVA of the GLMM was reported in Supplementary Table 2 (available at Rheumatology online). G–J. Comparison of efficacy endpoints between standard group and belimumab group. (G) Time to first PERR. (H) Time to first CRR. (I) Kaplan–Meier curve (P = 0.170 [log-rank test]). The end point is renal flare. (J) Kaplan–Meier curve (P = 0.064 [log-rank test]). The renal endpoint is doubling of serum creatinine, eGFR 30% decrease of the baseline or reaching end-stage renal disease or need for dialysis or renal transplantation or death. Statistical significance levels are indicated in the plot as ‘*’, which represents P < 0.05, and ‘**’, representing P < 0.01. C3: complement factor 3; C4: complement factor 4; CRR: complete renal response; PERR: primary efficacy renal response
Comparing the disease biomarkers of two groups of LN patients after treatment, the results showed that C3 (P < 0.001) and C4 levels (P < 0.001) were significantly higher in BLM group compared with control group at 1 year of follow-up after baseline, and C4 levels in BLM group was still higher than that in control group at last follow-up (P = 0.004). IgA (P = 0.001, P < 0.001), IgM (P = 0.002, P < 0.001) and IgG levels (P < 0.001, P < 0.001) were significantly lower in BLM group than in control group at the first 1 year of the follow-up and at the last follow-up. The proportion of patients with positive ANA (P = 0.005) was lower in BLM group compared with control group at the last follow-up (Table 2, Supplementary Table 4, available at Rheumatology online).
Comparison of clinical parameters and drug between patients treated with or without belimumab at first year and last follow-up
| Factor . | First year . | Last follow-up . | ||||
|---|---|---|---|---|---|---|
| Standard therapy . | Belimumab . | P value . | Standard therapy . | Belimumab . | P value . | |
| Number of patients | 112 | 112 | 112 | 112 | ||
| Laboratory examination | ||||||
| Serum creatinine, umol/L | 63.50 [51.75, 91.25] | 64.00 [55.00, 86.75] | 0.392 | 63.00 [54.75, 86.00] | 64.00 [54.00, 86.75] | 0.908 |
| eGFR | 107.61 [79.65, 121.11] | 108.07 [78.26, 120.89] | 0.929 | 105.46 [80.61, 119.17] | 108.87 [78.80, 121.20] | 0.390 |
| Serum albumin, g/L | 40.55 [36.48, 43.10] | 37.90 [33.48, 40.62] | <0.001 | 40.50 [36.93, 42.73] | 39.10 [35.70, 41.32] | 0.022 |
| C3, g/L | 0.93 [0.78, 1.07] | 1.07 [0.89, 1.29] | <0.001 | 1.03 [0.85, 1.17] | 1.03 [0.80, 1.25] | 0.670 |
| C4, g/L | 0.18 [0.13, 0.24] | 0.26 [0.19, 0.35] | <0.001 | 0.20 [0.14, 0.30] | 0.26 [0.19, 0.34] | 0.004 |
| IgA, g/L | 2.54 [1.94, 3.25] | 2.00 [1.63, 2.77] | 0.001 | 2.47 [2.02, 3.18] | 1.94 [1.59, 2.67] | <0.001 |
| IgG, g/L | 12.15 (4.01) | 10.12 (3.45) | <0.001 | 12.32 [10.04, 15.20] | 10.12 [7.96, 12.40] | <0.001 |
| IgM, g/L | 0.73 [0.55, 1.09] | 0.58 [0.39, 0.85] | 0.002 | 0.78 [0.50, 1.06] | 0.55 [0.34, 0.84] | <0.001 |
| Lupus anticoagulant | 1.15 [1.02, 1.44] | 1.12 [1.03, 1.29] | 0.375 | 1.15 [1.01, 1.33] | 1.16 [1.03, 1.32] | 0.819 |
| Proteinuria, g/24h | 0.60 [0.18, 1.77] | 0.55 [0.17, 1.73] | 0.831 | 0.34 [0.13, 0.84] | 0.38 [0.17, 1.49] | 0.294 |
| Urine WBC, WBC/ul | 9.00 [4.00, 23.00] | 11.59 [4.00, 39.00] | 0.402 | 13.29 [5.00, 48.61] | 9.00 [3.23, 29.01] | 0.015 |
| Urine RBC, RBC/ul | 10.62 [3.50, 21.75] | 7.50 [3.38, 20.00] | 0.343 | 9.00 [4.00, 20.12] | 8.00 [3.00, 19.25] | 0.422 |
| Positive ANA | 106 (94.6) | 101 (90.2) | 0.313 | 109 (97.3) | 97 (86.6) | 0.005 |
| Positive anti-dsDNA | 50 (44.6) | 48 (42.9) | 0.893 | 53 (47.3) | 50 (44.6) | 0.789 |
| Positive anti-Sm | 26 (23.2) | 24 (21.4) | 0.873 | 27 (24.1) | 15 (13.4) | 0.059 |
| Positive anti-ssA | 71 (63.4) | 56 (50.0) | 0.059 | 74 (66.1) | 52 (46.4) | 0.005 |
| Positive anti-ssB | 17 (15.2) | 13 (11.6) | 0.557 | 28 (25.0) | 14 (12.5) | 0.025 |
| Therapy | ||||||
| ATM (%) | 77 (68.8) | 71 (63.4) | 0.481 | 65 (58.0) | 61 (54.5) | 0.686 |
| AZA (%) | 1 (0.9) | 0 (0.0) | 1.000 | 3 (2.7) | 1 (0.9) | 0.622 |
| CNI (%) | 19 (17.0) | 34 (30.4) | 0.027 | 21 (18.8) | 32 (28.6) | 0.115 |
| CTX (%) | 1 (0.9) | 5 (4.5) | 0.212 | 1 (0.9) | 3 (2.7) | 0.622 |
| LEF (%) | 8 (7.1) | 6 (5.4) | 0.784 | 9 (8.0) | 6 (5.4) | 0.594 |
| MMF (%) | 48 (42.9) | 47 (42.0) | 1.000 | 34 (30.4) | 43 (38.4) | 0.260 |
| RAASi (%) | 46 (41.1) | 47 (42.0) | 1.000 | 28 (25.0) | 36 (32.1) | 0.301 |
| Steroid (%) | 75 (67.0) | 68 (60.7) | 0.404 | 70 (62.5) | 61 (54.5) | 0.278 |
| Dosage of steroid, mg/day | 5.00 [0.00, 10.00] | 5.00 [0.00, 10.00] | 0.269 | 5.00 [0.00, 8.12] | 4.38 [0.00, 8.12] | 0.643 |
| Factor . | First year . | Last follow-up . | ||||
|---|---|---|---|---|---|---|
| Standard therapy . | Belimumab . | P value . | Standard therapy . | Belimumab . | P value . | |
| Number of patients | 112 | 112 | 112 | 112 | ||
| Laboratory examination | ||||||
| Serum creatinine, umol/L | 63.50 [51.75, 91.25] | 64.00 [55.00, 86.75] | 0.392 | 63.00 [54.75, 86.00] | 64.00 [54.00, 86.75] | 0.908 |
| eGFR | 107.61 [79.65, 121.11] | 108.07 [78.26, 120.89] | 0.929 | 105.46 [80.61, 119.17] | 108.87 [78.80, 121.20] | 0.390 |
| Serum albumin, g/L | 40.55 [36.48, 43.10] | 37.90 [33.48, 40.62] | <0.001 | 40.50 [36.93, 42.73] | 39.10 [35.70, 41.32] | 0.022 |
| C3, g/L | 0.93 [0.78, 1.07] | 1.07 [0.89, 1.29] | <0.001 | 1.03 [0.85, 1.17] | 1.03 [0.80, 1.25] | 0.670 |
| C4, g/L | 0.18 [0.13, 0.24] | 0.26 [0.19, 0.35] | <0.001 | 0.20 [0.14, 0.30] | 0.26 [0.19, 0.34] | 0.004 |
| IgA, g/L | 2.54 [1.94, 3.25] | 2.00 [1.63, 2.77] | 0.001 | 2.47 [2.02, 3.18] | 1.94 [1.59, 2.67] | <0.001 |
| IgG, g/L | 12.15 (4.01) | 10.12 (3.45) | <0.001 | 12.32 [10.04, 15.20] | 10.12 [7.96, 12.40] | <0.001 |
| IgM, g/L | 0.73 [0.55, 1.09] | 0.58 [0.39, 0.85] | 0.002 | 0.78 [0.50, 1.06] | 0.55 [0.34, 0.84] | <0.001 |
| Lupus anticoagulant | 1.15 [1.02, 1.44] | 1.12 [1.03, 1.29] | 0.375 | 1.15 [1.01, 1.33] | 1.16 [1.03, 1.32] | 0.819 |
| Proteinuria, g/24h | 0.60 [0.18, 1.77] | 0.55 [0.17, 1.73] | 0.831 | 0.34 [0.13, 0.84] | 0.38 [0.17, 1.49] | 0.294 |
| Urine WBC, WBC/ul | 9.00 [4.00, 23.00] | 11.59 [4.00, 39.00] | 0.402 | 13.29 [5.00, 48.61] | 9.00 [3.23, 29.01] | 0.015 |
| Urine RBC, RBC/ul | 10.62 [3.50, 21.75] | 7.50 [3.38, 20.00] | 0.343 | 9.00 [4.00, 20.12] | 8.00 [3.00, 19.25] | 0.422 |
| Positive ANA | 106 (94.6) | 101 (90.2) | 0.313 | 109 (97.3) | 97 (86.6) | 0.005 |
| Positive anti-dsDNA | 50 (44.6) | 48 (42.9) | 0.893 | 53 (47.3) | 50 (44.6) | 0.789 |
| Positive anti-Sm | 26 (23.2) | 24 (21.4) | 0.873 | 27 (24.1) | 15 (13.4) | 0.059 |
| Positive anti-ssA | 71 (63.4) | 56 (50.0) | 0.059 | 74 (66.1) | 52 (46.4) | 0.005 |
| Positive anti-ssB | 17 (15.2) | 13 (11.6) | 0.557 | 28 (25.0) | 14 (12.5) | 0.025 |
| Therapy | ||||||
| ATM (%) | 77 (68.8) | 71 (63.4) | 0.481 | 65 (58.0) | 61 (54.5) | 0.686 |
| AZA (%) | 1 (0.9) | 0 (0.0) | 1.000 | 3 (2.7) | 1 (0.9) | 0.622 |
| CNI (%) | 19 (17.0) | 34 (30.4) | 0.027 | 21 (18.8) | 32 (28.6) | 0.115 |
| CTX (%) | 1 (0.9) | 5 (4.5) | 0.212 | 1 (0.9) | 3 (2.7) | 0.622 |
| LEF (%) | 8 (7.1) | 6 (5.4) | 0.784 | 9 (8.0) | 6 (5.4) | 0.594 |
| MMF (%) | 48 (42.9) | 47 (42.0) | 1.000 | 34 (30.4) | 43 (38.4) | 0.260 |
| RAASi (%) | 46 (41.1) | 47 (42.0) | 1.000 | 28 (25.0) | 36 (32.1) | 0.301 |
| Steroid (%) | 75 (67.0) | 68 (60.7) | 0.404 | 70 (62.5) | 61 (54.5) | 0.278 |
| Dosage of steroid, mg/day | 5.00 [0.00, 10.00] | 5.00 [0.00, 10.00] | 0.269 | 5.00 [0.00, 8.12] | 4.38 [0.00, 8.12] | 0.643 |
Values for numeric data are presented as mean (standard deviation) or median [interquartile range]; for categorical data, as count (percent).
ANA: antinuclear antibodies; ATM: hydroxychloroquine; AZA: azathioprine; C3: complement factor 3; C4: complement factor 4; CNI: calcineurin inhibitor; CTX: cyclophosphamide; dsDNA: double-stranded DNA; eGFR: estimated glomerular filtration rate; IgA: Immunoglobulin A; IgG: Immunoglobulin G; IgM: Immunoglobulin M; LEF: leflunomide; MMF: mycophenolate mofetil; RAASi: renin-angiotensin-aldosterone system inhibitors; RBC: red blood cells; ssA: Sjögren's-syndrome-related antigen A; ssB: Sjögren's-syndrome-related antigen B; WBC: white blood cells.
Comparison of clinical parameters and drug between patients treated with or without belimumab at first year and last follow-up
| Factor . | First year . | Last follow-up . | ||||
|---|---|---|---|---|---|---|
| Standard therapy . | Belimumab . | P value . | Standard therapy . | Belimumab . | P value . | |
| Number of patients | 112 | 112 | 112 | 112 | ||
| Laboratory examination | ||||||
| Serum creatinine, umol/L | 63.50 [51.75, 91.25] | 64.00 [55.00, 86.75] | 0.392 | 63.00 [54.75, 86.00] | 64.00 [54.00, 86.75] | 0.908 |
| eGFR | 107.61 [79.65, 121.11] | 108.07 [78.26, 120.89] | 0.929 | 105.46 [80.61, 119.17] | 108.87 [78.80, 121.20] | 0.390 |
| Serum albumin, g/L | 40.55 [36.48, 43.10] | 37.90 [33.48, 40.62] | <0.001 | 40.50 [36.93, 42.73] | 39.10 [35.70, 41.32] | 0.022 |
| C3, g/L | 0.93 [0.78, 1.07] | 1.07 [0.89, 1.29] | <0.001 | 1.03 [0.85, 1.17] | 1.03 [0.80, 1.25] | 0.670 |
| C4, g/L | 0.18 [0.13, 0.24] | 0.26 [0.19, 0.35] | <0.001 | 0.20 [0.14, 0.30] | 0.26 [0.19, 0.34] | 0.004 |
| IgA, g/L | 2.54 [1.94, 3.25] | 2.00 [1.63, 2.77] | 0.001 | 2.47 [2.02, 3.18] | 1.94 [1.59, 2.67] | <0.001 |
| IgG, g/L | 12.15 (4.01) | 10.12 (3.45) | <0.001 | 12.32 [10.04, 15.20] | 10.12 [7.96, 12.40] | <0.001 |
| IgM, g/L | 0.73 [0.55, 1.09] | 0.58 [0.39, 0.85] | 0.002 | 0.78 [0.50, 1.06] | 0.55 [0.34, 0.84] | <0.001 |
| Lupus anticoagulant | 1.15 [1.02, 1.44] | 1.12 [1.03, 1.29] | 0.375 | 1.15 [1.01, 1.33] | 1.16 [1.03, 1.32] | 0.819 |
| Proteinuria, g/24h | 0.60 [0.18, 1.77] | 0.55 [0.17, 1.73] | 0.831 | 0.34 [0.13, 0.84] | 0.38 [0.17, 1.49] | 0.294 |
| Urine WBC, WBC/ul | 9.00 [4.00, 23.00] | 11.59 [4.00, 39.00] | 0.402 | 13.29 [5.00, 48.61] | 9.00 [3.23, 29.01] | 0.015 |
| Urine RBC, RBC/ul | 10.62 [3.50, 21.75] | 7.50 [3.38, 20.00] | 0.343 | 9.00 [4.00, 20.12] | 8.00 [3.00, 19.25] | 0.422 |
| Positive ANA | 106 (94.6) | 101 (90.2) | 0.313 | 109 (97.3) | 97 (86.6) | 0.005 |
| Positive anti-dsDNA | 50 (44.6) | 48 (42.9) | 0.893 | 53 (47.3) | 50 (44.6) | 0.789 |
| Positive anti-Sm | 26 (23.2) | 24 (21.4) | 0.873 | 27 (24.1) | 15 (13.4) | 0.059 |
| Positive anti-ssA | 71 (63.4) | 56 (50.0) | 0.059 | 74 (66.1) | 52 (46.4) | 0.005 |
| Positive anti-ssB | 17 (15.2) | 13 (11.6) | 0.557 | 28 (25.0) | 14 (12.5) | 0.025 |
| Therapy | ||||||
| ATM (%) | 77 (68.8) | 71 (63.4) | 0.481 | 65 (58.0) | 61 (54.5) | 0.686 |
| AZA (%) | 1 (0.9) | 0 (0.0) | 1.000 | 3 (2.7) | 1 (0.9) | 0.622 |
| CNI (%) | 19 (17.0) | 34 (30.4) | 0.027 | 21 (18.8) | 32 (28.6) | 0.115 |
| CTX (%) | 1 (0.9) | 5 (4.5) | 0.212 | 1 (0.9) | 3 (2.7) | 0.622 |
| LEF (%) | 8 (7.1) | 6 (5.4) | 0.784 | 9 (8.0) | 6 (5.4) | 0.594 |
| MMF (%) | 48 (42.9) | 47 (42.0) | 1.000 | 34 (30.4) | 43 (38.4) | 0.260 |
| RAASi (%) | 46 (41.1) | 47 (42.0) | 1.000 | 28 (25.0) | 36 (32.1) | 0.301 |
| Steroid (%) | 75 (67.0) | 68 (60.7) | 0.404 | 70 (62.5) | 61 (54.5) | 0.278 |
| Dosage of steroid, mg/day | 5.00 [0.00, 10.00] | 5.00 [0.00, 10.00] | 0.269 | 5.00 [0.00, 8.12] | 4.38 [0.00, 8.12] | 0.643 |
| Factor . | First year . | Last follow-up . | ||||
|---|---|---|---|---|---|---|
| Standard therapy . | Belimumab . | P value . | Standard therapy . | Belimumab . | P value . | |
| Number of patients | 112 | 112 | 112 | 112 | ||
| Laboratory examination | ||||||
| Serum creatinine, umol/L | 63.50 [51.75, 91.25] | 64.00 [55.00, 86.75] | 0.392 | 63.00 [54.75, 86.00] | 64.00 [54.00, 86.75] | 0.908 |
| eGFR | 107.61 [79.65, 121.11] | 108.07 [78.26, 120.89] | 0.929 | 105.46 [80.61, 119.17] | 108.87 [78.80, 121.20] | 0.390 |
| Serum albumin, g/L | 40.55 [36.48, 43.10] | 37.90 [33.48, 40.62] | <0.001 | 40.50 [36.93, 42.73] | 39.10 [35.70, 41.32] | 0.022 |
| C3, g/L | 0.93 [0.78, 1.07] | 1.07 [0.89, 1.29] | <0.001 | 1.03 [0.85, 1.17] | 1.03 [0.80, 1.25] | 0.670 |
| C4, g/L | 0.18 [0.13, 0.24] | 0.26 [0.19, 0.35] | <0.001 | 0.20 [0.14, 0.30] | 0.26 [0.19, 0.34] | 0.004 |
| IgA, g/L | 2.54 [1.94, 3.25] | 2.00 [1.63, 2.77] | 0.001 | 2.47 [2.02, 3.18] | 1.94 [1.59, 2.67] | <0.001 |
| IgG, g/L | 12.15 (4.01) | 10.12 (3.45) | <0.001 | 12.32 [10.04, 15.20] | 10.12 [7.96, 12.40] | <0.001 |
| IgM, g/L | 0.73 [0.55, 1.09] | 0.58 [0.39, 0.85] | 0.002 | 0.78 [0.50, 1.06] | 0.55 [0.34, 0.84] | <0.001 |
| Lupus anticoagulant | 1.15 [1.02, 1.44] | 1.12 [1.03, 1.29] | 0.375 | 1.15 [1.01, 1.33] | 1.16 [1.03, 1.32] | 0.819 |
| Proteinuria, g/24h | 0.60 [0.18, 1.77] | 0.55 [0.17, 1.73] | 0.831 | 0.34 [0.13, 0.84] | 0.38 [0.17, 1.49] | 0.294 |
| Urine WBC, WBC/ul | 9.00 [4.00, 23.00] | 11.59 [4.00, 39.00] | 0.402 | 13.29 [5.00, 48.61] | 9.00 [3.23, 29.01] | 0.015 |
| Urine RBC, RBC/ul | 10.62 [3.50, 21.75] | 7.50 [3.38, 20.00] | 0.343 | 9.00 [4.00, 20.12] | 8.00 [3.00, 19.25] | 0.422 |
| Positive ANA | 106 (94.6) | 101 (90.2) | 0.313 | 109 (97.3) | 97 (86.6) | 0.005 |
| Positive anti-dsDNA | 50 (44.6) | 48 (42.9) | 0.893 | 53 (47.3) | 50 (44.6) | 0.789 |
| Positive anti-Sm | 26 (23.2) | 24 (21.4) | 0.873 | 27 (24.1) | 15 (13.4) | 0.059 |
| Positive anti-ssA | 71 (63.4) | 56 (50.0) | 0.059 | 74 (66.1) | 52 (46.4) | 0.005 |
| Positive anti-ssB | 17 (15.2) | 13 (11.6) | 0.557 | 28 (25.0) | 14 (12.5) | 0.025 |
| Therapy | ||||||
| ATM (%) | 77 (68.8) | 71 (63.4) | 0.481 | 65 (58.0) | 61 (54.5) | 0.686 |
| AZA (%) | 1 (0.9) | 0 (0.0) | 1.000 | 3 (2.7) | 1 (0.9) | 0.622 |
| CNI (%) | 19 (17.0) | 34 (30.4) | 0.027 | 21 (18.8) | 32 (28.6) | 0.115 |
| CTX (%) | 1 (0.9) | 5 (4.5) | 0.212 | 1 (0.9) | 3 (2.7) | 0.622 |
| LEF (%) | 8 (7.1) | 6 (5.4) | 0.784 | 9 (8.0) | 6 (5.4) | 0.594 |
| MMF (%) | 48 (42.9) | 47 (42.0) | 1.000 | 34 (30.4) | 43 (38.4) | 0.260 |
| RAASi (%) | 46 (41.1) | 47 (42.0) | 1.000 | 28 (25.0) | 36 (32.1) | 0.301 |
| Steroid (%) | 75 (67.0) | 68 (60.7) | 0.404 | 70 (62.5) | 61 (54.5) | 0.278 |
| Dosage of steroid, mg/day | 5.00 [0.00, 10.00] | 5.00 [0.00, 10.00] | 0.269 | 5.00 [0.00, 8.12] | 4.38 [0.00, 8.12] | 0.643 |
Values for numeric data are presented as mean (standard deviation) or median [interquartile range]; for categorical data, as count (percent).
ANA: antinuclear antibodies; ATM: hydroxychloroquine; AZA: azathioprine; C3: complement factor 3; C4: complement factor 4; CNI: calcineurin inhibitor; CTX: cyclophosphamide; dsDNA: double-stranded DNA; eGFR: estimated glomerular filtration rate; IgA: Immunoglobulin A; IgG: Immunoglobulin G; IgM: Immunoglobulin M; LEF: leflunomide; MMF: mycophenolate mofetil; RAASi: renin-angiotensin-aldosterone system inhibitors; RBC: red blood cells; ssA: Sjögren's-syndrome-related antigen A; ssB: Sjögren's-syndrome-related antigen B; WBC: white blood cells.
Comparison of renal parameters after treatment between two groups of LN patients showed that the results showed that urine WBC (P = 0.015) was lower in the BLM group than in control group at the last follow-up, and there was no significant difference in other renal parameters, such as eGFR, proteinuria and hematuria, between the two groups (Table 2).
Comparison of the major treatment regimens in two groups showed that the proportion of patients treated with steroid and the daily dose of steroid received in the BLM group were not significantly different between the two groups at 1-year follow-up after baseline and at the last follow-up (Table 2). However, the total steroid dose from baseline to PR was significantly lower in the BLM group compared with the control group (525.00 [280.00, 1050.00] mg vs 70.00 [35.00, 181.25] mg, P < 0.001).
Effect on renal outcomes
Comparing the first renal response achieved during follow-up between two groups of LN patients, the results showed that half of the patients had partial or complete renal response at 1 month after baseline, regardless of whether or not they were treated with belimumab. However, the BLM group experienced PRR (P < 0.001) or CRR (P < 0.001) significantly earlier than the control group (Fig. 2G and H). During the follow-up, there was no significant difference in the risk of renal flare and renal end point between the two groups of patients (Fig. 2I and J).
Adverse events
As shown in Table 3, the incidence of respiratory tract infection during follow-up in BLM group was significantly lower than that in control group (P = 0.015). The rate of the other common adverse events was not significantly different between the two groups.
Comparison of adverse event in the course of disease between patients treated with or without belimumab
| Factor . | Standard therapy . | Belimumab . | P value . |
|---|---|---|---|
| Number of patient | 112 | 112 | |
| Digestive tract infection | 60 (53.6) | 46 (41.1) | 0.082 |
| Respiratory tract infection | 58 (51.8) | 39 (34.8) | 0.015 |
| Urinary tract infection | 27 (24.1) | 15 (13.4) | 0.059 |
| Leukopenia | 13 (11.6) | 12 (10.7) | 1.000 |
| Mental and neurological adverse events | 28 (25.0) | 20 (17.9) | 0.254 |
| Factor . | Standard therapy . | Belimumab . | P value . |
|---|---|---|---|
| Number of patient | 112 | 112 | |
| Digestive tract infection | 60 (53.6) | 46 (41.1) | 0.082 |
| Respiratory tract infection | 58 (51.8) | 39 (34.8) | 0.015 |
| Urinary tract infection | 27 (24.1) | 15 (13.4) | 0.059 |
| Leukopenia | 13 (11.6) | 12 (10.7) | 1.000 |
| Mental and neurological adverse events | 28 (25.0) | 20 (17.9) | 0.254 |
Values for categorical data, as count (percent).
Comparison of adverse event in the course of disease between patients treated with or without belimumab
| Factor . | Standard therapy . | Belimumab . | P value . |
|---|---|---|---|
| Number of patient | 112 | 112 | |
| Digestive tract infection | 60 (53.6) | 46 (41.1) | 0.082 |
| Respiratory tract infection | 58 (51.8) | 39 (34.8) | 0.015 |
| Urinary tract infection | 27 (24.1) | 15 (13.4) | 0.059 |
| Leukopenia | 13 (11.6) | 12 (10.7) | 1.000 |
| Mental and neurological adverse events | 28 (25.0) | 20 (17.9) | 0.254 |
| Factor . | Standard therapy . | Belimumab . | P value . |
|---|---|---|---|
| Number of patient | 112 | 112 | |
| Digestive tract infection | 60 (53.6) | 46 (41.1) | 0.082 |
| Respiratory tract infection | 58 (51.8) | 39 (34.8) | 0.015 |
| Urinary tract infection | 27 (24.1) | 15 (13.4) | 0.059 |
| Leukopenia | 13 (11.6) | 12 (10.7) | 1.000 |
| Mental and neurological adverse events | 28 (25.0) | 20 (17.9) | 0.254 |
Values for categorical data, as count (percent).
Treatment response of patients with different levels of proteinuria
Univariate Cox regression model was constructed to analyse the relationship between clinical parameters and renal outcomes in patients receiving add-on therapy with belimumab. The results showed that proteinuria [1 g/24 h: hazard ratio (HR)=1.13, 95% confidence interval (CI) 1–1.27; P = 0.052] may be associated with renal end point of patients in the BLM group (Supplementary Table 5, available at Rheumatology online). Twenty-four hour proteinuria were further divided into two levels: <2.5 g/24 h and ≥2.5 g/24 h. The result of univariate Cox regression model showed that different proteinuria levels (<2.5 g/24 h/≥2.5 g/24 h: HR = 1.62, 95% CI 1.01–2.62; P = 0.047) was associated with renal end point of patients in the BLM group (Supplementary Table 6, available at Rheumatology online).
In conjunction with clinical practice, 24 h proteinuria was further divided into two levels: <3.5 g/24 h and ≥3.5 g/24 h, and the responses to treatment with belimumab was compared in patients with different proteinuria levels.
As shown in Table 4, in both groups with different proteinuria levels, serum albumin was higher (P < 0.001, P < 0.001) and proteinuria was lower (P = 0.001; P < 0.001) at the last follow-up compared with baseline. In the group of patients treated with belimumab with baseline proteinuria <3.5 g/24 h, C3 (P < 0.001) and C4 (P < 0.001) were significantly higher at the last follow-up than at baseline, IgA (P = 0.018), IgM (P = 0.014), urine RBC (P = 0.010) and positive anti-Sm (P = 0.045) were significantly lower at the last follow-up than at baseline, while there was no significant difference before and after treatment with belimumab in the group with proteinuria ≥3.5 g/24 h (Supplementary Table 7, available at Rheumatology online).
Stratification according to baseline proteinuria, comparison of baseline and last follow-up clinical parameters and drug in patients treated with belimumab
| Factor . | Proteinuria ≥ 3.5 g/24 h . | Proteinuria <3.5 g/24 h . | ||||
|---|---|---|---|---|---|---|
| Before treatment . | After treatment . | P value . | Before treatment . | After treatment . | P value . | |
| Number of patients | 17 | 17 | 95 | 95 | ||
| Laboratory examination | ||||||
| Serum creatinine, umol/L | 82.00 [62.00, 123.33] | 84.00 [65.25, 169.00] | 0.679 | 65.00 [54.00, 82.50] | 62.00 [53.00, 83.50] | 0.642 |
| eGFR | 86.53 [54.27, 119.71] | 87.62 [40.09, 106.46] | 0.757 | 110.80 [87.56, 120.49] | 111.01 [81.70, 121.66] | 0.614 |
| Serum albumin, g/L | 26.15 (6.62) | 35.96 (8.42) | <0.001 | 33.20 [29.70, 36.95] | 39.20 [35.70, 41.50] | <0.001 |
| C3, g/L | 0.95 (0.19) | 1.08 (0.23) | 0.096 | 0.90 (0.26) | 1.04 (0.28) | <0.001 |
| C4, g/L | 0.23 [0.14, 0.25] | 0.26 [0.22, 0.29] | 0.070 | 0.18 [0.11, 0.24] | 0.26 [0.18, 0.34] | <0.001 |
| IgA, g/L | 2.16 [1.75, 2.48] | 1.99 [1.63, 2.63] | 0.667 | 2.36 [1.75, 3.13] | 1.90 [1.58, 2.70] | 0.018 |
| IgG, g/L | 7.43 [4.95, 8.67] | 8.86 [7.04, 11.56] | 0.117 | 11.30 [8.68, 13.65] | 10.18 [8.45, 12.42] | 0.181 |
| IgM, g/L | 0.54 [0.44, 0.72] | 0.44 [0.33, 0.82] | 0.352 | 0.71 [0.46, 1.10] | 0.58 [0.34, 0.84] | 0.014 |
| Lupus anticoagulant | 1.11 [1.06, 1.24] | 1.18 [1.03, 1.41] | 0.617 | 1.10 [1.02, 1.22] | 1.16 [1.03, 1.31] | 0.142 |
| Proteinuria, g/24h | 4.42 [4.30, 5.59] | 0.80 [0.31, 3.35] | 0.001 | 0.94 [0.33, 1.99] | 0.31 [0.16, 1.28] | <0.001 |
| Urine WBC, WBC/ul | 24.00 [12.00, 45.00] | 18.57 [4.29, 31.00] | 0.134 | 12.00 [5.86, 40.00] | 9.00 [3.23, 28.75] | 0.100 |
| Urine RBC, RBC/ul | 20.00 [9.00, 59.00] | 16.00 [6.00, 25.00] | 0.407 | 11.00 [4.00, 49.00] | 7.00 [3.00, 17.72] | 0.010 |
| Positive ANA | 17 (100.0) | 16 (94.1) | 1.000 | 90 (94.7) | 81 (85.3) | 0.051 |
| Positive anti-dsDNA | 7 (41.2) | 5 (29.4) | 0.721 | 51 (53.7) | 45 (47.4) | 0.468 |
| Positive anti-Sm | 6 (35.3) | 2 (11.8) | 0.225 | 25 (26.3) | 13 (13.7) | 0.045 |
| Positive anti-ssA | 6 (35.3) | 10 (58.8) | 0.303 | 56 (58.9) | 42 (44.2) | 0.059 |
| Positive anti-ssB | 1 (5.9) | 3 (17.6) | 0.601 | 17 (17.9) | 11 (11.6) | 0.306 |
| Therapy | ||||||
| ATM (%) | 10 (58.8) | 8 (47.1) | 0.732 | 75 (78.9) | 53 (55.8) | 0.001 |
| AZA (%) | 17 (100.0) | 17 (100.0) | 1.000 | 1 (1.1) | 1 (1.1) | 1.000 |
| CNI (%) | 8 (47.1) | 4 (23.5) | 0.282 | 46 (48.4) | 28 (29.5) | 0.011 |
| CTX (%) | 5 (29.4) | 1 (5.9) | 0.175 | 8 (8.4) | 2 (2.1) | 0.100 |
| LEF (%) | 2 (11.8) | 2 (11.8) | 1.000 | 6 (6.3) | 4 (4.2) | 0.747 |
| MMF (%) | 4 (23.5) | 5 (29.4) | 1.000 | 47 (49.5) | 38 (40.0) | 0.243 |
| RAASi (%) | 13 (76.5) | 9 (52.9) | 0.282 | 44 (46.3) | 27 (28.4) | 0.016 |
| Steroid (%) | 17 (100.0) | 7 (41.2) | <0.001 | 91 (95.8) | 54 (56.8) | <0.001 |
| Dosage of steroid, mg/day | 28.33 [10.00, 38.00] | 0.00 [0.00, 10.00] | <0.001 | 12.50 [6.25, 22.25] | 5.00 [0.00, 7.50] | <0.001 |
| Factor . | Proteinuria ≥ 3.5 g/24 h . | Proteinuria <3.5 g/24 h . | ||||
|---|---|---|---|---|---|---|
| Before treatment . | After treatment . | P value . | Before treatment . | After treatment . | P value . | |
| Number of patients | 17 | 17 | 95 | 95 | ||
| Laboratory examination | ||||||
| Serum creatinine, umol/L | 82.00 [62.00, 123.33] | 84.00 [65.25, 169.00] | 0.679 | 65.00 [54.00, 82.50] | 62.00 [53.00, 83.50] | 0.642 |
| eGFR | 86.53 [54.27, 119.71] | 87.62 [40.09, 106.46] | 0.757 | 110.80 [87.56, 120.49] | 111.01 [81.70, 121.66] | 0.614 |
| Serum albumin, g/L | 26.15 (6.62) | 35.96 (8.42) | <0.001 | 33.20 [29.70, 36.95] | 39.20 [35.70, 41.50] | <0.001 |
| C3, g/L | 0.95 (0.19) | 1.08 (0.23) | 0.096 | 0.90 (0.26) | 1.04 (0.28) | <0.001 |
| C4, g/L | 0.23 [0.14, 0.25] | 0.26 [0.22, 0.29] | 0.070 | 0.18 [0.11, 0.24] | 0.26 [0.18, 0.34] | <0.001 |
| IgA, g/L | 2.16 [1.75, 2.48] | 1.99 [1.63, 2.63] | 0.667 | 2.36 [1.75, 3.13] | 1.90 [1.58, 2.70] | 0.018 |
| IgG, g/L | 7.43 [4.95, 8.67] | 8.86 [7.04, 11.56] | 0.117 | 11.30 [8.68, 13.65] | 10.18 [8.45, 12.42] | 0.181 |
| IgM, g/L | 0.54 [0.44, 0.72] | 0.44 [0.33, 0.82] | 0.352 | 0.71 [0.46, 1.10] | 0.58 [0.34, 0.84] | 0.014 |
| Lupus anticoagulant | 1.11 [1.06, 1.24] | 1.18 [1.03, 1.41] | 0.617 | 1.10 [1.02, 1.22] | 1.16 [1.03, 1.31] | 0.142 |
| Proteinuria, g/24h | 4.42 [4.30, 5.59] | 0.80 [0.31, 3.35] | 0.001 | 0.94 [0.33, 1.99] | 0.31 [0.16, 1.28] | <0.001 |
| Urine WBC, WBC/ul | 24.00 [12.00, 45.00] | 18.57 [4.29, 31.00] | 0.134 | 12.00 [5.86, 40.00] | 9.00 [3.23, 28.75] | 0.100 |
| Urine RBC, RBC/ul | 20.00 [9.00, 59.00] | 16.00 [6.00, 25.00] | 0.407 | 11.00 [4.00, 49.00] | 7.00 [3.00, 17.72] | 0.010 |
| Positive ANA | 17 (100.0) | 16 (94.1) | 1.000 | 90 (94.7) | 81 (85.3) | 0.051 |
| Positive anti-dsDNA | 7 (41.2) | 5 (29.4) | 0.721 | 51 (53.7) | 45 (47.4) | 0.468 |
| Positive anti-Sm | 6 (35.3) | 2 (11.8) | 0.225 | 25 (26.3) | 13 (13.7) | 0.045 |
| Positive anti-ssA | 6 (35.3) | 10 (58.8) | 0.303 | 56 (58.9) | 42 (44.2) | 0.059 |
| Positive anti-ssB | 1 (5.9) | 3 (17.6) | 0.601 | 17 (17.9) | 11 (11.6) | 0.306 |
| Therapy | ||||||
| ATM (%) | 10 (58.8) | 8 (47.1) | 0.732 | 75 (78.9) | 53 (55.8) | 0.001 |
| AZA (%) | 17 (100.0) | 17 (100.0) | 1.000 | 1 (1.1) | 1 (1.1) | 1.000 |
| CNI (%) | 8 (47.1) | 4 (23.5) | 0.282 | 46 (48.4) | 28 (29.5) | 0.011 |
| CTX (%) | 5 (29.4) | 1 (5.9) | 0.175 | 8 (8.4) | 2 (2.1) | 0.100 |
| LEF (%) | 2 (11.8) | 2 (11.8) | 1.000 | 6 (6.3) | 4 (4.2) | 0.747 |
| MMF (%) | 4 (23.5) | 5 (29.4) | 1.000 | 47 (49.5) | 38 (40.0) | 0.243 |
| RAASi (%) | 13 (76.5) | 9 (52.9) | 0.282 | 44 (46.3) | 27 (28.4) | 0.016 |
| Steroid (%) | 17 (100.0) | 7 (41.2) | <0.001 | 91 (95.8) | 54 (56.8) | <0.001 |
| Dosage of steroid, mg/day | 28.33 [10.00, 38.00] | 0.00 [0.00, 10.00] | <0.001 | 12.50 [6.25, 22.25] | 5.00 [0.00, 7.50] | <0.001 |
Values for numeric data are presented as mean (standard deviation) or median [interquartile range]; for categorical data, as count (percent).
AI: activity NIH index; ANA: antinuclear antibodies; ATM: hydroxychloroquine; AZA: azathioprine; C3: complement factor 3; C4: complement factor 4; CI: chronicity NIH index; CNI: calcineurin inhibitor; CTX: cyclophosphamide; dsDNA: double-stranded DNA; eGFR: estimated glomerular filtration rate; IgA: Immunoglobulin A; IgG: Immunoglobulin G; IgM: Immunoglobulin M; ISN/RPS: International Society of Nephrology/Renal Pathology Society; LEF: leflunomide; MMF: mycophenolate mofetil; RAASi: renin-angiotensin-aldosterone system inhibitors; RBC, red blood cells; ssA: Sjögren's-syndrome-related antigen A; ssB: Sjögren's-syndrome-related antigen B; WBC: white blood cells.
Stratification according to baseline proteinuria, comparison of baseline and last follow-up clinical parameters and drug in patients treated with belimumab
| Factor . | Proteinuria ≥ 3.5 g/24 h . | Proteinuria <3.5 g/24 h . | ||||
|---|---|---|---|---|---|---|
| Before treatment . | After treatment . | P value . | Before treatment . | After treatment . | P value . | |
| Number of patients | 17 | 17 | 95 | 95 | ||
| Laboratory examination | ||||||
| Serum creatinine, umol/L | 82.00 [62.00, 123.33] | 84.00 [65.25, 169.00] | 0.679 | 65.00 [54.00, 82.50] | 62.00 [53.00, 83.50] | 0.642 |
| eGFR | 86.53 [54.27, 119.71] | 87.62 [40.09, 106.46] | 0.757 | 110.80 [87.56, 120.49] | 111.01 [81.70, 121.66] | 0.614 |
| Serum albumin, g/L | 26.15 (6.62) | 35.96 (8.42) | <0.001 | 33.20 [29.70, 36.95] | 39.20 [35.70, 41.50] | <0.001 |
| C3, g/L | 0.95 (0.19) | 1.08 (0.23) | 0.096 | 0.90 (0.26) | 1.04 (0.28) | <0.001 |
| C4, g/L | 0.23 [0.14, 0.25] | 0.26 [0.22, 0.29] | 0.070 | 0.18 [0.11, 0.24] | 0.26 [0.18, 0.34] | <0.001 |
| IgA, g/L | 2.16 [1.75, 2.48] | 1.99 [1.63, 2.63] | 0.667 | 2.36 [1.75, 3.13] | 1.90 [1.58, 2.70] | 0.018 |
| IgG, g/L | 7.43 [4.95, 8.67] | 8.86 [7.04, 11.56] | 0.117 | 11.30 [8.68, 13.65] | 10.18 [8.45, 12.42] | 0.181 |
| IgM, g/L | 0.54 [0.44, 0.72] | 0.44 [0.33, 0.82] | 0.352 | 0.71 [0.46, 1.10] | 0.58 [0.34, 0.84] | 0.014 |
| Lupus anticoagulant | 1.11 [1.06, 1.24] | 1.18 [1.03, 1.41] | 0.617 | 1.10 [1.02, 1.22] | 1.16 [1.03, 1.31] | 0.142 |
| Proteinuria, g/24h | 4.42 [4.30, 5.59] | 0.80 [0.31, 3.35] | 0.001 | 0.94 [0.33, 1.99] | 0.31 [0.16, 1.28] | <0.001 |
| Urine WBC, WBC/ul | 24.00 [12.00, 45.00] | 18.57 [4.29, 31.00] | 0.134 | 12.00 [5.86, 40.00] | 9.00 [3.23, 28.75] | 0.100 |
| Urine RBC, RBC/ul | 20.00 [9.00, 59.00] | 16.00 [6.00, 25.00] | 0.407 | 11.00 [4.00, 49.00] | 7.00 [3.00, 17.72] | 0.010 |
| Positive ANA | 17 (100.0) | 16 (94.1) | 1.000 | 90 (94.7) | 81 (85.3) | 0.051 |
| Positive anti-dsDNA | 7 (41.2) | 5 (29.4) | 0.721 | 51 (53.7) | 45 (47.4) | 0.468 |
| Positive anti-Sm | 6 (35.3) | 2 (11.8) | 0.225 | 25 (26.3) | 13 (13.7) | 0.045 |
| Positive anti-ssA | 6 (35.3) | 10 (58.8) | 0.303 | 56 (58.9) | 42 (44.2) | 0.059 |
| Positive anti-ssB | 1 (5.9) | 3 (17.6) | 0.601 | 17 (17.9) | 11 (11.6) | 0.306 |
| Therapy | ||||||
| ATM (%) | 10 (58.8) | 8 (47.1) | 0.732 | 75 (78.9) | 53 (55.8) | 0.001 |
| AZA (%) | 17 (100.0) | 17 (100.0) | 1.000 | 1 (1.1) | 1 (1.1) | 1.000 |
| CNI (%) | 8 (47.1) | 4 (23.5) | 0.282 | 46 (48.4) | 28 (29.5) | 0.011 |
| CTX (%) | 5 (29.4) | 1 (5.9) | 0.175 | 8 (8.4) | 2 (2.1) | 0.100 |
| LEF (%) | 2 (11.8) | 2 (11.8) | 1.000 | 6 (6.3) | 4 (4.2) | 0.747 |
| MMF (%) | 4 (23.5) | 5 (29.4) | 1.000 | 47 (49.5) | 38 (40.0) | 0.243 |
| RAASi (%) | 13 (76.5) | 9 (52.9) | 0.282 | 44 (46.3) | 27 (28.4) | 0.016 |
| Steroid (%) | 17 (100.0) | 7 (41.2) | <0.001 | 91 (95.8) | 54 (56.8) | <0.001 |
| Dosage of steroid, mg/day | 28.33 [10.00, 38.00] | 0.00 [0.00, 10.00] | <0.001 | 12.50 [6.25, 22.25] | 5.00 [0.00, 7.50] | <0.001 |
| Factor . | Proteinuria ≥ 3.5 g/24 h . | Proteinuria <3.5 g/24 h . | ||||
|---|---|---|---|---|---|---|
| Before treatment . | After treatment . | P value . | Before treatment . | After treatment . | P value . | |
| Number of patients | 17 | 17 | 95 | 95 | ||
| Laboratory examination | ||||||
| Serum creatinine, umol/L | 82.00 [62.00, 123.33] | 84.00 [65.25, 169.00] | 0.679 | 65.00 [54.00, 82.50] | 62.00 [53.00, 83.50] | 0.642 |
| eGFR | 86.53 [54.27, 119.71] | 87.62 [40.09, 106.46] | 0.757 | 110.80 [87.56, 120.49] | 111.01 [81.70, 121.66] | 0.614 |
| Serum albumin, g/L | 26.15 (6.62) | 35.96 (8.42) | <0.001 | 33.20 [29.70, 36.95] | 39.20 [35.70, 41.50] | <0.001 |
| C3, g/L | 0.95 (0.19) | 1.08 (0.23) | 0.096 | 0.90 (0.26) | 1.04 (0.28) | <0.001 |
| C4, g/L | 0.23 [0.14, 0.25] | 0.26 [0.22, 0.29] | 0.070 | 0.18 [0.11, 0.24] | 0.26 [0.18, 0.34] | <0.001 |
| IgA, g/L | 2.16 [1.75, 2.48] | 1.99 [1.63, 2.63] | 0.667 | 2.36 [1.75, 3.13] | 1.90 [1.58, 2.70] | 0.018 |
| IgG, g/L | 7.43 [4.95, 8.67] | 8.86 [7.04, 11.56] | 0.117 | 11.30 [8.68, 13.65] | 10.18 [8.45, 12.42] | 0.181 |
| IgM, g/L | 0.54 [0.44, 0.72] | 0.44 [0.33, 0.82] | 0.352 | 0.71 [0.46, 1.10] | 0.58 [0.34, 0.84] | 0.014 |
| Lupus anticoagulant | 1.11 [1.06, 1.24] | 1.18 [1.03, 1.41] | 0.617 | 1.10 [1.02, 1.22] | 1.16 [1.03, 1.31] | 0.142 |
| Proteinuria, g/24h | 4.42 [4.30, 5.59] | 0.80 [0.31, 3.35] | 0.001 | 0.94 [0.33, 1.99] | 0.31 [0.16, 1.28] | <0.001 |
| Urine WBC, WBC/ul | 24.00 [12.00, 45.00] | 18.57 [4.29, 31.00] | 0.134 | 12.00 [5.86, 40.00] | 9.00 [3.23, 28.75] | 0.100 |
| Urine RBC, RBC/ul | 20.00 [9.00, 59.00] | 16.00 [6.00, 25.00] | 0.407 | 11.00 [4.00, 49.00] | 7.00 [3.00, 17.72] | 0.010 |
| Positive ANA | 17 (100.0) | 16 (94.1) | 1.000 | 90 (94.7) | 81 (85.3) | 0.051 |
| Positive anti-dsDNA | 7 (41.2) | 5 (29.4) | 0.721 | 51 (53.7) | 45 (47.4) | 0.468 |
| Positive anti-Sm | 6 (35.3) | 2 (11.8) | 0.225 | 25 (26.3) | 13 (13.7) | 0.045 |
| Positive anti-ssA | 6 (35.3) | 10 (58.8) | 0.303 | 56 (58.9) | 42 (44.2) | 0.059 |
| Positive anti-ssB | 1 (5.9) | 3 (17.6) | 0.601 | 17 (17.9) | 11 (11.6) | 0.306 |
| Therapy | ||||||
| ATM (%) | 10 (58.8) | 8 (47.1) | 0.732 | 75 (78.9) | 53 (55.8) | 0.001 |
| AZA (%) | 17 (100.0) | 17 (100.0) | 1.000 | 1 (1.1) | 1 (1.1) | 1.000 |
| CNI (%) | 8 (47.1) | 4 (23.5) | 0.282 | 46 (48.4) | 28 (29.5) | 0.011 |
| CTX (%) | 5 (29.4) | 1 (5.9) | 0.175 | 8 (8.4) | 2 (2.1) | 0.100 |
| LEF (%) | 2 (11.8) | 2 (11.8) | 1.000 | 6 (6.3) | 4 (4.2) | 0.747 |
| MMF (%) | 4 (23.5) | 5 (29.4) | 1.000 | 47 (49.5) | 38 (40.0) | 0.243 |
| RAASi (%) | 13 (76.5) | 9 (52.9) | 0.282 | 44 (46.3) | 27 (28.4) | 0.016 |
| Steroid (%) | 17 (100.0) | 7 (41.2) | <0.001 | 91 (95.8) | 54 (56.8) | <0.001 |
| Dosage of steroid, mg/day | 28.33 [10.00, 38.00] | 0.00 [0.00, 10.00] | <0.001 | 12.50 [6.25, 22.25] | 5.00 [0.00, 7.50] | <0.001 |
Values for numeric data are presented as mean (standard deviation) or median [interquartile range]; for categorical data, as count (percent).
AI: activity NIH index; ANA: antinuclear antibodies; ATM: hydroxychloroquine; AZA: azathioprine; C3: complement factor 3; C4: complement factor 4; CI: chronicity NIH index; CNI: calcineurin inhibitor; CTX: cyclophosphamide; dsDNA: double-stranded DNA; eGFR: estimated glomerular filtration rate; IgA: Immunoglobulin A; IgG: Immunoglobulin G; IgM: Immunoglobulin M; ISN/RPS: International Society of Nephrology/Renal Pathology Society; LEF: leflunomide; MMF: mycophenolate mofetil; RAASi: renin-angiotensin-aldosterone system inhibitors; RBC, red blood cells; ssA: Sjögren's-syndrome-related antigen A; ssB: Sjögren's-syndrome-related antigen B; WBC: white blood cells.
In terms of therapeutic medication, the proportion of patients receiving steroid (P < 0.001, P < 0.001) and the daily dose of steroid (P < 0.001, P < 0.001) at the last follow-up were significantly reduced in both groups with different proteinuria levels than at baseline. In the group with proteinuria <3.5 g/24 h, after receiving belimumab treatment, the proportion of patients who received hydroxychloroquine (P = 0.001) and daily therapeutic dose (P < 0.001), the proportion of patients who received CNI (P = 0.011) and daily therapeutic dose (P = 0.010), as well as the proportion of patients who received RAASI (P = 0.016) and daily therapeutic dose (P = 0.027) were significantly reduced from baseline, whereas there was no significant difference before and after treatment with belimumab in the group with proteinuria ≥3.5 g/24 h (Table 4).
Discussion
Despite receiving standard treatment, there are still some LN patients who cannot achieve complete remission and have poor long-term prognosis [17]. Multiple phase III clinical trials have confirmed that add-on treatment with belimumab improves renal response in patients [7, 18], and was approved for the treatment of adult patients with active lupus nephritis in 2019. However, clinical trials use strict inclusion and exclusion criteria, and real-world patients often struggle to fully meet the criteria. There are currently fewer studies of belimumab treatment in the real world. This study collected data from LN patients who received standard therapy and add-on treatment with belimumab at our hospital, providing real-world evidence of the efficacy and safety of add-on treatment with belimumab.
Our study showed that despite being relatively more severe at baseline (relatively low serum albumin and relatively high proteinuria), LN patients who received add-on treatment with belimumab had more significant improvement in overall disease activity indicators such as serum albumin and complement levels, significantly lower B-cell count, IgA, IgM, IgG, and earlier first attainment of partial and complete remission compared with patients who received standard treatment only, but there was no significant improvement in renal function during the 2-year follow-up period. This is not completely consistent with the previous studies. A post hoc study of BLISS-LN [19] showed that the addition of belimumab to standard therapy significantly controlled disease activity in lupus nephritis and reduced the risk of eGFR decline. However, Tan et al. [20] compared changes in relevant markers before and after treatment with belimumab and found that the biomarkers of disease activity, such as proteinuria, urine RBC and urine WBC, were significantly decreased, while markers of renal function, such as serum creatinine and eGFR, remained stable during the follow-up period. We believe that the inconsistency between the results of clinical trials and retrospective studies may be due to the short period of time that belimumab has been approved for the treatment of LN, and the short follow-up period of patients after receiving belimumab, which needs to be clarified by longer-term clinical practice and further studies.
A post-hoc pooled analysis of BLISS-52 and BLISS-76 by Dooley et al. found that within 52 weeks, patients receiving belimumab had numerically lower renal flare rates compared with patients in control group, but there was no significant difference between groups [21]. Our study similarly showed that add-on treatment with belimumab had no significant effect on reducing the risk of renal flare and kidney-related events or death during the 2-year follow-up period. However, BLISS-LN [7] and its post hoc study [19] found that patients treated with belimumab had a significantly lower risk of renal flare and kidney-related events or death than patients treated with placebo. This may be related to the different baseline characteristics of the study cohort: BLISS-LN only included patients with active lupus nephritis, whose baseline urinary protein-creatinine ratio was >1. The median level of proteinuria in the study population was relatively high, and all patients were initiated on treatment after a recent diagnosis of lupus nephritis, whereas our study included all patients receiving add-on therapy with belimumab in different states of disease activity, which was more in line with everyday clinical practice situations. Nearly half of patients in our study cohort achieved clinical remission with standard therapy and then received add-on therapy with belimumab due to relapse. In addition, in our study, patients receiving add-on therapy with belimumab were relatively more severe at baseline compared with the control group, which may have contributed to the nonsignificant improvement in renal end point in patients receiving add-on therapy with belimumab. However, our study has the shortcomings of small sample size and short follow-up time, the effect of belimumab treatment on renal flare in patients needs further large-sample and long-term follow-up studies.
Previous studies have shown that baseline proteinuria level can predict the prognosis of LN patients. The higher the baseline urinary protein level, the later the time to achieve renal remission, and the poorer the long-term prognosis of the patients [22]. Therefore, we further analysed the treatment response to belimumab in patients with different proteinuria levels, and found that belimumab had a more prominent treatment effect in patients with low level of proteinuria, such as significant increases in C3, C4, significant reductions in B-cell count, IgA, IgM and urine RBC compared with baseline, and significant reductions in the use of therapeutic drugs such as hydroxychloroquine, CNI and RAASI. This may be related to the fact that high levels of proteinuria are often indicative of severe renal disease and may lead to increased excretion of belimumab through the kidney, reducing the blood concentration of the patient and reducing curative efficacy [19]. However, the small number of patients with high baseline urinary protein levels in this study may have led to a non-significant difference in the findings, the exact mechanism needs to be further investigated.
This study also showed that patients receiving add-on treatment with belimumab had a lower proportion of respiratory infections, and other common adverse events such as gastrointestinal tract infections, urinary tract infections, and neuropsychiatric changes did not significantly increase after treatment with belimumab. This may be related to the reduced need for steroid in patients after add-on treatment with belimumab. We found that the total dose of steroid from baseline to the time of achieving PRR was significantly less in patients receiving add-on therapy with belimumab than in the control group, and that the daily dose of steroid was progressively reduced during the 2 years of follow-up, with the mean daily dose of steroid being numerically less at the 24 month of follow-up and at the last follow-up compared with that of patients who were receiving only the standard therapy, although the between-group difference was not significant. Previous studies have also found that the use of belimumab can reduce the dose of steroid or even discontinue steroid [20, 23]. This study confirms that belimumab treatment has a favorable safety profile in LN patients, reduces steroid dosage, and decreases the risk of respiratory tract infections.
There are several limitations in this study. First, this was a retrospective observational study, and even after matching patients for disease activity and renal function at baseline, there were some differences in the baseline characteristics of the two groups. In addition, due to the lack of SLEDAI, BILAG and PGA scores in some patients at follow-up, SLEDAI, BILAG and PGA scores at baseline were not compared between the two groups. Second, the small sample size of patients treated with belimumab and the fact that some patients were excluded from the study due to a follow-up period of <3 months and some patients were lost to follow-up may have had an impact on the study. In addition, some patients in this study were clinically diagnosed with lupus nephritis and lacked renal pathological data, so the diagnosis of lupus nephritis could not be completely clarified, and the therapeutic effects of belimumab on different pathological types could not be compared, which needs to be further studied in the future.
In summary, our study provides evidence for the effectiveness and safety of belimumab in real-world clinical practice, confirming that combined treatment with belimumab provides better disease remission without increasing the risk of renal flare and kidney-related events or death during the 2-year follow-up period. In patients with lower proteinuria levels, the treatment effect of belimumab is more significant. In addition, it has a favorable safety profile, reduces the dosage of steroid, and decreases the risk of respiratory tract infections.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
Due to ethical considerations, the data used in this study are not publicly available. For any further inquiries regarding the data, interested individuals can contact the corresponding author.
Contribution statement
S.L.: collected the clinical and pathological data, analysed and drafted the manuscript. J.Z., Y.B.: made contribution to the conception and design, analysed and interpreted the data and revised the manuscript. X.Y., B.C., Y.L., Y.Z., X.D., Y.L., H.Z., B.S.: acquired the data and material support. C.C.: supervised the study, made contribution to the conception and design, provided the project funding, revised the manuscript, and finally approved the version of the manuscript for publication.
Funding
This research was funded by a grant from the Natural Science Foundation of Zhejiang Province (No. LY22H050004).
Disclosure statement: The authors have declared no conflicts of interest.
Acknowledgements
The authors would like to thank their colleagues at the Department of Nephrology, the First Affiliated Hospital of Wenzhou Medical University, for their support and assistance during the study period.




Comments