-
PDF
- Split View
-
Views
-
Cite
Cite
Arthur Bouchut, Raphael Lhote, Philippe Maksud, Thouraya Ben Salem, Anne Fustier, Quentin Moyon, Julien Haroche, Michael Soussan, Alexis Mathian, Miguel Hie, Zahir Amoura, Fleur Cohen Aubart, Prognostic value of hypermetabolic bone sarcoidosis observed by 18F-fluorodeoxyglucose positron emission tomography, Rheumatology, Volume 64, Issue 2, February 2025, Pages 607–613, https://doi.org/10.1093/rheumatology/keae019
Close - Share Icon Share
Abstract
Sarcoidosis is a multisystemic granulomatosis diagnosed mainly in young adults. 18F-fluorodeoxyglucose (18F-FDG) PET/CT is useful in sarcoidosis cases to search for a biopsiable site or assess disease activity.18F-FDG PET/CT can reveal bone hypermetabolism in sarcoidosis patients, even in the absence of osteoarticular symptoms. The aim of this study was to describe metabolic bone involvement in sarcoidosis patients and to evaluate its prognostic impact.
This was an observational, comparative, retrospective, monocentric study. Inclusion criteria were a confirmed diagnosis of sarcoidosis according to the World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) criteria and at least one 18F-FDG PET/CT scan during follow-up. Metabolic bone involvement of sarcoidosis was defined as focal bone hypermetabolism with no argument for a differential diagnosis of bone 18F-FDG uptake. Patients with and without bone involvement were compared.
Among the 175 included patients, 32 (18%) had metabolic bone involvement of sarcoidosis. The metabolic bone involvement was mainly axial and mostly without bone abnormalities on CT. Metabolic bone involvement was associated with intrathoracic and extrathoracic lymph node involvement and with a greater number of organs involved. Patients with metabolic bone involvement more frequently received corticosteroids, methotrexate and TNF-α inhibitors and a greater number of treatments. Relapse of sarcoidosis occurred sooner in patients with metabolic bone involvement.
These results suggest that metabolic bone involvement is associated with more diffuse and more severe sarcoidosis.
Patients with metabolic bone involvement of sarcoidosis had a greater number of organs involved.
Patients with metabolic bone involvement of sarcoidosis received more treatments.
Patients with metabolic bone involvement of sarcoidosis had a shorter time to relapse.
Introduction
Sarcoidosis is a multisystemic granulomatosis primarily affecting the lungs and lymph nodes in young patients [1, 2]. Bone involvement has been reported in 3–30% of sarcoidosis patients, depending on the imaging modality and organ involvement definition [3, 4]. 18F-fluorodeoxyglucose (18F-FDG) PET/CT has emerged as a valuable tool for sarcoidosis assessment [5–7]. 18F-FDG PET/CT use in sarcoidosis includes identification of biopsiable lesions [1, 8], assessment of myocardial involvement [9–11] and evaluation of organ-specific disease activity [12–17]. 18F-FDG PET/CT can reveal bone involvement in sarcoidosis even in asymptomatic patients [4, 18–21]. Bone involvement of sarcoidosis was initially described as lytic, pseudocystic, permeative or destructive on X-ray imaging, primarily affecting distal bones of the hands and feet [22, 23]. 18F-FDG PET/CT evaluation of sarcoidosis patients revealed a higher prevalence of bone lesions than previously believed, presenting as bone hypermetabolism often without CT abnormalities, more often involving the axial skeleton and long bones [24–27]. Radiographic bone lesions are a recognized adverse prognostic factor during the sarcoidosis course, as they have been associated with a more chronic evolution of the disease [5, 28]. However, the prognostic value of bone hypermetabolism during sarcoidosis has not been well evaluated. In addition, few data on the evolution of bone hypermetabolism under treatment are available.
The aim of this study was to determine the prevalence of bone hypermetabolism, describe radiologic lesions associated with bone hypermetabolism, compare the characteristics of patients with and without metabolic bone involvement of sarcoidosis and evaluate the impact of metabolic bone involvement on treatments and outcomes.
Patients and methods
Patients and study design
This was an observational, comparative, retrospective, monocentric study of prospectively collected data. Patients followed in a single tertiary care centre specializing in sarcoidosis in France were screened for the diagnosis of sarcoidosis through the French information system medicalization program, which records the medical codes of hospitalized patients (PMSI codes D86, G532 and/or M633). Medical records were manually reviewed to assess the sarcoidosis diagnosis. Patients were included if they had a diagnosis of sarcoidosis according to the definition of the joint statement of the American Thoracic Society, European Respiratory Society and World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG), including histological documentation of non-caseating granuloma and at least one 18F-FDG PET/CT scan was available at any time in the sarcoidosis course [5]. Exclusion criteria were lack of sufficient data, lack of histological documentation of granuloma, Löfgren syndrome or absence of sarcoidosis. The year of diagnosis was defined as the year of histological documentation of granuloma. Demographic, clinical, biological and radiological data, treatments and follow-up data were extracted from medical records.
In France, retrospective and monocentric studies based on information drawn from medical records do not require approval by institutional review boards. The study was performed in compliance with the Helsinki Declaration and European legislation on personal data protection.
18F-FDG PET/CT data and analysis
All 18F-FDG PET/CT reports were manually reviewed for bone 18F-FDG uptake. 18F-FDG PET/CT exams with bone 18F-FDG uptake were reassessed by a nuclear medicine physician, allowing confirmation and classification of bone 18F-FDG uptake and exclusion of bone uptake differential diagnoses. Bone hypermetabolism was classified as focal or diffuse. Bone uptake differential diagnoses were defined by the presence of features typical of osteoarthritis, fracture, osteonecrosis or any otherwise documented bone disease. Standardized uptake values were not analysed because of the diversity of 18F-FDG PET/CT acquisition parameters.
18F-FDG PET/CT exams with focal bone hypermetabolism were further reassessed by an osteoarticular radiologist and screened for abnormalities in bone structure on CT images at hypermetabolic locations. Structural bone lesions were defined in the area of hypermetabolic locations as abnormal segmental hypodense and/or hyperdense bone images compared with the surrounding bone that did not qualify as bone uptake differential diagnoses.
Definition of metabolic and structural bone involvement in sarcoidosis
Metabolic bone involvement of sarcoidosis was defined as focal bone hypermetabolism with no bone uptake differential diagnosis, thereby fulfilling the criteria for ‘at least probable’ bone sarcoidosis according to the 2014 WASOG organ definition criteria [29]. Structural bone involvement of sarcoidosis was defined as the presence of structural bone lesions at hypermetabolic locations. Patients with diffuse bone hypermetabolism were not considered to have metabolic bone involvement of sarcoidosis.
Radiological features of the metabolic bone involvement of sarcoidosis were assessed. Metabolic bone involvement of sarcoidosis was classified as axial when involving the skull, spine, sacrum, pelvis, sternum, clavicle, scapula or ribs and as appendicular otherwise. It was classified as monostotic when it involved one bone and as polyostotic when it involved two or more bones. Structural bone involvement was further classified as lytic, condensing and mixed when hypodense and hyperdense images coexisted.
Comparison of patients with and without bone involvement of sarcoidosis
Patients with and without metabolic bone involvement at any point in the disease history were compared regarding extraosseous organ involvement of sarcoidosis according to the 2014 WASOG definitions for organ involvement at any time during the disease and treatments received at any time during the disease [29].
Systemic outcomes of bone sarcoidosis involvement
Patients with available follow-up data were assessed for sarcoidosis relapse. An event was considered as sarcoidosis relapse when it was considered and treated as a sarcoidosis relapse in the medical records, there was at least one objective (biological, radiological and/or histological) element of progression and appropriate explorations were performed to exclude differential diagnoses when relapse presentation differed from the initial presentation at diagnosis of sarcoidosis. The occurrence of sarcoidosis relapse and time from diagnosis to relapse were assessed. Patients with and without metabolic bone involvement were compared.
Metabolic outcomes of bone sarcoidosis involvement
Additionally, evolution of metabolic bone involvement of sarcoidosis under treatment was assessed in all patients with metabolic bone involvement of sarcoidosis and 18F-FDG PET/CT follow-up by comparing the first 18F-FDG PET/CT with metabolic bone involvement with the last 18F-FDG PET/CT available under treatment. Evolution was classified as complete remission, partial remission, stability or progression. The evolution of structural bone involvement of sarcoidosis under treatment in patients with 18F-FDG PET/CT follow-up and structural bone involvement of sarcoidosis at any time during the disease was similarly classified.
Statistical analysis
Categorical variables are presented as number (%) and numerical variables are presented as median [interquartile range (IQR)] or mean (s.d.). Patients with and without metabolic bone involvement of sarcoidosis were compared using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Comparisons were made using the Fisher’s test and Mann–Whitney test as appropriate. Odds ratios (ORs) and 95% CIs were calculated, with and without adjustment for gender, age and disease duration. Relapse-free survival was presented using the Kaplan–Meier method and compared between groups using the logrank test. All tests were bilateral and P-values <0.05 were considered significant.
Results
Patients and study design
Among 498 screened patients, 233 (47%) had a confirmed sarcoidosis diagnosis (Fig. 1). Among the 233 patients, 175 (75%) with at least one 18F-FDG PET/CT scan were included. Patient characteristics are presented in Table 1. The mean age at diagnosis was 42 years (s.d. 14) and the female:male ratio was 0.93.
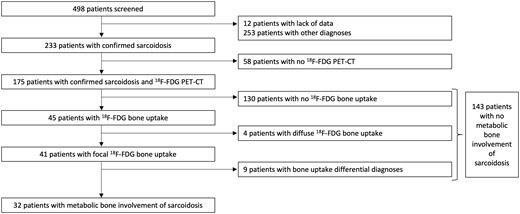
Flow chart of 498 patients screened for sarcoidosis through the French information system medicalization program
Demographic and follow-up characteristics of 175 patients with sarcoidosis and 18F-FDG PET/CT evaluation
| Characteristics . | Total (N = 175) . | Metabolic bone involvement (n = 32) . | No metabolic bone involvement (n = 143) . |
|---|---|---|---|
| Gender (male), n (%) | 91 (52) | 14 (44) | 77 (54) |
| Age at diagnosis (years), mean (s.d.) | 42 (14) | 46 (15) | 41 (14) |
| 18F-FDG PET/CT exams, n, mean (s.d.) | 2.3 (1.9) | 3.1 (2.6) | 2.1 (1.6) |
| Follow-upa, years, mean (s.d.) | 8.5 (7.4) | 8.4 (6.6) | 8.6 (7.6) |
| Characteristics . | Total (N = 175) . | Metabolic bone involvement (n = 32) . | No metabolic bone involvement (n = 143) . |
|---|---|---|---|
| Gender (male), n (%) | 91 (52) | 14 (44) | 77 (54) |
| Age at diagnosis (years), mean (s.d.) | 42 (14) | 46 (15) | 41 (14) |
| 18F-FDG PET/CT exams, n, mean (s.d.) | 2.3 (1.9) | 3.1 (2.6) | 2.1 (1.6) |
| Follow-upa, years, mean (s.d.) | 8.5 (7.4) | 8.4 (6.6) | 8.6 (7.6) |
Follow-up refers to the time from diagnosis to the last medical visit.
Demographic and follow-up characteristics of 175 patients with sarcoidosis and 18F-FDG PET/CT evaluation
| Characteristics . | Total (N = 175) . | Metabolic bone involvement (n = 32) . | No metabolic bone involvement (n = 143) . |
|---|---|---|---|
| Gender (male), n (%) | 91 (52) | 14 (44) | 77 (54) |
| Age at diagnosis (years), mean (s.d.) | 42 (14) | 46 (15) | 41 (14) |
| 18F-FDG PET/CT exams, n, mean (s.d.) | 2.3 (1.9) | 3.1 (2.6) | 2.1 (1.6) |
| Follow-upa, years, mean (s.d.) | 8.5 (7.4) | 8.4 (6.6) | 8.6 (7.6) |
| Characteristics . | Total (N = 175) . | Metabolic bone involvement (n = 32) . | No metabolic bone involvement (n = 143) . |
|---|---|---|---|
| Gender (male), n (%) | 91 (52) | 14 (44) | 77 (54) |
| Age at diagnosis (years), mean (s.d.) | 42 (14) | 46 (15) | 41 (14) |
| 18F-FDG PET/CT exams, n, mean (s.d.) | 2.3 (1.9) | 3.1 (2.6) | 2.1 (1.6) |
| Follow-upa, years, mean (s.d.) | 8.5 (7.4) | 8.4 (6.6) | 8.6 (7.6) |
Follow-up refers to the time from diagnosis to the last medical visit.
18F-FDG PE/-CT data and analysis
A total of 395 18F-FDG PET/CT exams of the 175 included patients were reviewed. Bone 18F-FDG uptake was found in 45 patients. Among these, 41 patients had focal bone hypermetabolism and 4 patients had diffuse bone hypermetabolism. Among the 41 patients with focal bone hypermetabolism, bone uptake differential diagnoses were found in 9 patients: 1 patient, Paget’s disease of bone; 1 patient, fibrous dysplasia of bone; 1 patient, lymphoma; 2 patients, hip osteonecrosis; 1 patient, rib fracture; 1 patient, synovial herniation pit; and 2 patients, osteoarthritis. Thus a total of 32 of 175 patients (18%) were diagnosed with bone involvement of sarcoidosis.
The characteristics of metabolic bone involvement in sarcoidosis are shown in Table 2. 18F-FDG PET/CT parameters included full-body acquisition in 17 of the 32 patients (53%). Among the 32 patients with metabolic bone involvement, axial involvement was found in 25 patients (78%) and appendicular involvement was found in 7 patients (22%). Metabolic bone involvement was polyostotic in 26 patients (81%). Nine of the 32 patients had structural bone involvement (28%). Lytic lesions were found in seven patients, condensing lesions in four patients and a mixed lesion in one patient. An example of a hypermetabolic and structural humeral lesion is shown in Fig. 2. Metabolic bone involvement of sarcoidosis was never isolated, as it coexisted with extraosseous 18F-FDG uptake in all patients, including 31 (97%) with lymph node 18F-FDG uptake.
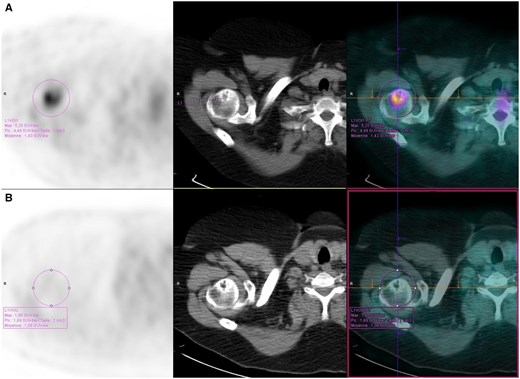
Hypermetabolic and osteolytic bone lesion of the proximal humerus on 18F-FDG PET/CT in a patient with sarcoidosis (A) before and (B) after treatment with infliximab. Left: 18F-FDG PET; middle: CT; right: 18F-FDG PET/CT
| Area . | Metabolic bone involvement (n = 32), n (%) . |
|---|---|
| Axial involvement | 25 (78) |
| Skull | 2 (6) |
| Spine | 15 (47) |
| Sacrum | 8 (25) |
| Pelvis | 15 (47) |
| Sternum | 4 (12) |
| Clavicle | 2 (6) |
| Scapula | 5 (16) |
| Ribs | 12 (37) |
| Appendicular involvement | 7 (22) |
| Humerus | 3 (9) |
| Femur | 5 (16) |
| Hands/feet | 4 (12) |
| Area . | Metabolic bone involvement (n = 32), n (%) . |
|---|---|
| Axial involvement | 25 (78) |
| Skull | 2 (6) |
| Spine | 15 (47) |
| Sacrum | 8 (25) |
| Pelvis | 15 (47) |
| Sternum | 4 (12) |
| Clavicle | 2 (6) |
| Scapula | 5 (16) |
| Ribs | 12 (37) |
| Appendicular involvement | 7 (22) |
| Humerus | 3 (9) |
| Femur | 5 (16) |
| Hands/feet | 4 (12) |
| Area . | Metabolic bone involvement (n = 32), n (%) . |
|---|---|
| Axial involvement | 25 (78) |
| Skull | 2 (6) |
| Spine | 15 (47) |
| Sacrum | 8 (25) |
| Pelvis | 15 (47) |
| Sternum | 4 (12) |
| Clavicle | 2 (6) |
| Scapula | 5 (16) |
| Ribs | 12 (37) |
| Appendicular involvement | 7 (22) |
| Humerus | 3 (9) |
| Femur | 5 (16) |
| Hands/feet | 4 (12) |
| Area . | Metabolic bone involvement (n = 32), n (%) . |
|---|---|
| Axial involvement | 25 (78) |
| Skull | 2 (6) |
| Spine | 15 (47) |
| Sacrum | 8 (25) |
| Pelvis | 15 (47) |
| Sternum | 4 (12) |
| Clavicle | 2 (6) |
| Scapula | 5 (16) |
| Ribs | 12 (37) |
| Appendicular involvement | 7 (22) |
| Humerus | 3 (9) |
| Femur | 5 (16) |
| Hands/feet | 4 (12) |
Description of patients with metabolic bone involvement of sarcoidosis
Six patients underwent bone or bone marrow biopsy: two had a bone biopsy, which revealed granulomas in both individuals, and four had an unguided bone marrow biopsy, which revealed granulomas in one individual (Supplementary Table S1, available at Rheumatology online). The indication for systemic treatment was extraosseous or multisystemic involvement of sarcoidosis in 30 patients (94%) and bone involvement in 2 patients (6%).
No fracture was reported on bone lesions in patients with metabolic bone involvement of sarcoidosis. However, one patient underwent preventive osteosynthesis of the femoral neck because of a lytic structural lesion considered at risk of fracture.
Comparison of patients with and without bone involvement of sarcoidosis
Sarcoidosis organ involvement and treatments received by patients with and without metabolic bone involvement of sarcoidosis are shown in Table 3. Intrathoracic lymph node involvement was more frequent in patients with metabolic bone involvement of sarcoidosis than in patients without [31 (97%) and 112 (78%), respectively; OR 7.5 (95% CI 1.5, 182.5), P < 0.001]. Extrathoracic lymph node involvement was also more frequent in this population [17 (53%) and 45 (31%); OR 2.5 (95% CI 1.1, 5.4), P < 0.04]. Other organ involvements did not differ significantly between the two groups. The number of organ involvements was significantly higher in patients with metabolic bone involvement than in patients without [median 5.5 (IQR 4.0–7.0) and 4.0 (3.0–5.0), respectively; P < 0.0001], as shown in Fig. 3.
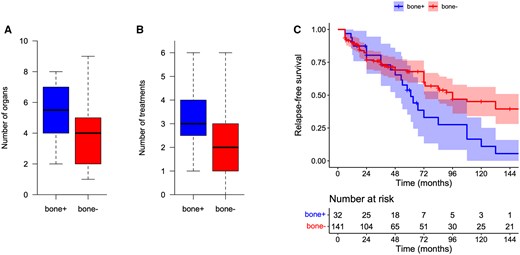
Comparison between patients with metabolic bone involvement of sarcoidosis and patients without metabolic bone involvement: (A) number of organ involvements, (B) number of treatments and (C) relapse-free survival. Bone+: patients with metabolic bone involvement; bone−: patients without metabolic bone involvement
Sarcoidosis organ involvement and treatments received in 175 patients with sarcoidosis
| Characteristic . | Total (N = 175) . | Metabolic bone involvement (n = 32) . | No metabolic bone involvement (n = 143) . | OR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| Number of organs, median (IQR) | 4.0 (3.0–5.2) | 5.5 (4.0–7.0) | 4.0 (2.0–5.0) | NA | <0.0001 |
| Intrathoracic lymph nodes | 143 (82) | 31 (97) | 112 (78) | 7.5 (1.5, 182.5) | <0.001 |
| Extrathoracic lymph nodes | 62 (25) | 17 (53) | 45 (31) | 2.5 (1.1, 5.4) | <0.04 |
| Lung (all stages) | 121 (69) | 22 (69) | 99 (69) | 1.0 (0.4, 2.3) | 0.96 |
| Lung (stage 4) | 15 (9) | 4 (13) | 11 (8) | 1.7 (0.4, 5.6) | 0.45 |
| Heart | 26 (15) | 7 (22) | 19 (13) | 1.8 (0.7, 4.7) | 0.29 |
| Muscle | 5 (3) | 2 (6) | 3 (2) | 3.1 (0.3, 21.6) | 0.36 |
| Eye | 39 (22) | 4 (13) | 35 (24) | 0.5 (0.1, 1.3) | 0.09 |
| Nervous system | 77 (44) | 13 (41) | 64 (45) | 0.8 (0.4, 1.8) | 0.67 |
| Skin | 45 (26) | 9 (28) | 36 (25) | 1.2 (0.5, 2.7) | 0.74 |
| Joints | 24 (14) | 8 (25) | 16 (11) | 2.6 (0.9, 6.8) | 0.10 |
| Liver | 33 (19) | 8 (25) | 25 (17) | 1.6 (0.6, 3.9) | 0.38 |
| Spleen | 18 (10) | 4 (13) | 14 (10) | 1.3 (0.3, 4.1) | 0.67 |
| Kidney | 14 (8) | 1 (3) | 13 (9) | 0.4 (0.0, 2.0) | 0.13 |
| Digestive tract | 5 (3) | 1 (3) | 4 (3) | 1.2 (0.0, 9.3) | 0.92 |
| Hypercalcemia | 10 (6) | 2 (6) | 8 (6) | 1.2 (0.2, 5.2) | 0.89 |
| Ear, nose, throat | 49 (28) | 13 (41) | 36 (25) | 2.0 (0.9, 4.5) | 0.11 |
| Number of treatments, median (IQR) | 2.0 (1.0–3.0) | 3.0 (2.7–4.0) | 2.0 (1.0–3.0) | NA | <0.0001 |
| Corticosteroids | 138 (79) | 32 (100) | 106 (74) | NA | <0.0001 |
| Hydroxychloroquine | 31 (18) | 8 (25) | 23 (16) | 1.7 (0.7, 4.3) | 0.29 |
| Methotrexate | 97 (55) | 28 (88) | 69 (48) | 7.2 (2.6, 25.9) | <0.0001 |
| Azathioprine | 27 (15) | 3 (9) | 24 (17) | 0.5 (0.1, 1.7) | 0.23 |
| Mycophenolate | 17 (10) | 2 (6) | 15 (10) | 0.6 (0.1, 2.3) | 0.40 |
| Ciclosporin | 3 (2) | 1 (3) | 2 (1) | 2.4 (0.1, 30.5) | 0.60 |
| Cyclophosphamide | 22 (13) | 5 (16) | 17 (12) | 1.4 (0.4, 3.9) | 0.60 |
| TNFi | 63 (36) | 22 (69) | 41 (29) | 5.4 (2.4, 12.9) | <0.0001 |
| Rituximab | 2 (1) | 0 (0) | 2 (1) | NA | 0.15 |
| Tocilizumab | 2 (1) | 1 (3) | 1 (1) | 4.5 (0.1, 179.4) | 0.45 |
| Characteristic . | Total (N = 175) . | Metabolic bone involvement (n = 32) . | No metabolic bone involvement (n = 143) . | OR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| Number of organs, median (IQR) | 4.0 (3.0–5.2) | 5.5 (4.0–7.0) | 4.0 (2.0–5.0) | NA | <0.0001 |
| Intrathoracic lymph nodes | 143 (82) | 31 (97) | 112 (78) | 7.5 (1.5, 182.5) | <0.001 |
| Extrathoracic lymph nodes | 62 (25) | 17 (53) | 45 (31) | 2.5 (1.1, 5.4) | <0.04 |
| Lung (all stages) | 121 (69) | 22 (69) | 99 (69) | 1.0 (0.4, 2.3) | 0.96 |
| Lung (stage 4) | 15 (9) | 4 (13) | 11 (8) | 1.7 (0.4, 5.6) | 0.45 |
| Heart | 26 (15) | 7 (22) | 19 (13) | 1.8 (0.7, 4.7) | 0.29 |
| Muscle | 5 (3) | 2 (6) | 3 (2) | 3.1 (0.3, 21.6) | 0.36 |
| Eye | 39 (22) | 4 (13) | 35 (24) | 0.5 (0.1, 1.3) | 0.09 |
| Nervous system | 77 (44) | 13 (41) | 64 (45) | 0.8 (0.4, 1.8) | 0.67 |
| Skin | 45 (26) | 9 (28) | 36 (25) | 1.2 (0.5, 2.7) | 0.74 |
| Joints | 24 (14) | 8 (25) | 16 (11) | 2.6 (0.9, 6.8) | 0.10 |
| Liver | 33 (19) | 8 (25) | 25 (17) | 1.6 (0.6, 3.9) | 0.38 |
| Spleen | 18 (10) | 4 (13) | 14 (10) | 1.3 (0.3, 4.1) | 0.67 |
| Kidney | 14 (8) | 1 (3) | 13 (9) | 0.4 (0.0, 2.0) | 0.13 |
| Digestive tract | 5 (3) | 1 (3) | 4 (3) | 1.2 (0.0, 9.3) | 0.92 |
| Hypercalcemia | 10 (6) | 2 (6) | 8 (6) | 1.2 (0.2, 5.2) | 0.89 |
| Ear, nose, throat | 49 (28) | 13 (41) | 36 (25) | 2.0 (0.9, 4.5) | 0.11 |
| Number of treatments, median (IQR) | 2.0 (1.0–3.0) | 3.0 (2.7–4.0) | 2.0 (1.0–3.0) | NA | <0.0001 |
| Corticosteroids | 138 (79) | 32 (100) | 106 (74) | NA | <0.0001 |
| Hydroxychloroquine | 31 (18) | 8 (25) | 23 (16) | 1.7 (0.7, 4.3) | 0.29 |
| Methotrexate | 97 (55) | 28 (88) | 69 (48) | 7.2 (2.6, 25.9) | <0.0001 |
| Azathioprine | 27 (15) | 3 (9) | 24 (17) | 0.5 (0.1, 1.7) | 0.23 |
| Mycophenolate | 17 (10) | 2 (6) | 15 (10) | 0.6 (0.1, 2.3) | 0.40 |
| Ciclosporin | 3 (2) | 1 (3) | 2 (1) | 2.4 (0.1, 30.5) | 0.60 |
| Cyclophosphamide | 22 (13) | 5 (16) | 17 (12) | 1.4 (0.4, 3.9) | 0.60 |
| TNFi | 63 (36) | 22 (69) | 41 (29) | 5.4 (2.4, 12.9) | <0.0001 |
| Rituximab | 2 (1) | 0 (0) | 2 (1) | NA | 0.15 |
| Tocilizumab | 2 (1) | 1 (3) | 1 (1) | 4.5 (0.1, 179.4) | 0.45 |
Data are expressed as n (%) unless stated otherwise.
NA: not applicable.
Sarcoidosis organ involvement and treatments received in 175 patients with sarcoidosis
| Characteristic . | Total (N = 175) . | Metabolic bone involvement (n = 32) . | No metabolic bone involvement (n = 143) . | OR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| Number of organs, median (IQR) | 4.0 (3.0–5.2) | 5.5 (4.0–7.0) | 4.0 (2.0–5.0) | NA | <0.0001 |
| Intrathoracic lymph nodes | 143 (82) | 31 (97) | 112 (78) | 7.5 (1.5, 182.5) | <0.001 |
| Extrathoracic lymph nodes | 62 (25) | 17 (53) | 45 (31) | 2.5 (1.1, 5.4) | <0.04 |
| Lung (all stages) | 121 (69) | 22 (69) | 99 (69) | 1.0 (0.4, 2.3) | 0.96 |
| Lung (stage 4) | 15 (9) | 4 (13) | 11 (8) | 1.7 (0.4, 5.6) | 0.45 |
| Heart | 26 (15) | 7 (22) | 19 (13) | 1.8 (0.7, 4.7) | 0.29 |
| Muscle | 5 (3) | 2 (6) | 3 (2) | 3.1 (0.3, 21.6) | 0.36 |
| Eye | 39 (22) | 4 (13) | 35 (24) | 0.5 (0.1, 1.3) | 0.09 |
| Nervous system | 77 (44) | 13 (41) | 64 (45) | 0.8 (0.4, 1.8) | 0.67 |
| Skin | 45 (26) | 9 (28) | 36 (25) | 1.2 (0.5, 2.7) | 0.74 |
| Joints | 24 (14) | 8 (25) | 16 (11) | 2.6 (0.9, 6.8) | 0.10 |
| Liver | 33 (19) | 8 (25) | 25 (17) | 1.6 (0.6, 3.9) | 0.38 |
| Spleen | 18 (10) | 4 (13) | 14 (10) | 1.3 (0.3, 4.1) | 0.67 |
| Kidney | 14 (8) | 1 (3) | 13 (9) | 0.4 (0.0, 2.0) | 0.13 |
| Digestive tract | 5 (3) | 1 (3) | 4 (3) | 1.2 (0.0, 9.3) | 0.92 |
| Hypercalcemia | 10 (6) | 2 (6) | 8 (6) | 1.2 (0.2, 5.2) | 0.89 |
| Ear, nose, throat | 49 (28) | 13 (41) | 36 (25) | 2.0 (0.9, 4.5) | 0.11 |
| Number of treatments, median (IQR) | 2.0 (1.0–3.0) | 3.0 (2.7–4.0) | 2.0 (1.0–3.0) | NA | <0.0001 |
| Corticosteroids | 138 (79) | 32 (100) | 106 (74) | NA | <0.0001 |
| Hydroxychloroquine | 31 (18) | 8 (25) | 23 (16) | 1.7 (0.7, 4.3) | 0.29 |
| Methotrexate | 97 (55) | 28 (88) | 69 (48) | 7.2 (2.6, 25.9) | <0.0001 |
| Azathioprine | 27 (15) | 3 (9) | 24 (17) | 0.5 (0.1, 1.7) | 0.23 |
| Mycophenolate | 17 (10) | 2 (6) | 15 (10) | 0.6 (0.1, 2.3) | 0.40 |
| Ciclosporin | 3 (2) | 1 (3) | 2 (1) | 2.4 (0.1, 30.5) | 0.60 |
| Cyclophosphamide | 22 (13) | 5 (16) | 17 (12) | 1.4 (0.4, 3.9) | 0.60 |
| TNFi | 63 (36) | 22 (69) | 41 (29) | 5.4 (2.4, 12.9) | <0.0001 |
| Rituximab | 2 (1) | 0 (0) | 2 (1) | NA | 0.15 |
| Tocilizumab | 2 (1) | 1 (3) | 1 (1) | 4.5 (0.1, 179.4) | 0.45 |
| Characteristic . | Total (N = 175) . | Metabolic bone involvement (n = 32) . | No metabolic bone involvement (n = 143) . | OR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| Number of organs, median (IQR) | 4.0 (3.0–5.2) | 5.5 (4.0–7.0) | 4.0 (2.0–5.0) | NA | <0.0001 |
| Intrathoracic lymph nodes | 143 (82) | 31 (97) | 112 (78) | 7.5 (1.5, 182.5) | <0.001 |
| Extrathoracic lymph nodes | 62 (25) | 17 (53) | 45 (31) | 2.5 (1.1, 5.4) | <0.04 |
| Lung (all stages) | 121 (69) | 22 (69) | 99 (69) | 1.0 (0.4, 2.3) | 0.96 |
| Lung (stage 4) | 15 (9) | 4 (13) | 11 (8) | 1.7 (0.4, 5.6) | 0.45 |
| Heart | 26 (15) | 7 (22) | 19 (13) | 1.8 (0.7, 4.7) | 0.29 |
| Muscle | 5 (3) | 2 (6) | 3 (2) | 3.1 (0.3, 21.6) | 0.36 |
| Eye | 39 (22) | 4 (13) | 35 (24) | 0.5 (0.1, 1.3) | 0.09 |
| Nervous system | 77 (44) | 13 (41) | 64 (45) | 0.8 (0.4, 1.8) | 0.67 |
| Skin | 45 (26) | 9 (28) | 36 (25) | 1.2 (0.5, 2.7) | 0.74 |
| Joints | 24 (14) | 8 (25) | 16 (11) | 2.6 (0.9, 6.8) | 0.10 |
| Liver | 33 (19) | 8 (25) | 25 (17) | 1.6 (0.6, 3.9) | 0.38 |
| Spleen | 18 (10) | 4 (13) | 14 (10) | 1.3 (0.3, 4.1) | 0.67 |
| Kidney | 14 (8) | 1 (3) | 13 (9) | 0.4 (0.0, 2.0) | 0.13 |
| Digestive tract | 5 (3) | 1 (3) | 4 (3) | 1.2 (0.0, 9.3) | 0.92 |
| Hypercalcemia | 10 (6) | 2 (6) | 8 (6) | 1.2 (0.2, 5.2) | 0.89 |
| Ear, nose, throat | 49 (28) | 13 (41) | 36 (25) | 2.0 (0.9, 4.5) | 0.11 |
| Number of treatments, median (IQR) | 2.0 (1.0–3.0) | 3.0 (2.7–4.0) | 2.0 (1.0–3.0) | NA | <0.0001 |
| Corticosteroids | 138 (79) | 32 (100) | 106 (74) | NA | <0.0001 |
| Hydroxychloroquine | 31 (18) | 8 (25) | 23 (16) | 1.7 (0.7, 4.3) | 0.29 |
| Methotrexate | 97 (55) | 28 (88) | 69 (48) | 7.2 (2.6, 25.9) | <0.0001 |
| Azathioprine | 27 (15) | 3 (9) | 24 (17) | 0.5 (0.1, 1.7) | 0.23 |
| Mycophenolate | 17 (10) | 2 (6) | 15 (10) | 0.6 (0.1, 2.3) | 0.40 |
| Ciclosporin | 3 (2) | 1 (3) | 2 (1) | 2.4 (0.1, 30.5) | 0.60 |
| Cyclophosphamide | 22 (13) | 5 (16) | 17 (12) | 1.4 (0.4, 3.9) | 0.60 |
| TNFi | 63 (36) | 22 (69) | 41 (29) | 5.4 (2.4, 12.9) | <0.0001 |
| Rituximab | 2 (1) | 0 (0) | 2 (1) | NA | 0.15 |
| Tocilizumab | 2 (1) | 1 (3) | 1 (1) | 4.5 (0.1, 179.4) | 0.45 |
Data are expressed as n (%) unless stated otherwise.
NA: not applicable.
Patients with metabolic bone involvement received more corticosteroids [32 (100%) and 106 (74%), respectively; P < 0.0001], more methotrexate [28 (88%) and 69 (48%), respectively; OR 7.2 (95% CI 2.6, 25.9), P < 0.0001] and more TNF-α inhibitors (TNFis) [22 (69%) and 41 (29%), respectively; OR 5.4 (95% CI 2.4, 12.9), P < 0.0001]. The number of treatments received by the patients with metabolic bone involvement was greater than that received by the patients without metabolic bone involvement [3.0 (IQR 2.7–4.0) and 2.0 (1.0–3.0), respectively; P < 0.001], as shown in Fig. 3.
Adjustment for gender, age and disease duration did not significantly alter the results. Adjusted ORs and coefficient plots are available in Supplementary Table S2 and Figure S1, both available at Rheumatology online.
Systemic outcomes of bone sarcoidosis involvement
Follow-up data were available for all patients but two (1%), both without metabolic bone involvement of sarcoidosis. Sarcoidosis relapse occurred during follow-up in 23 of the 32 patients (72%) with metabolic bone involvement of sarcoidosis and in 68 of the 141 patients without (48%) [OR 2.8 (95% CI 1.2, 6.8), P < 0.02]. Kaplan–Meier curves of relapse-free survival are shown in Fig. 3. The median time to the first relapse was 61 months (IQR 48–84) and 96 months (IQR 73–156), respectively (P = 0.01).
Metabolic outcomes of bone sarcoidosis involvement
Among the 32 patients with metabolic bone involvement of sarcoidosis, 16 had 18F-FDG PET/CT follow-ups. Complete remission of metabolic bone involvement on the last 18F-FDG PET/CT under treatment was observed in 12 of the 16 patients (75%) and partial remission was observed in 4 of them (25%). Structural bone involvement was present in six patients (37%), which was stable in five patients and progressed in one patient under treatment. Fig. 2 shows an example of hypermetabolic remission and structural stability of a humeral lesion after treatment with infliximab.
Discussion
In this retrospective study of 175 patients with sarcoidosis evaluated by 18F-FDG PET/CT, metabolic bone involvement of sarcoidosis was found in 18% of patients, mainly without structural bone lesions on CT, and these cases responded to sarcoidosis treatment. Metabolic bone involvement was found to be associated with more organ involvement, more systemic treatments (including methotrexate and TNFi, which are the main immunosuppressive drugs used in sarcoidosis) and more relapses and thus seems to have a strong prognostic value.
Characteristics of metabolic bone involvement
Metabolic bone involvement of sarcoidosis was found in 18% of patients, which is consistent with the estimated prevalence of 3–30% in recent studies [1–3]. It was predominantly axial, as reported in several studies [4, 19–21, 29]. However, only 53% of 18F-FDG PET/CT exams involved a full-body acquisition, which could have led to an underestimation of hand and foot involvement.
Structural lesions were found at the sites of bone 18F-FDG uptake in 31% of patients with metabolic bone involvement of sarcoidosis, which is slightly more than the 0–6% reported in a recent series of comparable size [4, 21, 30]. These structural bone changes are thought to result from perigranuloma osteoclastic activation [23, 31, 32]. The fact that most patients did not have any structural lesions suggests that osteoclastic activation occurs inconstantly in patients with bone sarcoidosis or only in patients with advanced disease [4, 21].
Diffuse bone hypermetabolism has been associated with various other conditions that may occur in sarcoidosis patients (anaemia, hyperleukocytosis, biological inflammation, cancer, immunosuppressive therapy) and is not specific to sarcoidosis involvement [33, 34]. Focal bone hypermetabolism is also not specific, and CT may be useful to differentiate sarcoidosis involvement from other bone-uptake differential diagnoses. In our series, reviewing individual imaging data of patients with focal bone hypermetabolism revealed differential diagnoses for bone uptake in 22% of them, including osseous side effects of treatments and bone diseases other than sarcoidosis.
Metabolic bone lesions improved under treatment, which was retrospectively in favour of granulomatous lesions despite the histological documentation of bone lesions in a few patients. Two studies of sarcoidosis patients with seven follow-up 18F-FDG PET/CT exams each, one retrospective and one prospective, also found results in favour of bone 18F-FDG uptake regression under treatment [18, 21]. Similar results have been reported for unbiopsied MRI bone lesions in patients with histologically proven sarcoidosis [3].
Prognostic outcomes
In this study, patients with metabolic bone involvement of sarcoidosis had more organ involvement, received more treatments and had more relapses. The higher number of organ involvements indicates a more multisystemic disease and has been reported in one of two other studies [19, 21]. However, in this study only intrathoracic and extrathoracic lymph node involvement were more frequent in patients with bone involvement, which was observed in one and four other series, respectively, but did not account for the received treatments [19–21, 30].
Patients with metabolic bone involvement received more corticosteroids, methotrexate, TNFi and other treatments than patients without bone involvement. One other study reported that patients with bone involvement were more likely to be treated with infliximab [19]. Bone sarcoidosis was the main indication of treatment in two patients, while in all other cases, treatments were guided by extraosseous or multisystemic organ involvement.
Patients with metabolic bone involvement had more sarcoidosis relapses, which could explain the greater number of treatments. Radiographic bone sarcoidosis documented on conventional X-ray imaging has been associated with chronic evolution of the disease for several years. Our results suggest that metabolic bone involvement in sarcoidosis, even without structural abnormalities on X-ray imaging, is an indicator of a more resistant and more chronic disease.
Limitations
Our study has several limitations. It was a retrospective, monocentric study. The study population notably involved 44% of patients with neurosarcoidosis. Only patients who had an indication for PET/CT were included, which constitutes a selection bias, as these patients often have a more severe disease.
There was histological evidence of bone sarcoidosis in only three patients. However, a recent study documented granulomas on bone biopsies in 35 of 35 patients with sarcoidosis who had positive 18F-FDG PET/CT or MRI findings evocative of bone involvement, which suggests that histology may not always be required [19].
Comparison of treatments was only made between molecules, which did not account for the heterogeneity of doses and durations of treatments. Finally, using stringent definition criteria for metabolic bone involvement could have underestimated its prevalence. However, it allowed the selection of patients with severe bone involvement.
Conclusion
In this retrospective study of 175 patients with sarcoidosis evaluated by 18F-FDG PET/CT, metabolic bone involvement of sarcoidosis, which was present in 18% of the patients, was associated with more organ involvement, more treatments and more relapses. Metabolic bone involvement of sarcoidosis could therefore reflect a more severe multisystemic involvement and chronic evolution of the disease. These findings could help clinicians adjust individual diagnostic and therapeutic strategies for patients with sarcoidosis.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Authors’ contributions
A.B. and F.C.A. designed the study and wrote the manuscript. All authors reviewed and approved the final manuscript.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.




Comments