-
PDF
- Split View
-
Views
-
Cite
Cite
Eugenio De Miguel, Pierluigi Macchioni, Edoardo Conticini, Corrado Campochiaro, Rositsa Karalilova, Sara Monti, Cristina Ponte, Giulia Klinowski, Irene Monjo-Henry, Paolo Falsetti, Zguro Batalov, Alessandro Tomelleri, Alojzija Hocevar, Prevalence and characteristics of subclinical giant cell arteritis in polymyalgia rheumatica, Rheumatology, Volume 63, Issue 1, January 2024, Pages 158–164, https://doi.org/10.1093/rheumatology/kead189
Close - Share Icon Share
Abstract
The main objective of this study was to analyse the prevalence and characteristics of subclinical GCA in patients with PMR.
This was a cross-sectional multicentre international study of consecutive patients with newly diagnosed PMR without symptoms or signs suggestive of GCA. All patients underwent US of the temporal superficial, common carotid, subclavian and axillary arteries. Patients with halo signs in at least one examined artery were considered to have subclinical GCA. The clinical, demographic and laboratory characteristics of the PMR group without subclinical vasculitis were compared with subclinical GCA, and the pattern of vessel involvement was compared with that of a classical single-centre GCA cohort.
We included 346 PMR patients, 267 (77.2%) without subclinical GCA and 79 (22.8%) with subclinical GCA. The PMR patients with subclinical GCA were significantly older, had a longer duration of morning stiffness and more frequently reported hip pain than PMR without subclinical GCA. PMR with subclinical GCA showed a predominant extracranial large vessel pattern of vasculitic involvement compared with classical GCA, where the cranial phenotype predominated. The patients with PMR in the classical GCA group showed a pattern of vessel involvement similar to classical GCA without PMR but different from PMR with subclinical involvement.
More than a fifth of the pure PMR patients had US findings consistent with subclinical GCA. This specific subset of patients showed a predilection for extracranial artery involvement. The optimal screening strategy to assess the presence of vasculitis in PMR remains to be determined.
Subclinical GCA in PMR without clinical features suggestive of concomitant GCA has a prevalence of 22.8%.
In PMR with subclinical GCA, large arteries are more often involved than in classical GCA.
The US screening of subclinical GCA in PMR is currently not standard of care; however, when performed it should include at least temporal, axillary and subclavian arteries.
Introduction
PMR and GCA are two frequently overlapping diseases. PMR is a relatively common inflammatory disease in populations aged above 50 years. Patients usually have pain and stiffness involving the shoulder, pelvic girdle and the neck, and elevations of acute-phase proteins such as ESR or CRP are the main clinical characteristics associated with the disease [1, 2].
GCA is a primary vasculitis that typically involves medium and large arteries, including the superficial temporal and other cranial arteries (‘cranial-GCA’ subset), but it also can involve the aorta and its major branches, such as the subclavian, axillary and carotid arteries, which are involved in a subset of patients with extracranial large-vessel GCA (LV-GCA).
In clinical practice, GCA and PMR can appear associated with each other or individually. Patients with GCA have manifestations of PMR in 40–60% of cases, whereas 10–16% of patients with PMR can have manifestations of GCA [3–6]. GCA is suspected in a patient with PMR when cranial features such as a new onset of headache, scalp tenderness, jaw claudication or visual disturbances are present. Solely, LV-GCA is more difficult to suspect because it frequently does not present with specific clinical manifestations. Current evidence shows that subclinical GCA can appear in patients with ‘pure’ PMR without clinical symptoms of GCA. However, there is a high discrepancy between different studies, with a low number of cases included in most studies and a prevalence of subclinical GCA reported between 0 and 92% [7–9]. In addition, the association of PMR with GCA may have significant therapeutic and prognostic consequences. The ischaemic complications and the potential damage occurrence (e.g. aortic aneurysm or dissection) leading to increased mortality in GCA are well known. Furthermore, higher doses of glucocorticoids are required in GCA than in PMR, and therefore the risk of developing or worsening associated comorbidities (e.g. diabetes mellitus, osteoporosis) may be higher. Nevertheless, it is still debatable which patients with PMR should be screened for GCA and how they should be screened. The aims of the present study were (i) to evaluate the frequency of subclinical GCA in patients with PMR in a well-defined multicentre prospective cohort using US as a screening tool and (ii) to study the characteristics of patients with subclinical GCA compared with patients with clinically overt GCA.
Methods
This was a cross-sectional analysis of a prospective, multicentre, international cohort of consecutive nonselected PMR patients without clinical features suggestive of concomitant GCA (‘pure’ PMR). Patients with newly diagnoses of PMR, without other rheumatic previous diseases, recruited between 1 July 2020 and 31 July 2022 were included and followed in one of the eight participating centres from five European countries (Bulgaria, Italy, Portugal, Slovenia and Spain). Patients met the ACR/EULAR 2012 classification criteria [10] and had been on glucocorticoid treatment for <14 days (<20 mg/day). We prospectively enrolled patients sent to our attention by local General Practitioners (GPs) or by other specialists (mainly other Rheumatologists) with a suspect or an established diagnosis of PMR. We did not inform GPs regarding the conducting of this study and therefore we did not solicit more patient referral. We only informed other Rheumatologists, but we asked them to send us patients with a diagnosis of PMR regardless of the severity of symptoms and only if there were no clinical symptoms of GCA. None of the patients was treated with tocilizumab or MTX before the assessment. Any patient who presented with symptoms suggestive of GCA (headache, visual disturbances, jaw or tongue claudication, scalp tenderness, or limb claudication) was excluded. Clinical, laboratory and imaging data were collected at baseline. In practice for a candidate patient, a Rheumatologist established the clinical diagnosis of PMR and excluded clinically the possibility of GCA (based on patient history and clinical examination). According to study protocol only then (if there were no signs/symptoms suggestive of concomitant GCA) the patient was referred to US investigation of arteries, as well as hips and shoulders. Ultrasonographers were blind to the patient’s clinical data at the time of performing examination. Weight loss was marked as present if patient reported weight change compared with pre-illness state (in kg). Fever was defined when the patient or the physician recorded the body temperature above 37°C. Arthritis (except in shoulders and hips) was assessed clinically. To analyse the differences between subclinical GCA in PMR and classical GCA, a cohort of 97 consecutive GCA patients was recruited through a fast-track pathway from a single centre (Madrid, Spain), and these patients’ demographic characteristics have been previously published [11].
Ultrasound assessment
US was performed on the temporal superficial arteries (including the common temporal, frontal and parietal branches) and the extracranial arteries (the bilateral common carotid, axillary and subclavian arteries) at baseline. The presence or absence of a halo sign [12] and the maximum value of the intima-media thickness (IMT) were recorded in all examined vascular segments. The IMT was measured in the longitudinal view in the wall distal to the US probe and in the area with greatest wall thickness without an atherosclerotic appearance. We used cut-off values of the halo thickness, which was measured from the luminal side of the intima to the adventitia: ≥0.34 mm for the frontal and parietal branches, ≥0.42 mm for the common trunks of the temporal arteries, and ≥1 mm for the axillary, subclavian and carotid arteries [13–16]. Patients with isolated involvement of the carotid arteries were excluded to improve the diagnostic validity of the study. An increased IMT in carotid arteries namely lacks the specificity for GCA compared with atherosclerosis as recently shown [16].
A high-quality US machine with linear high-frequency probes was used with the following settings: a greyscale frequency of ≥18 MHz for temporal arteries and 4–18 MHz for extracranial arteries, a colour Doppler pulse repetition frequency of 2–3.5 kHz for temporal arteries and 3–4 kHz for extracranial arteries, colour box with angle correction of ≤60° and the gain adjusted to fill only the lumen [17]. All examinations were performed by expert sonographers, who had performed >300 vascular US previously. Information on specific US machines used is available in Supplementary Data S1, available at Rheumatology online.
Statistical analysis
Descriptive analysis of the data was performed. The data are expressed as the mean (s.d.) for normally distributed continuous variables and as median (interquartile range) for non-normally distributed variables, and percentages and frequencies for categorical variables. Univariate analysis was performed using the χ2 test, Fischer’s exact test, Student’s t-test and Mann–Whitney U test, as appropriate. All the analyses were performed on complete data, without imputation, and the cut-off of P < 0.05 was adopted for defining statistical significance. The data were analysed using IBM SPSS Statistics 25.0.
Ethical aspects
The protocol of this present study was first approved by the Ethics Committee of the University Hospital ‘La Paz’ on 9 March 2020, code HULP: PI-3992, and was presented to the participant centres. All patients provided written informed consent prior to inclusion in the study. The study was not registered at the ClinicalTrials.gov database.
Results
We included 346 patients with new PMR without clinical data of GCA; the patients had a mean age of 73.8 (8.8) years, and there were 188 (54.3%) females. The mean CRP at diagnosis was 51 (49) mg/dl, and the mean ESR was 56 (30) mm/h. An additional group of 48 patients was excluded by possible bias and did not fulfil the protocol (see Supplementary Fig. S1, available at Rheumatology online). There were 267 patients (77.2%) without US halo sign that we name as ‘pure PMR’ and 79 patients who presented with a halo sign on vascular US, which was deemed as PMR with subclinical GCA (22.8%). Table 1 shows the main characteristics of the patients with PMR with and without subclinical GCA, as well as the differences in presentation between the subgroups of PMR patients, who were stratified according to the subtype of subclinical GCA: LV-GCA, cranial-GCA and mixed-GCA (i.e. cranial and extracranial large vessel GCA). Fourteen patients (18%) received treatment with glucocorticoids, prior to inclusion in the study (prior to baseline visit), with a mean prednisone dose of 17.45 (5.1) mg/day. In our analysis, we considered cases of vasculitis of the carotid artery when the carotid artery was affected concurrently with another vessel territory. If we considered both carotids diameter of ≥1 mm as a positive sign for vasculitis, the prevalence was 28.4%, but when considering only one of the carotids having such a diameter as sufficient for vasculitis, the frequency of vasculitis increased to 32.9%. If in the US examination protocol we did not included subclavian arteries we missed six cases of large vessel involvement, 8.2% of prevalence.
| . | PMR without GCA (n = 267) . | PMR with GCA (n = 79) . | PMR with isolated LV-GCA (n = 31) . | PMR with isolated cranial-GCA (n = 22) . | PMR with mixed-GCA (n = 26) . |
|---|---|---|---|---|---|
| Age, years, mean (s.d.) | 72.86 (8.73) | 77.16 (8.27) | 75.45 (8.1) | 75.82 (8.9) | 80.34 (7.1) |
| Female sex, n (%) | 147 (55.1) | 32 (51.9) | 18 (58.1) | 11 (50) | 12 (46.2) |
| Hip pain, n (%) | 212 (79.4) | 77 (97.5) | 30 (96.8) | 22 (100) | 25 (96.2) |
| Neck pain, n (%) | 160 (59.9) | 44 (55.7) | 20 (64.5) | 11 (50) | 13 (50) |
| Morning stiffness presence, n (%) | 228 (85.4) | 63 (79.7) | 25 (80.6) | 16 (72.7) | 22 (84.6) |
| Weight loss, n (%) | 69 (25.8) | 18 (22.8) | 7 (22.6) | 2 (9.1) | 9 (34.6) |
| Fever, n (%) | 27 (10.1) | 8 (10.1) | 7 (22.6) | 0 (0) | 1 (3.8) |
| Peripheral arthritis, n (%) | 77 (29.1) | 24 (30.4) | 10 (32.3) | 6 (27.3) | 8 (30.8) |
| CRP, mg/l, mean (s.d.) | 52.23 (48.97) | 47.65 (47.85) | 48.37 (52.94) | 41.26 (46.44) | 51.71 (43.66) |
| ESR, mm/h, mean (s.d.) | 56.12 (28.24) | 57.75 (34.53) | 57.74 (33.25) | 60.84 (41.08) | 55.42 (31.87) |
| . | PMR without GCA (n = 267) . | PMR with GCA (n = 79) . | PMR with isolated LV-GCA (n = 31) . | PMR with isolated cranial-GCA (n = 22) . | PMR with mixed-GCA (n = 26) . |
|---|---|---|---|---|---|
| Age, years, mean (s.d.) | 72.86 (8.73) | 77.16 (8.27) | 75.45 (8.1) | 75.82 (8.9) | 80.34 (7.1) |
| Female sex, n (%) | 147 (55.1) | 32 (51.9) | 18 (58.1) | 11 (50) | 12 (46.2) |
| Hip pain, n (%) | 212 (79.4) | 77 (97.5) | 30 (96.8) | 22 (100) | 25 (96.2) |
| Neck pain, n (%) | 160 (59.9) | 44 (55.7) | 20 (64.5) | 11 (50) | 13 (50) |
| Morning stiffness presence, n (%) | 228 (85.4) | 63 (79.7) | 25 (80.6) | 16 (72.7) | 22 (84.6) |
| Weight loss, n (%) | 69 (25.8) | 18 (22.8) | 7 (22.6) | 2 (9.1) | 9 (34.6) |
| Fever, n (%) | 27 (10.1) | 8 (10.1) | 7 (22.6) | 0 (0) | 1 (3.8) |
| Peripheral arthritis, n (%) | 77 (29.1) | 24 (30.4) | 10 (32.3) | 6 (27.3) | 8 (30.8) |
| CRP, mg/l, mean (s.d.) | 52.23 (48.97) | 47.65 (47.85) | 48.37 (52.94) | 41.26 (46.44) | 51.71 (43.66) |
| ESR, mm/h, mean (s.d.) | 56.12 (28.24) | 57.75 (34.53) | 57.74 (33.25) | 60.84 (41.08) | 55.42 (31.87) |
LV: large vessel; n: number of cases.
| . | PMR without GCA (n = 267) . | PMR with GCA (n = 79) . | PMR with isolated LV-GCA (n = 31) . | PMR with isolated cranial-GCA (n = 22) . | PMR with mixed-GCA (n = 26) . |
|---|---|---|---|---|---|
| Age, years, mean (s.d.) | 72.86 (8.73) | 77.16 (8.27) | 75.45 (8.1) | 75.82 (8.9) | 80.34 (7.1) |
| Female sex, n (%) | 147 (55.1) | 32 (51.9) | 18 (58.1) | 11 (50) | 12 (46.2) |
| Hip pain, n (%) | 212 (79.4) | 77 (97.5) | 30 (96.8) | 22 (100) | 25 (96.2) |
| Neck pain, n (%) | 160 (59.9) | 44 (55.7) | 20 (64.5) | 11 (50) | 13 (50) |
| Morning stiffness presence, n (%) | 228 (85.4) | 63 (79.7) | 25 (80.6) | 16 (72.7) | 22 (84.6) |
| Weight loss, n (%) | 69 (25.8) | 18 (22.8) | 7 (22.6) | 2 (9.1) | 9 (34.6) |
| Fever, n (%) | 27 (10.1) | 8 (10.1) | 7 (22.6) | 0 (0) | 1 (3.8) |
| Peripheral arthritis, n (%) | 77 (29.1) | 24 (30.4) | 10 (32.3) | 6 (27.3) | 8 (30.8) |
| CRP, mg/l, mean (s.d.) | 52.23 (48.97) | 47.65 (47.85) | 48.37 (52.94) | 41.26 (46.44) | 51.71 (43.66) |
| ESR, mm/h, mean (s.d.) | 56.12 (28.24) | 57.75 (34.53) | 57.74 (33.25) | 60.84 (41.08) | 55.42 (31.87) |
| . | PMR without GCA (n = 267) . | PMR with GCA (n = 79) . | PMR with isolated LV-GCA (n = 31) . | PMR with isolated cranial-GCA (n = 22) . | PMR with mixed-GCA (n = 26) . |
|---|---|---|---|---|---|
| Age, years, mean (s.d.) | 72.86 (8.73) | 77.16 (8.27) | 75.45 (8.1) | 75.82 (8.9) | 80.34 (7.1) |
| Female sex, n (%) | 147 (55.1) | 32 (51.9) | 18 (58.1) | 11 (50) | 12 (46.2) |
| Hip pain, n (%) | 212 (79.4) | 77 (97.5) | 30 (96.8) | 22 (100) | 25 (96.2) |
| Neck pain, n (%) | 160 (59.9) | 44 (55.7) | 20 (64.5) | 11 (50) | 13 (50) |
| Morning stiffness presence, n (%) | 228 (85.4) | 63 (79.7) | 25 (80.6) | 16 (72.7) | 22 (84.6) |
| Weight loss, n (%) | 69 (25.8) | 18 (22.8) | 7 (22.6) | 2 (9.1) | 9 (34.6) |
| Fever, n (%) | 27 (10.1) | 8 (10.1) | 7 (22.6) | 0 (0) | 1 (3.8) |
| Peripheral arthritis, n (%) | 77 (29.1) | 24 (30.4) | 10 (32.3) | 6 (27.3) | 8 (30.8) |
| CRP, mg/l, mean (s.d.) | 52.23 (48.97) | 47.65 (47.85) | 48.37 (52.94) | 41.26 (46.44) | 51.71 (43.66) |
| ESR, mm/h, mean (s.d.) | 56.12 (28.24) | 57.75 (34.53) | 57.74 (33.25) | 60.84 (41.08) | 55.42 (31.87) |
LV: large vessel; n: number of cases.
The patients with PMR without subclinical GCA were younger [mean 72.8 (8.7) years, P < 0.001] and had a longer duration of morning stiffness (mean 72.3 vs 34.5 min, P < 0.001) compared with the patients with PMR and subclinical GCA (Table 2). There were no significant differences in the values of CRP and ESR between the compared two groups. The PMR patients with subclinical GCA more frequently reported hip pain [97.5% vs 79.4%, P < 0.001; odds ratio (OR) 10.0, 95% CI 3.6–28.0] compared with the PMR group without subclinical GCA. Table 3 shows the main clinical differences between the patients with pure PMR and the patients with PMR with subclinical GCA according to the different subtypes of vessel involvement. The PMR patients with subclinical GCA, regardless of the vasculitic type of involvement, more frequently reported hip pain (P < 0.010). In addition, the patients with isolated LV-GCA subtype reported a shorter duration of morning stiffness (P < 0.05), and the patients with mixed-GCA were older (P < 0.01) than the patients with PMR without subclinical GCA.
Characteristics and differences in PMR patients with and without silent GCA
| . | PMR without GCA (n = 267) . | PMR with GCA (n = 79) . | P-value . |
|---|---|---|---|
| Age, years, mean (s.d.) | 72.86 (8.73) | 77.16 (8.27) | 0.001 |
| Morning stiffness presence, n (%) | 228 (85.4) | 63 (79.7) | 0.21 |
| Morning stiffness, mina, mean (s.d.) | 72.3 (80.9) | 34.6 (52.1) | 0.001 |
| Female sex, n (%) | 147 (55.1) | 32(51.9) | 0.70 |
| Hip paina, n (%) | 212 (79.4) | 77 (97.5) | 0.001 |
| Neck pain, n (%) | 160 (59.9) | 44 (55.7) | 0.52 |
| Weight loss, n (%) | 69 (25.8) | 18 (22.8) | 0.56 |
| Fever, n (%) | 27 (10.1) | 8 (10.1) | 0.69 |
| Peripheral arthritis, n (%) | 77 (29.1) | 24 (30.4) | 0.89 |
| CRP, mg/l, mean (s.d.) | 52.23 (48.97) | 47.65 (47.85) | 0.46 |
| ESR, mm/h, mean (s.d.) | 56.12 (28.24) | 57.75 (34.53) | 0.68 |
| . | PMR without GCA (n = 267) . | PMR with GCA (n = 79) . | P-value . |
|---|---|---|---|
| Age, years, mean (s.d.) | 72.86 (8.73) | 77.16 (8.27) | 0.001 |
| Morning stiffness presence, n (%) | 228 (85.4) | 63 (79.7) | 0.21 |
| Morning stiffness, mina, mean (s.d.) | 72.3 (80.9) | 34.6 (52.1) | 0.001 |
| Female sex, n (%) | 147 (55.1) | 32(51.9) | 0.70 |
| Hip paina, n (%) | 212 (79.4) | 77 (97.5) | 0.001 |
| Neck pain, n (%) | 160 (59.9) | 44 (55.7) | 0.52 |
| Weight loss, n (%) | 69 (25.8) | 18 (22.8) | 0.56 |
| Fever, n (%) | 27 (10.1) | 8 (10.1) | 0.69 |
| Peripheral arthritis, n (%) | 77 (29.1) | 24 (30.4) | 0.89 |
| CRP, mg/l, mean (s.d.) | 52.23 (48.97) | 47.65 (47.85) | 0.46 |
| ESR, mm/h, mean (s.d.) | 56.12 (28.24) | 57.75 (34.53) | 0.68 |
GCA: Giant cell arteritis, PMR: Polymyalgia rheumatica; LV: large vessel; n: number of cases; SD : standard deviation; CRP: C reactive protein; ESR: Erythrocyte sedimentation rate; Quantitative variables appear as median (interquartile); IQR: interquartile.
Values that achieved statistical significance (P > 0.05) appears in bold.
Characteristics and differences in PMR patients with and without silent GCA
| . | PMR without GCA (n = 267) . | PMR with GCA (n = 79) . | P-value . |
|---|---|---|---|
| Age, years, mean (s.d.) | 72.86 (8.73) | 77.16 (8.27) | 0.001 |
| Morning stiffness presence, n (%) | 228 (85.4) | 63 (79.7) | 0.21 |
| Morning stiffness, mina, mean (s.d.) | 72.3 (80.9) | 34.6 (52.1) | 0.001 |
| Female sex, n (%) | 147 (55.1) | 32(51.9) | 0.70 |
| Hip paina, n (%) | 212 (79.4) | 77 (97.5) | 0.001 |
| Neck pain, n (%) | 160 (59.9) | 44 (55.7) | 0.52 |
| Weight loss, n (%) | 69 (25.8) | 18 (22.8) | 0.56 |
| Fever, n (%) | 27 (10.1) | 8 (10.1) | 0.69 |
| Peripheral arthritis, n (%) | 77 (29.1) | 24 (30.4) | 0.89 |
| CRP, mg/l, mean (s.d.) | 52.23 (48.97) | 47.65 (47.85) | 0.46 |
| ESR, mm/h, mean (s.d.) | 56.12 (28.24) | 57.75 (34.53) | 0.68 |
| . | PMR without GCA (n = 267) . | PMR with GCA (n = 79) . | P-value . |
|---|---|---|---|
| Age, years, mean (s.d.) | 72.86 (8.73) | 77.16 (8.27) | 0.001 |
| Morning stiffness presence, n (%) | 228 (85.4) | 63 (79.7) | 0.21 |
| Morning stiffness, mina, mean (s.d.) | 72.3 (80.9) | 34.6 (52.1) | 0.001 |
| Female sex, n (%) | 147 (55.1) | 32(51.9) | 0.70 |
| Hip paina, n (%) | 212 (79.4) | 77 (97.5) | 0.001 |
| Neck pain, n (%) | 160 (59.9) | 44 (55.7) | 0.52 |
| Weight loss, n (%) | 69 (25.8) | 18 (22.8) | 0.56 |
| Fever, n (%) | 27 (10.1) | 8 (10.1) | 0.69 |
| Peripheral arthritis, n (%) | 77 (29.1) | 24 (30.4) | 0.89 |
| CRP, mg/l, mean (s.d.) | 52.23 (48.97) | 47.65 (47.85) | 0.46 |
| ESR, mm/h, mean (s.d.) | 56.12 (28.24) | 57.75 (34.53) | 0.68 |
GCA: Giant cell arteritis, PMR: Polymyalgia rheumatica; LV: large vessel; n: number of cases; SD : standard deviation; CRP: C reactive protein; ESR: Erythrocyte sedimentation rate; Quantitative variables appear as median (interquartile); IQR: interquartile.
Values that achieved statistical significance (P > 0.05) appears in bold.
Characteristics and differences of pure PMR and the subtypes of silent GCA in PMR
| . | PMR without GCA (n = 267) . | PMR with isolated LV-GCA (n = 31) . | P-value no GCA vs LV-GCA . | PMR with isolated cranial-GCA (n = 22) . | P-value no GCA vs isolated cranial-GCA . | PMR with mixed-GCA (n = 26) . | P-value no GCA vs mixed-GCA . |
|---|---|---|---|---|---|---|---|
| Age, years | 74 (68–78) | 72 (65–77) | 0.846 | 74 (70–78) | 0.86 | 81 (78–82) | 0.01 |
| Female sex, n (%) | 147 (55.1) | 18 (58.1) | 1 | 11 (50) | 1 | 12 (46.2) | 0.14 |
| Hip pain, n (%) | 212 (79.4) | 30 (96.8) | 0.001 | 22 (100) | 0.001 | 25 (96.2) | 0.01 |
| Neck pain, n (%) | 160 (59.9) | 20 (64.5) | 0.57 | 11 (50) | 0.49 | 13 (50) | 0.85 |
| Morning stiffness presence, n (%) | 228 (85.4) | 25 (80.6) | 0.23 | 16 (72.7) | 0.06 | 22 (84.6) | 0.37 |
| Morning stiffness, min | 60 (30–90) | 60 (0–115) | 0.045 | 75 (30–115) | 0.47 | 60 (60–96) | 0.241 |
| Weight loss, n (%) | 69 (25.8) | 7 (22.6) | 1 | 2 (9.1) | 0.12 | 9 (34.6) | 0.67 |
| Fever, n (%) | 27 (10.1) | 7 (22.6) | 0.37 | 0 (0) | 0.24 | 1 (3.8) | 0.75 |
| Peripheral arthritis, n (%) | 77 (29.1) | 10 (32.3) | 0.84 | 6 (27.3) | 1 | 8 (30.8) | 0.68 |
| CRP, mg/l | 39 (19–65) | 36 (13.6–82) | 0.765 | 28 (8–71) | 0.14 | 40 (14–72) | 0.995 |
| ESR, mm/h | 54.5 (40–72) | 60 (24–84) | 0.063 | 40 (22–85) | 0.15 | 55 (33–102) | 0.481 |
| . | PMR without GCA (n = 267) . | PMR with isolated LV-GCA (n = 31) . | P-value no GCA vs LV-GCA . | PMR with isolated cranial-GCA (n = 22) . | P-value no GCA vs isolated cranial-GCA . | PMR with mixed-GCA (n = 26) . | P-value no GCA vs mixed-GCA . |
|---|---|---|---|---|---|---|---|
| Age, years | 74 (68–78) | 72 (65–77) | 0.846 | 74 (70–78) | 0.86 | 81 (78–82) | 0.01 |
| Female sex, n (%) | 147 (55.1) | 18 (58.1) | 1 | 11 (50) | 1 | 12 (46.2) | 0.14 |
| Hip pain, n (%) | 212 (79.4) | 30 (96.8) | 0.001 | 22 (100) | 0.001 | 25 (96.2) | 0.01 |
| Neck pain, n (%) | 160 (59.9) | 20 (64.5) | 0.57 | 11 (50) | 0.49 | 13 (50) | 0.85 |
| Morning stiffness presence, n (%) | 228 (85.4) | 25 (80.6) | 0.23 | 16 (72.7) | 0.06 | 22 (84.6) | 0.37 |
| Morning stiffness, min | 60 (30–90) | 60 (0–115) | 0.045 | 75 (30–115) | 0.47 | 60 (60–96) | 0.241 |
| Weight loss, n (%) | 69 (25.8) | 7 (22.6) | 1 | 2 (9.1) | 0.12 | 9 (34.6) | 0.67 |
| Fever, n (%) | 27 (10.1) | 7 (22.6) | 0.37 | 0 (0) | 0.24 | 1 (3.8) | 0.75 |
| Peripheral arthritis, n (%) | 77 (29.1) | 10 (32.3) | 0.84 | 6 (27.3) | 1 | 8 (30.8) | 0.68 |
| CRP, mg/l | 39 (19–65) | 36 (13.6–82) | 0.765 | 28 (8–71) | 0.14 | 40 (14–72) | 0.995 |
| ESR, mm/h | 54.5 (40–72) | 60 (24–84) | 0.063 | 40 (22–85) | 0.15 | 55 (33–102) | 0.481 |
Quantitative variables appear as median (interquartile range). LV: large vessel; n: number of cases.
Values that achieved statistical significance (P > 0.05) appears in bold.
Characteristics and differences of pure PMR and the subtypes of silent GCA in PMR
| . | PMR without GCA (n = 267) . | PMR with isolated LV-GCA (n = 31) . | P-value no GCA vs LV-GCA . | PMR with isolated cranial-GCA (n = 22) . | P-value no GCA vs isolated cranial-GCA . | PMR with mixed-GCA (n = 26) . | P-value no GCA vs mixed-GCA . |
|---|---|---|---|---|---|---|---|
| Age, years | 74 (68–78) | 72 (65–77) | 0.846 | 74 (70–78) | 0.86 | 81 (78–82) | 0.01 |
| Female sex, n (%) | 147 (55.1) | 18 (58.1) | 1 | 11 (50) | 1 | 12 (46.2) | 0.14 |
| Hip pain, n (%) | 212 (79.4) | 30 (96.8) | 0.001 | 22 (100) | 0.001 | 25 (96.2) | 0.01 |
| Neck pain, n (%) | 160 (59.9) | 20 (64.5) | 0.57 | 11 (50) | 0.49 | 13 (50) | 0.85 |
| Morning stiffness presence, n (%) | 228 (85.4) | 25 (80.6) | 0.23 | 16 (72.7) | 0.06 | 22 (84.6) | 0.37 |
| Morning stiffness, min | 60 (30–90) | 60 (0–115) | 0.045 | 75 (30–115) | 0.47 | 60 (60–96) | 0.241 |
| Weight loss, n (%) | 69 (25.8) | 7 (22.6) | 1 | 2 (9.1) | 0.12 | 9 (34.6) | 0.67 |
| Fever, n (%) | 27 (10.1) | 7 (22.6) | 0.37 | 0 (0) | 0.24 | 1 (3.8) | 0.75 |
| Peripheral arthritis, n (%) | 77 (29.1) | 10 (32.3) | 0.84 | 6 (27.3) | 1 | 8 (30.8) | 0.68 |
| CRP, mg/l | 39 (19–65) | 36 (13.6–82) | 0.765 | 28 (8–71) | 0.14 | 40 (14–72) | 0.995 |
| ESR, mm/h | 54.5 (40–72) | 60 (24–84) | 0.063 | 40 (22–85) | 0.15 | 55 (33–102) | 0.481 |
| . | PMR without GCA (n = 267) . | PMR with isolated LV-GCA (n = 31) . | P-value no GCA vs LV-GCA . | PMR with isolated cranial-GCA (n = 22) . | P-value no GCA vs isolated cranial-GCA . | PMR with mixed-GCA (n = 26) . | P-value no GCA vs mixed-GCA . |
|---|---|---|---|---|---|---|---|
| Age, years | 74 (68–78) | 72 (65–77) | 0.846 | 74 (70–78) | 0.86 | 81 (78–82) | 0.01 |
| Female sex, n (%) | 147 (55.1) | 18 (58.1) | 1 | 11 (50) | 1 | 12 (46.2) | 0.14 |
| Hip pain, n (%) | 212 (79.4) | 30 (96.8) | 0.001 | 22 (100) | 0.001 | 25 (96.2) | 0.01 |
| Neck pain, n (%) | 160 (59.9) | 20 (64.5) | 0.57 | 11 (50) | 0.49 | 13 (50) | 0.85 |
| Morning stiffness presence, n (%) | 228 (85.4) | 25 (80.6) | 0.23 | 16 (72.7) | 0.06 | 22 (84.6) | 0.37 |
| Morning stiffness, min | 60 (30–90) | 60 (0–115) | 0.045 | 75 (30–115) | 0.47 | 60 (60–96) | 0.241 |
| Weight loss, n (%) | 69 (25.8) | 7 (22.6) | 1 | 2 (9.1) | 0.12 | 9 (34.6) | 0.67 |
| Fever, n (%) | 27 (10.1) | 7 (22.6) | 0.37 | 0 (0) | 0.24 | 1 (3.8) | 0.75 |
| Peripheral arthritis, n (%) | 77 (29.1) | 10 (32.3) | 0.84 | 6 (27.3) | 1 | 8 (30.8) | 0.68 |
| CRP, mg/l | 39 (19–65) | 36 (13.6–82) | 0.765 | 28 (8–71) | 0.14 | 40 (14–72) | 0.995 |
| ESR, mm/h | 54.5 (40–72) | 60 (24–84) | 0.063 | 40 (22–85) | 0.15 | 55 (33–102) | 0.481 |
Quantitative variables appear as median (interquartile range). LV: large vessel; n: number of cases.
Values that achieved statistical significance (P > 0.05) appears in bold.
When we compared our PMR patients with subclinical GCA cohort vs the classical GCA cohort, we found an ‘inverse’ pattern of vasculitic distribution between both groups. The PMR patients with associated subclinical GCA presented more frequently with isolated LV-GCA (39.24%), followed by a mixed-GCA pattern (32.91%) and a cranial-GCA pattern (27.85%). The patients from the classic GCA cohort were more likely to present with a cranial subtype (52.6%), followed by a mixed pattern (28.9%) and finally the isolated LV-GCA subtype (18.6%) (Fig. 1). In the classical GCA cohort, there were 47/97 (48.5%) patients with concomitant PMR. The analysis of this subgroup of GCA patients showed no major differences in age or levels of CRP compared with the PMR patients with subclinical GCA (Table 4). However, in the classical GCA cohort, the patients with PMR showed a pattern of vasculitic involvement similar to the patients without associated PMR but opposite to our PMR group with subclinical GCA (Table 4).
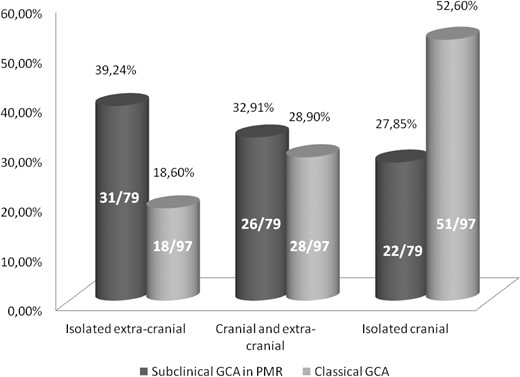
Prevalence of the subtypes of vessel affectation in PMR with subclinical GCA and classical GCA
Subanalysis of the influence of PMR on the vascular subtypes of subclinical GCA in PMR and classical GCA
| . | Subclinical GCA in PMR (n = 79) . | Classical GCA (n = 97) . | P-value subclinical GCA in PMR vs classical GCA . | Classical GCA with PMR (n = 47) . | P-value PMR/GCA vs classical GCA with PMR . |
|---|---|---|---|---|---|
| Age, years, mean (s.d.) | 77.16 (8.3) | 77.32 (7.9) | 0.17 | 77.74 (7.8) | 0.69 |
| Female sex, n (%) | 41 (51.9) | 53 (54.6) | 0.76 | 24 (51.1) | 0.93 |
| CRP, mg/l, mean (s.d.) | 47.65 (47.85) | 63.33 (58.13) | 0.06 | 64.48 (64.59) | 0.10 |
| GCA subtype, n (%) | |||||
| Isolated cranial | 20 (25.3) | 51 (52.6) | 0.001 | 24 (51.1) | 0.01 |
| Mixed | 28 (35.4) | 28 (28.9) | 0.35 | 13 (19.1) | 0.37 |
| Isolated large vessel | 31(39.2) | 18 (18.6) | 0.001 | 9 (19.1) | 0.02 |
| . | Subclinical GCA in PMR (n = 79) . | Classical GCA (n = 97) . | P-value subclinical GCA in PMR vs classical GCA . | Classical GCA with PMR (n = 47) . | P-value PMR/GCA vs classical GCA with PMR . |
|---|---|---|---|---|---|
| Age, years, mean (s.d.) | 77.16 (8.3) | 77.32 (7.9) | 0.17 | 77.74 (7.8) | 0.69 |
| Female sex, n (%) | 41 (51.9) | 53 (54.6) | 0.76 | 24 (51.1) | 0.93 |
| CRP, mg/l, mean (s.d.) | 47.65 (47.85) | 63.33 (58.13) | 0.06 | 64.48 (64.59) | 0.10 |
| GCA subtype, n (%) | |||||
| Isolated cranial | 20 (25.3) | 51 (52.6) | 0.001 | 24 (51.1) | 0.01 |
| Mixed | 28 (35.4) | 28 (28.9) | 0.35 | 13 (19.1) | 0.37 |
| Isolated large vessel | 31(39.2) | 18 (18.6) | 0.001 | 9 (19.1) | 0.02 |
n: number of cases; GCA: Giant cell arteritis.
Subanalysis of the influence of PMR on the vascular subtypes of subclinical GCA in PMR and classical GCA
| . | Subclinical GCA in PMR (n = 79) . | Classical GCA (n = 97) . | P-value subclinical GCA in PMR vs classical GCA . | Classical GCA with PMR (n = 47) . | P-value PMR/GCA vs classical GCA with PMR . |
|---|---|---|---|---|---|
| Age, years, mean (s.d.) | 77.16 (8.3) | 77.32 (7.9) | 0.17 | 77.74 (7.8) | 0.69 |
| Female sex, n (%) | 41 (51.9) | 53 (54.6) | 0.76 | 24 (51.1) | 0.93 |
| CRP, mg/l, mean (s.d.) | 47.65 (47.85) | 63.33 (58.13) | 0.06 | 64.48 (64.59) | 0.10 |
| GCA subtype, n (%) | |||||
| Isolated cranial | 20 (25.3) | 51 (52.6) | 0.001 | 24 (51.1) | 0.01 |
| Mixed | 28 (35.4) | 28 (28.9) | 0.35 | 13 (19.1) | 0.37 |
| Isolated large vessel | 31(39.2) | 18 (18.6) | 0.001 | 9 (19.1) | 0.02 |
| . | Subclinical GCA in PMR (n = 79) . | Classical GCA (n = 97) . | P-value subclinical GCA in PMR vs classical GCA . | Classical GCA with PMR (n = 47) . | P-value PMR/GCA vs classical GCA with PMR . |
|---|---|---|---|---|---|
| Age, years, mean (s.d.) | 77.16 (8.3) | 77.32 (7.9) | 0.17 | 77.74 (7.8) | 0.69 |
| Female sex, n (%) | 41 (51.9) | 53 (54.6) | 0.76 | 24 (51.1) | 0.93 |
| CRP, mg/l, mean (s.d.) | 47.65 (47.85) | 63.33 (58.13) | 0.06 | 64.48 (64.59) | 0.10 |
| GCA subtype, n (%) | |||||
| Isolated cranial | 20 (25.3) | 51 (52.6) | 0.001 | 24 (51.1) | 0.01 |
| Mixed | 28 (35.4) | 28 (28.9) | 0.35 | 13 (19.1) | 0.37 |
| Isolated large vessel | 31(39.2) | 18 (18.6) | 0.001 | 9 (19.1) | 0.02 |
n: number of cases; GCA: Giant cell arteritis.
Discussion
With the widespread use of advanced vascular imaging modalities, subclinical vasculitis has increasingly been recognized in patients with PMR without clinical features suggestive of GCA. However, the prevalence of subclinical GCA in PMR may vary considerably in the literature, with figures ranging from 0% to 92% of patients [7, 8]. Therefore, the real impact of this issue is still unknown, and consequently, there are currently no international guidelines/recommendations for screening PMR patients for vasculitic involvement.
In the present multicentre study, we included 346 patients with newly diagnosed PMR and found vasculitic changes with the US in more than a fifth of the patients (22.8%). To the best of our knowledge, this study includes the largest cohort of PMR patients in whom subclinical GCA was assessed. The recently published meta-analysis of Hemmig et al. [18], encompassing 13 studies with 566 patients, showed a pooled prevalence for subclinical GCA of 23% (95% CI 14–36%), which was very similar to our results. However, it included studies with different screening modalities: US in three studies (135 cases), temporal artery biopsy (TAB) in three studies (165 cases) and PET/CT in seven studies (266 patients). The studies using PET/CT revealed a higher pooled prevalence for subclinical GCA compared with TAB and US [29% (95% CI 13–53%) vs 20% (95% CI 7–46%) vs 15% (95% CI 3–50%), respectively]. Only one US study, reported as an abstract to the ACR meeting, performed US of more arteries than the temporal artery (including the occipital, carotid, vertebral, central retinal and axillary arteries), and its prevalence of subclinical GCA was 37% (n = 10/27), which was higher than most cohorts [19]. The remaining study, applying US, reported a prevalence of 8% (n = 8/102) [20], evaluating a large number of PMR patients screened for vasculitis, but only in the temporal arteries. Therefore, regardless of other biases, the quality of equipment in the early 21st century and the smaller number of vessels examined could account for the underestimation of prevalence.
Currently, further imaging and/or TAB is not recommended in patients with pure PMR. However, the prognostic implications of this subclinical vascular involvement have yet to be determined. If left untreated, GCA can result in severe acute and chronic vascular complications such as vision loss (up to 19% of cases), arterial stenosis (5% to 29%), stroke (2% to 7%) and the development of aortic aneurysms [21–24]. Therefore, when should subclinical GCA be suspected in patients with PMR? Hemmig et al. [18] reported an association between some clinical biomarkers and the occurrence of subclinical GCA in PMR. Inflammatory back pain (OR 2.73), absence of lower limb pain (OR 2.35), female sex (OR 2.31), temperature >37°C (OR 1.83), weight loss (OR 1.83), thrombocyte count (OR 1.51) and haemoglobin level (OR 0.80) were the most strongly associated markers with subclinical GCA in the univariable analysis, but CRP (OR 1.00) and ESR (OR 1.01) were not associated. In our study, the PMR patients with subclinical GCA were older, had a longer duration of morning stiffness and more frequently reported hip pain than PMR patients without subclinical GCA. Interestingly, we found no major differences in the levels of inflammatory markers (ESR and CRP) between the groups. However, it is important to highlight that the patients in our study could have been treated with glucocorticoids up to 2 weeks prior to inclusion in the study. Although this may have influenced the results, only 14 patients were under glucocorticoid treatment when they underwent vascular assessment.
Is the pattern of vascular involvement in subclinical GCA different from that in classical GCA? To answer this question, we compared our PMR cohort with subclinical GCA to a single-centre GCA cohort described previously [11]. Interestingly, we found an ‘inverse’ distribution of vascular involvement in our cohort compared with the classical GCA cohort, with extracranial large vessel involvement being the most prevalent and isolated cranial subtype being the least common. Therefore, one could speculate that the predominant affection of extracranial large arteries tends to be less symptomatic. Moreover, the observation of a distinct subtype of vasculitic distribution in PMR with subclinical GCA is interesting and has not been reported previously. Hence, based on our findings, one could recommend the screening of at least the superficial temporal, axillary and subclavian arteries in patients with PMR.
Our study has several strengths to highlight. In addition to the large sample size, with patients recruited from various international centres with expertise in vascular US, the study protocol foresaw rigorous selection for patient inclusion (i.e. the patients included were required to fulfil the criteria of the 2012 PMR classification criteria, and the patients lacked the presence of clinical features suggestive of GCA). This was in contrast to the studies included in the meta-analysis by Hemmig et al. [18] which had different inclusion criteria, which could explain the heterogeneity found in the prevalence of subclinical GCA. In addition, we examined both the cranial and large vessels bilaterally in every patient and not only the superficial temporal arteries, and this potentially increases the diagnostic sensitivity for subclinical GCA.
Our study has limitations. We narrowed the US investigation to a four-vessel territory, and we excluded carotid arteries from the prevalence results. In fact, not all arteries that may be involved in GCA are accessible to US examination (for example maxillary arteries, thoracic aorta) and these arteries could be depicted by other imaging modalities [e.g. fluorodeoxyglucose (FDG)-PET], as showing some recent studies, comparing US and PET/CT [25, 26]. For example the prevalence of aortitis in GCA has been estimated at 45–65% [27–29]. Although it is usually associated with involvement of other supra-aortic LVs, there are a small percentage of isolated aortic vasculitis. In our experience in a comparative study with 72 GCA patients with US and FDG-PET/CT, with a frequency of aortitis of 33.3% in only 2/24 cases (8.2%) had positive aortitis PET/CT and negative US halo sign in other large vessels (personal data, submitted to press). PET studies can show carotid affectation, but an US wall thickness ≥1 mm may also appear in patients with atherosclerosis and there is not accurate validity in this isolated vasculitis vessel in our experience [16] and the exclusion of carotid arteries could have excluded a few cases of subclinical GCA. A glucocorticoid treatment prior to baseline visit is another study limitation, as it may impact US musculoskeletal findigs but also the thickness of intima-media complex, but it is unlikely that US signs of potential vasculitis would completely resolve in <14 days treatment with prednisone doses used in PMR [30]. Regarding selection bias, we performed vascular US in consecutive patients with new-onset PMR. These patients were sent to our attention mainly or totally by local GPs. There was no selection bias based on clinical/laboratory inclusion features. In addition, there was no communication between us and local GPs with the aim of increasing the referral of patients with PMR and local GPs were not aware that we were conducting this study. As a limitation, our cohort was based on referral hospital centres, and this is probably a sample of the full PMR patients evaluated by GPs, but as far as possible we have tried to avoid selection bias.
In conclusion, our study shows that subclinical GCA is not infrequent in PMR patients and commonly affects the large extracranial arteries. The optimal screening protocol remains to be agreed upon.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Ethics: The study complies with the Declaration of Helsinki, and the locally appointed ethics committee has approved the research protocol.
Acknowledgements
We would like to thank the GCA/PMR study group for its contributions to the development of collaborative studies.




Comments