-
PDF
- Split View
-
Views
-
Cite
Cite
Jacopo Ciaffi, Sophie I E Liem, Nina M van Leeuwen, Cornelia F Allaart, Tom W J Huizinga, Jeska K de Vries-Bouwstra, Comment on: Glucocorticoids prescribing practices in systemic sclerosis: an analysis of the EUSTAR database, Rheumatology, Volume 62, Issue 8, August 2023, Pages e251–e253, https://doi.org/10.1093/rheumatology/kead120
Close - Share Icon Share
Dear Editor, We read, with great interest, the two recently published articles by Iudici et al. [1] and Yomono and Kuwana [2], highlighting the gradual decrease in the utilization of glucocorticoids and the existence of a ‘window of opportunity’ in SSc. We agree that the use of medications in SSc is changing, and tailoring treatment to each patient’s needs is challenging. This is one of the reasons why specific care pathways, such as the Leiden Combined Care in SSc (CCISS) cohort, have been instituted [3]. Therapeutic strategies in SSc aim to target disease manifestations to improve patients’ symptoms but also to avoid progression of organ damage. However, while recommendations and guidelines define first-line treatments [4, 5], the management of second-line options or combinations of therapies mainly relies on the rheumatologist’s experience and expertise rather than on specific treatment algorithms. Furthermore, evidence regarding the necessity of medication changes is scarce and it is unclear whether and how SSc patients need to be treated in the long term. Unlike what happens in other rheumatic diseases [6, 7], the feasibility of tapering and even withdrawing immunosuppressive therapies in patients with stable SSc is still an open question.
Using data from the CCISS cohort, our aim was to describe how often treatment is modified during a follow-up period of 10 years, with a special interest in the possibility of tapering the dosage of immunosuppressors in patients experiencing clinical improvement or persistently stable disease. We therefore analysed the use of and changes in immunosuppressive therapies in the cohort, excluding the cases in which tapering was driven by drug toxicity or side effects.
We included 708 SSc patients with at least one visit performed between 2009 and 2021, accounting for 2968 visits. All included patients fulfilled the 2013 ACR/EULAR classification criteria for SSc [8]. A description of tapering of immunosuppressive treatments at each visit year in all patients and separately for subgroups defined on the basis of sex and autoantibody status is provided in Table 1. Furthermore, we analysed differences in tapering stratifying the cohort for presence of interstitial lung disease (ILD), presence of synovitis and disease subset (Supplementary Data S1, S2, S3, available at Rheumatology online). At the time of referral to the care pathway (i.e. at the baseline visit), 31% of patients were receiving immunosuppressors and tapering was possible in 8% of them. During the follow-up, tapering was possible in a larger proportion of patients treated with immunosuppressive medications, up to 20% at the third-year visit and 26% at the sixth-year visit. Stratified for sex and autoantibody status, no significant differences emerged in the proportions of drug tapering between males and females or between patients with anti-centromere (ACA) and anti-topoisomerase (ATA) antibodies.
Tapering of immunosuppressive therapies in SSc patients from the Leiden CCISS cohort
| Visit year . | All patients . | Female patients . | Male patients . | ACA-positive patients . | ATA-positive patients . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | |
| 1 | 708 | 218/708 (31) | 17/218 (8) | 557/708 (79) | 164/557 (29) | 10/164 (6) | 151/708 (21) | 54/151 (36) | 7/54 (13) | 295/708 (42) | 47/295 (16) | 2/47 (4) | 160/708 (23) | 67/160 (42) | 6/67 (9) |
| 2 | 563 | 213/563 (38) | 24/213 (11) | 446/563 (79) | 162/446 (36) | 18/162 (11) | 117/563 (21) | 51/117 (44) | 6/51 (12) | 234/563 (42) | 48/234 (21) | 5/48 (10) | 129/563 (23) | 58/129 (45) | 4/58 (7) |
| 3 | 452 | 169/452 (37) | 34/169 (20) | 359/452 (79) | 129/359 (36) | 29/129 (22) | 93/452 (21) | 40/93 (43) | 5/40 (13) | 182/452 (40) | 34/182 (19) | 6/34 (18) | 108/452 (24) | 54/108 (50) | 8/54 (15) |
| 4 | 356 | 125/356 (35) | 19/125 (15) | 285/356 (80) | 101/285 (35) | 14/101 (14) | 71/356 (20) | 24/71 (34) | 5/24 (21) | 137/356 (38) | 28/137 (20) | 3/28 (11) | 89/356 (25) | 36/89 (40) | 5/36 (14) |
| 5 | 275 | 91/275 (33) | 11/91 (12) | 226/275 (82) | 76/226 (34) | 8/76 (11) | 49/275 (18) | 15/49 (31) | 3/15 (20) | 111/275 (40) | 24/111 (22) | 4/24 (17) | 79/275 (29) | 27/79 (34) | 3/27 (11) |
| 6 | 225 | 74/225 (33) | 19/74 (26) | 190/225 (84) | 63/190 (33) | 17/63 (27) | 35/225 (16) | 11/35 (31) | 2/11 (18) | 86/225 (38) | 19/86 (22) | 8/19 (42) | 65/225 (29) | 26/65 (40) | 6/26 (23) |
| 7 | 165 | 44/165 (27) | 7/44 (16) | 141/165 (85) | 40/141 (28) | 6/40 (15) | 24/165 (15) | 4/24 (17) | 1/4 (25) | 59/165 (36) | 11/59 (19) | 1/11 (9) | 53/165 (32) | 18/53 (34) | 3/18 (17) |
| 8 | 113 | 30/113 (27) | 2/30 (7) | 97/113 (86) | 28/97 (29) | 1/28 (4) | 16/113 (14) | 2/16 (13) | 1/2 (50) | 43/113 (38) | 7/43 (16) | 0/7 | 39/113 (35) | 11/39 (28) | 1/11 (9) |
| 9 | 74 | 21/74 (28) | 3/21 (14) | 60/74 (81) | 19/60 (32) | 3/19 (16) | 14/74 (19) | 2/14 (14) | 0/2 | 21/74 (28) | 3/21 (14) | 0/3 | 34/74 (46) | 11/34 (32) | 2/11 (18) |
| 10 | 38 | 13/38 (34) | 1/13 (8) | 27/38 (71) | 10/27 (37) | 1/10 (10) | 11/38 (29) | 3/11 (27) | 0/3 | 11/38 (29) | 2/11 (18) | 0/2 | 19/38 (50) | 8/19 (42) | 1/8 (13) |
| Visit year . | All patients . | Female patients . | Male patients . | ACA-positive patients . | ATA-positive patients . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | |
| 1 | 708 | 218/708 (31) | 17/218 (8) | 557/708 (79) | 164/557 (29) | 10/164 (6) | 151/708 (21) | 54/151 (36) | 7/54 (13) | 295/708 (42) | 47/295 (16) | 2/47 (4) | 160/708 (23) | 67/160 (42) | 6/67 (9) |
| 2 | 563 | 213/563 (38) | 24/213 (11) | 446/563 (79) | 162/446 (36) | 18/162 (11) | 117/563 (21) | 51/117 (44) | 6/51 (12) | 234/563 (42) | 48/234 (21) | 5/48 (10) | 129/563 (23) | 58/129 (45) | 4/58 (7) |
| 3 | 452 | 169/452 (37) | 34/169 (20) | 359/452 (79) | 129/359 (36) | 29/129 (22) | 93/452 (21) | 40/93 (43) | 5/40 (13) | 182/452 (40) | 34/182 (19) | 6/34 (18) | 108/452 (24) | 54/108 (50) | 8/54 (15) |
| 4 | 356 | 125/356 (35) | 19/125 (15) | 285/356 (80) | 101/285 (35) | 14/101 (14) | 71/356 (20) | 24/71 (34) | 5/24 (21) | 137/356 (38) | 28/137 (20) | 3/28 (11) | 89/356 (25) | 36/89 (40) | 5/36 (14) |
| 5 | 275 | 91/275 (33) | 11/91 (12) | 226/275 (82) | 76/226 (34) | 8/76 (11) | 49/275 (18) | 15/49 (31) | 3/15 (20) | 111/275 (40) | 24/111 (22) | 4/24 (17) | 79/275 (29) | 27/79 (34) | 3/27 (11) |
| 6 | 225 | 74/225 (33) | 19/74 (26) | 190/225 (84) | 63/190 (33) | 17/63 (27) | 35/225 (16) | 11/35 (31) | 2/11 (18) | 86/225 (38) | 19/86 (22) | 8/19 (42) | 65/225 (29) | 26/65 (40) | 6/26 (23) |
| 7 | 165 | 44/165 (27) | 7/44 (16) | 141/165 (85) | 40/141 (28) | 6/40 (15) | 24/165 (15) | 4/24 (17) | 1/4 (25) | 59/165 (36) | 11/59 (19) | 1/11 (9) | 53/165 (32) | 18/53 (34) | 3/18 (17) |
| 8 | 113 | 30/113 (27) | 2/30 (7) | 97/113 (86) | 28/97 (29) | 1/28 (4) | 16/113 (14) | 2/16 (13) | 1/2 (50) | 43/113 (38) | 7/43 (16) | 0/7 | 39/113 (35) | 11/39 (28) | 1/11 (9) |
| 9 | 74 | 21/74 (28) | 3/21 (14) | 60/74 (81) | 19/60 (32) | 3/19 (16) | 14/74 (19) | 2/14 (14) | 0/2 | 21/74 (28) | 3/21 (14) | 0/3 | 34/74 (46) | 11/34 (32) | 2/11 (18) |
| 10 | 38 | 13/38 (34) | 1/13 (8) | 27/38 (71) | 10/27 (37) | 1/10 (10) | 11/38 (29) | 3/11 (27) | 0/3 | 11/38 (29) | 2/11 (18) | 0/2 | 19/38 (50) | 8/19 (42) | 1/8 (13) |
Data are presented as n/N (%).
Tapering of immunosuppressive therapies in SSc patients from the Leiden CCISS cohort
| Visit year . | All patients . | Female patients . | Male patients . | ACA-positive patients . | ATA-positive patients . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | |
| 1 | 708 | 218/708 (31) | 17/218 (8) | 557/708 (79) | 164/557 (29) | 10/164 (6) | 151/708 (21) | 54/151 (36) | 7/54 (13) | 295/708 (42) | 47/295 (16) | 2/47 (4) | 160/708 (23) | 67/160 (42) | 6/67 (9) |
| 2 | 563 | 213/563 (38) | 24/213 (11) | 446/563 (79) | 162/446 (36) | 18/162 (11) | 117/563 (21) | 51/117 (44) | 6/51 (12) | 234/563 (42) | 48/234 (21) | 5/48 (10) | 129/563 (23) | 58/129 (45) | 4/58 (7) |
| 3 | 452 | 169/452 (37) | 34/169 (20) | 359/452 (79) | 129/359 (36) | 29/129 (22) | 93/452 (21) | 40/93 (43) | 5/40 (13) | 182/452 (40) | 34/182 (19) | 6/34 (18) | 108/452 (24) | 54/108 (50) | 8/54 (15) |
| 4 | 356 | 125/356 (35) | 19/125 (15) | 285/356 (80) | 101/285 (35) | 14/101 (14) | 71/356 (20) | 24/71 (34) | 5/24 (21) | 137/356 (38) | 28/137 (20) | 3/28 (11) | 89/356 (25) | 36/89 (40) | 5/36 (14) |
| 5 | 275 | 91/275 (33) | 11/91 (12) | 226/275 (82) | 76/226 (34) | 8/76 (11) | 49/275 (18) | 15/49 (31) | 3/15 (20) | 111/275 (40) | 24/111 (22) | 4/24 (17) | 79/275 (29) | 27/79 (34) | 3/27 (11) |
| 6 | 225 | 74/225 (33) | 19/74 (26) | 190/225 (84) | 63/190 (33) | 17/63 (27) | 35/225 (16) | 11/35 (31) | 2/11 (18) | 86/225 (38) | 19/86 (22) | 8/19 (42) | 65/225 (29) | 26/65 (40) | 6/26 (23) |
| 7 | 165 | 44/165 (27) | 7/44 (16) | 141/165 (85) | 40/141 (28) | 6/40 (15) | 24/165 (15) | 4/24 (17) | 1/4 (25) | 59/165 (36) | 11/59 (19) | 1/11 (9) | 53/165 (32) | 18/53 (34) | 3/18 (17) |
| 8 | 113 | 30/113 (27) | 2/30 (7) | 97/113 (86) | 28/97 (29) | 1/28 (4) | 16/113 (14) | 2/16 (13) | 1/2 (50) | 43/113 (38) | 7/43 (16) | 0/7 | 39/113 (35) | 11/39 (28) | 1/11 (9) |
| 9 | 74 | 21/74 (28) | 3/21 (14) | 60/74 (81) | 19/60 (32) | 3/19 (16) | 14/74 (19) | 2/14 (14) | 0/2 | 21/74 (28) | 3/21 (14) | 0/3 | 34/74 (46) | 11/34 (32) | 2/11 (18) |
| 10 | 38 | 13/38 (34) | 1/13 (8) | 27/38 (71) | 10/27 (37) | 1/10 (10) | 11/38 (29) | 3/11 (27) | 0/3 | 11/38 (29) | 2/11 (18) | 0/2 | 19/38 (50) | 8/19 (42) | 1/8 (13) |
| Visit year . | All patients . | Female patients . | Male patients . | ACA-positive patients . | ATA-positive patients . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | Whole group . | Patients on IS therapy . | Patients in which IS therapy is tapered . | |
| 1 | 708 | 218/708 (31) | 17/218 (8) | 557/708 (79) | 164/557 (29) | 10/164 (6) | 151/708 (21) | 54/151 (36) | 7/54 (13) | 295/708 (42) | 47/295 (16) | 2/47 (4) | 160/708 (23) | 67/160 (42) | 6/67 (9) |
| 2 | 563 | 213/563 (38) | 24/213 (11) | 446/563 (79) | 162/446 (36) | 18/162 (11) | 117/563 (21) | 51/117 (44) | 6/51 (12) | 234/563 (42) | 48/234 (21) | 5/48 (10) | 129/563 (23) | 58/129 (45) | 4/58 (7) |
| 3 | 452 | 169/452 (37) | 34/169 (20) | 359/452 (79) | 129/359 (36) | 29/129 (22) | 93/452 (21) | 40/93 (43) | 5/40 (13) | 182/452 (40) | 34/182 (19) | 6/34 (18) | 108/452 (24) | 54/108 (50) | 8/54 (15) |
| 4 | 356 | 125/356 (35) | 19/125 (15) | 285/356 (80) | 101/285 (35) | 14/101 (14) | 71/356 (20) | 24/71 (34) | 5/24 (21) | 137/356 (38) | 28/137 (20) | 3/28 (11) | 89/356 (25) | 36/89 (40) | 5/36 (14) |
| 5 | 275 | 91/275 (33) | 11/91 (12) | 226/275 (82) | 76/226 (34) | 8/76 (11) | 49/275 (18) | 15/49 (31) | 3/15 (20) | 111/275 (40) | 24/111 (22) | 4/24 (17) | 79/275 (29) | 27/79 (34) | 3/27 (11) |
| 6 | 225 | 74/225 (33) | 19/74 (26) | 190/225 (84) | 63/190 (33) | 17/63 (27) | 35/225 (16) | 11/35 (31) | 2/11 (18) | 86/225 (38) | 19/86 (22) | 8/19 (42) | 65/225 (29) | 26/65 (40) | 6/26 (23) |
| 7 | 165 | 44/165 (27) | 7/44 (16) | 141/165 (85) | 40/141 (28) | 6/40 (15) | 24/165 (15) | 4/24 (17) | 1/4 (25) | 59/165 (36) | 11/59 (19) | 1/11 (9) | 53/165 (32) | 18/53 (34) | 3/18 (17) |
| 8 | 113 | 30/113 (27) | 2/30 (7) | 97/113 (86) | 28/97 (29) | 1/28 (4) | 16/113 (14) | 2/16 (13) | 1/2 (50) | 43/113 (38) | 7/43 (16) | 0/7 | 39/113 (35) | 11/39 (28) | 1/11 (9) |
| 9 | 74 | 21/74 (28) | 3/21 (14) | 60/74 (81) | 19/60 (32) | 3/19 (16) | 14/74 (19) | 2/14 (14) | 0/2 | 21/74 (28) | 3/21 (14) | 0/3 | 34/74 (46) | 11/34 (32) | 2/11 (18) |
| 10 | 38 | 13/38 (34) | 1/13 (8) | 27/38 (71) | 10/27 (37) | 1/10 (10) | 11/38 (29) | 3/11 (27) | 0/3 | 11/38 (29) | 2/11 (18) | 0/2 | 19/38 (50) | 8/19 (42) | 1/8 (13) |
Data are presented as n/N (%).
Overall, immunosuppressive medications were tapered 137 times during the 2968 visits. Of the 107 patients who tapered medications at least once during the follow-up, in 64% of cases it happened during the first 3 years, suggesting that reducing the immunosuppressive burden in our patients might be feasible early during clinical follow-up. In 24 patients, tapering occurred at the last available visit, whereas in 83 patients the management of medications could be evaluated after tapering (246 follow-up visits). In 31 cases, therapies were not changed further. In the other 52 patients, the treatment regimen was modified again and in 31 cases we had to either increase the dosage of the current therapy or initiate a new immunosuppressive drug due to inadequate control of the disease. This indicates that, in our large cohort of SSc patients, tapering of immunosuppressors could be considered in 15% of cases (107/708) and it was successful in approximately two-thirds (52/83).
Analysing the type of immunosuppressive medications used (Fig. 1), we observed that treatment with MTX tended to decrease, while MMF gained favour over the years, in particular in women and in ATA-positive patients (Supplementary Data S4, available at Rheumatology online) but also in individuals with ILD or in those with more extensive skin involvement (Supplementary Data S5, available at Rheumatology online). The use of glucocorticoids remained stable in the overall cohort, but in analysing the different subgroups, we noticed that at the baseline visits, low-dose glucocorticoids were used mainly by males and patients with ATA positivity, diffuse cutaneous disease, ILD or synovitis (Supplementary Data S4 and S5, available at Rheumatology online), consistent with the findings of Iudici et al. [1].
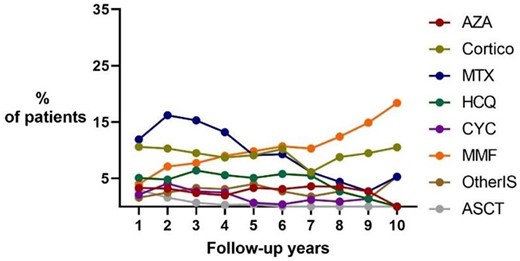
Medication use over time in 708 SSc patients from the Leiden CCISS cohort. ASCT: autologous stem cell transplantation; Cortico: corticosteroids; other IS: immunosuppressors including rituximab, tocilizumab and trial medications
In conclusion, data regarding the use of immunosuppressive medications and the possibilities of tapering in our prospective real-life cohort provide valuable insights into treatment trajectories of SSc patients. We confirm the increased use of MMF and we show stable use of low-dose glucocorticoids, particularly in male ATA-positive patients. If early therapeutic intervention might be beneficial and a window of opportunity exists in SSc, as suggested by Yomono and Kuwana [2], tapering immunosuppressive treatments might become feasible in a proportion of patients with stable disease. However, the potential occurrence of a relapse, as observed in 31 patients in our cohort, highlights that patients should be monitored closely after dose tapering or discontinuation of immunosuppressive therapies.
Moreover, prospective research with a longitudinal design is warranted to elucidate the characteristics of SSc patients who benefit from tapering strategies, but also to define the schedules of dose reduction and the optimal timing for tapering and discontinuation [9].
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Authors’ contributions
J.C. and S.I.E.L. contributed equally.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors declare no conflicts of interest.
Acknowledgements
All patients included in the cohort provided written informed consent prior to inclusion. The cohort was approved by the Leiden University Medical Center Ethics Committee (CME no. B16.037, REU 043/SH/sh, P09.003/SH/second).




Comments