-
PDF
- Split View
-
Views
-
Cite
Cite
Aurélie Daumas, Jérémy Magalon, Elisabeth Jouve, Dominique Casanova, Cécile Philandrianos, Maxime Abellan Lopez, Stéphanie Mallet, Julie Veran, Isabelle Auquit-Auckbur, Dominique Farge, Hervé Levesque, Ygal Benhamou, Laurent Arnaud, Laurent Giraudo, Chloé Dumoulin, Camille Giverne, Olivier Boyer, Alexandra Giuliani, Véronique Bourgarel, Jean-Robert Harlé, Nicolas Schleinitz, Julie Brunet, Yves-Marie Pers, Rosanna Ferreira, Audrey Cras, David Boccara, Jérome Larghero, Joseph Château, Arnaud Hot, Françoise Dignat-George, Guy Magalon, Florence Sabatier, Brigitte Granel, Adipose tissue-derived stromal vascular fraction for treating hands of patients with systemic sclerosis: a multicentre randomized trial Autologous AD-SVF versus placebo in systemic sclerosis, Rheumatology, Volume 61, Issue 5, May 2022, Pages 1936–1947, https://doi.org/10.1093/rheumatology/keab584
Close - Share Icon Share
Abstract
To assess the superiority of adipose tissue-derived stromal vascular fraction (AD-SVF) injection into the fingers vs placebo in reducing hand disability in systemic sclerosis (SSc) patients.
We performed a double-blind, multicentre, phase II trial from October 2015 to January 2018 in France. SSc patients with a Cochin Hand Function Scale (CHFS) ≥20/90 were randomized 1:1 to receive injection of AD-SVF or placebo. AD-SVF was obtained using the automated processing Celution 800/CRS system. The placebo was lactated Ringer’s solution. The primary efficacy end point was the change of the CHFS score from baseline to 3 months. Secondary efficacy endpoints included the CHFS score at 6 months, hand function, vasculopathy, hand pain, skin fibrosis, sensitivity of the finger pulps, Scleroderma Health Assessment Questionnaire, patients and physician satisfaction, and safety.
Forty patients were randomized. The AD-SVF and placebo groups were comparable for age, sex ratio, disease duration, skin fibrosis of the hands and main cause of hand disability. After 3 months’ follow-up, hand function significantly improved in both groups with no between-group difference of CHFS (mean change of −9.2 [12.2] in the AD-SVF group vs −7.6 [13.2] in the placebo group). At 6 months, hand function improved in both groups.
This study showed an improvement of hand function in both groups over time, with no superiority of the AD-SVF. Considering the limits of this trial, studies on a larger population of patients with homogeneous phenotype and hand handicap should be encouraged to accurately assess the benefit of AD-SVF therapy.
ClinicalTrials.gov, https://clinicaltrials.gov, NCT02558543. Registered on September 24, 2015.
Impaired hand function reduces quality of life in patients with systemic sclerosis.
Adipose tissue-derived stromal vascular fraction (AD-SVF) is an easily accessible source of regenerative cells offering therapeutic potential in systemic sclerosis.
At 3 months, no superiority of AD-SVF injection into the fingers in the improvement of hand function was observed.
Introduction
Systemic sclerosis (SSc) is a rare autoimmune disease characterized by a fibrotic process and peripheral vasculopathy impairing the function of many organs. Severe damage to the pulmonary, cardiac, digestive and renal systems represents the main cause of mortality in patients with SSc. However, on a daily quality of life and occupational basis, the greatest source of morbidity is hand handicap [1]. Hand involvement is also associated with substantial pain and psychological and social distress. The aetiology of hand involvement in SSc disease is complex and can be attributed to numerous factors, such as vascular damage, including Raynaud’s phenomenon and digital ulcers (DU), a fibrotic skin process leading to retraction of the fingers and muscular and joint inflammation. To date, the recently published worldwide Expert Agreement of the updated EULAR and European Scleroderma Trials and Research group recommendations for treatment indicates vasodilator therapy and physiotherapy associated with protective measures for the hands [2]. For many patients, the best available therapy provides only limited relief. There is therefore a need for novel therapies with potential pleiotropic efficacy to overcome the pathological process related to SSc disease.
Adipose tissue reconstruction is an important technique for soft tissue defects with expanding indications in plastic surgery. It is aimed at achieving volume recovery and tissue regeneration. In order to improve severe and refractory Raynaud’s phenomenon, Bank et al. [3] reported in a series of 13 patients (including nine patients with SSc), the benefit of the injection of autologous decanted fat into the dorsum and palmar face of the hands. Fat grafting showed improvement in pain and skin and soft-tissue texture, fewer cold attacks with a decrease in DU number and patient-reported improved function.
In the context of chronic ischaemic DU related to SSc, Del Papa et al. [4] performed a single-centre, randomized, controlled study investigating the therapeutic potential of regional injection of autologous adipose tissue. The grafting procedure consisted in the injection of adipose tissue obtained after centrifugation of fat aspirate, at the base of the affected finger after a small skin incision. The study was prematurely stopped after the enrolment of 25 and 13 patients receiving autologous fat grafting and saline solution, respectively. The results at 8 weeks strongly confirmed that autologous adipose tissue grafting was effective in inducing DU healing, as DU healed in 23/25 patients treated with fat grafting vs only 1/13 patients who received saline solution. Interestingly, all 12 patients who initially received saline solution underwent rescue with autologous adipose tissue grafting, and their DU healed.
In addition to adipocytes, adipose tissue contains the stromal vascular fraction (SVF), a cell population including adipose stromal/stem cells (ASCs), endothelial cells and their progenitors, haematopoietic and immune cells and pericytes [5]. Potential therapeutic use of adipose-derived SVF (AD-SVF) has been proposed based on the proangiogenic, anti-inflammatory and reparative properties of the component cell types with the aim of focusing on a trophic effect instead of a volumizing effect [6].
In the context of chronic autoimmune diseases, the use of autologous AD-SVF can be debated, as their properties could be hampered by the pathological context. Recently, our team showed that the proportion of endothelial, ASC, immune and pericyte cell subsets was preserved within the AD-SVF extracted from SSc patients compared with healthy donors [7]. We showed that AD-SVF from SSc patients sustained a functional vascular repair capacity [7]. Similarly, Capelli et al. [8] showed that in vitro-expanded ASCs isolated from patients with SSc maintained the same phenotypic pattern, proliferation and differentiation potential, proangiogenic activity, and immunosuppressive properties as those from healthy controls. Thus, autologous AD-SVF use for therapy appeared promising in SSc.
We previously performed a first-in-man, open-label, single-centre clinical trial, called Scleradec (NTC01813279), assessing the safety and potential effects of the subcutaneous injection of autologous AD-SVF into the fingers of 12 SSc patients at 6 months [9]. Despite the limits inherent to an open-label clinical trial performed on a low number of patients, the results of the trial showed that this cell-based therapy was feasible and well tolerated for SSc patients. The secondary efficacy endpoints suggested an improvement in hand disability, hand pain, severity of Raynaud's phenomenon, the number of digital ulcers, hand mobility and patient quality of life. These preliminary results encouraged us to propose a randomized, double-blind clinical trial on a higher number of patients to assess the efficacy and confirm the safety of this innovative therapy.
Methods
Study design
This was a randomized, phase II, placebo-controlled, double-blind, parallel-group study conducted in five centres in France between October 2015 and January 2018. Patients were randomly assigned 1:1 to receive either autologous AD-SVF or placebo injection into their 10 fingers. The design of the study and follow-up are illustrated in Fig. 1. The study remained double blinded up to 6 months. The study was approved by the Ethics Committee Sud Méditerranée V prior the data collection and the French Drug Agency (no. 2014-003023-22). An independent data monitoring committee ensured the integrity of the trial and safety of participants. All patients gave written informed consent before their participation in the trial.
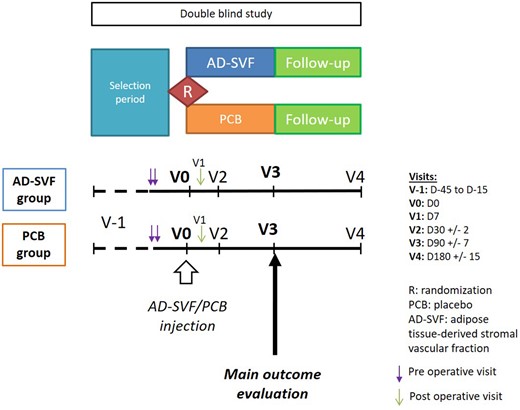
Study design
The study was designed as a multicentre, placebo-controlled, double-blind, superiority trial. Patients were randomly assigned 1:1 to receive either autologous AD-SVF or placebo injection into their 10 fingers and followed up to 6 months. AD-SVF: adipose tissue-derived stromal vascular fraction; PCB: placebo.
Randomization and masking
Randomization was centralized and implemented by the Cell Therapy Unit of Marseille on the day of the surgical procedure, when the adipose tissue sample was received by the Cell Therapy Unit. Dose preparation was performed by unblinded designated qualified staff not involved in study patient management and follow-up assessments. To maintain the blindness of the study, both AD-SVF and placebo were preconditioned using opaque syringes and the surgeons who performed the injections were not involved in the follow-up of the patients. In the case of patients randomized into the placebo group, AD-SVF was immediately cryopreserved.
Patient inclusion and exclusion criteria
Patients were eligible if they had a score ≥9 according to the 2013 EULAR/ACR Classification criteria for SSc [10], were aged older than 18 and had a Cochin Hand Function Scale (CHFS) score above 20/90 despite optimal treatment according to the EULAR recommendations [11]. CHFS is a validated scale assessing hand disability in SSc patients [12]. Patients with DU of ischaemic and mechanical origin could be enrolled. Active ulcer was defined as a full-thickness skin lesion with loss of epithelization.
The exclusion criteria of the study were a BMI <18 kg/m2; active infection in any finger; a modified Rodnan skin score focused on hands >16/18 [13]; severe retraction of the fingers objectified by a defect in extension >90° for at least two proximal interphalangeal joints; new vasodilators or immunosuppressive therapy for SSc in the 3 months prior to enrolment; the beginning or the modification of a hand physiotherapy programme within 90 days before enrolment; surgical contraindication; positive status for HIV, hepatitis B or C, HTLV1-2 or syphilis; lack of contraception for women of childbearing age; systemic steroids in doses exceeding the equivalent of 10 mg prednisone daily; any intravenous cyclophosphamide; methotrexate exceeding 25 mg/week; mycophenolate mofetil exceeding 3 g/day; azathioprine exceeding 300 mg/day or any other immunosuppressive medication in the 90 days prior to the inclusion visit; allergy to human albumin; pulmonary arterial hypertension; and severe and/or progressive and/or oxygen-dependent pulmonary fibrosis.
Skin thickening of the hands was assessed by the modified Rodnan skin score [13]. In this score, skin thickening was scored from 0 (normal thickness) to 3 (severe thickness with inability to pinch the skin into a fold). Skin thickening was measured on the back of the hand and the first and second phalanges of the most affected finger, with a maximum total score of 18. The patients were asked to maintain their regular medications during the study, and subjects receiving physiotherapy for hand dysfunction were asked to continue without any changes in therapeutic modalities during the course of the study.
Procedures are detailed in Supplementary Data S1 [14], available at Rheumatology online.
Endpoints
Efficacy assessments
The primary efficacy end point was the change in the CHFS score from baseline to 3 months. The secondary efficacy endpoints included the change in the CHFS score from baseline to 1 and 6 months. Other parameters were assessed at 1, 3 and 6 months [15–21]. (For details, see Supplementary Table S1, available at Rheumatology online.)
Assessment of safety and tolerability
At each visit, a standardized interview and physical examination collected information on adverse events (AEs). AEs were classified according to the medical dictionary for regulatory activity (MedDRA). Causality was assessed by the investigator at the time of the event based on the clinical judgement.
Statistical analysis
Sample size was based on previous results of the Scleradec study [9]; no further data on efficacy of AD-SVF in SSc was available at this time. We assumed that 30% of the effect observed could not be attributable to the product. Consequently, a 16-point difference on CHFS changes at 3 months post-treatment was expected. With a standard deviation of 16.4, a two-sided α level of 0.05 and a power of 80%, the number of patients was of 18 by group. Considering a risk of patients’ withdrawal, a total of 40 subjects were fixed to be included.
All subjects’ data related to the clinical trial were collected with an Electronic Data Capture (IBM Clinical Development, Morrisville, NC, USA) which were audited from source data by a clinical trial monitor. All data were reviewed and validated during the data blinded review meeting. Data consistency checks and statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA), according to the validated clinical data management plan and statistical analysis plan. Efficacy and safety were assessed on intention-to-treat population. Per-protocol analyses were done as sensitivity analysis. Continuous data were summarized by mean and s.d. and categorical variables by frequency, number and percentage of patients. Baseline characteristics of patients were compared using Student’s t-test or Mann–Whitney test and χ2 test or Fisher’s exact test. P-values <0.05 were considered significant.
Mixed models were performed to analyse repeated measures. Fixed effects were as follows: treatment group (AD-SVF vs Placebo), time (Baseline, M1, M3, M6), treatment group-by-time interaction, SSc cutaneous form (limited, diffuse) and treatment group-by-SSc cutaneous form, and centre. Random factors were intercept and subject. Treatment groups were compared at each time using F-tests of effect sliced by time. The primary analysis was the comparison at month 3. Tukey–Kramer adjustment was used for multiple comparisons.
Results
Description of the study population
Forty patients were enrolled (26 in Marseille, nine in Rouen, two in Paris, one in Montpellier and one in Lyon): 25 had the limited cutaneous form (lcSSc), and 15 had the diffuse form (dcSSc). Twenty were assigned to the AD-SVF group (including 10 dcSSc), and 20 were assigned to the placebo group (including 5 dcSSc). All 40 patients had surgery, and there were no dropouts or patients lost to follow-up before the primary end point at 3 months post-treatment (Fig. 2). One patient was prematurely withdrawn after the 4-month follow-up due to death from worsening pulmonary hypertension.

CONSORT flow diagram of the study
Group allocation, follow-up and data analysis of study participants summarized as a flow diagram. Twenty patients with scleroderma were assigned to the AD-SVF group and 20 were assigned to the placebo group. All 40 patients had surgery, and there were no dropouts or patients lost to follow-up before the primary endpoint 3 months post-treatment. Efficacy and safety were assessed on intention-to-treat population. Per-protocol analyses were done as sensitivity analysis. AD-SVF: adipose tissue-derived stromal vascular fraction; ITT: intention to treat. PP: per protocol.
The main demographic and clinical characteristics of the patients are summarized in Table 1. The AD-SVF and placebo groups were comparable in terms of age, sex ratio, disease duration, modified Rodnan skin score focused on the hands and main cause of hand disability (mainly vascular aspects). They were comparable in terms of visceral involvement except for total lung capacity, which was better in the AD-SVF group. Corticosteroids were more frequently observed in the placebo group (40% vs 10% for AD-SVF; P = 0.029). Calcium channel blockers were prescribed more in the AD-SVF group (55% vs 25% for placebo; P = 0.053).
| . | AD-SVF (n = 20) . | Placebo (n = 20) . | P-value . |
|---|---|---|---|
| Demographic | |||
| Female, n (%) | 20 (100) | 19 (95) | 0.999 |
| Age, mean (s.d.) [minimum and maximum], years | 53.9 (12.7) [30.0–73.0] | 57.2 (13.3) [27.0–77.0] | 0.421 |
| BMI, mean (s.d.) [minimum and maximum], kg/m² | 22.7 (3.3) [18.0–28.7] | 23.6 (3.5) [18.8–31.9] | 0.382 |
| Main causes of hand disability | |||
| Vascular aspects (Raynaud’s phenomenon/digital ulcers), n (%) | 9 (23) | 12 (30) | 0.527 |
| Skin fibrosis (sclerodactyly), n (%) | 12 (30) | 7 (18) | 0.205 |
| Musculoskeletal aspects (joint synovitis), n (%) | 0 | 3 (8) | 0.231 |
| Disease history | |||
| Disease duration from diagnosis, mean (s.d.) [minimum and maximum], years | 18.2 (4.5) [10–26] | 18.0 (4.9) [9–24] | 0.894 |
| Early SSc disease <4 years, n (%) | 7 (35) | 8 (40) | 0.744 |
| History of Raynaud’s phenomenon, mean (s.d.) [minimum and maximum], years | 13.2 (9.0) [1–32] | 14.3 (7.7) [2–56] | 0.731 |
| Limited/diffuse cutaneous form | 10/10 | 15/5 | 0.103 |
| Pulmonary arterial hypertension, n (%) | 0 | 2 (10) | 0.147 |
| Body skin thickness | |||
| Global modified Rodnan skin score, mean (s.d.) [minimum and maximum] | 13.2 (7.4) [4–30] | 10.5 (6.3) [0–20] | 0.230 |
| Gastrointestinal | |||
| Gastrointestinal reflux, n (%) | 16 (80) | 18 (90) | 0.376 |
| Hypotonia of the inferior oesophagus sphincter (manometry), n (%) | 8 (67)/12 tested | 6 (55)/11 tested | 0.552 |
| Lung | |||
| FVC, mean (s.d.) [minimum and maximum], % predicted | 101.7 (16.9) [67–133] | 100.7 (23.6) [57–139] | 0.883 |
| Total lung capacity, mean (s.d.) [minimum and maximum], % predicted | 103.9 (15.6) [82–140] | 91.8 (15.1) [68–115] | 0.023 |
| DLCO, mean (s.d.) [minimum and maximum], % predicted | 63.8 (12.2) [40–81] | 56.0 (16.2) [24–78] | 0.112 |
| Fibrosis on high-resolution chest tomography (bronchiectasia and/or honeycombing), n (%) | 5 (29)/17 tested | 5 (26)/19 tested | 0.999 |
| Immunological profile, n (%) | |||
| Anti-nuclear antibodies positive (indirect immunofluorescence on HEp2) | 20 (100) | 20 (100) | NA |
| Anti-topoisomerase I (Scl70) antibodies positive (ELIA) | 10 (50) | 6 (30) | 0.197 |
| Anti-centromere antibodies positive (ELIA) | 7 (35) | 6 (30) | 0.736 |
| Anti-RNA polymerase III | 1 (14)/7 tested | 4 (29)/14 tested | 0.624 |
| Ongoing systemic sclerosis medications, n (%) | |||
| Calcium channel blockers | 11 (55) | 5 (25) | 0.053 |
| Endothelin-1 receptor antagonist | 2 (10) | 7 (35) | 0.127 |
| Prednisone <10 mg/day | 2 (10) | 8 (40) | 0.029 |
| Methotrexate | 2 (10) | 4 (20) | 0.661 |
| Previous iloprost infusion | 0 | 0 | NA |
| Oral phosphodiesterase type 5 (PDE5) inhibitors | 0 | 3 (15) | 0.231 |
| Proton pump inhibitor | 18 (90) | 18 (90) | 0.999 |
| Physiotherapy for hands ongoing | 9 (45) | 7 (35) | 0.519 |
| Cardiovascular risks, n (%) | |||
| Tobacco ongoing | 0 | 1 | 0.999 |
| Diabetes | 0 | 0 | NA |
| Hypertension | 1 (5) | 2 (10) | 0.605 |
| Hypercholesterolaemia | 2 (10) | 4 (20) | 0.661 |
| . | AD-SVF (n = 20) . | Placebo (n = 20) . | P-value . |
|---|---|---|---|
| Demographic | |||
| Female, n (%) | 20 (100) | 19 (95) | 0.999 |
| Age, mean (s.d.) [minimum and maximum], years | 53.9 (12.7) [30.0–73.0] | 57.2 (13.3) [27.0–77.0] | 0.421 |
| BMI, mean (s.d.) [minimum and maximum], kg/m² | 22.7 (3.3) [18.0–28.7] | 23.6 (3.5) [18.8–31.9] | 0.382 |
| Main causes of hand disability | |||
| Vascular aspects (Raynaud’s phenomenon/digital ulcers), n (%) | 9 (23) | 12 (30) | 0.527 |
| Skin fibrosis (sclerodactyly), n (%) | 12 (30) | 7 (18) | 0.205 |
| Musculoskeletal aspects (joint synovitis), n (%) | 0 | 3 (8) | 0.231 |
| Disease history | |||
| Disease duration from diagnosis, mean (s.d.) [minimum and maximum], years | 18.2 (4.5) [10–26] | 18.0 (4.9) [9–24] | 0.894 |
| Early SSc disease <4 years, n (%) | 7 (35) | 8 (40) | 0.744 |
| History of Raynaud’s phenomenon, mean (s.d.) [minimum and maximum], years | 13.2 (9.0) [1–32] | 14.3 (7.7) [2–56] | 0.731 |
| Limited/diffuse cutaneous form | 10/10 | 15/5 | 0.103 |
| Pulmonary arterial hypertension, n (%) | 0 | 2 (10) | 0.147 |
| Body skin thickness | |||
| Global modified Rodnan skin score, mean (s.d.) [minimum and maximum] | 13.2 (7.4) [4–30] | 10.5 (6.3) [0–20] | 0.230 |
| Gastrointestinal | |||
| Gastrointestinal reflux, n (%) | 16 (80) | 18 (90) | 0.376 |
| Hypotonia of the inferior oesophagus sphincter (manometry), n (%) | 8 (67)/12 tested | 6 (55)/11 tested | 0.552 |
| Lung | |||
| FVC, mean (s.d.) [minimum and maximum], % predicted | 101.7 (16.9) [67–133] | 100.7 (23.6) [57–139] | 0.883 |
| Total lung capacity, mean (s.d.) [minimum and maximum], % predicted | 103.9 (15.6) [82–140] | 91.8 (15.1) [68–115] | 0.023 |
| DLCO, mean (s.d.) [minimum and maximum], % predicted | 63.8 (12.2) [40–81] | 56.0 (16.2) [24–78] | 0.112 |
| Fibrosis on high-resolution chest tomography (bronchiectasia and/or honeycombing), n (%) | 5 (29)/17 tested | 5 (26)/19 tested | 0.999 |
| Immunological profile, n (%) | |||
| Anti-nuclear antibodies positive (indirect immunofluorescence on HEp2) | 20 (100) | 20 (100) | NA |
| Anti-topoisomerase I (Scl70) antibodies positive (ELIA) | 10 (50) | 6 (30) | 0.197 |
| Anti-centromere antibodies positive (ELIA) | 7 (35) | 6 (30) | 0.736 |
| Anti-RNA polymerase III | 1 (14)/7 tested | 4 (29)/14 tested | 0.624 |
| Ongoing systemic sclerosis medications, n (%) | |||
| Calcium channel blockers | 11 (55) | 5 (25) | 0.053 |
| Endothelin-1 receptor antagonist | 2 (10) | 7 (35) | 0.127 |
| Prednisone <10 mg/day | 2 (10) | 8 (40) | 0.029 |
| Methotrexate | 2 (10) | 4 (20) | 0.661 |
| Previous iloprost infusion | 0 | 0 | NA |
| Oral phosphodiesterase type 5 (PDE5) inhibitors | 0 | 3 (15) | 0.231 |
| Proton pump inhibitor | 18 (90) | 18 (90) | 0.999 |
| Physiotherapy for hands ongoing | 9 (45) | 7 (35) | 0.519 |
| Cardiovascular risks, n (%) | |||
| Tobacco ongoing | 0 | 1 | 0.999 |
| Diabetes | 0 | 0 | NA |
| Hypertension | 1 (5) | 2 (10) | 0.605 |
| Hypercholesterolaemia | 2 (10) | 4 (20) | 0.661 |
AD-SVF: adipose-derived stromal vascular fraction; DLCO: diffusion lung capacity for carbon monoxide; FVC: forced vital capacity; NA: not assessed.
| . | AD-SVF (n = 20) . | Placebo (n = 20) . | P-value . |
|---|---|---|---|
| Demographic | |||
| Female, n (%) | 20 (100) | 19 (95) | 0.999 |
| Age, mean (s.d.) [minimum and maximum], years | 53.9 (12.7) [30.0–73.0] | 57.2 (13.3) [27.0–77.0] | 0.421 |
| BMI, mean (s.d.) [minimum and maximum], kg/m² | 22.7 (3.3) [18.0–28.7] | 23.6 (3.5) [18.8–31.9] | 0.382 |
| Main causes of hand disability | |||
| Vascular aspects (Raynaud’s phenomenon/digital ulcers), n (%) | 9 (23) | 12 (30) | 0.527 |
| Skin fibrosis (sclerodactyly), n (%) | 12 (30) | 7 (18) | 0.205 |
| Musculoskeletal aspects (joint synovitis), n (%) | 0 | 3 (8) | 0.231 |
| Disease history | |||
| Disease duration from diagnosis, mean (s.d.) [minimum and maximum], years | 18.2 (4.5) [10–26] | 18.0 (4.9) [9–24] | 0.894 |
| Early SSc disease <4 years, n (%) | 7 (35) | 8 (40) | 0.744 |
| History of Raynaud’s phenomenon, mean (s.d.) [minimum and maximum], years | 13.2 (9.0) [1–32] | 14.3 (7.7) [2–56] | 0.731 |
| Limited/diffuse cutaneous form | 10/10 | 15/5 | 0.103 |
| Pulmonary arterial hypertension, n (%) | 0 | 2 (10) | 0.147 |
| Body skin thickness | |||
| Global modified Rodnan skin score, mean (s.d.) [minimum and maximum] | 13.2 (7.4) [4–30] | 10.5 (6.3) [0–20] | 0.230 |
| Gastrointestinal | |||
| Gastrointestinal reflux, n (%) | 16 (80) | 18 (90) | 0.376 |
| Hypotonia of the inferior oesophagus sphincter (manometry), n (%) | 8 (67)/12 tested | 6 (55)/11 tested | 0.552 |
| Lung | |||
| FVC, mean (s.d.) [minimum and maximum], % predicted | 101.7 (16.9) [67–133] | 100.7 (23.6) [57–139] | 0.883 |
| Total lung capacity, mean (s.d.) [minimum and maximum], % predicted | 103.9 (15.6) [82–140] | 91.8 (15.1) [68–115] | 0.023 |
| DLCO, mean (s.d.) [minimum and maximum], % predicted | 63.8 (12.2) [40–81] | 56.0 (16.2) [24–78] | 0.112 |
| Fibrosis on high-resolution chest tomography (bronchiectasia and/or honeycombing), n (%) | 5 (29)/17 tested | 5 (26)/19 tested | 0.999 |
| Immunological profile, n (%) | |||
| Anti-nuclear antibodies positive (indirect immunofluorescence on HEp2) | 20 (100) | 20 (100) | NA |
| Anti-topoisomerase I (Scl70) antibodies positive (ELIA) | 10 (50) | 6 (30) | 0.197 |
| Anti-centromere antibodies positive (ELIA) | 7 (35) | 6 (30) | 0.736 |
| Anti-RNA polymerase III | 1 (14)/7 tested | 4 (29)/14 tested | 0.624 |
| Ongoing systemic sclerosis medications, n (%) | |||
| Calcium channel blockers | 11 (55) | 5 (25) | 0.053 |
| Endothelin-1 receptor antagonist | 2 (10) | 7 (35) | 0.127 |
| Prednisone <10 mg/day | 2 (10) | 8 (40) | 0.029 |
| Methotrexate | 2 (10) | 4 (20) | 0.661 |
| Previous iloprost infusion | 0 | 0 | NA |
| Oral phosphodiesterase type 5 (PDE5) inhibitors | 0 | 3 (15) | 0.231 |
| Proton pump inhibitor | 18 (90) | 18 (90) | 0.999 |
| Physiotherapy for hands ongoing | 9 (45) | 7 (35) | 0.519 |
| Cardiovascular risks, n (%) | |||
| Tobacco ongoing | 0 | 1 | 0.999 |
| Diabetes | 0 | 0 | NA |
| Hypertension | 1 (5) | 2 (10) | 0.605 |
| Hypercholesterolaemia | 2 (10) | 4 (20) | 0.661 |
| . | AD-SVF (n = 20) . | Placebo (n = 20) . | P-value . |
|---|---|---|---|
| Demographic | |||
| Female, n (%) | 20 (100) | 19 (95) | 0.999 |
| Age, mean (s.d.) [minimum and maximum], years | 53.9 (12.7) [30.0–73.0] | 57.2 (13.3) [27.0–77.0] | 0.421 |
| BMI, mean (s.d.) [minimum and maximum], kg/m² | 22.7 (3.3) [18.0–28.7] | 23.6 (3.5) [18.8–31.9] | 0.382 |
| Main causes of hand disability | |||
| Vascular aspects (Raynaud’s phenomenon/digital ulcers), n (%) | 9 (23) | 12 (30) | 0.527 |
| Skin fibrosis (sclerodactyly), n (%) | 12 (30) | 7 (18) | 0.205 |
| Musculoskeletal aspects (joint synovitis), n (%) | 0 | 3 (8) | 0.231 |
| Disease history | |||
| Disease duration from diagnosis, mean (s.d.) [minimum and maximum], years | 18.2 (4.5) [10–26] | 18.0 (4.9) [9–24] | 0.894 |
| Early SSc disease <4 years, n (%) | 7 (35) | 8 (40) | 0.744 |
| History of Raynaud’s phenomenon, mean (s.d.) [minimum and maximum], years | 13.2 (9.0) [1–32] | 14.3 (7.7) [2–56] | 0.731 |
| Limited/diffuse cutaneous form | 10/10 | 15/5 | 0.103 |
| Pulmonary arterial hypertension, n (%) | 0 | 2 (10) | 0.147 |
| Body skin thickness | |||
| Global modified Rodnan skin score, mean (s.d.) [minimum and maximum] | 13.2 (7.4) [4–30] | 10.5 (6.3) [0–20] | 0.230 |
| Gastrointestinal | |||
| Gastrointestinal reflux, n (%) | 16 (80) | 18 (90) | 0.376 |
| Hypotonia of the inferior oesophagus sphincter (manometry), n (%) | 8 (67)/12 tested | 6 (55)/11 tested | 0.552 |
| Lung | |||
| FVC, mean (s.d.) [minimum and maximum], % predicted | 101.7 (16.9) [67–133] | 100.7 (23.6) [57–139] | 0.883 |
| Total lung capacity, mean (s.d.) [minimum and maximum], % predicted | 103.9 (15.6) [82–140] | 91.8 (15.1) [68–115] | 0.023 |
| DLCO, mean (s.d.) [minimum and maximum], % predicted | 63.8 (12.2) [40–81] | 56.0 (16.2) [24–78] | 0.112 |
| Fibrosis on high-resolution chest tomography (bronchiectasia and/or honeycombing), n (%) | 5 (29)/17 tested | 5 (26)/19 tested | 0.999 |
| Immunological profile, n (%) | |||
| Anti-nuclear antibodies positive (indirect immunofluorescence on HEp2) | 20 (100) | 20 (100) | NA |
| Anti-topoisomerase I (Scl70) antibodies positive (ELIA) | 10 (50) | 6 (30) | 0.197 |
| Anti-centromere antibodies positive (ELIA) | 7 (35) | 6 (30) | 0.736 |
| Anti-RNA polymerase III | 1 (14)/7 tested | 4 (29)/14 tested | 0.624 |
| Ongoing systemic sclerosis medications, n (%) | |||
| Calcium channel blockers | 11 (55) | 5 (25) | 0.053 |
| Endothelin-1 receptor antagonist | 2 (10) | 7 (35) | 0.127 |
| Prednisone <10 mg/day | 2 (10) | 8 (40) | 0.029 |
| Methotrexate | 2 (10) | 4 (20) | 0.661 |
| Previous iloprost infusion | 0 | 0 | NA |
| Oral phosphodiesterase type 5 (PDE5) inhibitors | 0 | 3 (15) | 0.231 |
| Proton pump inhibitor | 18 (90) | 18 (90) | 0.999 |
| Physiotherapy for hands ongoing | 9 (45) | 7 (35) | 0.519 |
| Cardiovascular risks, n (%) | |||
| Tobacco ongoing | 0 | 1 | 0.999 |
| Diabetes | 0 | 0 | NA |
| Hypertension | 1 (5) | 2 (10) | 0.605 |
| Hypercholesterolaemia | 2 (10) | 4 (20) | 0.661 |
AD-SVF: adipose-derived stromal vascular fraction; DLCO: diffusion lung capacity for carbon monoxide; FVC: forced vital capacity; NA: not assessed.
Biological characteristics of the AD-SVF
Fat harvest and AD-SVF cell characterization for both groups are illustrated in Supplementary Table S2, available at Rheumatology online.
Safety and tolerability assessment
There were 35 AEs reported in 17 patients (42.5%); 19 AEs were reported in eight patients in the AD-SVF group, and 16 AEs were noted in eight patients in the placebo group. Twenty-seven were treatment emergent AEs (TEAEs) (deemed possibly, probably or definitely related to treatment): 18 in the AD-SVF group and nine in the placebo arm. Eight serious AEs (SAEs) were recorded: one in the AD-SVF group and seven in the placebo group (in four patients, P = 0.341). All SAEs were related to worsening of the underlying SSc. Only one SAE was attributed to the surgical procedure. This event (hypoxaemia during the surgical process) occurred in the placebo group (Supplementary Table S3, available at Rheumatology online.).
Efficacy parameters
The main efficacy end point outcomes are shown in Table 2. The CHFS score improved from baseline to 3 months post-treatment in both groups (P < 0.01): the changes were −9.2 (12.2) points in the AD-SVF group and −7.6 (13.2) in the placebo group. When the AD-SVF and placebo groups were compared, the CHFS outcome did not differ between the two groups (P = 0.274). At 6 months, the CHFS score was still improved compared with baseline in both groups (P < 0.01). As observed at 3 months, the between-group difference for the CHFS outcome was not significant at the 6-month follow-up (P = 0.833) (Fig. 3). At 3 months, the CHFS score did not correlate with the number of injected nucleated cells, cell viability percentage or CFU clonogenic assay.
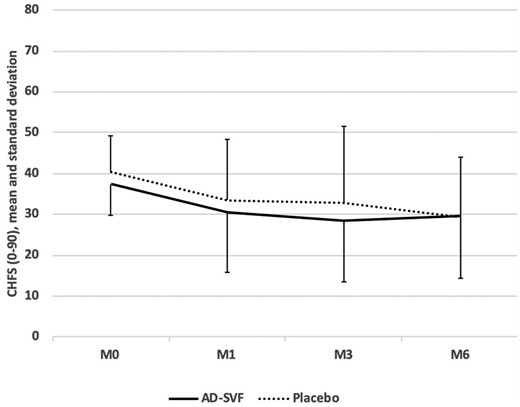
Cochin Hand Function Scale score outcome
CHFS scores are summarized by mean and standard deviation, from baseline to 6 months post-treatment in both groups. The CHFS score improved from baseline to 6 months post-treatment in both groups but the between-group difference for the CHFS outcome was not significant. AD-SVF: adipose-derived stromal vascular fraction; CHFS: Cochin Hand Function Scale; M: month.
| . | AD-SVF (n = 20) . | Placebob (n = 20) . | P-valuec . |
|---|---|---|---|
| CHFS score (0–90) | |||
| Baseline | 37.4 (7.8) | 40.4 (8.7) | |
| Change at M1 | −7.0 (11.1) | −6.9 (11.1) | Group: 0.415 |
| Change at M3 | −9.2 (12.2) | −7.6 (13.2) | Time: <0.001 |
| Change at M6 | −7.9 (14.4) | −10.8 (12) | |
| SHAQ Score (0–3) | |||
| Baseline | 1.32 (0.42) | 1.4 (0.37) | |
| Change at M1 | −0.16 (0.28) | −0.15 (0.37) | Group: 0.145 |
| Change at M3 | −0.2 (0.3) | −0.16 (0.41) | Time: <0.001 |
| Change at M6 | −0.23 (0.39) | −0.32 (0.36) | |
| Hand Pain VAS (0–100) | |||
| Baseline | 44.2 (20.7) | 54.4 (28.1) | |
| Change at M1 | −13.6 (21.8) | −20.4 (20.6) | Group: 0.209 |
| Change at M3 | −6.2 (28.7) | −12.5 (21.6) | Time: <0.001 |
| Change at M6 | −12.3 (29.4) | −23.9 (23.1) | |
| mRSS on right hand (0–18) | |||
| Baseline | 5.4 (1.7) | 5.3 (2.4) | |
| Change at M1 | −0.2 (1.1) | −0.7 (1.0) | Group: 0.384 |
| Change at M3 | −0.2 (1.2) | −0.7 (1.3) | Time: 0.013 |
| Change at M6 | −0.2 (1.3) | −1.1 (1.6) | |
| mRSS on left hand (0–18) | |||
| Baseline | 4.8 (1.7) | 5.1 (2.4) | |
| Change at M1 | −0.3 (0.8) | −0.8 (1.0) | Group: 0.591 |
| Change at M3 | −0.3 (1.2) | −1.1 (1.0) | Time: <0.001 |
| Change at M6 | −0.1 (0.9) | −1.7 (1.7) | |
| Number of days with at least one Raynaud’s crisisa | |||
| Baseline | 5.7 (2.3) | 5.6 (2.2) | |
| Change at M1 | −0.4 (2.7 | −0.1 (1.5) | Group: 0.255 |
| Change at M3 | −0.9 (2.3) | −1.0 (2.9) | Time: <0.001 |
| Change at M6 | −1.8 (2.8) | −2.1 (2.6) | |
| Mean intensity of Raynaud’s crisisa (0 = not severe to 10 = very severe) | |||
| Baseline | 5.5 (2.4) | 5.8 (2.1) | |
| Change at M1 | −0.8 (3.5) | −1.8 (3.2) | Group: 0.734 |
| Change at M3 | −1.6 (3.5) | −0.8 (2.7) | Time: 0.006 |
| Change at M6 | −1.8 (3.1) | −2.2 (3.1) | |
| Mean duration of Raynaud’s crisisa (min) | |||
| Baseline | 16.8 (12.3) | 18.9 (14.1) | |
| Change at M1 | −2.3 (6.6) | −9.3 (13.2) | Group: 0.767 |
| Change at M3 | −1.6 (13.9) | −4.2 (18.1) | Time: 0.015 |
| Change at M6 | −3.2 (13.9) | −10.2 (15.1) | |
| Mean number of Raynaud’s crisis by daya | |||
| Baseline | 4.2 (5.4) | 3.4 (2.8) | |
| Change at M1 | −1.0 (3.0) | −0.1 (3.0) | Group: 0.169 |
| Change at M3 | −0.3 (3.7) | −1.0 (3.2) | Time: 0.018 |
| Change at M6 | −1.1 (3.7) | −1.9 (3.0) | |
| . | AD-SVF (n = 20) . | Placebob (n = 20) . | P-valuec . |
|---|---|---|---|
| CHFS score (0–90) | |||
| Baseline | 37.4 (7.8) | 40.4 (8.7) | |
| Change at M1 | −7.0 (11.1) | −6.9 (11.1) | Group: 0.415 |
| Change at M3 | −9.2 (12.2) | −7.6 (13.2) | Time: <0.001 |
| Change at M6 | −7.9 (14.4) | −10.8 (12) | |
| SHAQ Score (0–3) | |||
| Baseline | 1.32 (0.42) | 1.4 (0.37) | |
| Change at M1 | −0.16 (0.28) | −0.15 (0.37) | Group: 0.145 |
| Change at M3 | −0.2 (0.3) | −0.16 (0.41) | Time: <0.001 |
| Change at M6 | −0.23 (0.39) | −0.32 (0.36) | |
| Hand Pain VAS (0–100) | |||
| Baseline | 44.2 (20.7) | 54.4 (28.1) | |
| Change at M1 | −13.6 (21.8) | −20.4 (20.6) | Group: 0.209 |
| Change at M3 | −6.2 (28.7) | −12.5 (21.6) | Time: <0.001 |
| Change at M6 | −12.3 (29.4) | −23.9 (23.1) | |
| mRSS on right hand (0–18) | |||
| Baseline | 5.4 (1.7) | 5.3 (2.4) | |
| Change at M1 | −0.2 (1.1) | −0.7 (1.0) | Group: 0.384 |
| Change at M3 | −0.2 (1.2) | −0.7 (1.3) | Time: 0.013 |
| Change at M6 | −0.2 (1.3) | −1.1 (1.6) | |
| mRSS on left hand (0–18) | |||
| Baseline | 4.8 (1.7) | 5.1 (2.4) | |
| Change at M1 | −0.3 (0.8) | −0.8 (1.0) | Group: 0.591 |
| Change at M3 | −0.3 (1.2) | −1.1 (1.0) | Time: <0.001 |
| Change at M6 | −0.1 (0.9) | −1.7 (1.7) | |
| Number of days with at least one Raynaud’s crisisa | |||
| Baseline | 5.7 (2.3) | 5.6 (2.2) | |
| Change at M1 | −0.4 (2.7 | −0.1 (1.5) | Group: 0.255 |
| Change at M3 | −0.9 (2.3) | −1.0 (2.9) | Time: <0.001 |
| Change at M6 | −1.8 (2.8) | −2.1 (2.6) | |
| Mean intensity of Raynaud’s crisisa (0 = not severe to 10 = very severe) | |||
| Baseline | 5.5 (2.4) | 5.8 (2.1) | |
| Change at M1 | −0.8 (3.5) | −1.8 (3.2) | Group: 0.734 |
| Change at M3 | −1.6 (3.5) | −0.8 (2.7) | Time: 0.006 |
| Change at M6 | −1.8 (3.1) | −2.2 (3.1) | |
| Mean duration of Raynaud’s crisisa (min) | |||
| Baseline | 16.8 (12.3) | 18.9 (14.1) | |
| Change at M1 | −2.3 (6.6) | −9.3 (13.2) | Group: 0.767 |
| Change at M3 | −1.6 (13.9) | −4.2 (18.1) | Time: 0.015 |
| Change at M6 | −3.2 (13.9) | −10.2 (15.1) | |
| Mean number of Raynaud’s crisis by daya | |||
| Baseline | 4.2 (5.4) | 3.4 (2.8) | |
| Change at M1 | −1.0 (3.0) | −0.1 (3.0) | Group: 0.169 |
| Change at M3 | −0.3 (3.7) | −1.0 (3.2) | Time: 0.018 |
| Change at M6 | −1.1 (3.7) | −1.9 (3.0) | |
Data represent mean (s.d.). Group (AD-SVF vs Placebo); time (Baseline, M1, M3, M6).
Assessed on the last week.
n = 19 in Placebo group at M6.
P-value from mixed model. AD-SVF: adipose-derived stromal vascular fraction; CHFS: Cochin Hand Function Scale; mRSS: modified Rodnan skin score focused on the hands; SHAQ: Scleroderma HAQ; VAS: visual analogue scale.
| . | AD-SVF (n = 20) . | Placebob (n = 20) . | P-valuec . |
|---|---|---|---|
| CHFS score (0–90) | |||
| Baseline | 37.4 (7.8) | 40.4 (8.7) | |
| Change at M1 | −7.0 (11.1) | −6.9 (11.1) | Group: 0.415 |
| Change at M3 | −9.2 (12.2) | −7.6 (13.2) | Time: <0.001 |
| Change at M6 | −7.9 (14.4) | −10.8 (12) | |
| SHAQ Score (0–3) | |||
| Baseline | 1.32 (0.42) | 1.4 (0.37) | |
| Change at M1 | −0.16 (0.28) | −0.15 (0.37) | Group: 0.145 |
| Change at M3 | −0.2 (0.3) | −0.16 (0.41) | Time: <0.001 |
| Change at M6 | −0.23 (0.39) | −0.32 (0.36) | |
| Hand Pain VAS (0–100) | |||
| Baseline | 44.2 (20.7) | 54.4 (28.1) | |
| Change at M1 | −13.6 (21.8) | −20.4 (20.6) | Group: 0.209 |
| Change at M3 | −6.2 (28.7) | −12.5 (21.6) | Time: <0.001 |
| Change at M6 | −12.3 (29.4) | −23.9 (23.1) | |
| mRSS on right hand (0–18) | |||
| Baseline | 5.4 (1.7) | 5.3 (2.4) | |
| Change at M1 | −0.2 (1.1) | −0.7 (1.0) | Group: 0.384 |
| Change at M3 | −0.2 (1.2) | −0.7 (1.3) | Time: 0.013 |
| Change at M6 | −0.2 (1.3) | −1.1 (1.6) | |
| mRSS on left hand (0–18) | |||
| Baseline | 4.8 (1.7) | 5.1 (2.4) | |
| Change at M1 | −0.3 (0.8) | −0.8 (1.0) | Group: 0.591 |
| Change at M3 | −0.3 (1.2) | −1.1 (1.0) | Time: <0.001 |
| Change at M6 | −0.1 (0.9) | −1.7 (1.7) | |
| Number of days with at least one Raynaud’s crisisa | |||
| Baseline | 5.7 (2.3) | 5.6 (2.2) | |
| Change at M1 | −0.4 (2.7 | −0.1 (1.5) | Group: 0.255 |
| Change at M3 | −0.9 (2.3) | −1.0 (2.9) | Time: <0.001 |
| Change at M6 | −1.8 (2.8) | −2.1 (2.6) | |
| Mean intensity of Raynaud’s crisisa (0 = not severe to 10 = very severe) | |||
| Baseline | 5.5 (2.4) | 5.8 (2.1) | |
| Change at M1 | −0.8 (3.5) | −1.8 (3.2) | Group: 0.734 |
| Change at M3 | −1.6 (3.5) | −0.8 (2.7) | Time: 0.006 |
| Change at M6 | −1.8 (3.1) | −2.2 (3.1) | |
| Mean duration of Raynaud’s crisisa (min) | |||
| Baseline | 16.8 (12.3) | 18.9 (14.1) | |
| Change at M1 | −2.3 (6.6) | −9.3 (13.2) | Group: 0.767 |
| Change at M3 | −1.6 (13.9) | −4.2 (18.1) | Time: 0.015 |
| Change at M6 | −3.2 (13.9) | −10.2 (15.1) | |
| Mean number of Raynaud’s crisis by daya | |||
| Baseline | 4.2 (5.4) | 3.4 (2.8) | |
| Change at M1 | −1.0 (3.0) | −0.1 (3.0) | Group: 0.169 |
| Change at M3 | −0.3 (3.7) | −1.0 (3.2) | Time: 0.018 |
| Change at M6 | −1.1 (3.7) | −1.9 (3.0) | |
| . | AD-SVF (n = 20) . | Placebob (n = 20) . | P-valuec . |
|---|---|---|---|
| CHFS score (0–90) | |||
| Baseline | 37.4 (7.8) | 40.4 (8.7) | |
| Change at M1 | −7.0 (11.1) | −6.9 (11.1) | Group: 0.415 |
| Change at M3 | −9.2 (12.2) | −7.6 (13.2) | Time: <0.001 |
| Change at M6 | −7.9 (14.4) | −10.8 (12) | |
| SHAQ Score (0–3) | |||
| Baseline | 1.32 (0.42) | 1.4 (0.37) | |
| Change at M1 | −0.16 (0.28) | −0.15 (0.37) | Group: 0.145 |
| Change at M3 | −0.2 (0.3) | −0.16 (0.41) | Time: <0.001 |
| Change at M6 | −0.23 (0.39) | −0.32 (0.36) | |
| Hand Pain VAS (0–100) | |||
| Baseline | 44.2 (20.7) | 54.4 (28.1) | |
| Change at M1 | −13.6 (21.8) | −20.4 (20.6) | Group: 0.209 |
| Change at M3 | −6.2 (28.7) | −12.5 (21.6) | Time: <0.001 |
| Change at M6 | −12.3 (29.4) | −23.9 (23.1) | |
| mRSS on right hand (0–18) | |||
| Baseline | 5.4 (1.7) | 5.3 (2.4) | |
| Change at M1 | −0.2 (1.1) | −0.7 (1.0) | Group: 0.384 |
| Change at M3 | −0.2 (1.2) | −0.7 (1.3) | Time: 0.013 |
| Change at M6 | −0.2 (1.3) | −1.1 (1.6) | |
| mRSS on left hand (0–18) | |||
| Baseline | 4.8 (1.7) | 5.1 (2.4) | |
| Change at M1 | −0.3 (0.8) | −0.8 (1.0) | Group: 0.591 |
| Change at M3 | −0.3 (1.2) | −1.1 (1.0) | Time: <0.001 |
| Change at M6 | −0.1 (0.9) | −1.7 (1.7) | |
| Number of days with at least one Raynaud’s crisisa | |||
| Baseline | 5.7 (2.3) | 5.6 (2.2) | |
| Change at M1 | −0.4 (2.7 | −0.1 (1.5) | Group: 0.255 |
| Change at M3 | −0.9 (2.3) | −1.0 (2.9) | Time: <0.001 |
| Change at M6 | −1.8 (2.8) | −2.1 (2.6) | |
| Mean intensity of Raynaud’s crisisa (0 = not severe to 10 = very severe) | |||
| Baseline | 5.5 (2.4) | 5.8 (2.1) | |
| Change at M1 | −0.8 (3.5) | −1.8 (3.2) | Group: 0.734 |
| Change at M3 | −1.6 (3.5) | −0.8 (2.7) | Time: 0.006 |
| Change at M6 | −1.8 (3.1) | −2.2 (3.1) | |
| Mean duration of Raynaud’s crisisa (min) | |||
| Baseline | 16.8 (12.3) | 18.9 (14.1) | |
| Change at M1 | −2.3 (6.6) | −9.3 (13.2) | Group: 0.767 |
| Change at M3 | −1.6 (13.9) | −4.2 (18.1) | Time: 0.015 |
| Change at M6 | −3.2 (13.9) | −10.2 (15.1) | |
| Mean number of Raynaud’s crisis by daya | |||
| Baseline | 4.2 (5.4) | 3.4 (2.8) | |
| Change at M1 | −1.0 (3.0) | −0.1 (3.0) | Group: 0.169 |
| Change at M3 | −0.3 (3.7) | −1.0 (3.2) | Time: 0.018 |
| Change at M6 | −1.1 (3.7) | −1.9 (3.0) | |
Data represent mean (s.d.). Group (AD-SVF vs Placebo); time (Baseline, M1, M3, M6).
Assessed on the last week.
n = 19 in Placebo group at M6.
P-value from mixed model. AD-SVF: adipose-derived stromal vascular fraction; CHFS: Cochin Hand Function Scale; mRSS: modified Rodnan skin score focused on the hands; SHAQ: Scleroderma HAQ; VAS: visual analogue scale.
The secondary efficacy endpoints, including global disability related to SSc (SHAQ), hand pain severity (VAS), dorsal hand and finger skin sclerosis (mRSS applied to hands) and Raynaud’s phenomenon severity, improved at 3 and 6 months of follow-up in the whole population (Table 2). This clinical benefit was similarly observed between the AD-SVF and placebo groups.
When analysing SSc patients according to the cutaneous form of the disease in both groups, no significant difference was observed for the five main parameter outcomes (CHFS score, SHAQ score, VAS for hand pain, mRSS applied to hands and Raynaud’s attack severity). However, the statistical power of these comparisons is very low given the lower sample size for each disease subtype and the imbalance between subtypes in the AD-SVF group (10 patients; 50% dcSSc) and the placebo group (only five patients; 25% dcSSc). Furthermore, no centre effect was observed.
Digital ulcer outcome was recorded and is illustrated in Fig. 4. At baseline, 15% of the patients in the AD-SVF group had at least one active DU, and 30% of the patients in the placebo group had at least one active DU. At 3 months, 20% of the patients in the AD-SVF group had at least one active DU, vs 25% in the placebo group. At 6 months, 25% of the patients in the AD-SVF group had at least one active DU, vs 28% in the placebo group. Moreover, the mean number of new ulcers/patient for the AD-SVF and placebo groups was 1 (1.6) and 1.8 (3), respectively, at 3 months (P = 0.402) and 0.9 (1.7) and 2.5 (3.2), respectively, at 6 months (P = 0.149). The mean number of healed ulcers/patient for the AD-SVF and placebo groups was 4.2 (4.1) and 5.9 (4.3), respectively, at 3 months (P = 0.302) and 3.9 (3.7) and 6.2 (6), respectively, at 6 months (P = 0.256).

Active digital ulcers outcome
The percentage of patients with at least one active DU in the AD-SVF and the placebo groups is represented on the bar chart respectively in orange and blue. The mean number of ulcer(s) per patient for the AD-SVF and placebo groups is represented by the yellow and navy-blue curves. AD-SVF: adipose-derived stromal vascular fraction; DU: digital ulcer.
We also performed functional tests on hand motion, hand strength and vasculopathy. These parameters did not change (Supplementary Table S4, available at Rheumatology online). During follow-up, 45% of the patients in the AD-SVF group reported improvement (from minimal to very much), and 60% of the patients in the placebo group.
Discussion
Encouraged by our previous uncontrolled clinical trial [9], we performed a randomized multicentre trial on a larger population of SSc patients with the aim of showing the benefit of AD-SVF therapy in hand handicap. The current study showed a significant improvement in hand handicap in both the AD-SVF and placebo groups through numerous analysed parameters: CHFS score, SHAQ score, hand pain severity, dorsal hand and finger skin sclerosis, and Raynaud’s phenomenon severity. However, the results of the present study do not show the superiority of autologous AD-SVF injection into the fingers in the improvement of hand function after a 3-month follow-up (which was the primary efficacy criterion).
In the literature, the minimal clinically important difference in patients with SSc has been reported to be −3.38 for the CHFS score and −0.13 for the SHAQ score at 12 months of follow-up [22]. According to these data, in our study the minimal clinically important difference for the CHFS score was reached at 6 months in 55% of cases in the AD-SVF group and 74% of cases in the placebo group (P = 0.224). Similarly, the minimal clinically important difference for the SHAQ score was reached at 6 months in 55% of cases in the AD-SVF group and 68% in the placebo group (P = 0.389).
The characteristics of SSc patients in our previous clinical trial [9] and the present study were similar for sex, mean age, BMI, disease duration, Raynaud’s phenomenon duration, cutaneous limited/diffuse form and early SSc disease (<4 years). It is worth noting that enrolled patients in our preliminary study had a higher hand handicap assessed by the CHFS score at baseline (48.5 [10.8]) than in the present study (38.9 [8.3], P = 0.012). As the CHFS score was our primary efficacy criterion, the inclusion of less severe patients could have minimized the room for improvement in the present trial.
In both studies, AD-SVF global characteristics (cell viability and CFU-F) and cell subpopulations (leucocytes, endothelial cells and ASCs) were very similar and thus could not account for the lack of efficacy in the present placebo-controlled study. Furthermore, the number of viable nucleated cells injected into each finger was the same in both trials (3.76 [1.85] vs 3.87 [1.32] × 106).
The main differences between the preliminary and present clinical trials rely on their design and on the number of enrolled patients: a phase I open-label study based on 12 SSc patients vs a multicentre, randomized, double-blind, placebo-controlled trial on 40 SSc patients. An improvement in parameters based on patient self-assessment was observed in both studies: CHFS score, global disability (SHAQ score), hand pain (visual analogue scale) and Raynaud’s severity. To account for the placebo effect and other confounding factors, our present study was placebo controlled and double blind. Under these conditions, we could not show the superiority of AD-SVF therapy in the improvement of hand handicap. Furthermore, objective parameters based on hand function, strength and motility, hand volume, finger circumference, and nailfold capillaroscopy did not change over time.
Although the CHFS score has been validated in SSc [23], we acknowledge that unlike osteoarthritis and rheumatoid arthritis, SSc disease hand disability is complex and relies on numerous kinds of damage, including skin thickening, vascular disorder, calcinosis and osteoarticular manifestations. Furthermore, sociodemographic and disease variables have recently been independently associated with hand function in SSc patients [24]. Based on a large number of patients enrolled in the Scleroderma Patient-centred Intervention Network Cohort, the authors showed that both patient-related factors (female sex, current smoking and higher BMI) and disease-related factors (diffuse cutaneous form, severity of Raynaud’s phenomenon, presence of digital ulcers, moderate or severe small joint contractures, rheumatoid arthritis and idiopathic inflammatory myositis) were independently associated with higher CHFS scores, indicating more impaired hand function.
In this study, the relatively short delay of follow-up (6 months) was not compatible with spontaneous natural improvement of the disease, and a seasonal effect was minimized as the patient’s enrolment was particularly long (2 years), thus including all seasons. No literature suggests a pharmacological effect of lactated Ringer’s solution. However, we do not know the mechanical effects of its injection along the fingers.
Our results highlight some needs for further studies on AD-SVF efficacy, including the choice of more targeted and objective criteria for efficacy, a homogeneous patient population in terms of disease features (patients with early disease and diffuse cutaneous), hand disability (severity and pattern of hand damage) and anti-inflammatory systemic treatment. A sham procedure should be more appropriate to avoid a mechanical effect of the placebo.
We note that while no report has been published in the peer-reviewed literature, data from the STAR clinical trial (an 88-patient, multicentre, randomized, placebo-controlled study of AD-SVF for the treatment of hand dysfunction in patients with SSc in the USA) were reported at the 5th Systemic Sclerosis World Congress in Bordeaux, France [25]. Patients with significant impairment of hand function (CHFS score >20) were randomized to receive AD-SVF (fixed dose of 40 million cells injected in the 10 fingers, n = 48) or placebo (lactated Ringer’s solution, n = 40). In this brief report, the authors noted a similar large placebo effect that was particularly strong in patients with lcSSc. The authors further noted that while no effect of AD-SVF treatment was evident in patients with lcSSc, a statistically and clinically significant improvement was evident in those with dcSSc. Until the full report of this study is available in the peer-reviewed literature, it is difficult to draw comparisons. However, the larger placebo effect reported in patients with lcSSc is of note given the over-representation of lcSSc patients in the placebo group of the current study (15/20; 75%). The small number (n = 15) of patients with dcSSc in the present study precluded meaningful post hoc statistical assessment of this subpopulation. The data from the current study are consistent with the preliminary data from STAR, indicating the absence of a meaningful advantage over placebo in patients with lcSSc.
To date, different cell preparations of autologous adipose tissue have been used in different studies concerning SSc patients. The use of adipose tissue can offer volumizing and trophic properties. For adipose tissue processing techniques, the most reported in clinical use are sedimentation (decantation), centrifugation and filtration. Bank et al. [3] used decanted whereas Del papa et al. [4] used centrifuged fat graft.
The risk of ischaemia related to the volume effect of adipose tissue with inherent increased pressure during injection supports our policy of using the AD-SVF for hand therapy instead of fat tissue. Furthermore the AD-SVF cell population can easily be extracted from adipose tissue using an automated processing system and resuspended in a final fluid solution. Another approach is to expand ASCs from the AD-SVF, as these cells demonstrate in vitro and in vivo proangiogenic, immunosuppressive and anti-fibrotic properties and appear to be a promising therapy, which may target the three pathogenic pathways of SSc disease [26, 27]. A multicentre randomized placebo-controlled clinical trial using autologous culture ASCs injected into the fingers with the aim of healing refractory ischaemic digital ulcers is ongoing in SSc patients in France (ADUSE NCT04356755). However, it is important to take into account that European regulations classify AD-SVF and ASCs as advanced therapy medicinal products that can be manufactured in compliance with good manufacturing practice in authorized cell therapy facilities.
In conclusion, the present study showed an improvement in numerous parameters related to hand function in both the AD-SVF and placebo groups, with no superiority of the AD-SVF. The number of enrolled patients, heterogeneity of the disease, low proportion of patients with severe hand handicap and imbalanced assignment of the cutaneous form into groups are the limitations of this study. At this step, these results do not prove the absence of efficacy of AD-SVF for SSc patients. Although they are difficult to conduct among patients with a rare disease, studies on a larger population of SSc patients with a homogeneous phenotype and hand handicap should be encouraged to accurately assess the benefit of AD-SVF therapy.
Acknowledgements
We thank Anne Bourgoin from the EMAI team who contributed to data collection.
We thank Julie Cernoia and Onja Rarison for their contribution to the monitoring of the study and Estelle Charles-Baumel and Olivier Blin for the data management.
We acknowledge Didier Samson, who anaesthetized the patients in Marseilles.
We thank John Fraser, Marc Revol, Farid Bekara and Christian Jorgensen for their help in setting up the study.
We thank the patients and other clinicians who participated in this study.
Funding: This trial was supported by Cytori Therapeutics, Inc. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and execution of a Data Sharing Agreement. The unique identifier for sharing the data is Brigitte Granel.
Supplementary data
Supplementary data are available at Rheumatology online.




Comments