-
PDF
- Split View
-
Views
-
Cite
Cite
Tommaso Schioppo, Lorenza Maria Argolini, Savino Sciascia, Francesca Pregnolato, Francesco Tamborini, Paolo Miraglia, Dario Roccatello, Renato Alberto Sinico, Roberto Caporali, Gabriella Moroni, Maria Gerosa, Clinical and peculiar immunological manifestations of SARS-CoV-2 infection in systemic lupus erythematosus patients, Rheumatology, Volume 61, Issue 5, May 2022, Pages 1928–1935, https://doi.org/10.1093/rheumatology/keab611
Close - Share Icon Share
Abstract
The impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients with SLE remains unclear and data on clinical manifestations after infection are lacking. The aim of this multicentre study is to describe the effect of SARS-CoV-2 in SLE patients.
SLE patients referring to four Italian centres were monitored between February 2020 and March 2021. All patients with SARS-CoV-2 infection were included. Disease characteristics, treatment, disease activity and SARS-CoV-2-related symptoms were recorded before and after the infection.
Fifty-one (6.14%) SLE patients were included among 830 who were regularly followed up. Nine (17.6%) had an asymptomatic infection and 5 (9.8%) out of 42 (82.6%) symptomatic patients developed interstitial pneumonia (no identified risk factor). The presence of SLE major organ involvement (particularly renal involvement) was associated with asymptomatic SARS-CoV-2 infection (P = 0.02). Chronic corticosteroid therapy was found to be associated with asymptomatic infection (P = 0.018). Three SLE flares (5.9%) were developed after SARS-CoV-2 infection: one of them was characterized by MPO-ANCA-positive pauci-immune crescentic necrotizing glomerulonephritis and granulomatous pneumonia.
SARS-CoV-2 infection determined autoimmune flares in a small number of patients. Our data seem to confirm that there was not an increased risk of SARS-CoV-2 in SLE. Patients with asymptomatic SARS-CoV-2 infections were those having major SLE organ involvement. This may be explained by the high doses of corticosteroids and immunosuppressive agents used for SLE treatment.
SLE patients with major organ involvement tended to have asymptomatic SARS-CoV-2 infection.
SARS-CoV-2 infection determined flares in a small number of patients.
SARS-CoV-2 infection can trigger new-onset autoimmune disease with atypical presentation.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China in December 2019 and then spread worldwide. The World Health Organization (WHO) declared SARS-CoV-2 a pandemic in March 2020. SARS-CoV-2 has so far infected >139 million people and caused the death of almost 3 million of them [1]. In the following months, some risk factors for SARS-CoV-2 infection were identified in the general population (e.g. older age, male sex, non-white race, obesity) [2].
It is well established that patients with inflammatory rheumatic diseases (RDs) are at higher risk of infections than the general population because of associated comorbidities, underlying disease activity and concomitant immunosuppressive therapies [3]. SARS-CoV-2 was shown to cause endothelitis resulting in vascular manifestations (e.g. thrombosis) and immune system activation via toll-like receptors and complement system [4, 5]. At the beginning, the current pandemic raised many questions for patients with RDs such as SLE. In these patients, many factors associated with the disease were a matter of concern, such as the well-established role of viruses (e.g. Epstein–Barr virus) in SLE pathogenesis [6–8], the greater susceptibility to infections [9], the risk of SLE flares after viral and bacterial infections [10], the presence of organ damage directly caused by SLE, the use of glucocorticoids (GCs) and immunosuppressive regimens.
Few new SLE cases triggered by SARS-CoV-2 infection have so far been reported [11]. Conversely, as of today, studies have been unable to detect an increased risk of SARS-CoV-2 in SLE patients [12]. This could be explained by the fact that SLE patients may have adopted more protective behaviour than the general population and therefore this could have protected them from the infection [13, 14]. Besides GCs (>10 mg/day), no SLE-related risk factors have been identified [15]. Moreover, some data about mortality in patients with RDs suggest caution regarding rituximab [15].
Various post-SARS-CoV-2 syndromes have been reported [16]. Patients with pre-existing RDs may have flares during or after SARS-CoV-2 infection and it is an emerging consideration that RD patients might develop new and adjunctive autoimmune features [17].
The aim of the study was to describe the SARS-CoV-2 infection course in SLE patients in a multicentre cohort of tertiary hospitals in northern Italy.
Materials and methods
Study design
We conducted a retrospective observational multicentre cohort study.
Settings
Data regarding SLE patients who had a SARS-CoV-2 infection between February 2020 and March 2021 were retrospectively collected. The patients included in the study were referred to four different SLE tertiary centres (Lupus Clinic of the Clinical Rheumatology Division of ASST Pini-CTO, Milan; IRCCS Policlinico, Milan; Ospedale Giovanni Bosco, Turin; Renal and Rheumatology Units, San Gerardo Hospital, Monza). The analysis is part of a study to collect observational data from SLE patients and was approved by the Ethics Committee Comitato Etico Milano Area 2 (approval no. 0002450/2020).
Participants
All consecutive adult patients (>18 years of age) referring to participant centres with a diagnosis of SLE who had SARS-CoV-2 infection were included in the study. All patients provided written informed consent. The SLE diagnosis was made in accordance with the 1997 SLE classification criteria of the ACR or the 2019 EULAR–ACR criteria [18, 19]. Patients were considered to have had SARS-CoV-2 infection if a direct PCR swab and/or a serological test for SARS-CoV-2 was positive. Patients were excluded if they had an overlap syndrome (e.g. RA and SLE).
Variables
Data regarding demographics and clinical and serological features of SLE, including organ involvement, disease duration, autoimmune profile, ongoing treatment, disease activity before and after SAR-CoV-2 infection, possible treatment modifications related to nasal swab positivity and comorbidities, were collected and gathered in a database for statistical analysis. Moreover, when available, laboratory parameters (CRP, ESR, ferritin, gamma globulins, IL-6) were collected at the last follow-up visit before the SARS-CoV-2 infection and at the first follow-up visit after the infection. IL-6 dosage was available only for those patients referring to one centre (Ospedale Giovanni Bosco, Turin). Serum IL-6 levels were measured by an immunoassay kit (Elecsys IL-6, Roche Diagnostics, Rotkreuz, Switzerland). Information about SARS-CoV-2 infection (i.e. clinical manifestations and hospitalization) were also recorded. Disease activity was assessed according to SLEDAI-2K [20]. SARS-CoV-2 infection severity was assessed as previously defined [21].
Statistical analysis
Descriptive statistics were used to summarize the patients’ demographic and clinical data by using median, interquartile range (IQR), absolute numbers and percentages. All these variables were then investigated as risk factors of the following outcomes: SARS-CoV-2 symptoms, coronavirus disease 2019 (COVID-19) pneumonia and SLE flare after SARS-CoV-2 infection. The comparisons of continuous variables between groups of patients were assessed through Welch two-sample t-test or Mann–Whitney non-parametric test, as appropriate. The association between categorical variables was assessed by performing chi-squared or Fisher’s exact test, as appropriate. In order to identify subjects with a similar profile and the association among the categorical variables, multiple correspondence analysis was carried out [22]. This multivariate analysis can also be seen as a generalization of principal component analysis when the variables to be analysed are categorical instead of quantitative [23]. No further modelling was conducted because of the limited number of individuals in each level of the investigated outcomes. A P-value <0.05 was considered significant. All analyses were performed using R software version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria) with package Rcmdr (version 2.5-1).
Results
Characteristics before SARS-CoV-2 infection
Fifty-one patients who had SARS-CoV-2 infection were included in the study among 830 regularly followed-up patients. Disease history, laboratory parameters, demographic characteristics and therapy collected at the last follow-up visit before SARS-CoV-2 infection are reported in Table 1. Included patients had a median age of 46.2 years with an SLE disease duration of 16.4 years. Most of the patients were female (92.2%) and displayed a history of ANA positivity (96.1%), anti-dsDNA positivity (88.2%) and low complement (52.9%). The median CRP was 0.20 mg/dl (IQR 0.09–0.38), ESR 16 mm/h (IQR 11–20), ferritin 152 mg/dl (IQR 128–175), gamma globulins 85% (IQR 14.35–17.58) and IL-6 24 pg/ml (IQR 19–27). Thirty-four (66.7%) patients had experienced major organ involvement (i.e. kidney, CNS, lung) during their SLE history. Thirty-one patients (60.8%) were taking immunosuppressive drugs, 19 (37.3%) on GCs and/or HCQ, and 1 patient was without any SLE therapy. At the time of SARS-CoV-2 infection, the majority of patients were in remission or had low disease activity with a median SLEDAI-2K score of 2.
Demographics, comorbidities, SLE disease characteristics and therapies at the moment of SARS-CoV-2 infection
| Demographics | |
| Age, years, median (25th–75th percentile) | 46.2 (40.5–52.7) |
| Disease duration, years, median (25th–75th percentile) | 16.4 (5.0–22.3) |
| Gender (female), n (%) | 47 (92.2) |
| Laboratory, median (25th–75th percentile) | |
| CRP, mg/dLa | 0.20 (0.09–0.38) |
| ESR, mm/hb | 16 (11–20) |
| Ferritin, mg/dLc | 152 (128–175) |
| Gamma globulins, %d | 15.85 (14.35–17.58) |
| IL-6, pg/mle | 24 (19–27) |
| Serology and organ involvement ever, n (%) | |
| ANA positivity | 49 (96.1) |
| Anti-dsDNA antibody positive | 45 (88.2) |
| Low complement (C3 and/or C4) | 27 (52.9) |
| aPL antibody positive | 16 (31.4) |
| Arthritis | 42 (82.4) |
| Skin involvement | 32 (62.7) |
| Renal involvement | 31 (60.8) |
| Haematological involvement | 18 (35.3) |
| Serositis | 10 (19.6) |
| Lung involvement | 5 (9.8) |
| Neurological involvement | 4 (7.8) |
| Pulmonary arterial hypertension | 1 (2.0) |
| Therapy, n (%) | |
| At least one immunosuppressantf | 31 (60.8) |
| Corticosteroids | 32 (62.7) |
| HCQ | 45 (88.2) |
| ACEI/ARB therapy | 21 (41.2) |
| Comorbidities, n (%) | |
| Arterial hypertension | 21 (41.2) |
| Cardiovascular disease | 6 (11.8) |
| Diabetes | 2 (3.9) |
| Demographics | |
| Age, years, median (25th–75th percentile) | 46.2 (40.5–52.7) |
| Disease duration, years, median (25th–75th percentile) | 16.4 (5.0–22.3) |
| Gender (female), n (%) | 47 (92.2) |
| Laboratory, median (25th–75th percentile) | |
| CRP, mg/dLa | 0.20 (0.09–0.38) |
| ESR, mm/hb | 16 (11–20) |
| Ferritin, mg/dLc | 152 (128–175) |
| Gamma globulins, %d | 15.85 (14.35–17.58) |
| IL-6, pg/mle | 24 (19–27) |
| Serology and organ involvement ever, n (%) | |
| ANA positivity | 49 (96.1) |
| Anti-dsDNA antibody positive | 45 (88.2) |
| Low complement (C3 and/or C4) | 27 (52.9) |
| aPL antibody positive | 16 (31.4) |
| Arthritis | 42 (82.4) |
| Skin involvement | 32 (62.7) |
| Renal involvement | 31 (60.8) |
| Haematological involvement | 18 (35.3) |
| Serositis | 10 (19.6) |
| Lung involvement | 5 (9.8) |
| Neurological involvement | 4 (7.8) |
| Pulmonary arterial hypertension | 1 (2.0) |
| Therapy, n (%) | |
| At least one immunosuppressantf | 31 (60.8) |
| Corticosteroids | 32 (62.7) |
| HCQ | 45 (88.2) |
| ACEI/ARB therapy | 21 (41.2) |
| Comorbidities, n (%) | |
| Arterial hypertension | 21 (41.2) |
| Cardiovascular disease | 6 (11.8) |
| Diabetes | 2 (3.9) |
Available for 47 patients. bAvailable for 47 patients. cAvailable for 25 patients. dAvailable for 40 patients. eAvailable for 21 patients. fMethotrexate, mycophenolate mofetil, ciclosporin A, azathioprine, belimumab, rituximab in the prior 12 months, IVIG, cyclophosphamide. ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Demographics, comorbidities, SLE disease characteristics and therapies at the moment of SARS-CoV-2 infection
| Demographics | |
| Age, years, median (25th–75th percentile) | 46.2 (40.5–52.7) |
| Disease duration, years, median (25th–75th percentile) | 16.4 (5.0–22.3) |
| Gender (female), n (%) | 47 (92.2) |
| Laboratory, median (25th–75th percentile) | |
| CRP, mg/dLa | 0.20 (0.09–0.38) |
| ESR, mm/hb | 16 (11–20) |
| Ferritin, mg/dLc | 152 (128–175) |
| Gamma globulins, %d | 15.85 (14.35–17.58) |
| IL-6, pg/mle | 24 (19–27) |
| Serology and organ involvement ever, n (%) | |
| ANA positivity | 49 (96.1) |
| Anti-dsDNA antibody positive | 45 (88.2) |
| Low complement (C3 and/or C4) | 27 (52.9) |
| aPL antibody positive | 16 (31.4) |
| Arthritis | 42 (82.4) |
| Skin involvement | 32 (62.7) |
| Renal involvement | 31 (60.8) |
| Haematological involvement | 18 (35.3) |
| Serositis | 10 (19.6) |
| Lung involvement | 5 (9.8) |
| Neurological involvement | 4 (7.8) |
| Pulmonary arterial hypertension | 1 (2.0) |
| Therapy, n (%) | |
| At least one immunosuppressantf | 31 (60.8) |
| Corticosteroids | 32 (62.7) |
| HCQ | 45 (88.2) |
| ACEI/ARB therapy | 21 (41.2) |
| Comorbidities, n (%) | |
| Arterial hypertension | 21 (41.2) |
| Cardiovascular disease | 6 (11.8) |
| Diabetes | 2 (3.9) |
| Demographics | |
| Age, years, median (25th–75th percentile) | 46.2 (40.5–52.7) |
| Disease duration, years, median (25th–75th percentile) | 16.4 (5.0–22.3) |
| Gender (female), n (%) | 47 (92.2) |
| Laboratory, median (25th–75th percentile) | |
| CRP, mg/dLa | 0.20 (0.09–0.38) |
| ESR, mm/hb | 16 (11–20) |
| Ferritin, mg/dLc | 152 (128–175) |
| Gamma globulins, %d | 15.85 (14.35–17.58) |
| IL-6, pg/mle | 24 (19–27) |
| Serology and organ involvement ever, n (%) | |
| ANA positivity | 49 (96.1) |
| Anti-dsDNA antibody positive | 45 (88.2) |
| Low complement (C3 and/or C4) | 27 (52.9) |
| aPL antibody positive | 16 (31.4) |
| Arthritis | 42 (82.4) |
| Skin involvement | 32 (62.7) |
| Renal involvement | 31 (60.8) |
| Haematological involvement | 18 (35.3) |
| Serositis | 10 (19.6) |
| Lung involvement | 5 (9.8) |
| Neurological involvement | 4 (7.8) |
| Pulmonary arterial hypertension | 1 (2.0) |
| Therapy, n (%) | |
| At least one immunosuppressantf | 31 (60.8) |
| Corticosteroids | 32 (62.7) |
| HCQ | 45 (88.2) |
| ACEI/ARB therapy | 21 (41.2) |
| Comorbidities, n (%) | |
| Arterial hypertension | 21 (41.2) |
| Cardiovascular disease | 6 (11.8) |
| Diabetes | 2 (3.9) |
Available for 47 patients. bAvailable for 47 patients. cAvailable for 25 patients. dAvailable for 40 patients. eAvailable for 21 patients. fMethotrexate, mycophenolate mofetil, ciclosporin A, azathioprine, belimumab, rituximab in the prior 12 months, IVIG, cyclophosphamide. ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Clinical manifestations, therapy modifications and outcomes of SARS-CoV-2 infection
The clinical manifestations of SARS-CoV-2 infection and its impact on SLE disease activity are reported in Table 2. Nine (17.6%) patients had an asymptomatic SARS-CoV-2 infection, while 34 (66.7%) patients had mild COVID-19 and 8 (15.7%) had moderate–severe COVID-19. Of 42 symptomatic patients, 5 (9.8%) developed interstitial pneumonia [21] and 3 (5.9%) of them were hospitalized. In all patients with pneumonia the steroid dose was increased. Outpatients were treated with an oral course of antibiotics (macrolide). Among hospitalized patients, two received conventional oxygen therapy and one needed non-invasive positive pressure ventilation. Moreover, all three were treated with low molecular weight heparin and antibiotics.
Clinical manifestations of SARS-CoV-2 infection and SLE disease activity after SARS-CoV-2 infection
| Clinical manifestations of SARS-CoV-2 infection, n (%) | |
| Asymptomatic | 9 (17.6) |
| Fever | 31 (60.8) |
| Anosmia and/or ageusia | 25 (49.0) |
| Cough | 23 (45.1) |
| Diarrhoea | 4 (7.8) |
| Dyspnoea | 5 (9.8) |
| Interstitial pneumonia | 5 (9.8) |
| Hospitalized | 3 (5.9) |
| SLE disease activity | |
| SLEDAI-2K before SARS-CoV-2 infection, median (25th–75th percentile) | 2 (0–2) |
| Remission or low disease activitya, n (%) | 47 (92.2) |
| Moderate or high disease activityb, n (%) | 4 (7.8) |
| SLEDAI-2K after SARS-CoV-2 infection, median (25th–75th percentile) | 2 (0-4) |
| Remission or low disease activitya, n (%) | 44 (86.3) |
| Moderate or high disease activityb, n (%) | 7 (13.7) |
| SLE flare, n (%) | |
| SARS-CoV-2 infection with SLE flare | 3 (5.9) |
| Asymptomatic SARS-CoV-2 infection with SLE flare | 1 (2.0) |
| Symptomatic SARS-CoV-2 infection with SLE flare | 2 (3.9) |
| Hospitalized SARS-CoV-2 infection with SLE flare | 1 (2.0) |
| Clinical manifestations of SARS-CoV-2 infection, n (%) | |
| Asymptomatic | 9 (17.6) |
| Fever | 31 (60.8) |
| Anosmia and/or ageusia | 25 (49.0) |
| Cough | 23 (45.1) |
| Diarrhoea | 4 (7.8) |
| Dyspnoea | 5 (9.8) |
| Interstitial pneumonia | 5 (9.8) |
| Hospitalized | 3 (5.9) |
| SLE disease activity | |
| SLEDAI-2K before SARS-CoV-2 infection, median (25th–75th percentile) | 2 (0–2) |
| Remission or low disease activitya, n (%) | 47 (92.2) |
| Moderate or high disease activityb, n (%) | 4 (7.8) |
| SLEDAI-2K after SARS-CoV-2 infection, median (25th–75th percentile) | 2 (0-4) |
| Remission or low disease activitya, n (%) | 44 (86.3) |
| Moderate or high disease activityb, n (%) | 7 (13.7) |
| SLE flare, n (%) | |
| SARS-CoV-2 infection with SLE flare | 3 (5.9) |
| Asymptomatic SARS-CoV-2 infection with SLE flare | 1 (2.0) |
| Symptomatic SARS-CoV-2 infection with SLE flare | 2 (3.9) |
| Hospitalized SARS-CoV-2 infection with SLE flare | 1 (2.0) |
Remission or low disease activity defined as SLEDAI-2K score ≤4. bModerate or high disease activity defined as SLEDAI-2K score >5.
Clinical manifestations of SARS-CoV-2 infection and SLE disease activity after SARS-CoV-2 infection
| Clinical manifestations of SARS-CoV-2 infection, n (%) | |
| Asymptomatic | 9 (17.6) |
| Fever | 31 (60.8) |
| Anosmia and/or ageusia | 25 (49.0) |
| Cough | 23 (45.1) |
| Diarrhoea | 4 (7.8) |
| Dyspnoea | 5 (9.8) |
| Interstitial pneumonia | 5 (9.8) |
| Hospitalized | 3 (5.9) |
| SLE disease activity | |
| SLEDAI-2K before SARS-CoV-2 infection, median (25th–75th percentile) | 2 (0–2) |
| Remission or low disease activitya, n (%) | 47 (92.2) |
| Moderate or high disease activityb, n (%) | 4 (7.8) |
| SLEDAI-2K after SARS-CoV-2 infection, median (25th–75th percentile) | 2 (0-4) |
| Remission or low disease activitya, n (%) | 44 (86.3) |
| Moderate or high disease activityb, n (%) | 7 (13.7) |
| SLE flare, n (%) | |
| SARS-CoV-2 infection with SLE flare | 3 (5.9) |
| Asymptomatic SARS-CoV-2 infection with SLE flare | 1 (2.0) |
| Symptomatic SARS-CoV-2 infection with SLE flare | 2 (3.9) |
| Hospitalized SARS-CoV-2 infection with SLE flare | 1 (2.0) |
| Clinical manifestations of SARS-CoV-2 infection, n (%) | |
| Asymptomatic | 9 (17.6) |
| Fever | 31 (60.8) |
| Anosmia and/or ageusia | 25 (49.0) |
| Cough | 23 (45.1) |
| Diarrhoea | 4 (7.8) |
| Dyspnoea | 5 (9.8) |
| Interstitial pneumonia | 5 (9.8) |
| Hospitalized | 3 (5.9) |
| SLE disease activity | |
| SLEDAI-2K before SARS-CoV-2 infection, median (25th–75th percentile) | 2 (0–2) |
| Remission or low disease activitya, n (%) | 47 (92.2) |
| Moderate or high disease activityb, n (%) | 4 (7.8) |
| SLEDAI-2K after SARS-CoV-2 infection, median (25th–75th percentile) | 2 (0-4) |
| Remission or low disease activitya, n (%) | 44 (86.3) |
| Moderate or high disease activityb, n (%) | 7 (13.7) |
| SLE flare, n (%) | |
| SARS-CoV-2 infection with SLE flare | 3 (5.9) |
| Asymptomatic SARS-CoV-2 infection with SLE flare | 1 (2.0) |
| Symptomatic SARS-CoV-2 infection with SLE flare | 2 (3.9) |
| Hospitalized SARS-CoV-2 infection with SLE flare | 1 (2.0) |
Remission or low disease activity defined as SLEDAI-2K score ≤4. bModerate or high disease activity defined as SLEDAI-2K score >5.
Laboratory parameters were recorded at the first available follow-up visit after the infection: median CRP was 0.23 mg/dl (IQR 0.04–0.45), ESR 19 mm/h (IQR 11–34.25), ferritin 216 mg/dl (IQR 185–244), gamma globulins 16.9% (IQR 15.6–17.9), and IL-6 56 pg/ml (IQR 31–64). CRP (P = 0.40) and gamma globulins (P = 0.19) were not different from before the infection, whereas the levels of IL-6 (P = 0.0003), ferritin (P = 0.00003) and ESR (P = 0.03) were statistically significantly higher after the infection.
Thirty-nine (76.5%) of 51 patients did not change therapy during the SARS-CoV-2 infection. Nine (17.6%) patients stopped immunosuppressant therapy (e.g. azathioprine, mycophenolate, belimumab) during the SARS-CoV-2 infection until a negative swab. An increased steroid dose was prescribed to four patients during the period of SARS-CoV-2 infection.
In our cohort, three patients had a disease flare after SARS-CoV-2 infection. One patient with severe COVID-19 developed a severe disease flare, characterized by a typical picture of ANCA-associated vasculitis on renal and lung biopsy (details are reported below). One patient, after SARS-CoV-2 asymptomatic infection, had a severe disease flare with arthralgia and cutaneous vasculitis that was successfully treated with GCs and azathioprine. One patient had a mild flare (transient proteinuria up to 3 g/day with recovery without specific therapy) after a symptomatic SARS-CoV-2 infection.
Risk factors for symptomatic SARS-CoV-2 infection in SLE patients
Table 3 illustrates the distribution of the main demographic and clinical characteristics of the SLE patients stratified according to symptomatic/asymptomatic SARS-CoV-2 infection. Major organ involvement, in particular the kidneys (P = 0.008), was significantly more frequent (P = 0.021) in asymptomatic patients [100% (n = 9/9)] when compared with individuals who had COVID-19 symptoms [59.5% (n = 25/42)]. Concurrently, ongoing treatment with GCs and immunosuppressants overlapped the distribution of the SLE organ involvement in the two groups, leading to a straightforward association between the anti-inflammatory/immunosuppressive therapy and asymptomatic infection (4 and 23 of 34 patients with organ involvement underwent GC and/or immunosuppressive therapy, respectively). In contrast, the remaining variables seem not to represent distinctive characteristics of the SARS-CoV-2 symptomatic profile. In this regard, and on a strictly descriptive level, the multivariate analysis provided an overall view of the relationships among all the clinical characteristics and identified patients with similar profiles (Supplementary Fig. S1, available at Rheumatology online). A substantial biologic variability of our cohort was explained by two underlying dimensions: the disease-related characteristics and the ongoing therapy. In this context, the major organ involvement and the treatment clearly defined the two profiles of SARS-CoV-2 infection.
Demographic and SLE patient characteristics according to SARS-CoV-2 infection symptoms profile
| Characteristics . | Symptomatic SARS-CoV-2 (n = 42) . | Asymptomatic SARS-CoV-2 (n = 9) . | P-value . |
|---|---|---|---|
| Age, years, median (25th–75th percentile) | 44.9 (40.1–52.7) | 46.7 (43.6–52.7) | 0.584 |
| Disease duration, years, median (25th–75th percentile) | 16.3 (5.5–21.9) | 17.7 (4.0–22.5) | 0.733 |
| Female | 92.9 (39) | 88.9 (8) | 0.552 |
| GCs | 54.8 (23) | 100 (9) | 0.018a |
| At least one immunosuppressanta | 54.8 (23) | 88.9 (8) | 0.072 |
| HCQ | 92.9 (39) | 66.7 (6) | 0.060 |
| anti-dsDNA positive | 88.1 (37) | 88.9 (8) | 1.000 |
| aPL positive | 33.3 (14) | 22.2 (2) | 0.701 |
| Hypocomplementemia | 52.4 (22) | 55.6 (5) | 1.000 |
| Arterial hypertension | 38.1 (16) | 55.6 (5) | 0.460 |
| ACEI | 42.9 (18) | 33.3 (3) | 0.720 |
| Major organ involvementb | 59.5 (25) | 100 (9) | 0.021a |
| Renal involvement | 52.4 (22) | 100 (9) | 0.008 |
| SLEDAI-2K score before the infection, median (25th–75th percentile) | 1 (0–2) | 2 (0–4) | 0.729 |
| Characteristics . | Symptomatic SARS-CoV-2 (n = 42) . | Asymptomatic SARS-CoV-2 (n = 9) . | P-value . |
|---|---|---|---|
| Age, years, median (25th–75th percentile) | 44.9 (40.1–52.7) | 46.7 (43.6–52.7) | 0.584 |
| Disease duration, years, median (25th–75th percentile) | 16.3 (5.5–21.9) | 17.7 (4.0–22.5) | 0.733 |
| Female | 92.9 (39) | 88.9 (8) | 0.552 |
| GCs | 54.8 (23) | 100 (9) | 0.018a |
| At least one immunosuppressanta | 54.8 (23) | 88.9 (8) | 0.072 |
| HCQ | 92.9 (39) | 66.7 (6) | 0.060 |
| anti-dsDNA positive | 88.1 (37) | 88.9 (8) | 1.000 |
| aPL positive | 33.3 (14) | 22.2 (2) | 0.701 |
| Hypocomplementemia | 52.4 (22) | 55.6 (5) | 1.000 |
| Arterial hypertension | 38.1 (16) | 55.6 (5) | 0.460 |
| ACEI | 42.9 (18) | 33.3 (3) | 0.720 |
| Major organ involvementb | 59.5 (25) | 100 (9) | 0.021a |
| Renal involvement | 52.4 (22) | 100 (9) | 0.008 |
| SLEDAI-2K score before the infection, median (25th–75th percentile) | 1 (0–2) | 2 (0–4) | 0.729 |
Values presented as % (n) unless stated otherwise. aMTX, mycophenolate mofetil, ciclosporin A, AZA, belimumab, rituximab in the prior 12 months, IVIG, cyclophosphamide. bKidney, CNS, lung involvement. P < 0.05 considered significant. ACEI: angiotensin-converting enzyme inhibitor.
Demographic and SLE patient characteristics according to SARS-CoV-2 infection symptoms profile
| Characteristics . | Symptomatic SARS-CoV-2 (n = 42) . | Asymptomatic SARS-CoV-2 (n = 9) . | P-value . |
|---|---|---|---|
| Age, years, median (25th–75th percentile) | 44.9 (40.1–52.7) | 46.7 (43.6–52.7) | 0.584 |
| Disease duration, years, median (25th–75th percentile) | 16.3 (5.5–21.9) | 17.7 (4.0–22.5) | 0.733 |
| Female | 92.9 (39) | 88.9 (8) | 0.552 |
| GCs | 54.8 (23) | 100 (9) | 0.018a |
| At least one immunosuppressanta | 54.8 (23) | 88.9 (8) | 0.072 |
| HCQ | 92.9 (39) | 66.7 (6) | 0.060 |
| anti-dsDNA positive | 88.1 (37) | 88.9 (8) | 1.000 |
| aPL positive | 33.3 (14) | 22.2 (2) | 0.701 |
| Hypocomplementemia | 52.4 (22) | 55.6 (5) | 1.000 |
| Arterial hypertension | 38.1 (16) | 55.6 (5) | 0.460 |
| ACEI | 42.9 (18) | 33.3 (3) | 0.720 |
| Major organ involvementb | 59.5 (25) | 100 (9) | 0.021a |
| Renal involvement | 52.4 (22) | 100 (9) | 0.008 |
| SLEDAI-2K score before the infection, median (25th–75th percentile) | 1 (0–2) | 2 (0–4) | 0.729 |
| Characteristics . | Symptomatic SARS-CoV-2 (n = 42) . | Asymptomatic SARS-CoV-2 (n = 9) . | P-value . |
|---|---|---|---|
| Age, years, median (25th–75th percentile) | 44.9 (40.1–52.7) | 46.7 (43.6–52.7) | 0.584 |
| Disease duration, years, median (25th–75th percentile) | 16.3 (5.5–21.9) | 17.7 (4.0–22.5) | 0.733 |
| Female | 92.9 (39) | 88.9 (8) | 0.552 |
| GCs | 54.8 (23) | 100 (9) | 0.018a |
| At least one immunosuppressanta | 54.8 (23) | 88.9 (8) | 0.072 |
| HCQ | 92.9 (39) | 66.7 (6) | 0.060 |
| anti-dsDNA positive | 88.1 (37) | 88.9 (8) | 1.000 |
| aPL positive | 33.3 (14) | 22.2 (2) | 0.701 |
| Hypocomplementemia | 52.4 (22) | 55.6 (5) | 1.000 |
| Arterial hypertension | 38.1 (16) | 55.6 (5) | 0.460 |
| ACEI | 42.9 (18) | 33.3 (3) | 0.720 |
| Major organ involvementb | 59.5 (25) | 100 (9) | 0.021a |
| Renal involvement | 52.4 (22) | 100 (9) | 0.008 |
| SLEDAI-2K score before the infection, median (25th–75th percentile) | 1 (0–2) | 2 (0–4) | 0.729 |
Values presented as % (n) unless stated otherwise. aMTX, mycophenolate mofetil, ciclosporin A, AZA, belimumab, rituximab in the prior 12 months, IVIG, cyclophosphamide. bKidney, CNS, lung involvement. P < 0.05 considered significant. ACEI: angiotensin-converting enzyme inhibitor.
Risk factors for COVID-19 pneumonia
In our cohort, only 5 patients (9.8%) developed SARS-CoV-2-related interstitial pneumonia. None of the considered parameters (e.g. gender, age, disease duration, SLE organ involvement, serological SLE characteristics, SLE therapy, SLEDAI-2K score before the infection or comorbidities) correlated with the risk of COVID-19 pneumonia. In the considered parameters, no difference was found between hospitalized and non-hospitalized patients.
Case report
A 56-year-old woman was diagnosed SLE in 2001 based on ANAs, anti-dsDNA antibodies, low complement and lupus anti-coagulant positivity (ANCA, anti-extractable nuclear antigens, aCL and anti-beta-2 glycoprotein I antibodies negative), malar rash, alopecia, arthritis and RP. She was treated with low-dose prednisone and HCQ. Two years later, corticosteroids and antimalarial treatments were progressively stopped as sustained remission had been achieved. Regular follow-up visits confirmed persistent clinical remission with mild complement reduction, ANA positivity and inconstant anti-DNA positivity since 2020.
In March 2020 she developed malaise, cough and fever (maximum 38.5°C, no swab for SARS-CoV-2 performed, no specific therapy prescribed). In May 2020 the fever and cough recurred and a lung CT showed bibasilar consolidations (swab for SARS-CoV-2 negative, SARS-CoV-2 IgG positive, CRP 6.4 mg/dl). The suspicion was SARS-CoV-2 pneumonia since the patient had been treated with several courses of antibiotic therapy with incomplete resolution of the clinical manifestations and persistence of bibasilar consolidations. In July 2020 she received a diagnosis of autoimmune thyroiditis and levothyroxine was started. At the end of November 2020, for a new recurrence of chest pain, fever and cough (swab for SARS-CoV-2 negative), a new lung CT was performed with evidence of multiple parenchymal consolidations and several nodules (Fig. 1). A transbronchial lung biopsy revealed severe granulomatous inflammation with some multinucleated giant cells. The clinical condition of the patient worsened, blood pressure increased and rapid progressive kidney dysfunction developed with severe haematuria, anaemia, increased acute phase reactants and MPO-ANCA positivity (Table 4). Kidney biopsy was then performed with evidence of diffuse pauci-immune extracapillary necrotizing glomerulonephritis (Fig. 2). The patient received three i.v. methylprednisolone pulses of 750 mg each and rituximab (1 g 14 days apart) followed by oral prednisone 0.5 mg/kg/day, with rapid resolution of the fever and the other symptoms. One month after discharge the patient was asymptomatic with good blood pressure control and a noticeable improvement of laboratory tests.
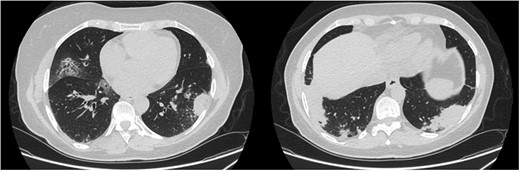
Pulmonary involvement: CT images performed in December 2020 before bronchoscopy
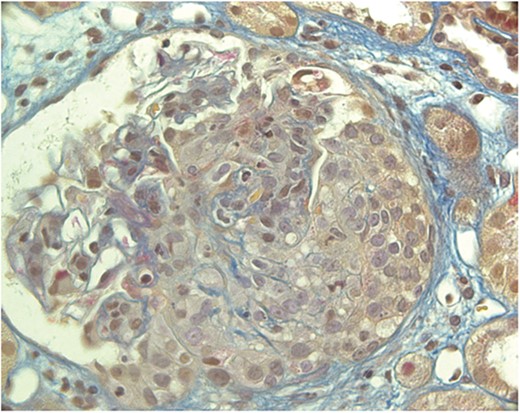
Kidney biopsy (Acid Fuchsin Orange G stain). The glomerulus shows a large, almost circumferential cellular crescent. Part of the Bowman capsule is disrupted
Laboratory blood test history of SLE patients who received the final diagnosis of ANCA-associated vasculitis after SARS-CoV-2 infection
| Laboratory test . | 10 July 2020 . | 5 November 2020 . | 11 January 2021 . | 5 March 2021 . |
|---|---|---|---|---|
| White blood cells, 109/L | 4.2 | 4.6 | 9.9 | 6.2 |
| Haemoglobin, g/dl | 12.0 | 13.7 | 8.8 | 11.9 |
| Platelets, 109/L | 237 | 235 | 307 | 187 |
| ESR, mm/h | 49 | 19 | 124 | 12 |
| CRP, mg/dL | 0.3 | 0.1 | 1.76 | 0.11 |
| C3 complement, mg/dL | – | 94 | 118 | 97 |
| C4 complement, mg/dL | – | 9 | 17 | 8 |
| TSH, mIU/L | 13.7 | – | 1.06 | – |
| Creatinine, mg/dL | 1.09 | 0.97 | 2.83 | 1.43 |
| ANA | 1/640a | – | 1/160 | 1/160 |
| aPL | – | Negative | Negative | – |
| Anti-dsDNA antibody | – | Negative | – | – |
| Urine sediment | Red blood cells | Normal | >100 urinary red blood cells/hpf, erythrocyte casts | 20 urinary red blood cells/hpf, no erythrocyte casts |
| Urine protein | 0.3 mg/dl | – | 2128 mg/24h | 1088 mg/24 h |
| Anti-GBM antibody | – | – | Negative | – |
| MPO-ANCA | – | – | Positive | Negative |
| Laboratory test . | 10 July 2020 . | 5 November 2020 . | 11 January 2021 . | 5 March 2021 . |
|---|---|---|---|---|
| White blood cells, 109/L | 4.2 | 4.6 | 9.9 | 6.2 |
| Haemoglobin, g/dl | 12.0 | 13.7 | 8.8 | 11.9 |
| Platelets, 109/L | 237 | 235 | 307 | 187 |
| ESR, mm/h | 49 | 19 | 124 | 12 |
| CRP, mg/dL | 0.3 | 0.1 | 1.76 | 0.11 |
| C3 complement, mg/dL | – | 94 | 118 | 97 |
| C4 complement, mg/dL | – | 9 | 17 | 8 |
| TSH, mIU/L | 13.7 | – | 1.06 | – |
| Creatinine, mg/dL | 1.09 | 0.97 | 2.83 | 1.43 |
| ANA | 1/640a | – | 1/160 | 1/160 |
| aPL | – | Negative | Negative | – |
| Anti-dsDNA antibody | – | Negative | – | – |
| Urine sediment | Red blood cells | Normal | >100 urinary red blood cells/hpf, erythrocyte casts | 20 urinary red blood cells/hpf, no erythrocyte casts |
| Urine protein | 0.3 mg/dl | – | 2128 mg/24h | 1088 mg/24 h |
| Anti-GBM antibody | – | – | Negative | – |
| MPO-ANCA | – | – | Positive | Negative |
aNuclear pattern.
TSH: thyroid-stimulating hormone; GBM: glomerular basement membrane; hpf: high-power field.
Laboratory blood test history of SLE patients who received the final diagnosis of ANCA-associated vasculitis after SARS-CoV-2 infection
| Laboratory test . | 10 July 2020 . | 5 November 2020 . | 11 January 2021 . | 5 March 2021 . |
|---|---|---|---|---|
| White blood cells, 109/L | 4.2 | 4.6 | 9.9 | 6.2 |
| Haemoglobin, g/dl | 12.0 | 13.7 | 8.8 | 11.9 |
| Platelets, 109/L | 237 | 235 | 307 | 187 |
| ESR, mm/h | 49 | 19 | 124 | 12 |
| CRP, mg/dL | 0.3 | 0.1 | 1.76 | 0.11 |
| C3 complement, mg/dL | – | 94 | 118 | 97 |
| C4 complement, mg/dL | – | 9 | 17 | 8 |
| TSH, mIU/L | 13.7 | – | 1.06 | – |
| Creatinine, mg/dL | 1.09 | 0.97 | 2.83 | 1.43 |
| ANA | 1/640a | – | 1/160 | 1/160 |
| aPL | – | Negative | Negative | – |
| Anti-dsDNA antibody | – | Negative | – | – |
| Urine sediment | Red blood cells | Normal | >100 urinary red blood cells/hpf, erythrocyte casts | 20 urinary red blood cells/hpf, no erythrocyte casts |
| Urine protein | 0.3 mg/dl | – | 2128 mg/24h | 1088 mg/24 h |
| Anti-GBM antibody | – | – | Negative | – |
| MPO-ANCA | – | – | Positive | Negative |
| Laboratory test . | 10 July 2020 . | 5 November 2020 . | 11 January 2021 . | 5 March 2021 . |
|---|---|---|---|---|
| White blood cells, 109/L | 4.2 | 4.6 | 9.9 | 6.2 |
| Haemoglobin, g/dl | 12.0 | 13.7 | 8.8 | 11.9 |
| Platelets, 109/L | 237 | 235 | 307 | 187 |
| ESR, mm/h | 49 | 19 | 124 | 12 |
| CRP, mg/dL | 0.3 | 0.1 | 1.76 | 0.11 |
| C3 complement, mg/dL | – | 94 | 118 | 97 |
| C4 complement, mg/dL | – | 9 | 17 | 8 |
| TSH, mIU/L | 13.7 | – | 1.06 | – |
| Creatinine, mg/dL | 1.09 | 0.97 | 2.83 | 1.43 |
| ANA | 1/640a | – | 1/160 | 1/160 |
| aPL | – | Negative | Negative | – |
| Anti-dsDNA antibody | – | Negative | – | – |
| Urine sediment | Red blood cells | Normal | >100 urinary red blood cells/hpf, erythrocyte casts | 20 urinary red blood cells/hpf, no erythrocyte casts |
| Urine protein | 0.3 mg/dl | – | 2128 mg/24h | 1088 mg/24 h |
| Anti-GBM antibody | – | – | Negative | – |
| MPO-ANCA | – | – | Positive | Negative |
aNuclear pattern.
TSH: thyroid-stimulating hormone; GBM: glomerular basement membrane; hpf: high-power field.
Discussion
Our data seem to confirm that there is not an increased risk of SARS-CoV-2 in SLE patients. In this cohort, SLE patients with major organ involvement (with lupus nephritis being most frequently observed) were those who experienced more often an asymptomatic form of the infection. SARS-CoV-2 infection determined flares in a small number of patients. In particular, a patient with long-standing SLE developed a renal and pulmonary syndrome, atypical for SLE, more closely resembling a vasculitis.
Although statistically significant, the difference found between IL-6, ferritin and ESR levels before and after the infection did not seem biologically relevant. Moreover, although increased with respect to pre-SARS-CoV-2 infection, ESR and ferritin values were still in the range of normality.
Considering the SLE intrinsic immunological abnormalities along with immunosuppressant therapies, the question of whether patients with SLE might have a different clinical SARS-CoV-2 infection outcome than the general population has arisen among clinicians worldwide. It now seems evident that SARS-CoV-2 infection is characterized by a viral phase and a subsequent immunological response, supporting the hypothesis that the clinical spectrum of COVID-19 is a result of the heterogeneity of the immune reaction to the virus itself. The critical aspect of the most severe form of COVID-19 is the loss of immune tolerance, leading to an exacerbation of the inflammatory components (the so-called cytokine storm) [24]. In this regard, several therapeutic immunomodulatory approaches (e.g. tocilizumab), usually administered in RDs, have been considered in the context of COVID-19 [25]. While overall results are still inconclusive, probably due to variability in dosing and heterogeneous inclusion criteria, it seems that some anti-rheumatic agents could have a role in the management of the immune response to SARS-CoV-2 infection.
In our cohort, patients with major organ involvement taking immunosuppressive regimens tended to experience asymptomatic SARS-CoV-2 infections; this fact might be explained by the concomitant ongoing SLE treatment (e.g. high-dose GCs) that could possibly have altered the immunological response to infection. This is only partially in line with what has been described in other inflammatory conditions. In a cohort of 65 patients with systemic vasculitis, mainly ANCA-associated vasculitis, GCs at presentation and concomitant lung conditions were both associated with severe outcomes in COVID-19, while vasculitis disease activity and non-GC immunosuppressants were not associated with a severe outcome [26].
The case of a male SLE patient has been reported with articular and haematological involvement who developed Coombs positive haemolytic anaemia and aPL antibody positivity after COVID-19 [27]. Here we reported the case of a female patient with a mild form of long-standing SLE in stable remission who developed a severe and atypical form of vasculitis after SARS-CoV-2 infection. This may suggest that in patients with an altered immunological substrate, SARS-CoV-2 may trigger immunological manifestations different from those expected.
Our study has some limitations, namely the number of patients included and the retrospective study design. Laboratory parameters were available only for some patients (e.g. IL-6 were available for less than half of the patients) and at different time points after the SARS-CoV-2 infection: these aspects prevented the drawing of conclusions about the impact of the infection on inflammatory markers (CRP, ESR, ferritin). In addition, because of outcoming information, behaviour (e.g. increased GCs, immunosuppressant suspension) may have changed over time and among centres towards SLE patients with SARS-CoV-2 infection.
Our data suggest a need to pay close attention to SLE patients with mild disease, considering that they usually do not take immunosuppressant therapy and therefore they could be more exposed to develop an intense response to SARS-CoV-2 infection. Furthermore, our case report should remind clinicians that SARS-CoV-2 infection can trigger new-onset autoimmune disease with atypical features in patients already prone to autoimmunity. The questions of whether these observations are reproducible in larger cohorts or, more critically, if they are somehow linked to immunological deregulations of SLE are still open.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data have been collected anonymously in an Excel file that is available upon request. Data about the case report is subject to some limitations due to privacy concerns.
Supplementary data
Supplementary data are available at Rheumatology online.
References
World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int (15 April
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ (13 August




Comments