-
PDF
- Split View
-
Views
-
Cite
Cite
Hideto Nagai, Yohei Kirino, Hiroto Nakano, Yosuke Kunishita, Riko Henmi, Ann Marie Szymanski, Ryusuke Yoshimi, Michael J Ombrello, Hideaki Nakajima, Elevated serum gasdermin D N-terminal implicates monocyte and macrophage pyroptosis in adult-onset Still's disease, Rheumatology, Volume 60, Issue 8, August 2021, Pages 3888–3895, https://doi.org/10.1093/rheumatology/keaa814
Close - Share Icon Share
Abstract
Elevation of serum IL-18 in adult-onset Still's disease (AOSD) and systemic JIA (sJIA) suggests the role of the inflammasome in these diseases. Gasdermin D is a pore-forming protein playing central roles in inflammasome-mediated inflammation, but its role in rheumatic disease is unknown. We aimed to elucidate the auto-inflammatory mechanisms in AOSD and sJIA.
Patients with AOSD, sJIA, hemophagocytic lymphohistiocytosis (HLH) and Behçet's disease followed at Yokohama City University (YCU), or US National Institutes of Health (NIH) were included in the study. Disease activity was evaluated by the modified Pouchot score. Ferritin and N-terminal gasdermin D levels in serum and culture supernatant were measured by ELISA. Primary monocytes (Mo) were stimulated with GM-CSF or M-CSF and differentiated into M1 macrophages (Mφ) or M2Mφ, respectively. The number of Mo/Mφ and their viability were monitored over time.
Patients with active AOSD and sJIA had increased levels of serum gasdermin D N-terminal, which correlated with serum ferritin and IL-18 levels. Mo-derived Mφ from active AOSD patients showed reduced cell viability and increased cell death. The number of cultured Mφ cells on day nine was negatively correlated with the serum ferritin and gasdermin D levels. Higher ferritin and gasdermin D levels were observed in the M1Mφ culture supernatant of active AOSD patients. Gasdermin D inhibitors reduced the pyroptosis-mediated ferritin release in Mo.
Elevation of serum gasdermin D N-terminal provides evidence for inflammasome activation triggering gasdermin D-mediated Mo and Mφ pyroptosis in AOSD and possibly sJIA.
Elevated serum gasdermin D N-terminal provides evidence for inflammasome activation in AOSD and sJIA
Gasdermin D-mediated Mo and Mφ pyroptosis may play important roles in AOSD and sJIA pathogenesis.
Introduction
Adult-onset Still's disease (AOSD) and systemic JIA (sJIA) are rare auto-inflammatory diseases of unknown cause that manifest with high-grade fever, arthritis, salmon-pink skin rash, serositis and sore throat [1]. Elucidation of the disease mechanisms is needed because some of the patients are refractory to treatments and develop a potentially life-threatening macrophage activation syndrome (MAS). Investigating the mechanisms underlying AOSD and sJIA has been difficult due to the low prevalence (the incidence of AOSD is 0.22–0.34 per 100 000 people per year in Japan [2] and 0.28–0.46 per 100 000 people per year in the United States [3]), and lacking animal models. Despite these obstacles, previous studies have revealed that abnormal macrophage activation and NK cell dysfunction triggered by a variety of stimuli, including microorganisms, play a key role in its pathogenesis [4–6].
In both AOSD and sJIA, extreme hyperferritinemia has long been considered a unique clinical phenotype [7]. Serum ferritin and glycosylated ferritin have been proposed for useful biomarkers for AOSD [7, 8], hemophagocytic lymphohistiocytosis (HLH) and sJIA, but the mechanism of hyperferrinemia in these diseases remains unknown.
In addition to ferritin, serum IL-18 has been reported to be elevated in AOSD and sJIA [9–11]. Because the cleaved form of IL-18 is produced by activated inflammasome [12], the elevation of serum IL-18 suggests inflammasome activation in these diseases. When the inflammasome is activated, full-length gasdermin D is cleaved into the N-terminal p30 fragment. The p30 forms a pore in the cell membrane [13], through which the activated IL-1β and IL-18 are exported from the cell. Eventually, pyroptosis is induced by this pore. Interestingly, a recent paper reported that ferritin is also exported through the gasdermin D pore [14]. Based on these data, we hypothesized a link between elevated serum ferritin and inflammasome activation in AOSD. As IL-1β and IL-18 are treatment targets of various auto-inflammatory diseases, including AOSD [15, 16], elucidation of their production mechanisms would lead to a novel treatment strategy for AOSD and sJIA. However, the role of inflammasome and gasdermin D in human inflammatory diseases has not yet been discovered in detail.
Here, we show for the first time that serum gasdermin D N-terminal is elevated in AOSD and sJIA and provide further evidence that macrophage pyroptosis is associated with hyperferritinemia in these disorders.
Patients and methods
Patients
Patients were recruited at Yokohama City University (YCU) Japan, and National Institutes of Health (NIH), MD. The study was approved by the ethics committees of YCU Hospital (B131107009) and NIH (18-AR-0081). All of the samples were collected after obtaining written informed consent. Criteria for AOSD, sJIA, MAS and BD are noted in the supplement material, available at Rheumatology online. Disease activity in AOSD and sJIA patients was assessed by a modified Pouchot score [17], which accounts for the presence of fever, arthralgia, skin rash, sore throat, lymphadenopathy or splenomegaly, myalgia, pleuritis, pericarditis, pneumonitis, hepatomegaly or abnormal liver function tests, elevated leukocyte count >15,000/µl, and serum ferritin level exceeding >3,000µg/l (score of 1 was assigned to each component). In this paper, a Pouchot score of 0 was considered remission and a score of 1 or more was considered active. Samples from HCs were obtained from young adults (mean age 28.2 (7.8)).
Measurement of cytokines, ferritin and gasdermin D N-terminal
Cytokines in serum and culture supernatants were measured using the LEGENDplex Human Inflammation Panel (BioLegend, San Diego, CA, USA). Serum ferritin was measured by the FER-latex NX kit (Denka, Niigata, Japan), which is government-approved for diagnostic testing in Japan. Ferritin levels in the culture supernatants were assessed with an ELISA kit (Assaypro, Saint Charles, MO, USA). N-terminal gasdermin D levels in serum and culture supernatant were measured with an ELISA kit, which only detects fragmented p30 form (Genway Biotech, San Diego, CA, USA). The medium was changed three times until day 9, and the medium was collected and stored at −80ºC until the measurement of ferritin and N-terminal gasdermin D at every medium change.
Isolation of peripheral monocytes and differentiation into macrophage
Human peripheal blood mononuclear cells (PBMC) from healthy controls (HC), AOSD, sJIA, and BD patients were collected by density gradient centrifugation. Subsequently, monocytes (Mo) were obtained by negative selection using the Monocyte isolation Kit II (Miltenyi Biotec, Bergisch, Germany). RPMI 1640 (Sigma Aldrich, Saint Louis, MO, USA) with 10% fetal bovine serum (MP Biomedicals, Santa Ana, CA, USA) and 1% penicillin-streptomycin (Gibco, Waltham, MA, USA) were used for cell culture throughout the experiments. 2.5× 105 Mo in 24 well dishes are supplemented with 20 ng/ml of GM-CSF (R&D Systems, Minneapolis, MN, USA), or 50 ng/ml of M-CSF (R&D Systems) for nine days as previously described [18]. In this study, GM-CSF-stimulated cells were defined as M1 and M-CSF-stimulated cells as M2. Medium changes were performed three times (days 3, 5 and 7) during the culture period. For evaluation of pyroptosis, monocytes were treated with 1 µg/ml lipopolysaccharide (LPS; L4391, Sigma Aldrich) for 14 h followed by 6.5 μM nigericin (SML1779, Sigma Aldrich) for another 2 h. Gasdermin D inhibitors 20 μM Necrosulfonamide [19] (Abcam, Cambridge, UK) and 30 μM disulfiram [20] (Merck, Darmstadt, Germany) were added at 0 h and 13 h after the start of culture.
Cell counting
M1 or M2Mφ cell numbers on day 9 of culture were air-dried, fixed with 4% paraformaldehyde and stained with the Diff-Quik staining kit (Sysmex Kokusai Shiyaku, Hyogo, Japan), according to a previously published protocol [18]. The number of Mφ cells in a high-power field (200×) visual field using BX53 (Olympus, Tokyo, Japan). Mean cell count was used in remission patients with multiple blood draws.
Cell viability assay
In total, 12 500 Mo were seeded onto 96-well plates. On days 3, 5 and 7, 10 μl of Cell Counting Kit-8 reagent (CCK-8, Dojindo Laboratories, Kumamoto, Japan) was added and incubated for 2 h at 37°C, 5% CO2. Thereafter, the absorbance at 450 nm was measured with a plate reader.
Cell death assay and live-cell imaging
50 000 Mo were seeded in triplicate on a 96-well plate with GM-CSF or M-CSF. CellTox Green (CTG) solution (Promega, Madison, WI, USA) was added at 100 μl per well on days 0, 4 or 7, and the fluorescence intensity was monitored for three days. BZ-9000 (Keyence, Osaka, Japan) was used for live-cell imaging.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism software (La Jolla, CA, USA). Student unpaired t-tests, one-way analysis of variance (ANOVA), and linear regression analysis were performed. A P-value of <0.05 after adjustment of multiple comparisons was considered significant.
Results
Elevated serum N-terminal gasdermin D in active AOSD and sJIA
Clinical features of AOSD and sJIA patients who participated in the study are summarized in Table 1.
Demographic characteristics of adult-onset Still's disease and systemic juvenile idiopathic arthritis patients included in the study
| (%) . | AOSD act . | AOSD rem . | sJIA act . | sJIA rem . | MAS . |
|---|---|---|---|---|---|
| n | 23 | 25 | 3 | 6 | 8* |
| Age | 48.7 (19.5) | 45.8 (20.2) | 6.0 (3.6) | 8.7 (3.9) | 46.1 (20) |
| Female n | 14 (60.1) | 15 (60.0) | 2 (66.7) | 5 (83.3) | 8 (100) |
| Fulfilling MAS criteria | 4 (17.3) | 0 0 | 0 0 | 0 0 | 8 (100) |
| Pouchot score | 5.1 (2.9) | 0.0 (0.0) | 2.0 (1.0) | 0.0 (0.0) | |
| High-grade fever | 16 (70.0) | 0 0 | 0 0 | 0 0 | 8 (100) |
| Evanescent rash | 16 (70.0) | 0 0 | 2 (66.7) | 0 0 | 0 0 |
| Arthralgia | 15 (65.2) | 0 0 | 0 0 | 0 0 | 0 0 |
| Leucocytosis | 7 (30.4) | 0 0 | 1 (33.3) | 0 0 | 0 0 |
| Sore throat | 9 (39.1) | 0 0 | 0 0 | 0 0 | 0 0 |
| Lymphadenopathy or splenomegaly | 10 (43.5) | 0 0 | 0 0 | 0 0 | 5 (62.5) |
| Liver dysfunction | 19 (82.6) | 0 0 | 0 0 | 0 0 | 7 (87.5) |
| RF/ANA negative | 22 (95.7) | 19 (76) | 3 (100) | 6 (100) | 3 (37.5) |
| Hyperferritinemia >1000 ng/ml | 15 (65.2) | 0 0 | 0 0 | 0 0 | 8 (100) |
| (%) . | AOSD act . | AOSD rem . | sJIA act . | sJIA rem . | MAS . |
|---|---|---|---|---|---|
| n | 23 | 25 | 3 | 6 | 8* |
| Age | 48.7 (19.5) | 45.8 (20.2) | 6.0 (3.6) | 8.7 (3.9) | 46.1 (20) |
| Female n | 14 (60.1) | 15 (60.0) | 2 (66.7) | 5 (83.3) | 8 (100) |
| Fulfilling MAS criteria | 4 (17.3) | 0 0 | 0 0 | 0 0 | 8 (100) |
| Pouchot score | 5.1 (2.9) | 0.0 (0.0) | 2.0 (1.0) | 0.0 (0.0) | |
| High-grade fever | 16 (70.0) | 0 0 | 0 0 | 0 0 | 8 (100) |
| Evanescent rash | 16 (70.0) | 0 0 | 2 (66.7) | 0 0 | 0 0 |
| Arthralgia | 15 (65.2) | 0 0 | 0 0 | 0 0 | 0 0 |
| Leucocytosis | 7 (30.4) | 0 0 | 1 (33.3) | 0 0 | 0 0 |
| Sore throat | 9 (39.1) | 0 0 | 0 0 | 0 0 | 0 0 |
| Lymphadenopathy or splenomegaly | 10 (43.5) | 0 0 | 0 0 | 0 0 | 5 (62.5) |
| Liver dysfunction | 19 (82.6) | 0 0 | 0 0 | 0 0 | 7 (87.5) |
| RF/ANA negative | 22 (95.7) | 19 (76) | 3 (100) | 6 (100) | 3 (37.5) |
| Hyperferritinemia >1000 ng/ml | 15 (65.2) | 0 0 | 0 0 | 0 0 | 8 (100) |
Act: active; ANA: antinuculear antibody; AOSD: adult-onset Still's disease; MAS: macrophage activation syndrome; rem: remission. *includes patients with systemic lupus erythematosus (SLE; n = 4), virus associated hemophagocytic syndrome (HPS; n = 3) and lymphoma associated HPS (n = 1).
Demographic characteristics of adult-onset Still's disease and systemic juvenile idiopathic arthritis patients included in the study
| (%) . | AOSD act . | AOSD rem . | sJIA act . | sJIA rem . | MAS . |
|---|---|---|---|---|---|
| n | 23 | 25 | 3 | 6 | 8* |
| Age | 48.7 (19.5) | 45.8 (20.2) | 6.0 (3.6) | 8.7 (3.9) | 46.1 (20) |
| Female n | 14 (60.1) | 15 (60.0) | 2 (66.7) | 5 (83.3) | 8 (100) |
| Fulfilling MAS criteria | 4 (17.3) | 0 0 | 0 0 | 0 0 | 8 (100) |
| Pouchot score | 5.1 (2.9) | 0.0 (0.0) | 2.0 (1.0) | 0.0 (0.0) | |
| High-grade fever | 16 (70.0) | 0 0 | 0 0 | 0 0 | 8 (100) |
| Evanescent rash | 16 (70.0) | 0 0 | 2 (66.7) | 0 0 | 0 0 |
| Arthralgia | 15 (65.2) | 0 0 | 0 0 | 0 0 | 0 0 |
| Leucocytosis | 7 (30.4) | 0 0 | 1 (33.3) | 0 0 | 0 0 |
| Sore throat | 9 (39.1) | 0 0 | 0 0 | 0 0 | 0 0 |
| Lymphadenopathy or splenomegaly | 10 (43.5) | 0 0 | 0 0 | 0 0 | 5 (62.5) |
| Liver dysfunction | 19 (82.6) | 0 0 | 0 0 | 0 0 | 7 (87.5) |
| RF/ANA negative | 22 (95.7) | 19 (76) | 3 (100) | 6 (100) | 3 (37.5) |
| Hyperferritinemia >1000 ng/ml | 15 (65.2) | 0 0 | 0 0 | 0 0 | 8 (100) |
| (%) . | AOSD act . | AOSD rem . | sJIA act . | sJIA rem . | MAS . |
|---|---|---|---|---|---|
| n | 23 | 25 | 3 | 6 | 8* |
| Age | 48.7 (19.5) | 45.8 (20.2) | 6.0 (3.6) | 8.7 (3.9) | 46.1 (20) |
| Female n | 14 (60.1) | 15 (60.0) | 2 (66.7) | 5 (83.3) | 8 (100) |
| Fulfilling MAS criteria | 4 (17.3) | 0 0 | 0 0 | 0 0 | 8 (100) |
| Pouchot score | 5.1 (2.9) | 0.0 (0.0) | 2.0 (1.0) | 0.0 (0.0) | |
| High-grade fever | 16 (70.0) | 0 0 | 0 0 | 0 0 | 8 (100) |
| Evanescent rash | 16 (70.0) | 0 0 | 2 (66.7) | 0 0 | 0 0 |
| Arthralgia | 15 (65.2) | 0 0 | 0 0 | 0 0 | 0 0 |
| Leucocytosis | 7 (30.4) | 0 0 | 1 (33.3) | 0 0 | 0 0 |
| Sore throat | 9 (39.1) | 0 0 | 0 0 | 0 0 | 0 0 |
| Lymphadenopathy or splenomegaly | 10 (43.5) | 0 0 | 0 0 | 0 0 | 5 (62.5) |
| Liver dysfunction | 19 (82.6) | 0 0 | 0 0 | 0 0 | 7 (87.5) |
| RF/ANA negative | 22 (95.7) | 19 (76) | 3 (100) | 6 (100) | 3 (37.5) |
| Hyperferritinemia >1000 ng/ml | 15 (65.2) | 0 0 | 0 0 | 0 0 | 8 (100) |
Act: active; ANA: antinuculear antibody; AOSD: adult-onset Still's disease; MAS: macrophage activation syndrome; rem: remission. *includes patients with systemic lupus erythematosus (SLE; n = 4), virus associated hemophagocytic syndrome (HPS; n = 3) and lymphoma associated HPS (n = 1).
A recent paper reported that ferritin is exported from the cell through gasdermin D pore [14]. To test the possibility that the inflammasome is activated in AOSD, we measured gasdermin D N-terminal, which is a marker of gasdermin D pore formation, in the serum of the patients.
We found that the serum level of gasdermin D N-terminal from active AOSD and sJIA patients is significantly higher compared with HC and patients in remission (Fig. 1A). Serum from BD and MAS (six out of eight patients also fulfilled criteria for the hemophagocytic syndrome [21]) were comparable to HC. The level of gasdermin D correlated with serum IL-18 and ferritin (Fig. 1B–D). Serum ferritin levels of AOSD/sJIA and MAS were similar, but serum IL-18 levels were lower in MAS patients (Supplementary Fig. 1, available at Rheumatology online). Although the number of sJIA samples was small, there was a trend for elevated serum gasdermin D in active sJIA samples (Supplementary Fig. 2, available at Rheumatology online). These results show that serum gasdermin D N-terminal is a potential biomarker for AOSD and possibly sJIA.
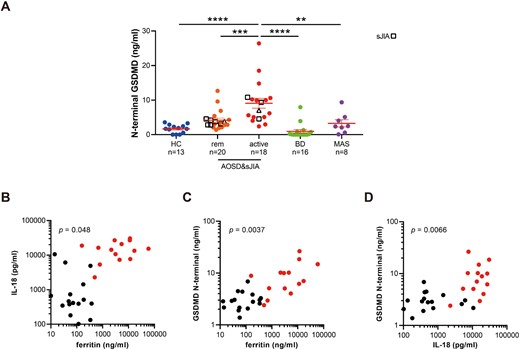
Elevated serum gasdermin D N-terminal and its correlation with serum ferritin and IL-18 in AOSD and sJIA
(A) Serum level of gasdermin D (GSDMD) N-terminal was measured in 13 healthy controls (HC), 20 adult-onset Still's disease (AOSD) and systemic JIA (sJIA) patients in remission (rem), 18 active AOSD and sJIA patients, 16 Behçet's disease (BD) patients, and eight macrophage activation syndrome (MAS) in Yokohama City University and US National Institutes of Health. sJIA patients are shown as box shapes. Samples from the same patient (n =1) with AOSD in active and remission phases are shown as triangles. Error bars indicate s.e. the mean. ****P <0.0001, ***P <0.001, **P <0.01, *P <0.05 by one-way ANOVA. (B–D) Correlation between serum ferritin, IL-18 and GSDMD N-terminal levels in AOSD patients in active (red dots, Pouchot score ≥1), and remission (black dots, Pouchot score=0). P-value was calculated by linear regression analysis.
Increased cell death of Mo-derived M1/M2 Mφ from AOSD and sJIA patients
Because AOSD has been classified as a macrophage-activation syndrome [6], we hypothesized that inflammasome activation of Mφ that form gasdermin D pores may be involved in the pathogenesis of AOSD. However, because Mφ are present in organs such as the skin and spleen, it is difficult to directly assess the function of macrophages in AOSD patients. Therefore, we decided to differentiate Mφ from monocytes obtained from AOSD patients in vitro.
To ask whether increased gasdermin D pores leads to Mo/Mφ pyroptosis in AOSD and sJIA, we counted the numbers of Mo-derived M1 and M2Mφ on the ninth day of culture according to previous protocol [18, 22]. Previously published data on BD, a disease also classified as an autoinflammatory disease, was used as a disease control [18, 23].
In both M1 and M2 Mφ, cell numbers at day 9 were significantly reduced in active AOSD and sJIA but not in patients in remission (Fig. 2A and B). There was a significant negative correlation between serum ferritin levels or serum gasdermin D levels at the time of blood draw and the number of cultured Mφ cells on day 9 (Fig. 2C and Supplementary Fig. 3, available at Rheumatology online).
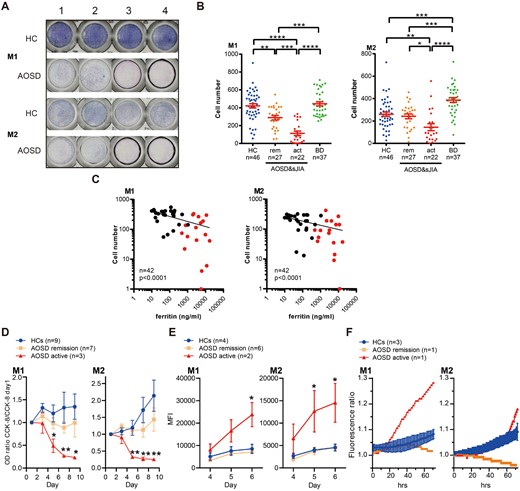
Increased cell death in monocyte-derived macrophages from AOSD and sJIA
(A) Monocytes from four healthy controls (HC) and four patients with adult-onset Still's disease (AOSD) in high disease activity stimulated with M-CSF or GM-CSF in 96-well dishes for nine days and stained with DiffQuik. (B) Numbers of M1 and M2 macrophage (Mφ) after nine day-culture of monocytes from HC, AOSD (rem: remission; act: active), and Behçet's disease (BD). (C) Correlation between serum ferritin level at the time of blood collection and the number of M1 or M2 Mφ on day 9 of culture. Red dots indicate active AOSD patients, whereas black dots represent patients in remission. (D) M1 and M2 Mφ cell viability after the start of culture evaluated with CCK-8 assay. (E) Amount of cell death under M1 or M2 culture conditions in HC and AOSD from day 4 to 6 as assessed by CellTox green (CTG) staining. MFI: mean fluorescence intensity. (F) Time-lapse imaging data of cell death in M1 and M2 Mφ stained with CTG. Error bars indicate s.e. of the mean. *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001 by one-way ANOVA.
Next, we performed the CCK-8 assay to monitor cell viability over time. Cell viability of Mo stimulated with either GM-CSF or M-CSF was significantly decreased over time compared with HC or AOSD in remission (Fig. 2D). Vice versa, cell-death was increased over time (Fig. 2E). Live-cell imaging showed increased cell death of Mo-derived Mφ in active AOSD samples (Fig. 2F). These results suggest that Mo-derived Mφ cell death is more prominent in active AOSD.
Increased ferritin and gasdermin D in the culture supernatant of Mo-derived M1/M2 Mφ from AOSD and sJIA patients
Mo cell death in patients with active AOSD/sJIA may result in intracellular ferritin being released into the culture medium via gasdermin D pores. Indeed, imaging of Mo from an active AOSD patient with M1Mφ-differentiation condition showed ballooning CTG-positive cells suggestive of pyroptosis (Supplementary Fig. 4, available at Rheumatology online). We measured the amount of ferritin, and gasdermin D N-terminal in the culture supernatant of M1 and M2Mφ derived from HC or AOSD/sJIA. In HC, ferritin was found to be measurable in the medium from day 3 of monocyte culture but decreased during the follow-up period. However, in M1Mφ from active AOSD/sJIA, ferritin levels were increased at day 5, 7 and 9 (Fig. 3A and B). Likewise, the gasdermin D N-terminal level in the medium was relatively higher in active AOSD patients (Fig. 3C and D).
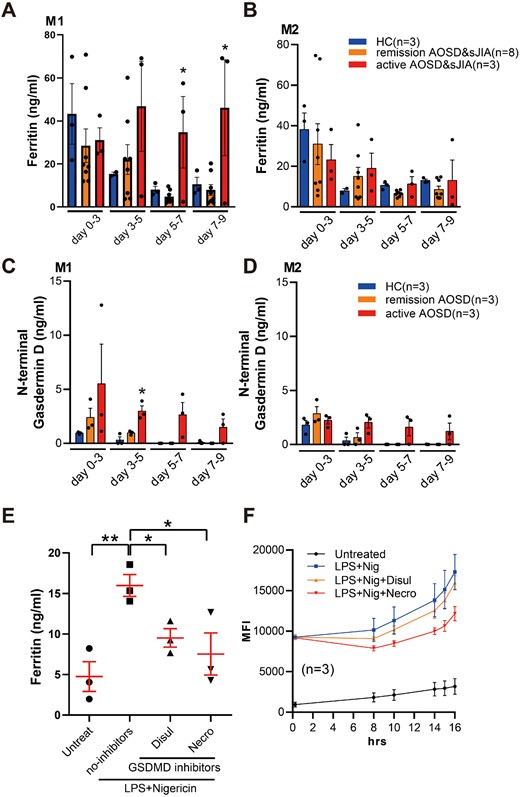
Increased ferritin and gasdermin D N-terminal concentration in culture supernatants of monocyte/macrophage from AOSD and sJIA
(A) Ferritin concentration in culture supernatant of monocytes incubated under M1 or (B) M2 macrophage differentiation conditions. Supernatants were collected every 3 days. (C) Gasdermin D N-terminal concentrations of culture supernatant of monocytes incubated under M1 or (D) M2 Mφ differentiation conditions. HC: healthy controls; AOSD: adult-onset Still's disease; sJIA: systemic JIA. Error bars indicate s.e. of the mean. *P <0.05 active vs remission by one-way ANOVA. (E) Supernatants’ ferritin levels and (F) cell death in cultured monocyte supernatants from HCs. Cells were divided into unstimulated (untreat), lipopolysaccharide (LPS) plus nigericin (Nig) with or without gasdermin D inhibitors necrosulfonamide (necro), or disulfiram (disul) groups. *P <0.05 by unpaired t-test.
To further investigate the association between pyroptosis and ferritin release, Mo from HC were stimulated with LPS and the pyroptosis inducer nigericin, assuming that inflammasome is activated in AOSD monocytes. LPS-pretreated cells induced significant cell death and IL-1β secretion after addition of nigericin in human monocytes, consistent with pyroptosis (Supplementary Fig. 5A and B, available at Rheumatology online). Gasdermin D N-terminal levels were also elevated in culture supernatants under this condition (Supplementary Fig. 5C, available at Rheumatology online). To evaluate the connection between ferritin elevation and pyroptosis in human cells, ferritin levels in supernatants were evaluated. As shown in Fig. 3E, ferritin was elevated in LPS and nigericin-treated group, but was partially inhibited by gasdermin D inhibitors necrosulfonamide or disulfiram. Cell death was also reduced in necrosulfonamide or disulfiram-treated cells (Fig. 3F). Taken together, our data suggest that increased Mo and Mφ pyroptosis may be associated with elevated serum ferritin levels in AOSD.
Discussion
Hyperferritinemia has long been used as a biomarker for in AOSD and sJIA [7, 24, 25], but its exact mechanism has not been fully elucidated. Macrophages have been suggested as a source of ferritin in AOSD [26], but direct evidence to connect Mo/Mφ in the pathogenesis of AOSD has been scarce. We previously reported that serum heme oxygenase (HO)- 1, a heme-degrading enzyme highly expressed in Mo/Mφ serves as a biomarker for AOSD [27]. CD163, a heme receptor expressed on macrophages, is also elevated in the serum of AOSD patients [28]. We here report that the N-terminal gasdermin D level is elevated in active AOSD and sJIA. Moreover, we showed that the Mo/Mφ cultured in the presence of M-CSF/GM-CSF undergoes more cell death resulting in ferritin release from those cells. This accumulating evidence suggests that Mo/Mφ pyroptosis may play roles in AOSD/sJIA pathogenesis.
It is interesting that there was no increase in serum gasdermin D in MAS patients (Fig. 1A). Although serum ferritin levels were comparable between AOSD and MAS, serum IL-18 levels were lower than in MAS samples (Supplementary Fig. 2, available at Rheumatology online). Further case accumulation is needed to determine whether serum gasdermin D is specific for AOSD compared with MAS.
The mechanism of ferritin release in normal conditions has also been discussed for a long time. As noted above, ferritin is mainly present in Mo/Mφ lineage among the leukocytes [29]. Multiple stimuli, such as iron load, oxidative stress, inflammatory cytokines and bacterial toxins stimulate translation of L- or H- ferritin, contribute to the accumulation of ferritin within the Mφ, thereby depleting iron from the serum [29]. Accumulated ferritin is secreted through the non-classical vesicular pathways rather than a cytosolic leak from damaged cells [30, 31]. In our previous studies, serum HO-1 and ferritin were both highly elevated in AOSD, but only the ferritin was high in patients with transfusion-induced iron overload [32], suggesting that the mechanisms of serum ferritin elevation during iron-rich status and inflammation are distinct. The consequences of ferritin release by gasdermin D in AOSD are interesting. Ferritin released from by Mφ pyroptosis may sequester free iron from the serum, resulting in iron-deficiency to combat bacteria. Further studies are needed for precise ferritin release mechanisms in various clinical settings.
The next important step is to clarify why the pyroptosis is activated in Mo and Mφ of AOSD/sJIA. A transient viral or bacterial infection that produces large amounts of danger-associated signals may play potent roles in AOSD/sJIA [33], which may lead to pyroptosis via the gasdermin D-dependent pathway.
In this paper, we have used M-CSF/GM-CSF to differentiate ‘M1’ and ‘M2’ Mφ according to previous literature [22]. Recently, however, LPS and interferon gamma for M1 and IL-4 for M2 Mφ differentiation protocol is more commonly employed [34]. Comparative studies using several Mφ differentiation protocols should be conducted in the future to clarify the role of M1 and M2 Mφ in AOSD.
In conclusion, the current study showed that elevation of serum gasdermin D N-terminal provides evidence for inflammasome activation triggering gasdermin D-mediated monocytes and macrophage pyroptosis in AOSD and possibly sJIA. Understanding the mechanisms of hyperferritinemia will ultimately lead to an understanding of the disease-specific mechanisms that lead to the development of disease-specific treatments for AOSD and sJIA.
Acknowledgements
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Y.K. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: H.Nag., H.Nakan., M.J.O., Y.Kirino. Acquisition of data: H.Nag., H.Nakan., Y.Kirino., R.H., M.S., M.J.O., Y.Kuni. Analysis and interpretation of data: H.Nag., H.Nakan., Y.Kirino., R.H., R.Y., M.J.O., Y.Kuni., H.Nakaj.
Funding: This study was supported by grants from the Japanese Society for the Promotion of Science Grants-in-Aid for Scientific Research #15K15374 (Y.K.) and NIH grant # Z01-AR-041198 (M.J.O.).
Disclosure statement: The authors declare no financial support or other benefits from commercial sources for this work. Y. Kirino received a consultant fee from Amgen, and grant support from Chugai Pharma Manufacturing Co., Ltd and Ono Pharmaceutical Co., Ltd.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on a reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.




Comments