-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel Li, Kazuki Yoshida, Candace H Feldman, Cameron Speyer, Medha Barbhaiya, Hongshu Guan, Daniel H Solomon, Brendan M Everett, Karen H Costenbader, Initial disease severity, cardiovascular events and all-cause mortality among patients with systemic lupus erythematosus, Rheumatology, Volume 59, Issue 3, March 2020, Pages 495–504, https://doi.org/10.1093/rheumatology/kez288
Close - Share Icon Share
Abstract
SLE is associated with high risks of cardiovascular disease (CVD) and mortality, and has a wide spectrum of presentations. We investigated whether SLE severity at diagnosis was associated with CVD or mortality risk.
Within Medicaid (2000–10), we identified patients 18–65 years of age with incident SLE. Initial SLE severity was classified—mild, moderate, or severe—during the baseline year prior to the start of follow-up (incident index date) using a published algorithm based on SLE-related medications and diagnoses. Patients were followed from the index date to the first CVD event or death, disenrollment, loss to follow-up or end of follow-up period. Cox and Fine–Gray regression models, adjusted for demographics and comorbidities accounting for the competing risk of death (for CVD), estimated CVD and mortality risks by baseline SLE severity.
Of 15 120 incident SLE patients, 48.7% had mild initial SLE severity, 33.9% moderate and 17.4% severe. Mean (s.d.) follow-up was 3.3 (2.4) years. After multivariable adjustment, CVD subdistribution hazard ratios (HRSD) were higher for initially severe [HRSD 1.64 (95% CI 1.32, 2.04)] and moderate [HRSD 1.19 (95% CI 1.00, 1.41)] SLE vs mild SLE. Mortality HRs were also higher for initially severe [HR 3.11 (95% CI 2.49, 3.89)] and moderate [HR 1.61 (95% CI 1.29, 2.01)] SLE vs mild SLE.
SLE patients with high initial severity had elevated mortality and CVD events risks compared with those who presented with milder disease. This has implications for clinical care and risk stratification of newly diagnosed SLE patients.
CVD and mortality risks are elevated in SLE patients with more severe disease at presentation.
Elevated risks persist after adjustment for known mortality and CVD comorbidities.
CVD and mortality risk assessment algorithms should incorporate this heterogeneity in SLE.
Introduction
SLE is a heterogeneous, often severe multisystem autoimmune disease with high risks of cardiovascular (CVD) disease and mortality [1–4]. Many efforts have been made to classify subtypes of SLE based on disease activity, severity, manifestations, autoantibodies and cytokines, and more recently gene expression profiles [5, 6]. SLE can have wide variation in severity even at initial presentation; some patients present with only rashes and arthritis requiring HCQ. In other cases, SLE can have life-threatening manifestations such as lupus nephritis and vasculitis requiring high-dose steroids and immunosuppression [7].
In past large cohort studies, overall myocardial infarction (MI) and stroke risks have been at least two to three times higher than in the general population, and heart failure risks were also elevated compared with age-matched controls [1–4, 8]. After controlling for known CVD risk factors, SLE has been independently associated with increased CVD risk [9]. This has been attributed to SLE’s pro-inflammatory state, although the exact mechanisms involved in accelerating atherogenesis remain unclear [10]. Past studies have shown that sustained high disease activity and severity are related to organ damage and mortality [11, 12]. In one study higher SLE disease activity scores among prevalent patients were positively associated with CVD risk [13]. Another study reported that prevalent SLE patients with SLEDAI-2000 (SLEDAI-2K) scores >6 were five times as likely to have CVD in the following 2 years as patients with a SLEDAI-2K score <3 [12].
Given the wide range of severity at presentation in SLE, we aimed to investigate associations between initial SLE severity within the first year and future risks of CVD and death. We examined this relationship within the US Medicaid population, which includes a large SLE population of lower socioeconomic status individuals from wide-ranging racial-ethnic backgrounds. We hypothesized that SLE patients with more severe early disease were more likely to have higher ensuing mortality and CVD risk.
Methods
Study population
Medicaid is the US public health insurance programme for individuals with low incomes that provides coverage for prescription drugs and medical expenses. Compared with the entire US population, the Medicaid population has a greater proportion of non-White people (41% White, 20% Black, 30% Hispanic) [14] with serious health conditions, and thus rather severe SLE with poor outcomes [15–22]. We analysed data from the Medicaid Analytic eXtract, an administrative database containing all billing claims for Medicaid patients, from the 48 most populated US states from 1 January 2000 through 31 December 2006, and the 29 most populated US states (includes 94% of SLE patients in the US) between 1 January 2007 and 31 December 2010 [20]. We included adults aged ⩾18 years to 65 years and excluded patients >65 years of age, as >90% are dually enrolled in Medicare and not all their claims are captured in Medicaid [23]. The Partners Institutional Review Board approved all aspects of this study.
As in previous work, we identified SLE as having three or more International Classification of Diseases, Ninth Revision (ICD-9) codes for SLE (710.0) from hospital discharge diagnoses or physician visit claims ⩾30 days apart [21]. Analyses were restricted to patients with ⩾24 months of continuous Medicaid enrolment prior to their first SLE code. Among these SLE patients, we identified incident SLE patients (no SLE-related codes for ⩾24 continuous months before the first SLE code and no end-stage renal disease codes before first SLE code, as these patients were less likely to have incident SLE). The index date was defined as the date of the third SLE billing code. The baseline period for SLE severity and covariate assessment was the 12 months prior to the index date (third SLE code). Fig. 1 illustrates the inclusion of patients.

Study design timeline
Study patients were required to have ≥24 months of continuous Medicaid enrolment, as well as no SLE codes (International Classification of Diseases, Ninth Revision 710.0) for ≥24 months before the first SLE code. The third SLE code was defined as the index date. We also required ≥30 days between SLE codes. The baseline period for SLE severity and covariate assessment was the 12 months prior the index date (third SLE code). Follow-up began after the index date.
Definition of SLE severity
We employed a published SLE severity classification algorithm that uses ICD-9, current procedural terminology and National Drug Code codes to categorize patients as mild, moderate or severe SLE at onset [24]. Patients were categorized based on filled prescriptions or the presence SLE-related medical conditions during the first year of SLE (supplementary Table S1, available at Rheumatology online). For example, acute psychosis and prescriptions for CYC are severe criteria, while pericarditis and prescriptions for MTX are moderate criteria. Severe SLE patients met at least one severe criterion, moderate patients at least one moderate but no severe criterion, and mild patients neither. Initial SLE severity classification was based on data from 12 months before the index date through the index date. In a separate study, we validated this algorithm compared with SLEDAI scores in a Brigham and Women’s Hospital SLE cohort of 100 patients with a mean of 3.77 SLEDAI scores per person over a 1-year period. The C-statistics showed good performance: 0.74 for mild vs moderate or severe SLE and 0.76 for mild or moderate vs severe SLE [25].
Outcome measures
The primary outcome measures were defined as all-cause death and the first CVD event after the index date. CVD events were defined as acute MI [ST elevation and non-ST elevation, positive predictive value (PPV): 94.1%], cerebrovascular accidents consisting of haemorrhagic and ischaemic stroke (PPV: 92.6%), heart failure (PPV: ⩾87%), percutaneous coronary intervention (PPV: 85%) and coronary artery bypass graft (PPV: 100%). Code-based algorithms have been validated and used in the past [15–19, 22, 26–33]. CVD outcomes were based on primary and secondary hospital discharge diagnosis codes, accounting for the possibility that SLE was billed as the primary diagnosis and a CVD event as a secondary diagnosis. Subjects were followed from the index date to the first CVD event, death, loss to follow-up, Medicaid disenrollment or end of follow-up period. Deaths were reported directly to Medicaid and obtained from the National Death Index. Cause of death was not available in this dataset.
Covariates
Baseline data from 12 months prior to the index date include demographic and CVD-related covariates. Demographic variables were age, sex, race/ethnicity, US Census-based region of residence and area-based socioeconomic status (SES). Race/ethnicity in the Medicaid Analytic eXtract database is self-reported. Region of residence was determined by zip codes. For area-based SES, we used a validated composite index of seven zip code SES indicators from 2000 US Census data [34] and divided area-level SES data into quartiles. Baseline comorbidities including diabetes, hyperlipidaemia, hypertension, obesity and smoking were identified using ICD-9 and/or current procedural terminology and/or Diagnosis-related group codes [35–37].
Statistical analysis
For CVD, we restricted analyses to incident SLE patients without a CVD event prior to the index date. For all-cause mortality, we included all incident SLE patients regardless of prior CVD events. We calculated unadjusted incidence rates and incidence rate ratios (IRRs) stratified by SLE severity with 95% CIs. We estimated cumulative incidence functions stratified by SLE severity and compared them using Gray’s test.
For CVD events, Cox subdistribution regression models estimated the subdistribution hazard ratios (HRSD) while accounting for the competing risk of death [38]. For all-cause mortality, we employed Cox regression models to estimate HRs. To control for confounding, we fit three models for each outcome. In each of our models (A through C), initial SLE severity was the predictor of interest. Model A included age (continuous) and sex. Model B added race, region and year to Model A. For CVD, Model C added cardiac-specific risk comorbidities to Model B. For mortality, Model C added an adjusted Charlson comorbidity index (without codes for stroke/ transient ischaemic attack as these codes are included in the Garris SLE severity algorithm). We did not include medications as covariates as the SLE severity stratification incorporates this information. We tested the proportional hazards assumption using Kaplan–Meier curves and cumulative incidence functions and observed no significant deviations.
We performed five sensitivity analyses. First, we included all incident SLE patients in predicting CVD. Second, since we hypothesized that patients with severe SLE at onset would be more likely to have many initial codes close in time, we stratified patients by the time between their first and third SLE codes (⩽365 days vs >365 days) in predicting CVD and mortality events. Third, we replaced the Charlson comorbidity index with the Ward SLE-specific risk adjustment index for mortality in predicting mortality [39]. Fourth, we created another SLE severity variable excluding ‘aortitis’ as a severe disease criterion (observed in 367 patients) as there are few case reports of lupus aortitis in the literature [40]. Fifth, we used Cox regression instead of a competing risk model to predict CVD events while censoring mortality events. As data on cause of mortality are not available in Medicaid, to estimate the proportion of deaths potentially attributable to CVD, we also identified deaths occurring within the same hospitalization or at any time in follow-up after the first CVD event. All statistical analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC, USA). Data were obtained from Centers for Medicare and Medicaid Services through an approved Data Use Agreement and are presented in accordance with their policies (cell sizes of <11 individuals have been suppressed).
Results
We identified 15 120 patients with incident SLE from 2000 to 2010. These patients had a mean age of 45.3 (s.d. 13.4) years and were mainly women (94.8%). The largest proportion resided in the US South (36.3%). The breakdown of SLE severity was mild 48.7%, moderate 33.9% and severe 17.5%. Among mild SLE patients, White (39.5%) and then Black (37.6%) race were most common, but among severe SLE patients, Black (46.3%) was more prevalent than White (32.0%) race. More severe SLE patients had ⩽365 days between their first and third SLE codes (78.6%) compared with mild SLE patients (55.5%). Table 1 shows baseline characteristics by initial SLE severity and supplementary Table S2, available at Rheumatology online, shows the frequencies of various items used in the Garris algorithm to classify initial SLE severity.
Baselinea characteristics of incident SLE patients enrolled in Medicaid, 2000–10 by SLE severity
| . | Mild . | Moderate . | Severe . |
|---|---|---|---|
| . | N = 7361 (48.7%) . | N = 5120 (33.9%) . | N = 2639 (17.4%) . |
| Women, n (%) | 7043 (95.7) | 4838 (94.5) | 2446 (92.7) |
| Age, mean (s.d.) years | 39.8 (12.0) | 39.0 (12.3) | 38.9 (12.9) |
| Race/ethnicity, n (%) | |||
| White | 2909 (39.5) | 1757 (34.3) | 844 (32.0) |
| Black | 2766 (37.6) | 2, 101 (41.0) | 1221 (46.3) |
| Hispanic | 1172 (15.9) | 849 (16.6) | 381 (14.4) |
| Asian | 212 (2.9) | 192 (3.8) | 77 (2.9) |
| American Indian | 94 (1.3) | 73 (1.4) | 34 (1.3) |
| Other | 208 (2.8) | 148 (2.9) | 82 (3.1) |
| Residential region, n (%) | |||
| Midwest | 1345 (18.3) | 901 (17.6) | 582 (22.1) |
| Northeast | 1603 (21.8) | 1150 (22.5) | 570 (21.6) |
| South | 2648 (36.0) | 1847 (36.1) | 993 (37.6) |
| West | 1765 (24.0) | 1222 (23.9) | 494 (18.7) |
| Time between 1st and 3rd SLE code, n (%) | |||
| <=365 days | 4086 (55.5) | 3691 (72.1) | 2073 (78.6) |
| Glucocorticoid dose, mean over 12 months, n (%) | |||
| None | 4494 (62.1) | 1739 (34.8) | 907 (35.5) |
| <0–10 mg/day | 2743 (37.9) | 2634 (52.8) | 1345 (52.7) |
| >10–20 mg/day | 0 (0.0) | 502 (10.1) | 240 (9.4) |
| >20 mg/day | 0 (0.0) | 116 (2.3) | 60 (2.4) |
| Medications, n (%) | |||
| Immunosuppressant | 13 (0.2) | 1956 (38.2) | 650 (24.6) |
| HCQ | 2770 (37.6) | 2470 (48.2) | 977 (37.0) |
| NSAID | 3969 (53.9) | 3038 (59.3) | 1370 (51.9) |
| ACE inhibitor/ARB | 1204 (16.4) | 1379 (26.9) | 862 (32.7) |
| Beta-blocker | 745 (10.1) | 832 (16.3) | 584 (22.1) |
| Statin | 781 (10.6) | 679 (13.3) | 451 (17.1) |
| Comorbidities,b n (%) | |||
| Diabetes | 767 (10.4) | 843 (16.5) | 567 (21.5) |
| Obesity | 408 (5.5) | 377 (7.4) | 273 (10.3) |
| Smoking | 502 (6.8) | 522 (10.2) | 330 (12.5) |
| Hypertension | 1859 (25.3) | 1985 (38.8) | 1378 (52.2) |
| Hyperlipidaemia | 920 (12.5) | 858 (16.8) | 495 (18.8) |
| Charlson comorbidity index, mean (s.d.) | 1.38 (1.13) | 1.92 (1.48) | 2.46 (1.82) |
| . | Mild . | Moderate . | Severe . |
|---|---|---|---|
| . | N = 7361 (48.7%) . | N = 5120 (33.9%) . | N = 2639 (17.4%) . |
| Women, n (%) | 7043 (95.7) | 4838 (94.5) | 2446 (92.7) |
| Age, mean (s.d.) years | 39.8 (12.0) | 39.0 (12.3) | 38.9 (12.9) |
| Race/ethnicity, n (%) | |||
| White | 2909 (39.5) | 1757 (34.3) | 844 (32.0) |
| Black | 2766 (37.6) | 2, 101 (41.0) | 1221 (46.3) |
| Hispanic | 1172 (15.9) | 849 (16.6) | 381 (14.4) |
| Asian | 212 (2.9) | 192 (3.8) | 77 (2.9) |
| American Indian | 94 (1.3) | 73 (1.4) | 34 (1.3) |
| Other | 208 (2.8) | 148 (2.9) | 82 (3.1) |
| Residential region, n (%) | |||
| Midwest | 1345 (18.3) | 901 (17.6) | 582 (22.1) |
| Northeast | 1603 (21.8) | 1150 (22.5) | 570 (21.6) |
| South | 2648 (36.0) | 1847 (36.1) | 993 (37.6) |
| West | 1765 (24.0) | 1222 (23.9) | 494 (18.7) |
| Time between 1st and 3rd SLE code, n (%) | |||
| <=365 days | 4086 (55.5) | 3691 (72.1) | 2073 (78.6) |
| Glucocorticoid dose, mean over 12 months, n (%) | |||
| None | 4494 (62.1) | 1739 (34.8) | 907 (35.5) |
| <0–10 mg/day | 2743 (37.9) | 2634 (52.8) | 1345 (52.7) |
| >10–20 mg/day | 0 (0.0) | 502 (10.1) | 240 (9.4) |
| >20 mg/day | 0 (0.0) | 116 (2.3) | 60 (2.4) |
| Medications, n (%) | |||
| Immunosuppressant | 13 (0.2) | 1956 (38.2) | 650 (24.6) |
| HCQ | 2770 (37.6) | 2470 (48.2) | 977 (37.0) |
| NSAID | 3969 (53.9) | 3038 (59.3) | 1370 (51.9) |
| ACE inhibitor/ARB | 1204 (16.4) | 1379 (26.9) | 862 (32.7) |
| Beta-blocker | 745 (10.1) | 832 (16.3) | 584 (22.1) |
| Statin | 781 (10.6) | 679 (13.3) | 451 (17.1) |
| Comorbidities,b n (%) | |||
| Diabetes | 767 (10.4) | 843 (16.5) | 567 (21.5) |
| Obesity | 408 (5.5) | 377 (7.4) | 273 (10.3) |
| Smoking | 502 (6.8) | 522 (10.2) | 330 (12.5) |
| Hypertension | 1859 (25.3) | 1985 (38.8) | 1378 (52.2) |
| Hyperlipidaemia | 920 (12.5) | 858 (16.8) | 495 (18.8) |
| Charlson comorbidity index, mean (s.d.) | 1.38 (1.13) | 1.92 (1.48) | 2.46 (1.82) |
Baseline data from 12 months prior to index date.
Collected at any time up to and including index date. Cell sizes of <11 individuals were suppressed in accordance with Federal reporting requirements. ACE: angiotensin-converting-enzyme; ARB: angiotensin receptor blocker.
Baselinea characteristics of incident SLE patients enrolled in Medicaid, 2000–10 by SLE severity
| . | Mild . | Moderate . | Severe . |
|---|---|---|---|
| . | N = 7361 (48.7%) . | N = 5120 (33.9%) . | N = 2639 (17.4%) . |
| Women, n (%) | 7043 (95.7) | 4838 (94.5) | 2446 (92.7) |
| Age, mean (s.d.) years | 39.8 (12.0) | 39.0 (12.3) | 38.9 (12.9) |
| Race/ethnicity, n (%) | |||
| White | 2909 (39.5) | 1757 (34.3) | 844 (32.0) |
| Black | 2766 (37.6) | 2, 101 (41.0) | 1221 (46.3) |
| Hispanic | 1172 (15.9) | 849 (16.6) | 381 (14.4) |
| Asian | 212 (2.9) | 192 (3.8) | 77 (2.9) |
| American Indian | 94 (1.3) | 73 (1.4) | 34 (1.3) |
| Other | 208 (2.8) | 148 (2.9) | 82 (3.1) |
| Residential region, n (%) | |||
| Midwest | 1345 (18.3) | 901 (17.6) | 582 (22.1) |
| Northeast | 1603 (21.8) | 1150 (22.5) | 570 (21.6) |
| South | 2648 (36.0) | 1847 (36.1) | 993 (37.6) |
| West | 1765 (24.0) | 1222 (23.9) | 494 (18.7) |
| Time between 1st and 3rd SLE code, n (%) | |||
| <=365 days | 4086 (55.5) | 3691 (72.1) | 2073 (78.6) |
| Glucocorticoid dose, mean over 12 months, n (%) | |||
| None | 4494 (62.1) | 1739 (34.8) | 907 (35.5) |
| <0–10 mg/day | 2743 (37.9) | 2634 (52.8) | 1345 (52.7) |
| >10–20 mg/day | 0 (0.0) | 502 (10.1) | 240 (9.4) |
| >20 mg/day | 0 (0.0) | 116 (2.3) | 60 (2.4) |
| Medications, n (%) | |||
| Immunosuppressant | 13 (0.2) | 1956 (38.2) | 650 (24.6) |
| HCQ | 2770 (37.6) | 2470 (48.2) | 977 (37.0) |
| NSAID | 3969 (53.9) | 3038 (59.3) | 1370 (51.9) |
| ACE inhibitor/ARB | 1204 (16.4) | 1379 (26.9) | 862 (32.7) |
| Beta-blocker | 745 (10.1) | 832 (16.3) | 584 (22.1) |
| Statin | 781 (10.6) | 679 (13.3) | 451 (17.1) |
| Comorbidities,b n (%) | |||
| Diabetes | 767 (10.4) | 843 (16.5) | 567 (21.5) |
| Obesity | 408 (5.5) | 377 (7.4) | 273 (10.3) |
| Smoking | 502 (6.8) | 522 (10.2) | 330 (12.5) |
| Hypertension | 1859 (25.3) | 1985 (38.8) | 1378 (52.2) |
| Hyperlipidaemia | 920 (12.5) | 858 (16.8) | 495 (18.8) |
| Charlson comorbidity index, mean (s.d.) | 1.38 (1.13) | 1.92 (1.48) | 2.46 (1.82) |
| . | Mild . | Moderate . | Severe . |
|---|---|---|---|
| . | N = 7361 (48.7%) . | N = 5120 (33.9%) . | N = 2639 (17.4%) . |
| Women, n (%) | 7043 (95.7) | 4838 (94.5) | 2446 (92.7) |
| Age, mean (s.d.) years | 39.8 (12.0) | 39.0 (12.3) | 38.9 (12.9) |
| Race/ethnicity, n (%) | |||
| White | 2909 (39.5) | 1757 (34.3) | 844 (32.0) |
| Black | 2766 (37.6) | 2, 101 (41.0) | 1221 (46.3) |
| Hispanic | 1172 (15.9) | 849 (16.6) | 381 (14.4) |
| Asian | 212 (2.9) | 192 (3.8) | 77 (2.9) |
| American Indian | 94 (1.3) | 73 (1.4) | 34 (1.3) |
| Other | 208 (2.8) | 148 (2.9) | 82 (3.1) |
| Residential region, n (%) | |||
| Midwest | 1345 (18.3) | 901 (17.6) | 582 (22.1) |
| Northeast | 1603 (21.8) | 1150 (22.5) | 570 (21.6) |
| South | 2648 (36.0) | 1847 (36.1) | 993 (37.6) |
| West | 1765 (24.0) | 1222 (23.9) | 494 (18.7) |
| Time between 1st and 3rd SLE code, n (%) | |||
| <=365 days | 4086 (55.5) | 3691 (72.1) | 2073 (78.6) |
| Glucocorticoid dose, mean over 12 months, n (%) | |||
| None | 4494 (62.1) | 1739 (34.8) | 907 (35.5) |
| <0–10 mg/day | 2743 (37.9) | 2634 (52.8) | 1345 (52.7) |
| >10–20 mg/day | 0 (0.0) | 502 (10.1) | 240 (9.4) |
| >20 mg/day | 0 (0.0) | 116 (2.3) | 60 (2.4) |
| Medications, n (%) | |||
| Immunosuppressant | 13 (0.2) | 1956 (38.2) | 650 (24.6) |
| HCQ | 2770 (37.6) | 2470 (48.2) | 977 (37.0) |
| NSAID | 3969 (53.9) | 3038 (59.3) | 1370 (51.9) |
| ACE inhibitor/ARB | 1204 (16.4) | 1379 (26.9) | 862 (32.7) |
| Beta-blocker | 745 (10.1) | 832 (16.3) | 584 (22.1) |
| Statin | 781 (10.6) | 679 (13.3) | 451 (17.1) |
| Comorbidities,b n (%) | |||
| Diabetes | 767 (10.4) | 843 (16.5) | 567 (21.5) |
| Obesity | 408 (5.5) | 377 (7.4) | 273 (10.3) |
| Smoking | 502 (6.8) | 522 (10.2) | 330 (12.5) |
| Hypertension | 1859 (25.3) | 1985 (38.8) | 1378 (52.2) |
| Hyperlipidaemia | 920 (12.5) | 858 (16.8) | 495 (18.8) |
| Charlson comorbidity index, mean (s.d.) | 1.38 (1.13) | 1.92 (1.48) | 2.46 (1.82) |
Baseline data from 12 months prior to index date.
Collected at any time up to and including index date. Cell sizes of <11 individuals were suppressed in accordance with Federal reporting requirements. ACE: angiotensin-converting-enzyme; ARB: angiotensin receptor blocker.
Unadjusted incidence rates
Of the 13 255 incident SLE patients with no CVD events prior to the index date, mean follow-up was 3.20 (s.d. 2.40) years. There were 681 CVD events and an annual incidence rate of 16.1 events per 1000 person-years (95% CI 14.9, 17.3) (Table 2). Mild SLE severity patients had the lowest CVD incidence rate of 13.3 events per 1000 person-years (95% CI 11.9, 15.0), and severe SLE patients had the highest CVD incidence rate of 24.6 events per 1000 person-years (95% CI 20.6, 29.2). Compared with mild SLE patients, severe SLE patients had increased CVD rates [IRR 1.84 (95% CI 1.62, 2.10)]. Severe SLE patients also had a lower mean age at CVD event (43.3, s.d. 13.8 years) compared with mild SLE patients (47.0, s.d. 12.1 years).
Annual rates of mortality and CVD events among patients with incident SLE in Medicaid 2000–10a
| Baseline SLE severity . | Total patients, n . | Age at event, years, mean (s.d.) . | No. of events . | Person-years follow-up, mean (s.d.) . | IR per 1000 person-years (95% CI) . | IRR (95% CI) . |
|---|---|---|---|---|---|---|
| CVD | ||||||
| All patients | 13 255 | 45.7 (12.9) | 681 | 3.20 (2.40) | 16.1 (14.9, 17.3) | – |
| Mild | 7032 | 47.0 (12.1) | 288 | 3.08 (2.34) | 13.3 (11.9, 15.0) | 1.0 (ref) |
| Moderate | 4586 | 45.5 (13.2) | 267 | 3.40 (2.45) | 17.1 (15.2, 19.3) | 1.29 (1.16, 1.42) |
| Severe | 1637 | 43.3 (13.8) | 126 | 3.13 (2.49) | 24.6 (20.6, 29.2) | 1.84 (1.62, 2.10) |
| Mortality | ||||||
| All patients | 15 120 | 45.3 (13.4) | 529 | 3.31 (2.44) | 10.6 (9.7, 11.5) | – |
| Mild | 7361 | 46.6 (13.5) | 139 | 3.17 (2.37) | 6.0 (5.0, 7.0) | 1.0 (ref) |
| Moderate | 5120 | 44.7 (14.2) | 187 | 3.53 (2.49) | 10.4 (9.0, 12.0) | 1.74 (1.54, 1.97) |
| Severe | 2639 | 45.0 (12.6) | 203 | 3.26 (2.54) | 23.6 (20.5, 27.1) | 3.96 (3.50, 4.48) |
| Baseline SLE severity . | Total patients, n . | Age at event, years, mean (s.d.) . | No. of events . | Person-years follow-up, mean (s.d.) . | IR per 1000 person-years (95% CI) . | IRR (95% CI) . |
|---|---|---|---|---|---|---|
| CVD | ||||||
| All patients | 13 255 | 45.7 (12.9) | 681 | 3.20 (2.40) | 16.1 (14.9, 17.3) | – |
| Mild | 7032 | 47.0 (12.1) | 288 | 3.08 (2.34) | 13.3 (11.9, 15.0) | 1.0 (ref) |
| Moderate | 4586 | 45.5 (13.2) | 267 | 3.40 (2.45) | 17.1 (15.2, 19.3) | 1.29 (1.16, 1.42) |
| Severe | 1637 | 43.3 (13.8) | 126 | 3.13 (2.49) | 24.6 (20.6, 29.2) | 1.84 (1.62, 2.10) |
| Mortality | ||||||
| All patients | 15 120 | 45.3 (13.4) | 529 | 3.31 (2.44) | 10.6 (9.7, 11.5) | – |
| Mild | 7361 | 46.6 (13.5) | 139 | 3.17 (2.37) | 6.0 (5.0, 7.0) | 1.0 (ref) |
| Moderate | 5120 | 44.7 (14.2) | 187 | 3.53 (2.49) | 10.4 (9.0, 12.0) | 1.74 (1.54, 1.97) |
| Severe | 2639 | 45.0 (12.6) | 203 | 3.26 (2.54) | 23.6 (20.5, 27.1) | 3.96 (3.50, 4.48) |
Cell sizes of <11 individuals suppressed in accordance with Federal reporting requirements. CVD excludes patients with CVD event before baseline. Mortality includes all incident SLE patients. CVD: cardiovascular disease (including coronary artery bypass graft, heart failure, myocardial infarction, stroke, percutaneous coronary intervention); IR: incidence rate; IRR: incidence rate ratio.
Annual rates of mortality and CVD events among patients with incident SLE in Medicaid 2000–10a
| Baseline SLE severity . | Total patients, n . | Age at event, years, mean (s.d.) . | No. of events . | Person-years follow-up, mean (s.d.) . | IR per 1000 person-years (95% CI) . | IRR (95% CI) . |
|---|---|---|---|---|---|---|
| CVD | ||||||
| All patients | 13 255 | 45.7 (12.9) | 681 | 3.20 (2.40) | 16.1 (14.9, 17.3) | – |
| Mild | 7032 | 47.0 (12.1) | 288 | 3.08 (2.34) | 13.3 (11.9, 15.0) | 1.0 (ref) |
| Moderate | 4586 | 45.5 (13.2) | 267 | 3.40 (2.45) | 17.1 (15.2, 19.3) | 1.29 (1.16, 1.42) |
| Severe | 1637 | 43.3 (13.8) | 126 | 3.13 (2.49) | 24.6 (20.6, 29.2) | 1.84 (1.62, 2.10) |
| Mortality | ||||||
| All patients | 15 120 | 45.3 (13.4) | 529 | 3.31 (2.44) | 10.6 (9.7, 11.5) | – |
| Mild | 7361 | 46.6 (13.5) | 139 | 3.17 (2.37) | 6.0 (5.0, 7.0) | 1.0 (ref) |
| Moderate | 5120 | 44.7 (14.2) | 187 | 3.53 (2.49) | 10.4 (9.0, 12.0) | 1.74 (1.54, 1.97) |
| Severe | 2639 | 45.0 (12.6) | 203 | 3.26 (2.54) | 23.6 (20.5, 27.1) | 3.96 (3.50, 4.48) |
| Baseline SLE severity . | Total patients, n . | Age at event, years, mean (s.d.) . | No. of events . | Person-years follow-up, mean (s.d.) . | IR per 1000 person-years (95% CI) . | IRR (95% CI) . |
|---|---|---|---|---|---|---|
| CVD | ||||||
| All patients | 13 255 | 45.7 (12.9) | 681 | 3.20 (2.40) | 16.1 (14.9, 17.3) | – |
| Mild | 7032 | 47.0 (12.1) | 288 | 3.08 (2.34) | 13.3 (11.9, 15.0) | 1.0 (ref) |
| Moderate | 4586 | 45.5 (13.2) | 267 | 3.40 (2.45) | 17.1 (15.2, 19.3) | 1.29 (1.16, 1.42) |
| Severe | 1637 | 43.3 (13.8) | 126 | 3.13 (2.49) | 24.6 (20.6, 29.2) | 1.84 (1.62, 2.10) |
| Mortality | ||||||
| All patients | 15 120 | 45.3 (13.4) | 529 | 3.31 (2.44) | 10.6 (9.7, 11.5) | – |
| Mild | 7361 | 46.6 (13.5) | 139 | 3.17 (2.37) | 6.0 (5.0, 7.0) | 1.0 (ref) |
| Moderate | 5120 | 44.7 (14.2) | 187 | 3.53 (2.49) | 10.4 (9.0, 12.0) | 1.74 (1.54, 1.97) |
| Severe | 2639 | 45.0 (12.6) | 203 | 3.26 (2.54) | 23.6 (20.5, 27.1) | 3.96 (3.50, 4.48) |
Cell sizes of <11 individuals suppressed in accordance with Federal reporting requirements. CVD excludes patients with CVD event before baseline. Mortality includes all incident SLE patients. CVD: cardiovascular disease (including coronary artery bypass graft, heart failure, myocardial infarction, stroke, percutaneous coronary intervention); IR: incidence rate; IRR: incidence rate ratio.
The cumulative incidence curves for CVD events are shown in Fig. 2A. Patients with mild severity had the lowest cumulative incidence of CVD events in follow-up at 8 years [11.6% (95% CI 8.4, 15.4%)]. Those with severe disease had the highest cumulative incidence [16.3% (95% CI 12.8, 20.3%)]. There was a statistically significant difference in the CVD cumulative incidence curves by SLE severity (Gray’s test, P < 0.001).
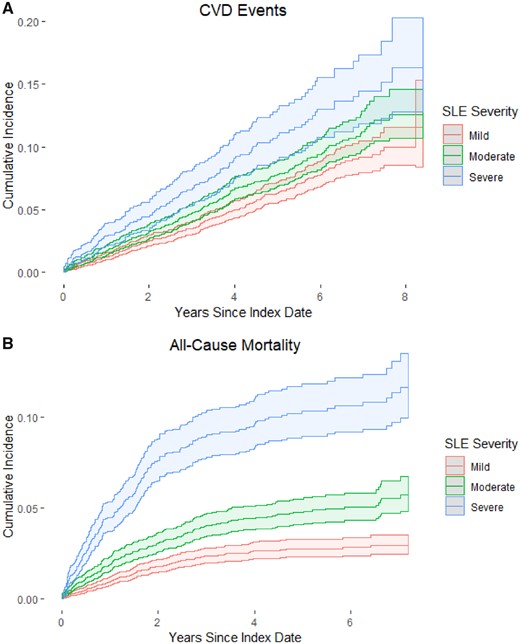
Cumulative incidence function for CVD and mortality among incident SLE patients, US Medicaid 2000–10
(A) Cumulative incidence function (CIF) estimates for cardiovascular disease (CVD) and (B) estimated CIF estimates for all-cause mortality. CIF estimates are stratified by SLE severity. Dotted lines are 95% CI estimates. Gray’s test for a difference in CIF curves by SLE severity for both CVD and all-cause mortality were statistically significant (both P<0.001).
Of all 15 120 incident SLE patients, mean follow-up was 3.31 (s.d. 2.44) years. There were 529 mortality events and an annual incidence rate of 10.6 events per 1000 person-years (95% CI 9.7, 11.5) (Table 2). Patients with initial mild SLE severity had the lowest mortality incidence rate of 6.0 events per 1000 person-years (95% CI 5.0, 7.0), and those with severe SLE severity had the highest rate of 23.6 events per 1000 person-years (95% CI 20.5, 27.1). Compared with mild SLE patients, severe patients had a higher unadjusted mortality rate [IRR 3.96 (95% CI 3.50, 4.48)].
The cumulative incidence curves for all-cause mortality are shown in Fig. 2B. Patients with mild severity had the lowest cumulative incidence of mortality events in follow-up at 7 years [2.9% (95% CI 2.4, 3.5%)]. Those with severe SLE had the highest cumulative incidence of death [11.6 (95% CI 9.9, 13.5%)]. There was a statistically significant difference in the mortality cumulative incidence curves by initial SLE severity (Gray’s test, P < 0.001).
Multivariable models—adjusted estimates
Table 3 shows the estimated HRSD for CVD risk according to SLE severity. After adjusting for age, sex and race (Model A), moderate patients [HRSD 1.31 (95% CI 1.11, 1.55)] and severe patients [HRSD 1.91 (95% CI 1.54, 2.36)] had elevated risks of CVD compared with mild patients. Adjustment for sociodemographic factors and comorbidities (Model C) reduced these risks marginally: those with moderate [HRSD 1.19 (95% CI 1.00, 1.41)] and severe [HRSD 1.64 (95% CI 1.32, 2.04)] SLE at onset still were seen to have an increased risk of CVD compared with those with mild onset.
| Baseline SLE disease severityc . | Model Ad . | Model Be . | Model Cf . |
|---|---|---|---|
| CVD (HRSD, N = 13 255) | |||
| Mild | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Moderate | 1.31 (1.11, 1.55) | 1.29 (1.09, 1.52) | 1.19 (1.00, 1.41) |
| Severe | 1.91 (1.54, 2.36) | 1.87 (1.51, 2.31) | 1.64 (1.32, 2.04) |
| Mortality (HR, N = 15 120) | |||
| Mild | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Moderate | 1.87 (1.50, 2.33) | 1.85 (1.48, 2.30) | 1.61 (1.29, 2.01) |
| Severe | 4.14 (3.34, 5.15) | 4.02 (3.23, 4.99) | 3.11 (2.49, 3.89) |
| Baseline SLE disease severityc . | Model Ad . | Model Be . | Model Cf . |
|---|---|---|---|
| CVD (HRSD, N = 13 255) | |||
| Mild | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Moderate | 1.31 (1.11, 1.55) | 1.29 (1.09, 1.52) | 1.19 (1.00, 1.41) |
| Severe | 1.91 (1.54, 2.36) | 1.87 (1.51, 2.31) | 1.64 (1.32, 2.04) |
| Mortality (HR, N = 15 120) | |||
| Mild | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Moderate | 1.87 (1.50, 2.33) | 1.85 (1.48, 2.30) | 1.61 (1.29, 2.01) |
| Severe | 4.14 (3.34, 5.15) | 4.02 (3.23, 4.99) | 3.11 (2.49, 3.89) |
HRSD: subdistribution hazard ratio (95% CI) based on competing risk analysis accounting for the competing risk of death from the Fine–Gray model; HR: hazard ratio from the Cox proportional hazards model.
Bold values indicates P < 0.05.
See reference [26]. Baseline was defined as 1 year prior to index date.
Adjusted for age (continuous), sex and race.
Adjusted for Model A plus region of residence, area-based socioeconomic status quartile and year.
Adjusted for Model B plus (i) comorbidities at study index date, including history of diabetes mellitus, hyperlipidaemia, hypertension, obesity and smoking for CVD, and (ii) Charlson comorbidity index for mortality. CVD: cardiovascular disease.
| Baseline SLE disease severityc . | Model Ad . | Model Be . | Model Cf . |
|---|---|---|---|
| CVD (HRSD, N = 13 255) | |||
| Mild | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Moderate | 1.31 (1.11, 1.55) | 1.29 (1.09, 1.52) | 1.19 (1.00, 1.41) |
| Severe | 1.91 (1.54, 2.36) | 1.87 (1.51, 2.31) | 1.64 (1.32, 2.04) |
| Mortality (HR, N = 15 120) | |||
| Mild | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Moderate | 1.87 (1.50, 2.33) | 1.85 (1.48, 2.30) | 1.61 (1.29, 2.01) |
| Severe | 4.14 (3.34, 5.15) | 4.02 (3.23, 4.99) | 3.11 (2.49, 3.89) |
| Baseline SLE disease severityc . | Model Ad . | Model Be . | Model Cf . |
|---|---|---|---|
| CVD (HRSD, N = 13 255) | |||
| Mild | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Moderate | 1.31 (1.11, 1.55) | 1.29 (1.09, 1.52) | 1.19 (1.00, 1.41) |
| Severe | 1.91 (1.54, 2.36) | 1.87 (1.51, 2.31) | 1.64 (1.32, 2.04) |
| Mortality (HR, N = 15 120) | |||
| Mild | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Moderate | 1.87 (1.50, 2.33) | 1.85 (1.48, 2.30) | 1.61 (1.29, 2.01) |
| Severe | 4.14 (3.34, 5.15) | 4.02 (3.23, 4.99) | 3.11 (2.49, 3.89) |
HRSD: subdistribution hazard ratio (95% CI) based on competing risk analysis accounting for the competing risk of death from the Fine–Gray model; HR: hazard ratio from the Cox proportional hazards model.
Bold values indicates P < 0.05.
See reference [26]. Baseline was defined as 1 year prior to index date.
Adjusted for age (continuous), sex and race.
Adjusted for Model A plus region of residence, area-based socioeconomic status quartile and year.
Adjusted for Model B plus (i) comorbidities at study index date, including history of diabetes mellitus, hyperlipidaemia, hypertension, obesity and smoking for CVD, and (ii) Charlson comorbidity index for mortality. CVD: cardiovascular disease.
Table 3 also shows the estimated HR for all-cause mortality risk according to SLE severity. After adjusting for age, sex and race (Model A), moderate patients [HR 1.87 (95% CI 1.50, 2.33)] and severe patients [HR 4.14 (95% CI 3.34, 5.15)] had elevated risks of mortality events compared with mild patients. Adjustment for sociodemographic factors and the Charlson comorbidity index (Model C) reduced these risks marginally: those with moderate [HR 1.61 (95% CI 1.29, 2.01)] and severe [HR 3.11 (95% CI 2.49, 3.89)] SLE still had an increased risk of mortality compared with those with mild disease.
Sensitivity analyses
All sensitivity analysis results presented below adjust for age, sex, sociodemographic factors and comorbidities. In the first sensitivity analysis, including all incident SLE patients, SLE severity was associated with CVD risk during the study period. Moderate patients [HRSD 1.33 (95% CI 1.15, 1.53)] and severe patients [HRSD 2.03 (95% CI 1.74, 2.36)] had an increased risk of CVD compared with mild patients (supplementary Table S3, available at Rheumatology online). Among all incident SLE patients, 1119 had a CVD event after the index date, of whom 19 (1.7%) had a death within 30 days of the first CVD event, and 114 (10.2%) had a death any time after the first CVD event.
In the second sensitivity analysis, in patients with ⩽365 days between codes, initial SLE severity was associated with CVD and mortality. Severe patients [HRSD 1.84 (95% CI 1.42, 2.39)] had an increased risk of CVD compared with mild patients (N = 8475). Moderate [HR 1.98 (95% CI 1.46, 2.69)] and severe [HR 3.70 (95% CI 2.73, 5.01)] patients had an increased risk of mortality compared with mild patients (N = 9850). However, in patients with slower onset of SLE and >365 days between first and third SLE codes, initial SLE severity was associated with mortality but not CVD. Severe patients [HR 2.69 (95% CI 1.83, 3.95)] had an increased risk of mortality events compared with mild patients (N = 5270; supplementary Table S3, available at Rheumatology online).
In the third sensitivity analysis, initial SLE severity was associated with mortality even after adjustment with the Ward risk adjustment index. Moderate [HR 1.50 (95% CI 1.20, 1.88] and severe patients [HR 2.44 (95% CI 1.92, 3.09)] had an increased risk of mortality compared with mild patients (supplementary Table S3, available at Rheumatology online).
In the fourth sensitivity analysis, we found that SLE severity was associated with CVD and mortality after excluding aortitis codes. Moderate [HRSD 1.19 (95% CI 1.01, 1.41)] and severe patients [HRSD 1.67 (95% CI 1.33, 2.10)] had an increased risk of CVD compared with mild patients. Moderate [HR 1.57 (95% CI 1.26, 1.96)] and severe patients [HR 3.20 (95% CI 2.56, 4.01)] had an increased risk of mortality compared with mild patients (supplementary Table S3, available at Rheumatology online).
In the fifth sensitivity analysis, in traditional Cox models censoring for death produced risk estimates that were very similar to the competing risk model. Moderate [HR 1.20 (95% CI 1.01, 1.42] and severe [HR 1.72 (95% CI 1.39, 2.13)] patients had an increased risk of CVD compared with mild patients (supplementary Table S3, available at Rheumatology online).
Discussion
Within a cohort of >15 000 racially, ethnically and geographically diverse incident SLE patients with US Medicaid health insurance from 2000–10, we investigated associations between initial SLE severity within the first year and future risks of CVD and death. Such associations could have important implications for clinical care and risk stratification of newly diagnosed SLE patients. We found that patients who initially had severe and moderate SLE had increased risks of CVD and all-cause mortality compared with patients who initially had mild SLE.
In our past studies, we have studied MI, stroke, heart failure and mortality risks in the US Medicaid SLE population, and compared these SLE patients to those with diabetes mellitus and the general population [15–17, 22]. Our work has demonstrated that disparities in CVD and other outcomes in SLE are partially explained by limited access to indicated care, substandard quality of care and poor adherence [18–21]. However, these studies have not investigated SLE severity in relation to CVD or mortality risk. The current study reveals that even within the early days of SLE, there is heterogeneity in disease severity that is a strong risk factor for future CVD and overall mortality. These relationships persist even after accounting for CVD comorbidities, the Charlson comorbidity index and the Ward SLE-specific risk adjustment index.
The relationship between initial SLE severity and CVD was stronger when we included patients with prior CVD, as these patients are at a higher risk of future CVD. The main analysis obtains HRSD for only incident CVD, whereas the first sensitivity analysis obtains HRSD for incident and recurring CVD combined. As is observed in clinical practice, we hypothesized that patients with more severe SLE at presentation would have a more rapid accumulation of SLE billing codes for their inpatient, outpatient and emergency care, and the rapidity of SLE onset would be associated with subsequent outcomes, including cardiovascular disease and overall mortality, which indeed was observed. The stronger relationship between SLE severity and CVD and mortality among patients with a shorter time between the first and third SLE codes suggests that the rapidity of SLE onset has additional prognostic information. There is great variability in the rapidity of SLE onset [41].
General population CVD risk stratification tools perform poorly in SLE, likely because SLE severity and medications influence risk [9, 41, 42]. Esdaile et al. found that the Framingham risk model vastly underestimates CVD risk in SLE [9] and Jafri et al. confirmed that both Framingham and 2013 American College of Cardiology/American Heart Association perform poorly in SLE as well [41]. Urowitz et al. attempted to address this by doubling Framingham estimates to improve performance, but results were still poor (sensitivity 32%; specificity 81%) [42]. A recent general population CVD prediction algorithm, the 2017 QRISK3, developed in a large general outpatient register in the UK, has for the first time included SLE as a CVD risk factor. However, again, there is a uniform doubling of risk for all SLE patients [43].
The lack of a well-calibrated CVD risk prediction algorithm that incorporates heterogeneity in disease severity creates uncertainty and anxiety for patients and providers in estimating individualized risk. Finely tuned SLE CVD risk stratification is essential to clinical care and to prevention studies, trial design and interpretation. Results of the current study may guide efforts to improve the prevention of CVD in newly diagnosed SLE patients and also provide rationale for ‘treat-to-target’ recommendations [44, 45]. Understanding how SLE severity affects health outcomes will allow improved risk stratification and possibly prevention strategies.
In this SLE Medicaid population, <50% of all patients were prescribed HCQ. This is consistent with previous work where we have documented poor access to care and poor adherence in the SLE Medicaid population for: infection prevention, adherence to indicated medications including HCQ, treatment for lupus nephritis and choices in renal replacement therapies [46]. For example, HCQ prescription rates were only 48% among incident lupus nephritis patients in our past studies, despite much higher rates of adverse outcomes than in academic cohort studies of SLE [47]. Additionally, our prior studies assessed HCQ prescriptions during the year following (rather than the year prior to) meeting our definition of SLE, so it is anticipated that prescription rates would be somewhat lower in the current studies.
Accelerated atherosclerosis and early age at CVD events among SLE patients are well recognized in academic studies [4]. The mean age of first CVD event in this cohort of Medicaid patients (46 years) was similar to, but slightly younger than, the mean age at first CVD event (49 years) in the SLICC Inception cohort, for example [42]. A cohort in Toronto also observed a mean age of 49 years at first MI [48]. Medicaid patients are low-income and likely have more severe disease and more comorbidities than privately insured patients. Additionally, the current study excluded patients ages >65 years because of potential dual enrolment with US Medicare coverage for the elderly, potentially reducing the mean age at CVD event somewhat.
Limitations of the current study include the potential for misclassification of diagnoses, medications and particularly comorbidities through ICD-9 codes. Additionally, it is acknowledged that there may have been incomplete or delayed reporting to the National Death Index and thus incomplete ascertainment of deaths. However, a past report comparing the Social Security Death Index to the National Death Index found that all deaths in the Social Security Death Index were also found in the National Death Index, and the National Death Index was more accurate on the date of death [46].
Laboratory values are also not readily in the Medicaid Analytic eXtract. The SLE severity algorithm we employed has the potential to include non-SLE related diseases, such as aortitis and pancreatitis for example. The algorithm also incorporates known predictors of CVD (stroke, thromboses, end-stage renal disease), which could inflate effect estimates when estimating future CVD risks. However, both after excluding those with baseline CVD and in analyses controlling for multiple known CVD comorbidities, initial SLE severity by this algorithm was still strongly associated with future CVD and mortality risks. Without cause of death data, we could not estimate mortality due to CVD and may have underestimated CVD events. Of those who died in follow-up, 113 of 529 (21.6%) had a preceding CVD event, and thus their death was likely to have been due to CVD. Additionally, our CVD risks estimates did not include CVD events without hospitalization, such as sudden cardiac deaths, which can occur without prior CVD hospitalization [47].
There are several strengths to our study. We had a specific scientific hypothesis focusing on patients with new-onset SLE, and we examined data from >15 000 incident SLE patients across a decade. Data were available on sociodemographic factors, SLE-related medications, and SLE- and CVD-related comorbidities, and we adjusted for potential confounders that might influence the risk of CVD events and all-cause mortality. We performed a rigorous code-by-code examination of an existing SLE severity classification algorithm and compared this algorithm to well established tools such as the Charlson comorbidity and Ward SLE-specific indices. We also performed several sensitivity analyses to further assess the validity of our primary analysis results.
In conclusion, we report that increased initial SLE severity classified using an administrative algorithm was associated with elevated CVD event risk and all-cause mortality. These findings point to the need for SLE-specific CVD and survival risk algorithms that incorporate SLE’s inherent heterogeneity. Clinicians should be aware that SLE severity has a great influence on CVD risk and thus a diagnosis of SLE in one person may carry very different implications for CVD risk than it does in the next person.
Funding: This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, K24 AR066109. The funders had no role in study design, data collection, analysis, decision to publish or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Disclosure statement: B.E. has received research support from the National Heart, Lung, and Blood Institute, Novartis and Roche Diagnostics, and has consulted for Roche Diagnostics, Amgen, Novartis, the Food and Drug Administration and the National Institute of Diabetes and Digestive and Kidney Diseases. K.H.C. has received grant funding from the National Institutes of Health (R01 AR057327 and K24 AR066109), the Lupus Foundation of America, Lupus Research Alliance, Merck, Astra-Zeneca and GlaxoSmithKline.
References
Distribution of the Nonelderly with Medicaid by Race/Ethnicity. Henry J Kaiser Family Foundation. https://www.kff.org (20 February 2019, date last accessed).
CMS Medicare-Medicaid Coordination Office. Data analysis brief: Medicare-Medicaid dual enrollment. Centers for Medicare & Medicaid Services 2016. https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-Coordination/Medicare-Medicaid-Coordination-Office/DataStatisticalResources/Downloads/Eleven-YearEver-EnrolledTrendsReport_2006-2016.pdf (2 July 2019, date last accessed).




Comments