-
PDF
- Split View
-
Views
-
Cite
Cite
Frej Tulin, Keeping quiet: cell cycle regulator PIKMIN1 helps maintain the quiescent center, Plant Physiology, Volume 191, Issue 3, March 2023, Pages 1468–1469, https://doi.org/10.1093/plphys/kiac597
Close - Share Icon Share
The decision between proliferation and differentiation is a carefully regulated process in the life of a cell. One of the major molecular gatekeepers of this decision is a large E3 ubiquitin ligase, ANAPHASE-PROMOTING COMPLEX/CYCLOSOME (APC/C) (Alfieri et al., 2017), found in all eukaryotes from humans to plants. The mechanistic basis for how the APC/C regulates the cell cycle is especially well understood in budding yeast (Saccharomyces cerevisiae). In this system, substrate recognition and degradation depend on two adaptor proteins, Cdc20 and Cdh1, with distinct but overlapping specificity. The Cdc20 adaptor is critical for exit from mitosis by promoting chromosome segregation and initiating degradation of mitotic cyclins. When cell division is complete, Cdh1 takes over and is responsible for keeping the level of mitosis-promoting proteins low. This prevents the cell from precocious entry into the next cell cycle and gives the cell time to respond to various developmental and environmental signals (Wäsch et al., 2010). Identification of APC/C substrates has been fundamental to our understanding of how the eukaryotic cell cycle is regulated (Thornton and Toczyski, 2003).
The plant homolog of Cdh1 is called CCS52 (CELL CYCLE SWITCH 52) and was identified in alfalfa (Medicago sativa) based on its elevated transcript level in developing root nodules, where the protein helps promote colonization by nitrogen-fixing bacteria (Cebolla et al., 1999). In two Arabidopsis (Arabidopsis thaliana) CCS52 family members, CCS52A1 and CCS52A2, are important for restraining cell division in the root transition zone and the quiescent center (QC), respectively (Vanstraelen et al., 2009). Previous studies have shown how CCS52A2 contributes to QC maintenance (Heyman et al., 2013), but the full repertoire of APC/CCCS52 substrates is still unknown. In this issue of Plant Physiology, Willems et al. present evidence that PIKMIN1 (PKN1), a previously uncharacterized, plant-specific protein, is an APC/CCCS52A2 substrate that regulates cell division rate in the root (Willems et al., 2022).
To identify PKN1, the authors looked for suppressors of the ccs52a2-1 mutant, which has a severely stunted root due to a distorted QC during early development, presumably due to overaccumulation of proteins normally targeted for destruction by the APC/CCCS52A2 complex. Overall root growth and QC morphology were noticeably improved in the ccs52a2-1 pkm1-1 double mutant compared to those in ccs52a2-1 alone during the first nine days of development (Figure 1). The authors then noted that PKN1 contains several short amino acid motifs (D-boxes and KEN-boxes) that are usually necessary for recognition by APC/CCCS52 complexes and that the protein was stabilized by treatment with a proteasome inhibitor. Moreover, the PKN1 promoter was active in several tissues containing dividing cells, such as shoot and root meristems, lateral root initiation sites, ovules, and embryos, suggesting a function in cell division throughout the plant. These observations are all consistent with PKN1 being an APC/C substrate that may accumulate to an unhealthy level in the root tip of plants with compromised CCS52A2 function.
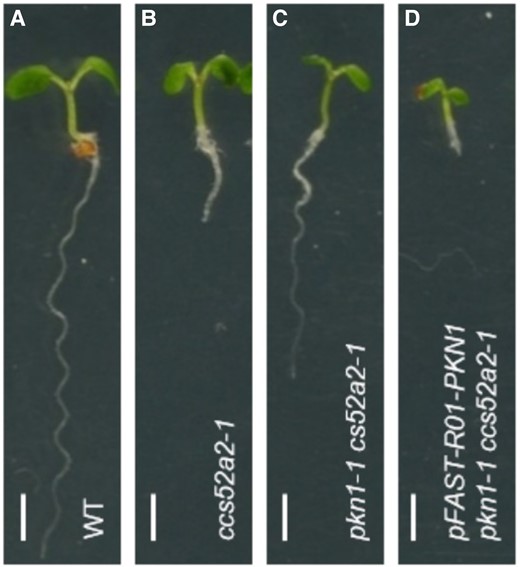
Suppression of the ccs52a2-1 mutation by pkn1-1: A, B Loss of the APC/C adapter CCS52A2 causes a short-root phenotype. C, The pkn1-1 mutation partially rescues root growth in ccs52a2-1 pants. D, The ccs52a2-1 mutant is sensitive to PKN1 overexpression. Scale bar: 1 mm.
How does PKN1 contribute to cell cycle control and development? Most likely by promoting proliferation in the root meristem. However, the loss of PKN1 function had no effect on QC organization, perhaps because CCS52A2 already maintains PKN1 at a low level there.
If a low level of PKN1 is important for proper QC development, then one might expect overexpression would cause a distorted QC phenotype similar to the one observed in the ccs52-a2 mutant. But this was not the case. PKN1 expression from a strong promoter affected neither root QC organization nor meristem size. To explain this, the authors suggested that the promoter they used may not be active in the relevant cell type (e.g. in the QC). Another possibility is that the level of ectopic PKN1 produced in the overexpression line is below some threshold capacity of the APC/C. Such threshold effects are common in the cell cycle, a highly non-linear system with multiple layers of feedback regulation. One example of this is the sudden lethality of yeast cells caused by a two-fold increase in mitotic cyclin Clb2 (Cross et al., 2005). When the level of Clb2 is below the threshold, the viability of the cells strictly depends on the normally non-essential Cdh1 (CCS52 homolog). Thus, yeast cells become hypersensitive to Clb2 overaccumulation in the absence of Cdh1. Perhaps, Arabidopsis roots would become hypersensitive to PKN1 in a similar fashion. This seemed to be the case since increased PKN1 dosage enhanced the short root phenotype of ccs52-a2 (Figure 1).
The results presented here by Willems et al. pave the way for many interesting future studies into the molecular basis of PKN1 function in cell division. One question is why PKN1 (in the absence of CCS52A2) causes defects in QC cell division patterns leading to the distorted QC phenotype described in the paper. One possibility is that PKN1 directly affects division plane orientation, which is plausible since PKN1 appeared to localize to mitotic microtubular arrays, such as the pre-prophase band and phragmoplast. Alternatively, distorted QC divisions may be a more indirect consequence of deregulated cell cycle progression. It will also be important to find out if the predicted D-boxes in PKN1 are actually required for APC/CCCS52A2-mediated degradation.
PKN1 joins a growing list of likely plant APC/C substrates (for a review, see the study by Willems and De Veylder, 2022), making it an important contribution to plant cell cycle research. Importantly, PKN1 homologs are found throughout land plants, and the authors showed that deletion of the liverwort (Marchantia polymorpha) homolog reduces the rate of cell proliferation, indicating that the function of PKN1 may be broadly conserved in the plant kingdom.
References
Author notes
Conflict of interest statement. None declared.



