-
PDF
- Split View
-
Views
-
Cite
Cite
Amy Lanctot, A lotus by any other color: Gene regulation divergence in lotus petal development, Plant Physiology, Volume 191, Issue 3, March 2023, Pages 1470–1472, https://doi.org/10.1093/plphys/kiac596
Close - Share Icon Share
Phenotypic diversity among individuals and species is largely generated by differences in gene expression. As changes in a gene's spatial or temporal expression profile are less likely to cause detrimental effects on fitness compared with changes in protein structure and function, such expression changes also often spur evolutionary innovation (Yocca and Edger, 2022). Divergence in gene expression can be caused by differences in the cis-regulatory sequence of a gene, which includes the non-coding regions of DNA to which transcriptional regulators bind and affect expression, as well as by differences in these trans-acting factors themselves. To what extent cis- and trans-regulatory divergences impact phenotype is still an open question in biology.
In this issue of Plant Physiology, Gao et al. (2022) investigate this question of cis- and trans-regulatory divergence in the context of lotus petal development. Using a global transcriptomic approach, the authors surveyed gene expression at four stages of petal development in two lotus species, Nelumbo nucifera and Nelumbo lutea, as well as in their F1 hybrid progeny. Notably, these two species have different color petals, providing a potential physical readout of gene expression differences (Figure 1A).
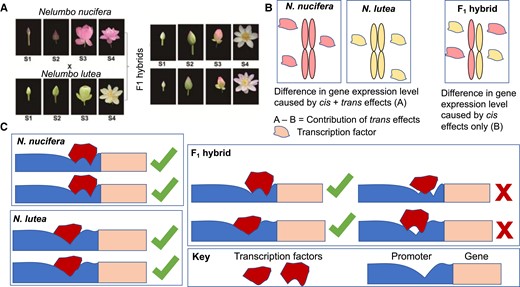
Comparative gene expression analyses in lotus reveal cis- and trans-regulatory divergences. A, Crossing scheme in this article: N. nucifera was crossed to N. lutea to generate two unique F1 hybrid lines. Gene expression was analyzed at four stages of petal development (S1–S4) in both parental genotypes and in both hybrid lines. B, Determination of cis- versus trans-regulatory gene expression divergence: For every gene, expression values were compared between the two parental lines (A) and between the two parental alleles within the F1 hybrid (B). Cis-regulatory changes and trans-regulatory changes contribute to the value (A), as both cis- and trans-regulation are diverged within species, whereas only cis-regulatory changes contribute to the value (B), as trans-regulation in the hybrid is shared. Consequently, the contribution of cis-regulation on gene expression can be quantified as (B), and the contribution of trans-regulation on gene expression can be quantified as (A, B). C, Potential mechanism by which transgressive gene expression can occur in F1 hybrids. In the parent species, cis- and trans-regulators have co-evolved to maintain their interactions with each other that regulate gene expression. In F1 hybrid lines, mutations that have accumulated in the parental alleles are mismatched between the species, preventing interaction between the cis-regulatory interactor of one species and the trans-regulatory interactor of the other species. This decreases the hybrid's capacity for transcriptional regulation, which can either decrease expression, in the case of activating interactions, or increase expression, in the case of repressing interactions. Figures adapted from Gao et al. (2022).
The authors classified the differential expression of genes (DEGs), both between the parental lines and between the parental alleles within the F1 hybrid using allele-specific expression (ASE) analyses (Signor and Nuzhdin, 2018). Differences in expression between the two parents, which the authors termed A, were caused by both cis- and trans-regulatory factors, which differed between the two species. Differences in gene expression between the parental alleles in the F1 hybrid, which the authors termed B, were caused only by cis-regulatory differences, as the trans-acting factors in the hybrid were shared (Figure 1B). Consequently, the cis-regulatory contribution to DEG is the value of expression difference in the hybrid (B), whereas the trans-regulatory contribution to DEG is the difference in the values of expression differences between the parents and the parental alleles in the hybrid (A and B). While cis-regulatory changes more commonly caused DEGs, trans-regulatory changes caused more severe changes, although this pattern is not maintained in other species (Metzger et al., 2016), suggesting that patterns of gene expression divergence are specific to the genetic context in which they arise.
The authors went on to characterize the modes of inheritance of different regulatory mutations. If the sum of the ASEs in the hybrid equaled the average of the alleles in the parents, inheritance was considered to be additive. The authors found that 80% of genes in the cis-regulatory category showed additive inheritance. If the expression in the hybrid corresponded to that of one parent, inheritance was dominant. For genes in the trans-regulatory category, 60% of the inheritance was dominant, and only 30% was additive. This pattern is in line with genetic theory, which posits that the effects of cis-regulatory changes are largely combinatorial unlike changes in trans-acting factors (McManus et al., 2010).
One of the most interesting categories of DEGs identified in this study were genes that showed transgressive expression in the F1 hybrid, that is, their expression levels in the hybrid differed from that in either parent. These genes mostly showed equivalent, that is, conserved, expression in the two parents, but some genes showed compensatory expression patterns, meaning that neither was their expression conserved between the parents nor was either parental allele's expression conserved in the hybrid. Such expression patterns can be explained by the evolution of both cis- and trans-regulatory sequences after the two species diverged—either mutations in a transcription factor binding site or the binding domain of the transcription factor that binds it prevents the interaction between cis- and trans-regulators in the hybrid (Figure 1C) (Payne and Wagner, 2019). This could cause either decreased gene expression in the hybrid, if this regulatory interaction originally activated transcription, or increased expression if the interaction originally repressed transcription. The authors found more instances where transgressive expression in the hybrid was decreased, suggesting that more activating interactions were affected by evolutionary divergence. An area of future research could explore whether this pattern is a general principle—that activating regulatory modules are more likely to be subject to developmental degradation and evolutionary drift than repressing modules. The cause of this developmental drift is also an interesting area of study, as many molecular mechanisms could underpin these patterns of evolutionary drift.
The authors also looked at the pleiotropic effects of these different regulatory mutations by analyzing which types of DEGs fell into the cis- and trans-only regulatory categories. Interestingly, the authors found a strong negative correlation between cis- and trans-effects, suggesting that these changes are occurring in different genes. They found that trans-regulated DEGs were enriched in “signaling” genes under higher pleiotropic constraints due to their multiple functions in development. These patterns of which genes are under cis- or trans-regulatory divergence could provide general rules as to which regulatory changes to expect for a given gene in a species-agnostic manner. Such predictability would be helpful as fine-tuning and modulation of gene expression changes are increasingly used targets in crop engineering. Trans-regulated DEGs also more frequently displayed broader expression patterns, another indication of pleiotropic function, and genes derived from whole-genome duplication events were also more frequently in the category of trans-regulated DEGs. Gene duplication can relieve pleiotropic constraints on the resulting paralogs because one paralog can preserve the original gene function, while the other copy can neo- or subfunctionalize. Finally, trans-regulatory DEGs were associated with genes with lower mutational frequency, again suggesting more stringent evolutionary constraints and pleiotropic pressure on these genes. These results also conformed with theoretical principles that posit that cis-regulatory divergence, as compared to trans-regulatory differences, often affects the expression of fewer genes in fewer tissues at fewer time points, allowing such genetic changes to circumvent pleiotropy (Prud’homme et al., 2007).
This comprehensive work takes advantage of the wealth of genetic tools available to analyze allele-specific gene expression to address multiple questions as to how cis- and trans-regulatory changes affect gene expression divergence across species. In the genomic era, our capacity for specificity in transcriptomic studies has hugely increased, with transcriptomes being produced at incredibly fine temporal and spatial scales. An underutilized tool in the genetics arsenal is the generation of hybrids, which allows for a level of dissection of interspecific gene expression divergence that is normally reserved for in-species analyses. Given the importance of hybrid breeding in agriculture (Labroo et al., 2021), such analyses have far-reaching implications. Gao et al. present a compelling example of such analyses, and this work illustrates the variety and depth of questions that can be addressed using such an approach.
Funding
No funding information for this article.
References
Author notes
Conflict of interest statement. None declared.



