-
PDF
- Split View
-
Views
-
Cite
Cite
Yannik Müllers, Johannes A Postma, Hendrik Poorter, Dagmar van Dusschoten, Stomatal conductance tracks soil-to-leaf hydraulic conductance in faba bean and maize during soil drying, Plant Physiology, Volume 190, Issue 4, December 2022, Pages 2279–2294, https://doi.org/10.1093/plphys/kiac422
Close - Share Icon Share
Abstract
Although regulation of stomatal conductance is widely assumed to be the most important plant response to soil drying, the picture is incomplete when hydraulic conductance from soil to the leaf, upstream of the stomata, is not considered. Here, we investigated to what extent soil drying reduces the conductance between soil and leaf, whether this reduction differs between species, how it affects stomatal regulation, and where in the hydraulic pathway it occurs. To this end, we noninvasively and continuously measured the total root water uptake rate, soil water potential, leaf water potential, and stomatal conductance of 4-week-old, pot-grown maize (Zea mays) and faba bean (Vicia faba) plants during 4 days of water restriction. In both species, the soil–plant conductance, excluding stomatal conductance, declined exponentially with soil drying and was reduced to 50% above a soil water potential of −0.1 MPa, which is far from the permanent wilting point. This loss of conductance has immediate consequences for leaf water potential and the associated stomatal regulation. Both stomatal conductance and soil–plant conductance declined at a higher rate in faba bean than in maize. Estimations of the water potential at the root surface and an incomplete recovery 22 h after rewatering indicate that the loss of conductance, at least partly, occurred inside the plants, for example, through root suberization or altered aquaporin gene expression. Our findings suggest that differences in the stomatal sensitivity among plant species are partly explained by the sensitivity of root hydraulic conductance to soil drying.
Introduction
To describe plant responses to soil drying, Feddes et al. (1978) proposed the concept of a water stress curve which still is the basis for most current root water uptake (RWU) models (Dos Santos et al., 2017). In this approach, a reduction factor of the transpiration rate is related to the soil water potential (Ψsoil; see Table 1 for abbreviations). Within a specific range of reducing Ψsoil, the transpiration rate is sustained implying a steadily reduced plant water potential to compensate for the reduced soil water potential. Below a critical Ψsoil, the transpiration rate is linearly reduced due to a partial closure of stomata avoiding a too strong decrease of the plant water potential. At the permanent wilting point, usually assumed to be −1.5 MPa, transpiration ceases. The exact shape of such a water stress curve depends on the extent of stomatal closure at a given level of soil drying. Variation of this stomatal sensitivity among species led to the classification in iso- and anisohydric species (Tardieu and Simmoneau, 1998), which is used to explain varying plant responses to soil drying (Pou et al., 2012; Sade et al., 2012; Hochberg et al., 2013; Attia et al., 2015). However, characterizing the hydraulic response of plants to soil drying by stomatal sensitivity only comes with limitations as stomatal control and leaf water potential regulation are not necessarily related when compared across various species (Martínez-Vilalta and Garcia-Forner, 2017).
| Term . | Meaning . | Unit . |
|---|---|---|
| gs | Stomatal conductance | mol H2O m−2 s−1 |
| h | Soil matric potential | cm |
| KRL | Hydraulic conductance between root and leaf | cm h−1 Mpa−1 |
| Ksat | Saturated soil hydraulic conductivity | cm s−1 |
| KSL | Hydraulic conductance between soil and leaf | cm h−1 Mpa−1 |
| KSR | Hydraulic conductance between bulk soil and root surface | cm h−1 Mpa−1 |
| Ksoil | Soil hydraulic conductivity | cm d−1 |
| L | Root length | m |
| A | Leaf area | cm2 |
| r0 | Root radius | cm |
| rb | Radial distance from the root center defining the start of the bulk soil | cm |
| RWU | RWU rate | mL h−1 |
| SWaP | Soil water profiler | |
| UP | Plant-driven RWU distribution with depth | mL cm−3 h−1 |
| Normalized plant-driven RWU distribution with depth | ||
| US | Soil driven RWU redistribution | mL cm−3 h−1 |
| Utot | Total RWU rate | mL h−1 |
| Zi | Depth of soil layer i | cm |
| Α | Inverse of the air entry pressure | cm−1 |
| θ | Volumetric soil water content | mL cm−3 |
| Soil water depletion rate | mL cm−3 h−1 | |
| λ | Rate constant of the exponential relation between KSL or gs and Ψseq | Mpa−1 |
| λb | Dimensionless pore size index of the Brooks–Corey model | |
| τ | Brooks–Corey parameter with τ = −2–3 λb | |
| φ | Matrix flux potential | cm2 s−1 |
| Ψseq | Equivalent water potential in the bulk soil | Mpa |
| Ψseq_50 | Equivalent soil water potential at which the conductance (KSL or gs) was reduced to 50% of its initial value | Mpa |
| Ψsoil | Water potential in the bulk soil | Mpa |
| Ψsr | Water potential at the soil–root interface | Mpa |
| Ψseq, sr | Equivalent water potential at the soil–root interface | Mpa |
| Term . | Meaning . | Unit . |
|---|---|---|
| gs | Stomatal conductance | mol H2O m−2 s−1 |
| h | Soil matric potential | cm |
| KRL | Hydraulic conductance between root and leaf | cm h−1 Mpa−1 |
| Ksat | Saturated soil hydraulic conductivity | cm s−1 |
| KSL | Hydraulic conductance between soil and leaf | cm h−1 Mpa−1 |
| KSR | Hydraulic conductance between bulk soil and root surface | cm h−1 Mpa−1 |
| Ksoil | Soil hydraulic conductivity | cm d−1 |
| L | Root length | m |
| A | Leaf area | cm2 |
| r0 | Root radius | cm |
| rb | Radial distance from the root center defining the start of the bulk soil | cm |
| RWU | RWU rate | mL h−1 |
| SWaP | Soil water profiler | |
| UP | Plant-driven RWU distribution with depth | mL cm−3 h−1 |
| Normalized plant-driven RWU distribution with depth | ||
| US | Soil driven RWU redistribution | mL cm−3 h−1 |
| Utot | Total RWU rate | mL h−1 |
| Zi | Depth of soil layer i | cm |
| Α | Inverse of the air entry pressure | cm−1 |
| θ | Volumetric soil water content | mL cm−3 |
| Soil water depletion rate | mL cm−3 h−1 | |
| λ | Rate constant of the exponential relation between KSL or gs and Ψseq | Mpa−1 |
| λb | Dimensionless pore size index of the Brooks–Corey model | |
| τ | Brooks–Corey parameter with τ = −2–3 λb | |
| φ | Matrix flux potential | cm2 s−1 |
| Ψseq | Equivalent water potential in the bulk soil | Mpa |
| Ψseq_50 | Equivalent soil water potential at which the conductance (KSL or gs) was reduced to 50% of its initial value | Mpa |
| Ψsoil | Water potential in the bulk soil | Mpa |
| Ψsr | Water potential at the soil–root interface | Mpa |
| Ψseq, sr | Equivalent water potential at the soil–root interface | Mpa |
| Term . | Meaning . | Unit . |
|---|---|---|
| gs | Stomatal conductance | mol H2O m−2 s−1 |
| h | Soil matric potential | cm |
| KRL | Hydraulic conductance between root and leaf | cm h−1 Mpa−1 |
| Ksat | Saturated soil hydraulic conductivity | cm s−1 |
| KSL | Hydraulic conductance between soil and leaf | cm h−1 Mpa−1 |
| KSR | Hydraulic conductance between bulk soil and root surface | cm h−1 Mpa−1 |
| Ksoil | Soil hydraulic conductivity | cm d−1 |
| L | Root length | m |
| A | Leaf area | cm2 |
| r0 | Root radius | cm |
| rb | Radial distance from the root center defining the start of the bulk soil | cm |
| RWU | RWU rate | mL h−1 |
| SWaP | Soil water profiler | |
| UP | Plant-driven RWU distribution with depth | mL cm−3 h−1 |
| Normalized plant-driven RWU distribution with depth | ||
| US | Soil driven RWU redistribution | mL cm−3 h−1 |
| Utot | Total RWU rate | mL h−1 |
| Zi | Depth of soil layer i | cm |
| Α | Inverse of the air entry pressure | cm−1 |
| θ | Volumetric soil water content | mL cm−3 |
| Soil water depletion rate | mL cm−3 h−1 | |
| λ | Rate constant of the exponential relation between KSL or gs and Ψseq | Mpa−1 |
| λb | Dimensionless pore size index of the Brooks–Corey model | |
| τ | Brooks–Corey parameter with τ = −2–3 λb | |
| φ | Matrix flux potential | cm2 s−1 |
| Ψseq | Equivalent water potential in the bulk soil | Mpa |
| Ψseq_50 | Equivalent soil water potential at which the conductance (KSL or gs) was reduced to 50% of its initial value | Mpa |
| Ψsoil | Water potential in the bulk soil | Mpa |
| Ψsr | Water potential at the soil–root interface | Mpa |
| Ψseq, sr | Equivalent water potential at the soil–root interface | Mpa |
| Term . | Meaning . | Unit . |
|---|---|---|
| gs | Stomatal conductance | mol H2O m−2 s−1 |
| h | Soil matric potential | cm |
| KRL | Hydraulic conductance between root and leaf | cm h−1 Mpa−1 |
| Ksat | Saturated soil hydraulic conductivity | cm s−1 |
| KSL | Hydraulic conductance between soil and leaf | cm h−1 Mpa−1 |
| KSR | Hydraulic conductance between bulk soil and root surface | cm h−1 Mpa−1 |
| Ksoil | Soil hydraulic conductivity | cm d−1 |
| L | Root length | m |
| A | Leaf area | cm2 |
| r0 | Root radius | cm |
| rb | Radial distance from the root center defining the start of the bulk soil | cm |
| RWU | RWU rate | mL h−1 |
| SWaP | Soil water profiler | |
| UP | Plant-driven RWU distribution with depth | mL cm−3 h−1 |
| Normalized plant-driven RWU distribution with depth | ||
| US | Soil driven RWU redistribution | mL cm−3 h−1 |
| Utot | Total RWU rate | mL h−1 |
| Zi | Depth of soil layer i | cm |
| Α | Inverse of the air entry pressure | cm−1 |
| θ | Volumetric soil water content | mL cm−3 |
| Soil water depletion rate | mL cm−3 h−1 | |
| λ | Rate constant of the exponential relation between KSL or gs and Ψseq | Mpa−1 |
| λb | Dimensionless pore size index of the Brooks–Corey model | |
| τ | Brooks–Corey parameter with τ = −2–3 λb | |
| φ | Matrix flux potential | cm2 s−1 |
| Ψseq | Equivalent water potential in the bulk soil | Mpa |
| Ψseq_50 | Equivalent soil water potential at which the conductance (KSL or gs) was reduced to 50% of its initial value | Mpa |
| Ψsoil | Water potential in the bulk soil | Mpa |
| Ψsr | Water potential at the soil–root interface | Mpa |
| Ψseq, sr | Equivalent water potential at the soil–root interface | Mpa |
One potential cause for these inconsistencies is that not only the stomatal conductance, but also the hydraulic conductance upstream toward the stomata, from soil to leaf (KSL) can be affected by soil drying. For each part of this pathway, namely leaves (Cochard, 2002; Ryu et al., 2016), stems (Cochard, 2006; Li et al., 2009), and the soil root system (Saliendra and Meinzer, 1989; Nobel and Cui, 1992; Sperry and Saliendra, 1994; Cochard et al., 1996; Bourbia et al., 2021), a loss of conductance at a reduced soil water potential could be demonstrated. Potential reasons are air gaps between soil and root (North and Nobel, 1997), altered root aquaporin gene expression (Vandeleur et al., 2009; Grondin et al., 2016), suberization of the root epi-, endo-, and exodermis (North and Nobel, 1991; Cruz et al., 1992), or xylem embolisms (Cochard, 2006; Ryu et al., 2016). Assessing the importance of a declining KSL for plant responses to soil drying requires data on characteristic parameters notably the decline rate, and the critical soil water potential at which the decline starts. Depending on these parameters a declining KSL might contribute to varying plant responses to soil drying among species and thus account for the reduced validity of stomatal sensitivity in this context (Martínez-Vilalta et al., 2014; Martínez-Vilalta and Garcia-Forner, 2017).
During soil drying, Ψseq, which depends on the soil water content, is reduced. According to Equation (1), this would lead to a drop in Ψleaf. A reduction of the stomatal conductance, and thus Utot, would dampen this drop whereas a reduction of KSL would increase it. In other words, a declining KSL during soil drying potentially triggers stomatal closure by amplifying the drop in Ψleaf. Evidence for such a coupling comes from two recent studies reporting a parallel decline of the soil–plant hydraulic conductance and the stomatal conductance during soil drying (Rodriguez-Dominguez and Brodribb, 2020; Bourbia et al., 2021). Another study proposed a strong water depletion zone around the roots to directly trigger stomatal closure (Carminati and Javaux, 2020).
Based on these findings, our study aims at answering the following questions:
Does soil drying cause a reduction in KSL?
Does the sensitivity of KSL to soil drying vary among species?
Does this variation partly account for the varying stomatal sensitivity among species?
Does the variation in KSL sensitivity occur in the soil or in the plant hydraulic pathway?
We hypothesize that the differences in the stomatal response to a reduced Ψseq between two species are associated with differences in the reduction of KSL. We tested this hypothesis for two species, faba bean (Vicia faba), a dicot, and maize (Zea mays), a monocot, with different root systems and water uptake rates per unit root length. Using a highly precise soil water sensor, we continuously scanned soil water profiles during several days of progressive soil drying and derived Ψseq and Utot. In combination with Ψleaf, which was measured with psychrometers, we could derive KSL and compare it with measurements of the stomatal conductance (Figure 1). To evaluate whether the conductance between bulk soil and root surface (KSR), or the conductance inside the plant, from root surface to leaf (KRL), caused the decline in KSL, we estimated the water potential at the root surface (Ψsr) using a model (van Lier et al., 2006, 2013; Carminati and Javaux, 2020).
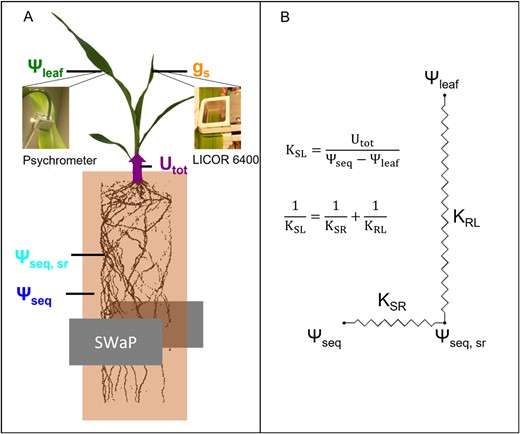
Experimental setup and hydraulic model used in this study. A, Scheme of the experimental setup and the different water potentials used for the data analysis. The equivalent water potential in the bulk soil (Ψseq), at the root surface (Ψseq, sr), and the total root water uptake rate (Utot) were derived from the SWaP measurements. The leaf water potential (Ψleaf) was measured with psychrometers, the stomatal conductance with a LICOR 6400. Root length was determined with MRI. B, Scheme of the hydraulic network from the bulk soil to the leaf. The overall conductance from bulk soil to leaf (KSL) can be separated into the conductance from bulk soil to the root surface (KSR) and from root surface to the leaf (KRL).
Results
On the first day, maize plants were significantly larger than faba bean plants regarding both leaf area (A, 1.5-fold) and root length (L, four-fold) (Table 2). Compared with maize, the smaller faba bean plants had significantly greater water uptake rates per leaf area (two-fold) and root length (six-fold). The total water uptake rate was also slightly (1.2-fold), albeit not significantly greater in faba bean. The hydraulic conductance between soil and leaf (KSL [mL h−1 MPa−1]) was similar for faba bean and maize. Assuming that in wet soil most of the hydraulic resistance occurs in the radial pathway of the roots, the radial root conductivity can be approximated by the ratio of KSL and L which was four times greater in faba bean compared with maize. As determined in a previous measurement, average root diameter was significantly greater in faba bean (0.04 cm) than in maize (0.02 cm).
Characteristic plant parameters at the beginning of the water restriction period as medians ± median absolute deviation among all measured replicates
| Parameter . | Faba bean . | Maize . |
|---|---|---|
| A (m2) | 0.036 ± 0.006*** | 0.063 ± 0.007 |
| L (m) | 41 ± 9*** | 162 ± 8 |
| Utot (mL h−1) | 5.0 ± 0.7 | 4.1 ± 0.9 |
| KSL (mL h−1 MPa−1) | 8.3 ± 1.2 | 7.8 ± 2.5 |
| UtotA−1 (mL h−1 m−2) | 138 ± 9*** | 64 ± 11 |
| UtotL−1 (mL h−1 m−1) | 0.119 ± 0.022*** | 0.022 ± 0.005 |
| KSLL−1 (mL h−1 MPa−1 m−1) | 0.20 ± 0.02*** | 0.05 ± 0.02 |
| Parameter . | Faba bean . | Maize . |
|---|---|---|
| A (m2) | 0.036 ± 0.006*** | 0.063 ± 0.007 |
| L (m) | 41 ± 9*** | 162 ± 8 |
| Utot (mL h−1) | 5.0 ± 0.7 | 4.1 ± 0.9 |
| KSL (mL h−1 MPa−1) | 8.3 ± 1.2 | 7.8 ± 2.5 |
| UtotA−1 (mL h−1 m−2) | 138 ± 9*** | 64 ± 11 |
| UtotL−1 (mL h−1 m−1) | 0.119 ± 0.022*** | 0.022 ± 0.005 |
| KSLL−1 (mL h−1 MPa−1 m−1) | 0.20 ± 0.02*** | 0.05 ± 0.02 |
Leaf area (A) and root length (L) were determined before the start of the measurements. Utot and KSL were averaged across the first day of measurement (four data points per light period, excluding data from the night) for each plant. Asterisks indicate significant difference between faba bean and maize. P-values were derived with a Mann–Whitney U test. P-values ˂0.05 are indicated as *, ˂0.01 as ** and ˂0.005 as ***.
Characteristic plant parameters at the beginning of the water restriction period as medians ± median absolute deviation among all measured replicates
| Parameter . | Faba bean . | Maize . |
|---|---|---|
| A (m2) | 0.036 ± 0.006*** | 0.063 ± 0.007 |
| L (m) | 41 ± 9*** | 162 ± 8 |
| Utot (mL h−1) | 5.0 ± 0.7 | 4.1 ± 0.9 |
| KSL (mL h−1 MPa−1) | 8.3 ± 1.2 | 7.8 ± 2.5 |
| UtotA−1 (mL h−1 m−2) | 138 ± 9*** | 64 ± 11 |
| UtotL−1 (mL h−1 m−1) | 0.119 ± 0.022*** | 0.022 ± 0.005 |
| KSLL−1 (mL h−1 MPa−1 m−1) | 0.20 ± 0.02*** | 0.05 ± 0.02 |
| Parameter . | Faba bean . | Maize . |
|---|---|---|
| A (m2) | 0.036 ± 0.006*** | 0.063 ± 0.007 |
| L (m) | 41 ± 9*** | 162 ± 8 |
| Utot (mL h−1) | 5.0 ± 0.7 | 4.1 ± 0.9 |
| KSL (mL h−1 MPa−1) | 8.3 ± 1.2 | 7.8 ± 2.5 |
| UtotA−1 (mL h−1 m−2) | 138 ± 9*** | 64 ± 11 |
| UtotL−1 (mL h−1 m−1) | 0.119 ± 0.022*** | 0.022 ± 0.005 |
| KSLL−1 (mL h−1 MPa−1 m−1) | 0.20 ± 0.02*** | 0.05 ± 0.02 |
Leaf area (A) and root length (L) were determined before the start of the measurements. Utot and KSL were averaged across the first day of measurement (four data points per light period, excluding data from the night) for each plant. Asterisks indicate significant difference between faba bean and maize. P-values were derived with a Mann–Whitney U test. P-values ˂0.05 are indicated as *, ˂0.01 as ** and ˂0.005 as ***.
Figure 2, A, C, and E, shows boxplots of Ψseq, Utot, and Ψleaf at selected time points on each day during soil drying for faba bean and maize. For Utot and Ψleaf each, one time point at low and one at high light were considered per day due to the strong light response of these two parameters. Figure 2, B, D, and F, shows the continuous time courses of the three parameters for one exemplary faba bean plant. The red vertical lines mark those time points selected for the boxplots. For faba bean, Ψseq decreased overall from −0.015 MPa on the first day to −0.12 MPa on the last day (Figure 2A). For maize, the reduction was lower, ranging from −0.015 to −0.06 MPa. The reduction of Ψseq mostly happened during the diurnal period, at a rate increasing from the first to the last day (Figure 2B). During the nights, Ψseq even increased slightly, resulting from a redistribution of soil water which Ψseq is sensitive to. Utot decreased in faba bean from the first day on (Figure 2C) from 5.7 (high light) and 4.7 mL h−1 (low light) to 2.2 and 1.8 mL h−1 on the last day. In maize, Utot was initially lower (4.7 mL h−1 at high light and 3.6 mL h−1 at low light) compared with faba bean and remained constant until the second day. From the third day on it also decreased to ultimately 3.4 and 2.8 mL h−1 and thus remained higher compared with faba bean. During the day, Utot alternated between a higher and a lower level in response to the two different light levels. The differences in Utot between the two light levels were lower in faba bean, especially during the last 2 days, indicating a reduced response to varying light (Figure 2D). During the night, Utot remained constant at a low level but never zero. Also, note that Utot at the first high light period of a day was similar to the last high light period of the previous day. This indicates that Utot declined during the day and not at night, and thus followed the dynamics of Ψseq. Ψleaf decreased gradually in faba bean (Figure 2E) from −0.7 (high light) and −0.6 MPa (low light) on the first day to −1.1 MPa and −1.0 MPa on the last day. In maize, the initial values were slightly higher (−0.6 MPa at high light and −0.5 at low light) compared with faba bean. During the following 2 days, Ψleaf only decreased slightly but more pronounced until the last day to −1.1 and −0.9 MPa. Like Utot, Ψleaf changed with the alternating light levels (Figure 2F). In contrast to Utot, however, Ψleaf did not remain constant but steadily increased during the nights. This steady increase was faster during the first compared with the last night. The first light period of a day, Ψleaf was similar (second day) or even higher (third and fourth day) compared with the last light period from the previous day.
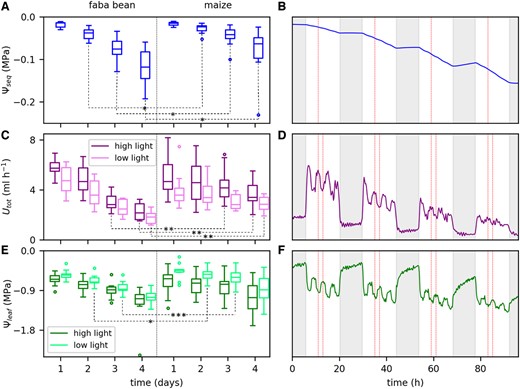
Reduction of the equivalent soil water potential (Ψseq), total root water uptake rate (Utot), and leaf water potential (Ψleaf) during 4 days of soil drying. A, C, and E, Data of all replicates (faba bean n = 12, maize n = 10) as boxplots at selected time points. Different colors in (C) and (E) refer to the two different light levels. Horizontal lines are medians, boxes reach from the first to the third quartile. Whiskers mark the minimal (lower whisker) and maximal (upper whisker) data points within 1.5 times the interquartile range from the first and third quartile, respectively. Circles are outliers beyond the whiskers. We tested for significant differences in ΨseqUtot, and Ψleaf between faba bean and maize at each selected time point. P-values were derived with a Mann–Whitney U test. P-values ˂0.05 are indicated by *, ˂0.01 by **, and ˂0.005 by ***. B, D, and F, The continuous time courses of the three parameters during the 4 days of soil drying for one example faba bean plant. Fluctuations of the observed parameters are caused by the fluctuating light intensity. Red dashed lines mark those points used for the boxplots. Shaded areas indicate the nonilluminated periods.
In a next step, we analyzed the effect of soil drying on Ψseq and the hydraulic conductance between soil and leaf, KSL. KSL was derived by rearranging Equation (1). Figure 3A shows an example KSL (black dots) of a faba bean as a function of Ψseq. For the analysis, we considered four measurement points per light period (28 points per day) excluding data measured at night. After a short, initial phase of increase, we observed an exponential decrease of KSL. Among all replicates, we found a Ψseq of −0.025 MPa as a consistent, critical point at which the exponential decline had started. For selected plants, we additionally measured the stomatal conductance gs with a portable LiCor 6400 photosynthesis system. During the 4 days of soil drying, gs (orange dots in Figure 3A) showed a similar dependence on Ψseq as KSL: it decreased exponentially below a Ψseq of −0.025 MPa. Note that gs was only measured once per light period, at least four times a day. We determined the rate constant λ of the decline by exponentially fitting the data starting from the critical Ψseq of −0.025 MPa: or . Note that Ψseq is negative and thus a positive λ implies a decline of KSL. Measured data on KSL and gs together with the exponential fit and the resulting λ are shown in Supplemental Figures S1 and S2 for each replicate separately. Both, KSL and gs, declined at a higher rate in faba bean compared with maize (Figure 3B). One maize replicate (bottom left panel in Supplemental Figure S2) had a very low initial Utot (2.5 mL h−1) leading to only moderate soil water depletion and thus a narrow range of Ψseq along which KSL and gs were fitted. This caused the strong outlier in Figure 3B. Excluding this outlier, the differences in λ for KSL between faba bean and maize were significant (P-value < 0.05, derived with a Mann–Whitney U test). Among faba bean replicates, λ was similar for KSL (14.5 MPa−1) and gs (16.6 MPa−1). Note that for KSL the median shown in Figure 3B was calculated among all 12 replicates. Stomatal conductance, however, was only measured for six of these replicates. Considering those six replicates only, the median of λ for KSL is 16.0 MPa−1, which was not significantly different from the λ of gs. In maize, λ for gs (4.7 MPa−1, Figure 3B) was lower than for KSL (9.5 MPa−1). Considering only replicates for which stomatal conductance was measured results in a λ of 7.8 MPa−1 for KSL. This was significantly higher (P-value < 0.05) than λ of gs when the outlier mentioned above was excluded.

KSL and gs drop exponentially during soil drying at higher rates in faba bean than in maize. A, Example data of KSL (black) and gs (orange) at reducing Ψseq for one faba bean plant. Per light period we considered four measured data points of KSL (black dots) and one measured data points of gs (orange dots). Dashed lines follow an exponential fit of the form or starting at a Ψseq of −0.025 MPa. Vertical dotted lines mark the values of Ψseq at which KSL or gs were reduced to 50% of their initial values at a Ψseq of −0.025 MPa. B, Variation of the rate constants λ obtained from the exponential fits of KSL and gs among faba bean and maize replicates. λ is a measure for the sensitivity of the conductance to soil drying. Characteristics of the boxplots are similar to Figure 2, A, C, and E. Numbers on the boxes indicate the median values. The inserted figure includes all outliers which are only partly shown in the main panel. gs was only measured for six replicates while KSL was measured for 12 (faba bean) and 10 (maize) replicates. Asterisks indicate significant differences (*P-value < 0.05, **P-value < 0.01, ***P-value < 0.005) between faba bean and maize, tested with a Mann–Whitney U test.
For faba bean, KSL and gs were both reduced by 50% at a Ψseq of −0.07 MPa compared with the initial value at a Ψseq of −0.025 MPa (Table 3). Maize with generally lower λs, showed lower (more negative) values: Ψseq_50 was −0.10 MPa for KSL and −0.13 MPa for gs.
Equivalent soil water potential at which KSL and gs were reduced to 50% of its initial value
| Parameter . | Faba bean . | Maize . |
|---|---|---|
| Ψseq_50 (MPa) for KSL | −0.07 ± 0.01 | −0.10 ± 0.03 |
| Ψseq_50 (MPa) for gs | −0.07 ± 0.02 | −0.13 ± 0.08 |
| Parameter . | Faba bean . | Maize . |
|---|---|---|
| Ψseq_50 (MPa) for KSL | −0.07 ± 0.01 | −0.10 ± 0.03 |
| Ψseq_50 (MPa) for gs | −0.07 ± 0.02 | −0.13 ± 0.08 |
Note that “initial” refers to the start of the exponential decay at a Ψseq of −0.025 MPa. Values are medians ± median absolute deviations.
Equivalent soil water potential at which KSL and gs were reduced to 50% of its initial value
| Parameter . | Faba bean . | Maize . |
|---|---|---|
| Ψseq_50 (MPa) for KSL | −0.07 ± 0.01 | −0.10 ± 0.03 |
| Ψseq_50 (MPa) for gs | −0.07 ± 0.02 | −0.13 ± 0.08 |
| Parameter . | Faba bean . | Maize . |
|---|---|---|
| Ψseq_50 (MPa) for KSL | −0.07 ± 0.01 | −0.10 ± 0.03 |
| Ψseq_50 (MPa) for gs | −0.07 ± 0.02 | −0.13 ± 0.08 |
Note that “initial” refers to the start of the exponential decay at a Ψseq of −0.025 MPa. Values are medians ± median absolute deviations.
To analyze how KSL behaves on a daily scale, we determined KSL as the slope of the relation between Utot and Ψleaf at morning, afternoon, and evening separately (Supplemental Figure S3A). For faba bean, KSL declined continuously during the day and was significantly lower in the evening compared with the morning on each day (Supplemental Figure S3B). However, each morning, KSL tended to be greater than on the previous evening, consistent with the increasing Ψseq during the nights (Figure 2B). For maize, we also observed consistently lower KSL in the evening compared with the morning of the same day, but on some days KSL slightly increased from morning to afternoon or from afternoon to evening.
The hydraulic pathway from bulk soil to the leaf can be separated into a soil part (from bulk soil to the root surface) and a plant part (from root surface to the leaf). Here, we want to estimate whether the observed reduction in KSL mostly happened in the soil or in the plant pathway. Deriving the hydraulic conductance of each part separately requires the water potential at the root surface Ψseq, sr to be known. Ψseq, sr can differ from Ψseq (referring to the bulk soil) due to a water depletion zone around the roots which can be estimated using a model (Carminati and Javaux, 2020). We estimated Ψseq, sr for two different scenarios: (1) the full root length is actively involved in water uptake. (2) Only 50% of the root length takes up water. Figure 4 shows an example calculated Ψseq, sr in comparison to Ψseq for one faba bean (Figure 4A) and one maize plant (Figure 4B). Generally, the difference between Ψseq, sr and Ψseq increases with increasing water uptake rates per unit root length and decreasing Ψseq. For faba bean, considering 100% root length (cyan), there was only a marginal difference between Ψseq, sr and Ψseq (black 1:1 line) (Figure 4A). Conservatively assuming that only 50% of the root length is active in water uptake led to a generally lower Ψseq, sr (blue). In this scenario, Ψseq, sr showed some fluctuations at the end of the measurement (Figure 4A) due to the dependency on the transpiration rate and thus the alternating light intensity. The difference between Ψseq, sr and Ψseq was still low (˂0.02 MPa) until a Ψseq of −0.10 MPa. At the end of the measurement, Ψseq, sr was 0.05 MPa lower than Ψseq. For maize, in both scenarios, the differences between Ψseq, sr and Ψseq were negligible.
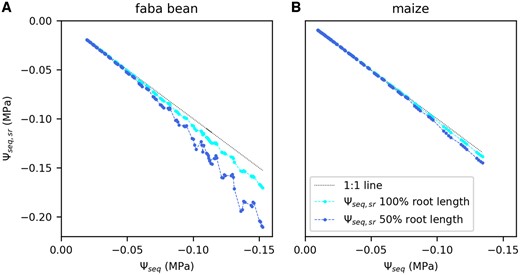
Estimated water potential at the root surface (Ψseq, sr) as a function of the bulk soil water potential (Ψseq). Data are an example shown for one faba bean (A) and one maize plant (B). Water potential at the root surface was calculated for the full measured root length and half of the measured root length. Dotted line is the 1:1 line.
Using the calculated Ψseq, sr, we could derive the conductance between bulk soil and root surface and between root surface and leaf (KRL) separately. For this, we divided the total water uptake rate by the water potential difference between bulk soil and root surface and root surface and leaf, respectively. Since the estimation of Ψseq, sr does not account for a loss of soil–root contact, the conductance of the interface between soil and root is included in KRL. In the following, we compare KRL to the overall conductance KSL. For one faba bean replicate, Figure 5A shows an example KSL (black, half-filled circles) and KRL for 100% root length (cyan, half-filled circles) and 50% root length (blue, nonfilled circles). Over a broad range of Ψseq, KSL and KRL were almost identical, both showing the above-described exponential decay. Only below a Ψseq of −0.18 MPa, KRL (50% root length) remained considerably higher than KSL and even increased slightly. Note that at this point, KSL was already reduced by 75%. Analogously to KSL we quantified the decay of KRL by determining the decay rate λ of an exponential fit. λ of KRL was close to that of KSL in all crops and simulated scenarios (Figure 5B).
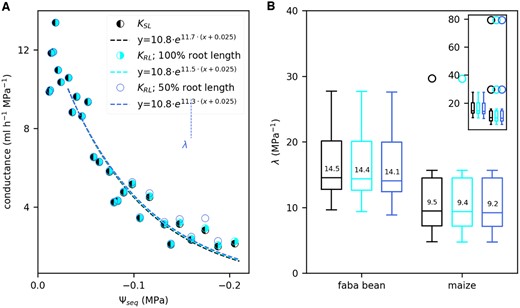
Comparison between the hydraulic conductance between root surface and leaf (KRL) and KSL throughout the soil drying period. A, KRL compared with KSL, as a function of Ψseq, example shown for one faba bean plant (same plant as in Figure 3A). KRL was determined using the calculated water potential at the root surface for the full root length (cyan) or half of the root length (blue). We exponentially fitted the data (dashed lines) to determine the rate constants λ starting at a Ψseq of −0.025 MPa. For reasons of clarity, we plotted only one data point per light period here but derived the exponential fit using four data points per light period, analogously to Figure 3. B, Boxplots of the rate constants λ obtained from the exponential fits of KSL (black, same data as in Figure 3B) and KRL considering the full root length (cyan) or half of the full root length (blue) for faba bean (n = 12) and maize (n = 10). Characteristics of the boxplots are similar to Figure 2, A, C, and E. The main panel does not include all outliers which are shown in the inserted figure.
For this scenario, KRL was assumed to be constant and approximated by the initial KSL at Ψseq = −0.025 MPa. The calculation shows that if KRL was constant, Ψseq, sr would need to decrease ˂−0.6 MPa at a Ψseq of −0.1 MPa and ˂−1.2 MPa at a Ψseq of −0.2 MPa to account for the reduction in KSL. Then, we tested how close the estimated Ψseq, sr, using the water depletion model, could get to this hypothetical line by considering only a reduced fraction of root length or root diameter (Supplemental Figure S4). For none of the tested fractions, the estimated Ψseq, sr was comparable to the theoretical one at constant KRL either in terms of amplitude or shape of the decline. This analysis supports our conclusion that most of the decline in KSL did not occur between bulk soil and root surface.
We compared the root architectures between faba bean and maize to further elucidate the different responses to soil drying between the species. As an example shown in Supplemental Figure S5A, faba bean had a greater fraction of root length in the top 10 cm while in maize the fraction ˂30 cm was greater. For quantification, we determined the depth D50, at which 50% of the total root length was reached, which was significantly deeper in maize (20 cm, Supplemental Figure S5D) than in faba bean (12 cm). The resulting pattern of water uptake rates () was initially contrasting, with a greater fraction of water uptake in shallow layers for maize compared with faba bean (Supplemental Figure S5B). This changed toward the last day of measurement when the fraction of ˂30 cm was greater in maize than in faba bean (Supplemental Figure S5C). These observations were confirmed by the D_50 of , which was deeper in faba bean for the first day but shifted to a significantly deeper layer in maize for the last day (Supplemental Figure S5D).
After 4 days of soil drying, we rewatered four of the faba bean plants to analyze how the measured parameters would recover. The example in Figure 6 shows the data for one faba bean plant. Within 30 min after rewatering, Ψseq increased from −0.14 to −0.01 MPa (Figure 6A) which is comparable to the initial value on the first day. Within 1 h after rewatering, Ψleaf increased from −1.3 to −0.8 MPa (Figure 6B) which was only slightly lower compared with the initial Ψleaf of −0.7 MPa. During the next 20 h, Ψleaf further increased resulting in values of −0.6 MPa which is even lower compared with the start of the measurement. In contrast, the recovery of Utot was slower (Figure 6C): 5 h after rewatering, Utot at high light level was similar to the high light level before rewatering (around 1.9 mL h−1). Four hours later, Utot had increased to 3.1 mL h−1. During the following night, Utot further increased steadily and reached around 4.0 mL h−1 on the next morning (21 h after rewatering) which is around 40% lower compared with the initial values on the first day (6.5 mL h−1). Compared with the value at a Ψseq of −0.025 (horizontal dotted line in Figure 6D), KSL had declined to around 17% before rewatering (Figure 6D). Upon rewatering, KSL steadily increased, up to 50% of the initial value after 8 h. The next morning, within 20 h after rewatering, KSL had recovered to around 85%. For the other three rewatered faba bean plants, measurements were taken only up to 5.5 h after rewatering. Nevertheless, trends were similar (Supplemental Figure S6, A–F): Ψleaf reached the initial value within several hours after rewatering while Utot recovered much slower. Data from a separate experiment on two 6-weeks-old faba bean plants indicate that full recovery of Utot took 40 h after rewatering (Supplemental Figure S6, G–H).
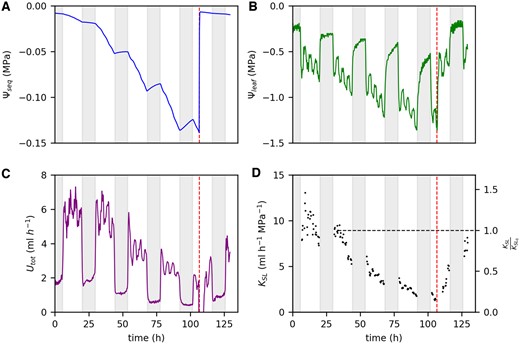
Recovery of different hydraulic parameters upon rewatering after 4 days of water restriction for one faba bean plant. Parameters are Ψseq (A), Ψleaf (B), Utot (C), and KSL (D). Vertical dashed lines mark the time point of rewatering. Horizontal dashed line in (D) marks the initial value of KSL at a Ψseq of −0.025 MPa (KSL,0). Nights are indicated by the shaded areas.
Discussion
The hydraulic conductance between soil and leaf declined exponentially with reducing soil water potential. In faba bean, this decline occurred at a higher rate than in maize which partly explains that faba bean closed its stomata more quickly. Estimations of the water potential at the root surface suggest that not only the soil conductance but also the conductance between root surface and leaf dropped. This was supported by an incomplete recovery, 22 h after rewatering.
We analyzed to what extent the hydraulic conductance between soil and leaf (KSL) is affected by soil drying. For that we continuously and noninvasively measured the total root water uptake rate (Utot) and the equivalent water potential in the bulk soil (Ψseq) with the soil water profiler (SWaP) and the leaf water potential (Ψleaf) on faba bean and maize during 4 days of soil drying. The initial conductance at the beginning of soil drying was higher in faba bean (8.3 mL h−1 MPa−1, or 3.5 mmol m−2 s−1 MPa−1 when normalized by leaf area) than in maize (7.8 mL h−1 MPa−1 or 1.9 mmol m−2 s−1 MPa−1). These values fit in the range reported in the literature for lupine (Lupinus albus L.) (13.7 mL h−1 MPa−1; Hayat et al., 2019) Tanacetum cinerariifolium and Callitris rhomboidea (both around 4.5 mmol m−2 s−1 MPa−1; Bourbia et al., 2021) or olive (Olea europaea L. var. arbequina) (0.7 mmol m−2 s−1 MPa−1; Rodriguez-Dominguez and Brodribb, 2020). Among replicates, KSL consistently declined exponentially as a function of the equivalent soil water potential starting at a Ψseq of −0.025 MPa (Figure 3A). We determined the rate constant λ of this decline by exponentially fitting KSL (Ψseq). The rate constant is a measure for how sensitive the conductance is to soil drying: A high λ reflects a fast decline of the conductance and thus high sensitivity to a reducing Ψseq. Among faba bean replicates we found a λ of 15 MPa−1. This is equivalent to 50% reduction of KSL within a range of Ψseq from −0.025 to −0.07 MPa. In maize, λ was lower (10 MPa−1) and KSL dropped to 50% only at a Ψseq of −0.10 MPa. These high decline rates are probably caused by the sandy soil substrate. Hayat et al. (2019) reported similar values for lupine plants in a sandy soil (90% loss of conductance at a Ψseq of −0.17 MPa). A study among different sugarcane (Saccharum spp. hybrid) cultivars reports an even faster decline, with an almost complete loss of conductance before Ψsoil reached −0.1 MPa (Saliendra and Meinzer, 1989). On the other hand, slower declines have been observed as well, ranging from a 50% loss of conductance below a Ψsoil of −1.0 MPa in T. cinerariifolium and Callitris rhomboidei (Bourbia et al., 2021) to a 30%–60% loss only below a Ψsoil of −10 MPa in desert succulents (Nobel and Cui, 1992). The rate constants observed in our study highlight the impact of the KSL decline on the plant response to soil drying: For faba bean, sustaining the initial transpiration rate, and thus stomatal opening, would lead to a drop in Ψleaf as little as −0.045 MPa to compensate the reduced Ψseq from −0.025 to −0.07 MPa. Due to the 50% reduction of KSL, however, keeping the stomata open would result in a much greater drop in Ψleaf by −0.6 MPa (see Equation (1)). Given these values, it is highly likely that the drop of KSL affects stomatal conductance. Note that for the considerations described above, we referred to the water potential in the bulk soil. Whether a drop of the water potential around the roots accounted for the observed drop in KSL is discussed further below.
The stomatal conductance (gs), measured with a portable LiCor 6400 photosynthesis system for selected plants, also declined exponentially starting at a Ψseq of −0.025 MPa (Figure 3A). Again, we quantified the exponential decay by determining the rate constant λ. In the case of gs, λ is an estimate for how strong the stomata respond to soil drying. A higher λ indicates a higher reduction of the stomatal conductance at a given Ψseq, independent of how fast Ψseq was reduced. Like for KSL, the decline rate for gs was lower in maize compared with faba bean. This can be interpreted as follows: at a given level of soil drying, measured as Ψseq, faba bean experienced a stronger reduction of water availability than maize due to the stronger decline of the hydraulic conductance between bulk soil and leaf. This led to a stronger reduction of the stomatal conductance. The exact mechanism which couples KSL and gs is still unclear. Different, nonhydraulic signaling cascades such as enhanced abscisic acid biosynthesis (Liang et al., 1997), reduced cytokinin supply (Blackman and Davies, 1985), or suppressed stringolactone biosynthesis (Visentin et al., 2016) have been suggested to propagate from dehydrated roots to the shoot and initiate stomatal closure. In this case, KSL and gs would be linked. A recent review, however, concludes that most of the stomatal regulation happens hydraulically via the leaf water potential (Buckley, 2019). We, therefore, postulate that KSL and gs are indirectly linked by the balancing of Ψleaf and the transpiration rate (Equation (1)) although our data do not allow a clear distinction between transpiration rate and gs. Both were closely linked since the VPD was kept constant by the climate chamber control and water-cooling of the LED panel. Irrespective of the above, our data suggest that the variation of stomatal sensitivity among species can partly be attributed to a variation of the KSL sensitivity. Potential causes for the variation of the KSL sensitivity among species are discussed below. However, while in faba bean, KSL and gs, in agreement with several recent studies (Rodriguez-Dominguez and Brodribb, 2020; Abdalla et al., 2021; Bourbia et al., 2021), declined almost in parallel, in maize gs declined at a 50% lower rate than KSL (Figure 3B). This indicates that in addition to its dependence on KSL, stomatal sensitivity is partly a species-inherent trait as commonly assumed (Tardieu and Simmoneau, 1998; Klein, 2014).
We used Ψseq as a measure for the extent of soil drying that the plant is exposed to. Ψseq is the distribution of soil water potential weighted by the distribution of root conductance () which is directly linked to the root distribution. During the 4 days of soil drying, Ψseq was generally lower in faba bean than in maize, especially on the last 2 days (Figure 1A). Since the total water uptake rates were comparable or, during the last 2 days even greater in maize (Figure 1C), the differences in Ψseq are most likely explained by the different root architectures between the two species: maize had a higher fraction of deep roots than faba bean (Supplemental Figure S5, A and D) and thus was able to acquire a higher fraction of water from deeper layers at the later stages of the experiment (Supplemental Figure S5, C and D). Since the soil water potential usually is less negative in those deeper layers, the weighted Ψseq was less negative in maize than in faba bean. Note that by using Ψseq, we account for the effect of root distribution on KSL which is not case when the more common average soil water potential or soil water content are used as a measure for soil drying.
Explaining the different rate constants between faba bean and maize starts with determining which part of the hydraulic pathway between bulk soil and leaf caused the observed drop in KSL. Recently, a water depletion zone around the roots has been proposed to account for a major loss of hydraulic conductance from soil to plant which could initiate stomatal closure (Carminati and Javaux, 2020). The water depletion zone would cause a much lower water potential at the root surface Ψseq, sr than in the bulk soil Ψseq. Based on a model by Carminati and Javaux (2020), Ψseq, sr can be calculated for given soil hydraulic properties. The difference between Ψseq, sr and Ψseq increases with increasing water uptake rate per root length, decreasing root radius, and decreasing Ψseq. We observed a steeper decline of Ψseq, sr with Ψseq in faba bean than in maize. Since the total water uptake rates were comparable between the two species, the differences in Ψseq, sr are caused by differences in the total root length and the average root diameter. Faba bean had a greater average root radius but a smaller total root length than maize (Table 2) which is typical since dicots usually have a lower specific root length than monocots (Read et al., 2010). As demonstrated in Supplemental Figure S4, root length had a stronger impact on the estimated Ψseq, sr than diameter. Therefore, the steeper decline of Ψseq, sr in faba bean is explained by the much lower total root length compared with maize. Nevertheless, the calculated difference between Ψseq, sr and Ψseq (Figure 4) and thus the impact on the decline of KSL was marginal for both faba bean and maize (Figure 5). In another study, the drop of Ψseq, sr could explain experimental data on the loss of soil–plant conductance when only 0.7%–2.5% of the measured root length was considered in the water uptake process (Hayat et al., 2020). From the magnetic resonance imaging (MRI) images, we know that parts of the root system were close to the pot borders in our experiment. This might have limited the access to soil water leading to a reduction of the active root length, however, not to such a drastic extent. Nevertheless, we tested the effect of halving the measured root length which resulted in a considerably lower Ψseq, sr for faba bean at the end of the measurements (Figure 4) but had no remarkable impact on KSL (Figure 5). This indicates that even though the soil conductivity around the roots drops sharply (Supplemental Figure S7) at the measured soil water regimes, it stays considerably greater than KRL. Therefore, our data suggest that most of the KSL decline did not occur in the soil zone close to the roots. Note that we used a total root length and average root radius for the estimation of Ψseq, sr and did not consider the spatial root distributions. Nevertheless, our analysis shows that even though we probably overestimate local root length densities or root radius, the model estimations of Ψseq, sr are far from explaining the decline in KSL (Supplemental Figure S4).
To further clarify whether the drop of KSL occurred inside or outside the plant, we need to consider a potential loss of the soil–root contact. Dehydration of root tissue in drying soils can lead to root shrinkage and thus enhance the reduction of the soil–root contact (Nobel and Cui, 1992; North and Nobel, 1997; Carminati et al., 2009). The estimation of Ψseq, sr does not account for a reduced root soil contact. Therefore, the estimated decline of KRL (Figure 5) includes the decreasing conductance resulting from a potential shrinkage of roots. Rodriguez-Dominguez and Brodribb (2020) observed a reduced conductance of the soil–root interface, attributed to root shrinkage, to mainly cause a 74% drop in the overall plant conductance during soil drying. Compared with our study, this drop is in the same order of magnitude but occurred at a much lower water potential (Ψstem between −1.0 and −4.0 MPa (Rodriguez-Dominguez and Brodribb, 2020) compared with Ψleaf between −0.6 and −1.1 MPa in our study (Figure 2)). However, X-ray CT studies on lupin (Carminati et al., 2013), faba bean (Koebernick et al., 2018), and maize (Duddek et al., 2022) revealed substantial root shrinkage leading to air gaps between soil and root starting already at a relatively high Ψsoil of −0.01 to −0.02 MPa. This is comparable to the critical Ψseq of −0.025 MPa at which the decline of KSL started in our study. Carminati et al. (2013) suggested that a slightly reduced soil conductivity led to the initial dehydration and shrinkage of the roots, which then, in a self-enhancing process, would cause an additional drop of the conductance and thus additional root shrinkage. In turn, the temporary recovery of KSL that we observed during the night (Supplemental Figure S3) could result from root rehydration and thus a recovery of soil–root contact. This would fit the observation of a diurnal variation of root diameter with shrinkage during the day, and swelling during the night (Huck et al., 1970). However, in faba bean, root shrinkage was shown to be almost fully reversed within 3 h after rewatering (Koebernick et al., 2018). In our study, the KSL of faba bean had not fully recovered within 20 h after rewatering (Figure 6D and Supplemental Figure S6) while Ψseq was almost fully recovered within 30 min (Figure 6A). This suggests that a reduction of the conductance between bulk soil and root surface was not the only reason for the reduced KSL.
It is, therefore, likely that the drop of KSL partly occurred inside the plant including the radial pathway from root surface into the root xylem and the axial pathway from root xylem into the leaf. Root aquaporin activity has been shown to modulate the loss of root hydraulic conductance during soil drying for various species (Martre et al., 2001; Aroca et al., 2006; Galmés et al., 2007; Perrone et al., 2012; Grondin et al., 2016; Rodríguez-Gamir et al., 2019). Other studies report a close linkage between a reduced radial root conductivity and suberization of the root endodermis (Cruz et al., 1992; Lo Gullo et al., 1998) or lacunae formation in the tissue of fine roots (Cuneo et al., 2016) or both (North and Nobel, 1991). Whereas the aquaporin contribution to root conductance was shown to fully recover within 5 h after rewatering (Rodríguez-Gamir et al., 2019), lacunae formation (Cuneo et al., 2016) and suberization (Lo Gullo et al., 1998) are permanent and require growth of new roots to restore KSL. This could explain the incomplete recovery of KSL 20 h after rewatering (Figure 6D) in our study. The extent of reduced conductance caused by changes in gene expression and root morphology varies among species. This is highlighted by two grapevine cultivars, for which differences in the reduction of root hydraulic conductance could be assigned to a difference in aquaporin expression during drought (Vandeleur et al., 2009). Another study on two different grapevine rootstocks revealed a stronger decline of root hydraulic conductance to correspond to a faster suberization (Barrios-Masias et al., 2015). If such variations occur even within the same species, it is likely that the different rate constants of KSL between faba bean and maize in our study are partly caused by differences in root morphological changes upon soil drying.
Although xylem embolism is predominantly observed in trees below a stem water potential of −2.0 MPa (Cochard, 2006), some studies suggest that it also needs to be considered in crops: in maize, xylem embolism was shown to cause a 25% loss of conductance in leaves at a Ψsoil of −0.25 MPa (Ryu et al., 2016) and 23% loss of conductance in stems at a Ψstem of −1.0 MPa (Li et al., 2009). Another study on maize leaves, however, shows that the conductance loss due to xylem embolism is less than 15% until a Ψstem of −1.5 MPa is reached (Cochard, 2002). Since in our experiments, Ψleaf mostly remained >−1.2 MPa (Figure 2E) and the decline of KSL started at a Ψseq of −0.025 MPa, we conclude that a reduced xylem conductance due to embolism did not cause the decline in KSL.
In summary, our estimations of Ψseq, sr and the slow recovery after rewatering indicate that the decrease in KSL partly occurred in the hydraulic pathway between root surface and leaf, and thus inside the plant. This should not obscure the fact that a reduction in the soil water potential around the roots with, however only a marginal effect on KSL, is likely to be the initial cause leading to a decreasing plant hydraulic conductance. The greater extent of water depletion around faba bean roots compared with maize roots at a given Ψseq (Figure 4) could have led to a stronger response of KSL in faba bean. From this perspective, the significant differences in root length densities (Table 2) might be the crucial factor to explain the differences of the decline in KSL, and ultimately in gs, between the two species. Additionally, faba bean generally had a greater initial stomatal conductance than maize (Supplemental Figures S1 and S2) which also contributed to the initially greater water uptake rates per unit root length (Table 2). The resulting dehydration of the root tissue could then have triggered a decrease of radial root conductance by alterations in the gene expression (aquaporins) or in root morphology (suberization) which amplifies the reduction of KSL. But why would the plant initiate such a drastic decline of KSL when the water supply toward the roots is only moderately constrained? As suggested by Carminati et al. (2020), stomatal closure at an early stage of water stress can prevent a severe drop of the water potential around the roots. Since leaf water status seems to be the principal factor regulating stomatal conductance (Buckley, 2019), we speculate that the decrease in KSL is a mechanism to force stomatal closure by enhancing the drop in Ψleaf. Such a strategy would amplify the initial water stress but could be beneficial in the long-term by avoiding a steep water potential gradient toward the root surface. In addition, the decrease of KSL in roots in drier soil layers could be accompanied by root growth in wetter soil layers. This would lead to lower water uptake rates per unit root length and thus help to avoid excessive rhizosphere drying. However, it should be kept in mind that due to the potential loss of soil–root contact, it remains difficult to evaluate to what extent the plant is in control of the KSL decline. Despite this speculative aspect, our study clearly highlights that how a plant responds to water stress, strongly depends on how sensitive KSL is to soil drying. This has different implications on the widely used Feddes model (Feddes et al., 1978; Feddes et al., 2001), at least when plants grow in a loamy sand:
The range of Ψseq at which the transpiration rate is sustained is very narrow because KSL starts already declining at Ψseq = −0.025 MPa.
The slope of the declining part of the water stress curve is not expected to be constant anymore since it depends on KSL (Couvreur et al., 2015).
The point at which transpiration reaches zero is much higher (less negative) than the permanent wilting point of −1.5 MPa.
The extent of reduced water availability at a given Ψseq is not constant anymore but varies among species due to a varying KSL sensitivity to soil drying.
To obtain a more precise description of plant responses to soil drying, the reduction of KSL should be taken into account when calculating water stress curves. This could be realized by the rate constant λ of the declining KSL(Ψseq) which, however, varies among species.
Conclusion
We observed a strong reduction of the hydraulic conductance between soil and leaf, KSL, at even moderately low soil water potentials. This implies that the main hydraulic impairment during soil drying does not only result from the reduced soil water potential but from the reduced hydraulic conductance between soil and leaves. Therefore, how plants respond to a gradually reduced soil water potential strongly depends on the extent of the KSL reduction. Here, we show that between two species, faba bean and maize the sensitivity of KSL differs as quantified by the rate constant of the exponential decline. In faba bean, this rate constant is higher than in maize implying a faster reduction of the water availability at a given soil water potential and thus a stronger impulse for stomatal closure. In agreement with that, also the stomatal conductance declined at a higher rate in faba bean than in maize suggesting that varying stomatal sensitivity among species partly arises from a varying susceptibility of KSL. A potential origin for the differences in the KSL susceptibility could be the initial water uptake rate per root length which was six times higher in faba bean than in maize. Our data suggest that the KSL decline partly occurs inside the plant which could be a strategy to avoid a severe water depletion zone around the roots and thus improve plant performance during a longer drought. However, additional studies are needed to further disentangle the role of the soil and plant in response to drought. This could be realized by comparing the KSL decline among plants grown in different soils with varying hydraulic properties.
Materials and methods
Plant growing conditions and experimental design
Faba bean (V. faba, n = 12) and maize (Z. mays, n = 10) plants were grown in PVC pipes (50 cm high, inner diameter: 8.1 cm) filled with a sandy loam containing 73.3% sand, 23.1% silt, 3.6% clay, as reported by Pohlmeier et al. (2009), mixed with 20% (v) coarse sand (0.7–1.4 mm). The water retention curve of the substrate is shown in Supplemental Figure S7. A total substrate volume of 2.32 L was filled into the pots to a height of 45 cm resulting in a bulk density of 1.47 kg/L. Plants were grown in a climate chamber at the Research Centre Jülich under a constant temperature of 21.5°C ± 0.2°C and a VPDair of 1.49 kPa. Until the start of the measurements, plants were regularly watered to maintain an average volumetric soil water content (θ) of around 20%. Once a week, plants were fertilized using an NPK nutrient salt (Hakaphos Red; Compo Expert; 8% N, 12% P, 24% K), diluted in water at 0.3% (v/v). Plants were illuminated using a water-cooled LED panel (3200K, 5 × 5 LEDs 20W each) for 14 h during the day. Within these 14 h, light intensity was regulated to alternate in 2-h periods of a high (PPFD of 1,000 µmol m−2 s−1) and a low light intensity (PPFD of 500 µmol m−2 s−1) resulting in a daily light integral of 39.6 mol m−2 d−1. The alternating light pattern enabled us to derive water uptake profiles with the SWaP as described below. At an age between 4 and 5 weeks after sowing, selected according to a preferably similar water uptake rate among replicates, root system of the plants were imaged with MRI. Then, water supply was withheld for 4 days. During these 4 days Utot and Ψseq were determined with the SWaP. Simultaneously, Ψleaf and the leaf gas exchange were measured with a psychrometer and a LiCor 6400, respectively. After the 4 days of soil drying, some of the plants were rewatered to observe if Utot, Ψseq, and Ψleaf recovered.
Root length measurement with MRI
Right before the start of the soil drying experiment, root length distributions of the plants were determined using noninvasive imaging with MRI. A 4.7T vertical wide bore (310 mm) magnet (Magnex, Oxford, UK) and a gradient coil (ID 205 mm [MR Solutions]) generating gradients up to 400 mT/m were used in our setup. An MR Solutions (Guildford, UK) console was used to control the measurements. MRI data were analyzed with NMRooting software (van Dusschoten et al., 2016) yielding the total root length and root length distributions at a 1-cm vertical resolution. For a similar MRI setup, roots with a diameter ˂200–300 µm were below the detection limit (van Dusschoten et al., 2016). In an earlier experiment with 4-weeks-old faba bean and maize plants, grown under the same conditions, we compared a destructive measurement (harvest and scanning roots) of the total root length to the noninvasive measurement with MRI and NMRooting. For faba bean, 70.5% of the destructively determined root length was detected with MRI. For maize with generally thinner roots, 18.0% were detected. To correct for that, we multiplied the total root length measured with MRI in this study by the respective correction factors (1.4 for faba bean and 5.6 for maize). Additionally, we obtained the average root radius (r0) of both species from the scanning of harvested roots in the earlier experiment.
SWaP measurements of Utot and Ψseq
We used the recently developed SWaP (van Dusschoten et al., 2020) to continuously scan the profile of the volumetric soil water content (θ) which enabled us to derive both Utot and Ψseq. In principle, the SWaP measurement is based on integrating the pots with the soil columns into a resonator circuit and then determining the resonance frequency which largely depends on θ. To this end, a sensor with two opposing copper plates (7 × 5 cm2) coupled to a coil partially encloses the pots with soil. The resonance frequency is determined by applying a frequency sweep between 150 and 220 MHz using a virtual network analyzer (DG8SAQ, VNWA3, SDR-Kits, UK). The sensor moves upward the pots in 1-cm steps and determines the resonance frequency at each step. This yields a vertical profile of the resonance frequency consisting of 45 values. The profiles were measured every 15 min which, for four pots simultaneously, took around 11 min. The sensors were calibrated using soil samples with a defined θ ranging from 2% to 30% in 2% steps which enabled us to transfer the resonance frequency profiles into θ profiles. Since the sensors have a height of 12 cm and measurements were taken in 1-cm steps, the measured θ value in each layer is a convolution of the sensors’ field strength distribution and the θ values from the adjacent layers. We, therefore, applied a deconvolution of the measured θ profiles. To avoid an error amplification by the deconvolution, we used a regularization term to constrain the deconvolved profiles.
Note that Ψseq is defined for the pot as a whole and does not depend on z. in Equation (4) is equivalent to the standard sink fraction used by Couvreur et al. (2012) which is the profile of RWU rates under conditions of uniformly distributed soil water potential. Note that is normalized and thus . From Equation (4), we see that the Ψsoil in layers with a higher root conductance (roughly corresponding to a higher root length) contributes more to Ψseq than the Ψsoil in layers with a lower root conductance. In the case of a uniform Ψsoil distribution, Ψseq and Ψsoil are equal. In the following paragraphs, we explain how we obtained Ψsoil and to calculate Ψseq.
Brooks–Corey parameters of our soil substrate derived from the water retention curve
| Parameter . | Value . |
|---|---|
| θs | 0.4 mL cm−3 |
| θr | 0.0 mL cm−3 |
| α | 0.072 cm−1 |
| λb | 0.43 |
| Ksat | 113.7 cm d−1 |
| τ | −3.29 |
| Parameter . | Value . |
|---|---|
| θs | 0.4 mL cm−3 |
| θr | 0.0 mL cm−3 |
| α | 0.072 cm−1 |
| λb | 0.43 |
| Ksat | 113.7 cm d−1 |
| τ | −3.29 |
Brooks–Corey parameters of our soil substrate derived from the water retention curve
| Parameter . | Value . |
|---|---|
| θs | 0.4 mL cm−3 |
| θr | 0.0 mL cm−3 |
| α | 0.072 cm−1 |
| λb | 0.43 |
| Ksat | 113.7 cm d−1 |
| τ | −3.29 |
| Parameter . | Value . |
|---|---|
| θs | 0.4 mL cm−3 |
| θr | 0.0 mL cm−3 |
| α | 0.072 cm−1 |
| λb | 0.43 |
| Ksat | 113.7 cm d−1 |
| τ | −3.29 |
Measurements of Ψleaf and stomatal conductance
We continuously measured Ψleaf on the youngest fully developed leaf using a thermocouple leaf psychrometer (ICT International, Armidale, Australia). Before attaching the psychrometer, the leaf cuticle was carefully removed with abrasive paper. For synchronization with SWaP measurements, Ψleaf was recorded every 15 min. Stomatal conductance (gs) was measured using a portable LiCor 6400 photosynthesis system (LiCOR Inc., Lincoln, Nebraska, USA) with a transparent cuvette head. The cuvette was attached to a leaf adjacent to the leaf used for the psychrometer measurements. Since we measured multiple plants at once with only one LiCor 6400, measurements of gs were not performed continuously but only once per light period at least 45 min after a change in light intensity to allow gs to reach steady state. Stomatal conductance was measured at least during four light periods a day and for six replicates per species only.
Calculating the water potential at the root surface
Statistical analyses
We used Mann–Whitney U tests (Mann and Whitney, 1947) to test for statistical differences in several parameters between faba bean and maize. The Mann–Whitney U test is a nonparametric test for two independent samples, testing the null hypothesis that each of two randomly selected values from two different samples have the same probability of being greater than the other value. Mann–Whitney U test were performed using the SciPy package (Virtanen et al., 2020) in Python.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Relation between KSL (black) and Ψseq and gs (orange) and Ψseq for all measured faba bean plants separately.
Supplemental Figure S2. Relation between KSL (black) and Ψseq and gs (orange) and Ψseq for all measured maize plants separately.
Supplemental Figure S3. Daily trend of KSL.
Supplemental Figure S4. Water potential at the root surface (Ψseq, sr) as a function of the bulk soil water potential (Ψseq) for different scenarios.
Supplemental Figure S5. Distribution of root length (L) and RWU rates ().
Supplemental Figure S6. Recovery of Utot and Ψleaf upon rewatering for different faba bean plants.
Supplemental Figure S7. Water retention curve of the soil substrate used in the experiments.
Supplemental Figure S8. Determination of profiles from the SWaP data on the local soil water depletion rate and the total root water uptake rate Utot (t).
Acknowledgments
We thank Johannes Kochs for setting up the LED panel which was required for the light modulation in our measurements. Additionally, we would like to thank Dr. Carel Windt and Congcong Zheng for commenting on the manuscript. We gratefully acknowledge the group of Mathieu Javaux at the Université catholique de Louvain, Earth and Life Institute, for deriving the water retention curve of our soil.
Funding
This study was institutionally funded by the Helmholtz Association, Germany—POF4-899.
Conflict of interest statement. The authors declare no conflict of interests.
Y.M., J.A.P., H.P., and D.v.D. designed the research. Y.M. and D.v.D. performed the research. Y.M. analyzed the data and wrote the manuscript. All authors contributed to the discussion and revision of the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://dbpia.nl.go.kr/plphys/pages/general-instructions) is: Dagmar van Dusschoten ([email protected]).



