-
PDF
- Split View
-
Views
-
Cite
Cite
Li-Jie Zhou, Yuxi Wang, Yiguang Wang, Aiping Song, Jiafu Jiang, Sumei Chen, Baoqing Ding, Zhiyong Guan, Fadi Chen, Transcription factor CmbHLH16 regulates petal anthocyanin homeostasis under different lights in Chrysanthemum, Plant Physiology, Volume 190, Issue 2, October 2022, Pages 1134–1152, https://doi.org/10.1093/plphys/kiac342
Close - Share Icon Share
Abstract
Light is essential to plant survival and elicits a wide range of plant developmental and physiological responses under different light conditions. A low red-to-far red (R/FR) light ratio induces shade-avoidance responses, including decreased anthocyanin accumulation, whereas a high R/FR light ratio promotes anthocyanin biosynthesis. However, the detailed molecular mechanism underpinning how different R/FR light ratios regulate anthocyanin homeostasis remains elusive, especially in non-model species. Here, we demonstrate that a low R/FR light ratio induced the expression of CmMYB4, which suppressed the anthocyanin activator complex CmMYB6-CmbHLH2, leading to the reduction of anthocyanin accumulation in Chrysanthemum (Chrysanthemum morifolium) petals. Specifically, CmMYB4 recruited the corepressor CmTPL (TOPLESS) to directly bind the CmbHLH2 promoter and suppressed its transcription by impairing histone H3 acetylation. Moreover, the low R/FR light ratio inhibited the PHYTOCHROME INTERACTING FACTOR family transcription factor CmbHLH16, which can competitively bind to CmMYB4 and destabilize the CmMYB4–CmTPL protein complex. Under the high R/FR light ratio, CmbHLH16 was upregulated, which impeded the formation of the CmMYB4-CmTPL complex and released the suppression of CmbHLH2, thus promoting anthocyanin accumulation in Chrysanthemum petals. Our findings reveal a mechanism by which different R/FR light ratios fine-tune anthocyanin homeostasis in flower petals.
Introduction
Plant growth and development depend on light. They develop elaborate and unique adaptive strategies in response to different light conditions due to their sessile life style (Nakayama et al., 2017). Depending on whether grown in light or dark, angiosperm seedlings can follow two basic developmental trajectories:photomorphogenesis and skotomorphogenesis. When plants are under photomorphogenesis, they display open apical hook with fully expanded green cotyledons and short hypocotyl. However, they favor elongation and form apical hook when growing in the dark, which facilitate young and growing tissues to emerge from the soil (Josse and Halliday, 2008; Tripathi et al., 2019). At later stages of their development, plants also exhibit differential responses to light signals from natural environment as light filter through vegetation with a reduction of the red (R; 600–700 nm, peak at 660 nm) to far red (FR; 700–800 nm, peak at 730 nm) ratio due to selective absorption of R and blue light, but not FR, by the photosynthesis pigments (Possart et al., 2014). Species intolerant to shade display dramatic shade avoidance responses (SARs), including stem elongation, suppressed branching, and accelerated transition to flowering, so-called SAR (Ruberti et al., 2012; Sessa et al., 2018). Besides the changes of growth patterns, the accumulation of anthocyanin in plant vegetative tissues and flowers also depends on light conditions. Anthocyanins are a group of natural pigments in plants with important physiological functions, such as increasing light saturation and light compensation points (Albert et al., 2009) and eliminating excess reactive oxygen species in vegetative tissues in response to various abiotic stresses, such as cold, drought, high light, and salinity (Lotkowska et al., 2015; Schulz et al., 2015; Li et al., 2016, 2017). In addition, anthocyanin pigmentation pattern in flowers is a vital breeding target and plays a major role in determining the commercial value of many ornamental plants. Despite the importance of anthocyanins to plant adaptations and their vital economic values, unlike the molecular mechanisms underpinning plant photomorphogenesis and SAR, which have been intensively investigated (Ciolfi et al., 2013; Hersch et al., 2014; Ma and Li, 2019; Tripathi et al., 2019), the detailed molecular mechanism underlying the light-induced anthocyanin biosynthesis remains largely unexplored except for a few model species, such as Arabidopsis and petunia (Albert et al., 2009; Petroni and Tonelli, 2011).
Plants perceive light quality through photoreceptors known as phytochrome (phy), cryptochrome (cry), and UV-B photoreceptor UVR8. Phys are chromophore-conjugated dimeric proteins responsible for detecting R and FR light (Kong and Okajima, 2016). They exist in two interconvertible forms, Pr (R-absorbing) and Pfr (FR-absorbing). Upon R light exposure, phys are converted to the biologically active Pfr form and enter the nucleus to promote photomorphogenesis partially by inhibiting transcription factors, such as PHYTOCHROME INTERACTING FACTORS (PIFs). PIFs possess a phyA binding active phytochrome A (APA) motif or phyB binding active phytochrome B (APB) motif. They bind to G-box (CACGTG) motifs either alone or together with other transcription factors to regulate gene expression (Zhou et al., 2017b). Phys can inhibit PIFs in part by interacting and promoting PIFs protein degradation by 26S proteasomes (Bae and Choi, 2008; Leivar and Quail, 2011). For instance, light-induced phys actively exclude the CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) from the nucleus, thereby disrupting the formation of the ubiquitin E3 ligase complex, which degrades photomorphogenesis-promoting factors including ELONGATED HYPOCOTYL5 (HY5) in the dark, thus allowing the accumulation of photomorphogenesis-promoting transcription factors, which ultimately lead to adaptive changes at both the cellular and organismal levels (Podolec and Ulm, 2018). Additionally, phys can suppress PIFs by sequestering them from the promoters of their target genes (Park et al., 2012, 2018).
Interestingly, perturbations of genes associated with photomorphogenesis and SAR alter anthocyanin biosynthesis as well. Arabidopsis (Arabidopsis thaliana) AtHY5 directly activates the key members of the MYB-bHLH-WD40 (MBW) complex, AtPAP1(AtMYB75). AtMYB75 can also be phosphorated and stabilized by the MPK KINASE 4 (AtMPK4) in the light, leading to the increase of anthocyanin biosynthesis (Shin et al., 2013; Li et al., 2016). In the dark, AtCOP1 interacts with AtPAP1 and AtPAP2, leading to their degradation (Maier et al., 2013). The HD-ZIP II transcription factor AtHAT1 negatively regulates anthocyanin biosynthesis by interacting with AtMYB75, thereby destabilizing the MBW protein complex. The ERF-associated amphiphilic repression (EAR) motif residing in the N-terminus of the AtHAT1 interacts with the corepressor TOPLESS (TPL), which recruits histone deacetylase (HDAC) AtHDA6 (HISTONE DEACETYLASE 6) to epigenetically modulate gene expression via chromatin modification (Zheng et al., 2019). The bHLH protein AtEGL3 (ENHANCER OF GLABRA3) can also be ubiquitinated and phosphorylated by Ubiquitin protein ligase 3 (AtUPL3) (Patra et al., 2013) and GSK3-like kinase AtBIN2 (Cheng et al., 2014), respectively. Other anthocyanin transcription repressors in Arabidopsis, such as CAPRICE (CPC), SQUAMOSA PROMOTER BINDING PROTEIN-LIKE family member (SPL9), and Jasmonate ZIM-Domain (JAZs) proteins suppress anthocyanin accumulation by disrupting the formation of the MBW protein complex (Zhu et al., 2009; Gou et al., 2011; Qi et al., 2011). Under the continuous FR light conditions, AtPIF3 and AtHY5 also independently activate anthocyanin biosynthesis (Kim et al., 2003; Shin et al., 2007). It has been shown that AtPIF1,3,4,5 regulate anthocyanin biosynthesis by regulating anthocyanin biosynthesis structural genes (Liu et al., 2015; Pacín et al., 2016). This raises the question of whether other PIFs also play a role in regulating anthocyanin biosynthesis.
Chrysanthemum (Chrysanthemum morifolium) is one of the most important horticultural crops with high anthocyanin pigmentation pattern diversity, which has substantially contributed to its commercial value (Dai and Hong, 2017). However, under the artificial high-density growing condition, many flowers are shaded by the neighboring plants, leading to the reduction of anthocyanin accumulation in petals, which has greatly affected the commercial value of the flowers. To gain a better understanding of how different light conditions affect the anthocyanin accumulation in petals, we chose Chrysanthemum “Nannong Fencui” cultivar as our model system. We found that a low R/FR light ratio induced the expression of CmMYB4, which directly bound to the promoter of CmbHLH2—a component of the MBW complex that positively regulates anthocyanin biosynthesis. CmMYB4 interacted with CmTPL to form a transcriptional repressor complex that inhibited CmbHLH2 transcription, leading to the suppression of anthocyanin biosynthesis. Moreover, the low R/FR light ratio inhibited the expression of the PIF family transcription factor CmbHLH16, which can competitively bind to CmMYB4 and destabilize the CmMYB4–CmTPL protein complex, contributing to lower anthocyanin biosynthesis, suggesting that CmbHLH16 is a positive anthocyanin regulator. By contrast, a high R/FR light ratio induced CmbHLH16, which interacted with CmMYB4, thereby impeding the formation of the CmMYB4–CmTPL protein complex and releasing CmbHLH2, which lead to higher anthocyanin biosynthesis. Our results not only provide a better understanding of the molecular mechanism underpinning light-regulated anthocyanin biosynthesis, but also offer a means to improve the quality of ornamental traits in plants.
Results
Low R/FR light ratio inhibits and high R/FR light ratio promotes anthocyanin biosynthesis by regulating multiple anthocyanin-associated structural genes
Optimal light condition is essential for plants to reach the maximum growth potential and ensure their reproductive success. Many plants exhibit a quick response to light conditions and can adjust their physiology accordingly to adapt to the changing environment. In direct sunlight the R/FR light ratio is above 1, while under deep shade it can drop below 0.1 (Franklin, 2008; Ballaré, 2009). To test if Chrysanthemum is responsive to different light conditions, the Chrysanthemum “Nannong Fencui” cultivar was treated under white light (WL) (with an R/FR light ratio of approximately 1.09), low R/FR light ratio (approximately 0.22), or high R/FR light ratio (approximately 7.18) conditions at the budding stage (Supplemental Figure S1A). After blooming, we found that under the low R/FR light ratio, its ray flower petals had accumulated significantly lower anthocyanin content than the plants under the WL. On the contrary, the ray flower petal anthocyanin content was significantly higher under the high R/FR light ratio (Figure 1, A and B). The change of anthocyanin content was strongly associated with the expression of several anthocyanin-associated structural genes in the ray flower petals, including CmCHS, CmCHI, CmF3H, CmDFR, CmANS, and CmUFGT (Hong et al., 2015). We found that, compared with the WL treatment, these structural genes were expressed at a significantly lower level when flower buds at the blooming stage were treated for 6 h under the low R/FR light ratio treatment, and the opposite was true when plants were subjected to the high R/FR light ratio treatment (Figure 1C). The concomitant change of gene expression levels in all anthocyanin structural genes suggests that different R/FR light ratios affect anthocyanin accumulation by regulating some common upstream transcriptional factors. CmMYB6 and CmbHLH2 are the two prime candidates, which have been previously identified to promote anthocyanin accumulation in Chrysanthemum petals (Xiang et al., 2015). To test whether the two transcription factors are involved in the light-mediated anthocyanin biosynthesis, we first assayed their transcript levels in the ray flower petals under different light conditions with RT-qPCR and found that CmbHLH2 transcription was significantly inhibited by the low R/FR light ratio and promoted by the high R/FR light ratio, consistent with its role as a positive anthocyanin regulator. However, CmMYB6 transcript level was not significantly changed under either low or high R/FR light ratio treatment (Figure 1D). It is worth noting that the photosynthetic photon flux density (PPFD) value for the high R/FR light ratio treatment (∼146.84 ± 1.41–191.50 ± 0.94 µmol m−2 s−1) was slightly higher than that under the WL treatment (∼135.29 ± 1.46–173.98 ± 1.20 µmol m−2 s−1). It is known that high light intensity promotes anthocyanin biosynthesis in Arabidopsis (Li et al., 2016). To exclude the possibility that the upregulation of CmbHLH2 at transcriptional level under the high R/FR light ratio treatment was due to the enhancement of light intensity, we examined the transcription of CmbHLH2 in the ray flower petals of “Nannong Fencui” cultivar under two different light intensities (175 µmol m−2 s−1 versus 200 µmol m−2 s−1). We found that the increase of light intensity did not statistically significantly affect the transcription of CmbHLH2 (Supplemental Figure S1B). Therefore, we conclude that the increase of anthocyanin content in the ray flower petals under the high R/FR light ratio treatment is likely due to the alteration of the R/FR light ratio itself, rather than the change of light intensity.
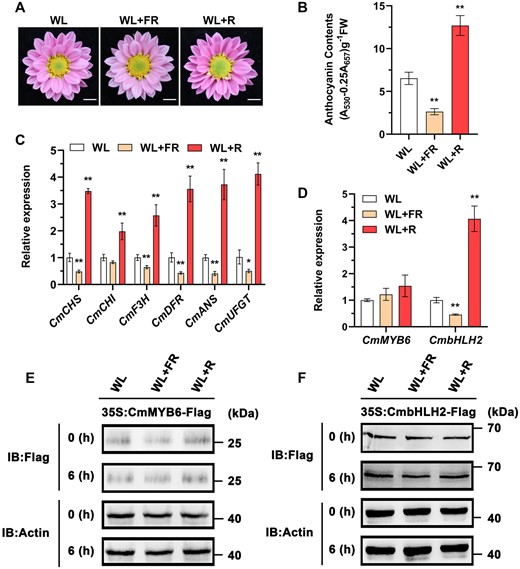
Low R/FR light ratio inhibits and high R/FR light ratio promotes anthocyanin biosynthesis by regulating multiple anthocyanin-associated structural genes. A, Phenotypes of flower color in plants treated under WL, low R/FR light ratio (WL+FR), and high R/FR light ratio (WL+R). Scale bars, 1 cm. B, Anthocyanin content in the ray flower petals. Error bars indicate the sd of three biological replicates. Significant differences are indicated with asterisks (**P < 0.01, ANOVA, Tukey’s correction). C and D, Relative expression of CmCHS, CmCHI, CmF3H, CmDFR, CmANS, CmUFGT, CmMYB6, and CmbHLH2 in the ray flower petals of plants treated under different light conditions for 6 h. Error bars indicate the sd of three biological replicates. Significant differences are indicated with asterisks (*P < 0.05, **P < 0.01, ANOVA, Tukey’s correction). CmEF1α and CmActin were used as the internal control. E and F, Protein abundance of CmMYB6 and CmbHLH2 in the ray flower petals of flowers transiently overexpressing CmMYB6 and CmbHLH2 with pSAK277-CmMYB6 (35S:CmMYB6-Flag) and pSAK277-CmbHLH2 (35S:CmbHLH2-Flag), respectively, as determined using an anti-Flag antibody. Actin in total protein extracts was used as the loading control. IB, immunoblot.
In addition, we found that the CmMYB6 and CmbHLH2 protein stability, determined by western blotting assay, was not affected by either low or high R/FR light ratio in the ray flower petals of the transiently transformed flowers (Figure 1, E and F), suggesting that light signaling is unlikely to modify either protein. Taken together, different R/FR light ratios likely affect anthocyanin biosynthesis by regulating CmbHLH2 at the transcriptional level in Chrysanthemum.
Low R/FR light ratio induces CmMYB4 to suppress CmbHLH2 and negatively regulates anthocyanin biosynthesis
To investigate how CmbHLH2 was regulated under different light ratios, we carried out a yeast one-hybrid (Y1H) screening. The 2-kb-long CmbHLH2 promoter was isolated and inserted into the pHIS2 vector as the bait, and several positive colonies containing parts of the CmMYB4 cDNA fragments were identified. In the ray flower petals, CmMYB4 was significantly upregulated by five-fold under the low R/FR light ratio, inversely associated with the reduction of anthocyanin content, suggesting that CmMYB4 might function as an anthocyanin biosynthesis repressor. However, CmMYB4 was only slightly, yet statistically significantly down regulated under the high R/FR light ratio (Supplemental Figure S2A), suggesting that CmMYB4 might regulate anthocyanin biosynthesis differently, depending on light conditions.
To further investigate the function of CmMYB4 in Chrysanthemum, we first performed a phylogenetic analysis and found that CmMYB4 was most closely related to AtMYB4 (Supplemental Figure S2B), which is a known anthocyanin biosynthesis repressor (Wang et al., 2020). CmMYB4 is localized to the nucleus of Nicotiana benthamiana leaf epidermal cells (Supplemental Figure S2C). Moreover, CmMYB4 harbors a conserved EAR motif (LNLELR) (Supplemental Figure S2D), which is a well-known transcriptional repression motif (Zheng et al., 2019). It has been reported that subgroup 4 (SG4) R2R3 MYB proteins exert their suppression by interacting with bHLH proteins (Wang et al., 2020). However, to our surprise, our yeast two hybrid (Y2H) and biomolecular fluorescence complementation (BiFC) assays both showed that CmMYB4 did not interact with the MBW complex component CmbHLH2 (Supplemental Figure S3, A and B), suggesting that CmMYB4 likely functions differently to regulate anthocyanin biosynthesis in Chrysanthemum.
In Arabidopsis, AtMYB4 binds to three cis-elements, namely ACCTACC (AC-I), ACCAACC (AC-II), and ACCTAAC (AC-III) to repress target gene expression (Zhao et al., 2007). To investigate whether CmMYB4 can repress the expression of CmbHLH2 through a similar mechanism, we first bioinformatically predicted two AC-II elements within the 2-kb-long CmbHLH2 promoter using PlantCARE (Lescot et al., 2002). To test whether CmMYB4 directly binds the promoter of CmbHLH2 in planta, we conducted a chromatin immunoprecipitation (ChIP)-PCR assay. We prepared chromatin samples from the leaves of the stably overexpressing CmMYB4 (35S:CmMYB4-GFP) plants (Supplemental Figure S2E) and performed ChIP with an anti-GFP antibody, subsequent qPCR determination showed that the first AC-II fragment localized between −1737 and −1593 bp was significantly enriched. However, the second AC-II element (−900 bp to −753bp), compared with the control (−1,328 to −1,193 bp), was not significantly enriched (Figure 2A), indicating that CmMYB4 can specifically recognize the AC-II element (ACCAACC, −1,702 to −1,695 bp) in the CmbHLH2 promoter and the nucleotides surrounding the AC-II element might play a role in determining the binding specificity.
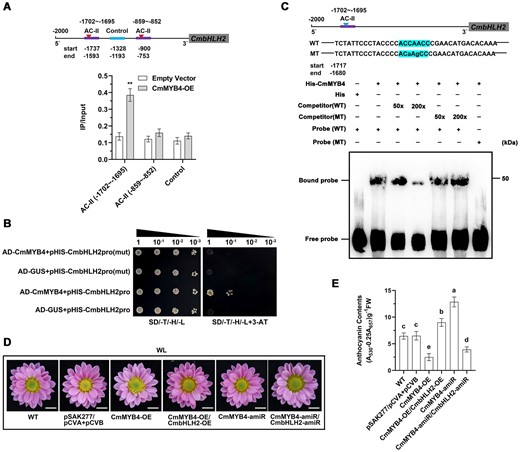
Low R/FR light ratio induces CmMYB4 to suppress CmbHLH2 and negatively regulates anthocyanin biosynthesis. A, ChIP-PCR of the enrichment of DNA fragments AC-II (−1702 to −1695 bp) and AC-II (−859 to −852 bp) in the CmbHLH2 promoter. The fragment (−1328 to −1193 bp) was used as a control. Error bars indicate the sd of three biological replicates. Significant differences are indicated with asterisks (**P < 0.01, ANOVA, Tukey’s correction). B, Interaction of CmMYB4 with the CmbHLH2 promoter and its mutant CmbHLH2pro(mut) in yeast cells. pGADT7-GUS was used as the negative control. SD/-T/-H/-L indicates Trp, His, and Leu synthetic dropout medium. 80 mM 3-AT was used. C, Interaction of CmMYB4 with labeled DNA probes for the AC-II element of the CmbHLH2 promoter in EMSA. His protein was used as the negative control. Increasing amounts (50× and 200×) of the unlabeled wild-type probes or the unlabeled mutant probes were added as cold competitors. “WT” indicates wild-type DNA probes and “MT” indicates mutated probes. “+” indicates presence and “−” indicates absence. D, Phenotypes of flower color in plants transiently overexpressing or suppressing genes denoted in the figure. Scale bars, 1 cm. E, Anthocyanin content in the ray flower petals. Error bars indicate the sd of three biological replicates. Samples denoted by different letters are significantly different (P < 0.01, ANOVA, Tukey’s correction).
To further confirm the binding of CmMYB4 to the CmbHLH2 promoter, we performed a Y1H assay, we found that the co-transformation of CmMYB4 and CmbHLH2 promoter enabled the yeast to grow on the selective medium, while the co-transformation of either the empty vector with the CmbHLH2 promoter or CmMYB4 with the mutated CmbHLH2 promoter lacking the AC-II elements failed to do so, confirming the binding specificity of CmMYB4 to the AC-II cis-element (Figure 2B). To further confirm this in vitro, an electrophoretic mobility shift assay (EMSA) was performed. A biotin-labeled probe containing the AC-II element was incubated with the affinity-purified His-CmMYB4 fusion protein. The unlabeled wild-type probe and probe with the AC-II element mutated at increasing concentrations were used as competitors. We found shifted bands only when the labeled DNA probe was mixed with His-CmMYB4, indicating a binding of the protein to the AC-II element. By contrast, no shifted band was detected when the His protein alone was used. The decreased binding capability with higher competitor concentrations demonstrated that CmMYB4 specifically binds the AC-II element in vitro (Figure 2C). These findings indicate the direct binding of CmMYB4 to the AC-II element at −1,702 to −1,695 bp in the CmbHLH2 promoter.
To test if CmMYB4 plays a role in regulating anthocyanin biosynthesis, we silenced CmMYB4 in the flowers of Chrysanthemum “Nannong Fencui” cultivar using virus-induced gene silencing (VIGS) technology with a modified cabbage leaf-curl geminivirus vector (pCVA) (Xu et al., 2020) containing the artificial micro-RNA-CmMYB4 (pCVA-CmMYB4-amiR, pCVB was used as an auxiliary vector). RT-qPCR assays of the ray flower petals demonstrated that expression of CmMYB4 was significantly reduced in the pCVA-CmMYB4-amiR lines (Supplemental Figure S4A). Compared with the empty vector control, CmMYB4 gene silencing resulted in higher anthocyanin accumulation in the ray flower petals (Figure 2, D and E). On the contrary, when CmMYB4 was transiently overexpressed with a modified overexpression vector pSAK277 (Hellens et al., 2005; Supplemental Figure S4A), the amount of anthocyanin accumulated in the ray flower petals has been reduced by >50% (Figure 2, D and E). Together, these results indicate that CmMYB4 negatively regulates anthocyanin accumulation in the ray flower petals in vivo. Concomitantly, virus-induced silencing of CmMYB4 and transiently overexpression of CmMYB4 led to the upregulation and downregulation of CmbHLH2 in the ray flower petals, respectively (Supplemental Figure S4, A and B), suggesting that CmMYB4 likely regulates anthocyanin accumulation by modulating the expression levels of CmbHLH2 in the Chrysanthemum ray flower petals. Consistently, transiently overexpressing CmbHLH2 in the CmMYB4 overexpression background had successfully quelled the suppressive effect of CmMYB4 on the ray flower coloration. In addition, when CmMYB4 and CmbHLH2 are simultaneously transiently silenced, the anthocyanin content in the ray flower petals fail to accumulate to a higher level than silencing the CmMYB4 alone (Figure 2, D and E and Supplemental Figure S4, A and B), further suggesting that CmMYB4 and CmbHLH2 likely function in the same pathway and CmMYB4 may act as an upstream regulator to directly repress the expression of CmbHLH2 and negatively regulate anthocyanin biosynthesis in the ray flower petals.
CmMYB4 interacts with CmTPL to impair CmbHLH2 histone H3 acetylation
CmMYB4 possesses a conserved EAR motif at the C-terminus. Transcription factors harboring the EAR motif repress the transcription of downstream genes by interacting with TPL corepressor (Kagale and Rozwadowski, 2011). This repressor complex can suppress target gene expression by recruiting HDACs to modify histone H3 acetylation level at the transcription start sites (TSSs) of the target genes (Zheng et al., 2019). To investigate if the same mechanism also operates in Chrysanthemum, we first analyzed whether the CmbHLH2 histone H3 can be deacetylated. ChIP-PCR assays were performed using antibodies against acetylated histone H3 in the ray flower petals of the transiently transformed flowers of CmMYB4 overexpression and knockdown (CmMYB4-amiR) plants. Compared with the control flowers (pSAK277 empty vector), CmMYB4-OE transgenic flowers showed decreased histone H3 acetylation at the TSS of CmbHLH2. In contrast, CmMYB4-amiR transgenic flowers exhibited elevated histone H3 acetylation (Figure 3A). The negative association between the expression levels of CmMYB4 and the H3 acetylation levels of CmbHLH2 suggests that CmMYB4 is likely involved in modulating the histone H3 acetylation levels of CmbHLH2.
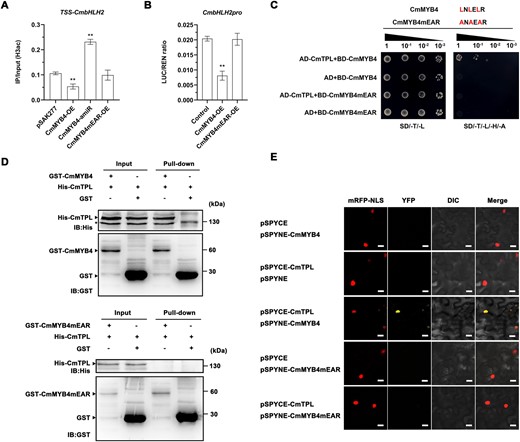
CmMYB4 interacts with CmTPL to impair CmbHLH2 histone H3 acetylation. A, ChIP qPCR analysis of histone H3 acetylation level in the TSS of CmbHLH2 in the ray flower petals of transgenic flowers. pSAK277 empty vector was used as a control. Error bars indicate the sd of three biological replicates. Significant differences are indicated with asterisks (**P < 0.01, ANOVA, Tukey’s correction). B, The ratio of LUC to Renilla (REN) activity. Error bars indicate the sd of six biological replicates. Significant differences are indicated with asterisks (**P < 0.01, ANOVA, Tukey’s correction). C, Interaction between CmMYB4 or CmMYB4mEAR and CmTPL in yeast cells. SD/-T/-L indicates Trp and Leu synthetic dropout medium; SD/-T/-L/-H/-A indicates Trp, Leu, His, and Ade synthetic dropout medium. CmMYB4mEAR: mutated CmMYB4 in which the core Leu residues of the EAR motif were substituted to Ala residues. D, Interaction between CmMYB4 or CmMYB4mEAR and CmTPL in an in vitro pull-down assay. In vitro-translated GST protein was used as the negative control. “Input” indicates protein mixtures before the experiments and “Pull-down” indicates purified protein mixture. “+” indicates presence and “−” indicates absence. IB, immunoblot. Asterisks indicate nonspecific binds. E, Interaction between CmMYB4 or CmMYB4mEAR and CmTPL in BiFC assay. pSPYCE and pSPYNE empty vectors were used as the negative controls. mRFP-NLS, nuclear marker co-expressing the 35S:D53-RFP construct; YFP, images obtained in the yellow fluorescence channel; DIC, images obtained in bright light; and Merged, overlay plots. Scale bars, 20 μm.
Next, we tested if the EAR motif in the CmMYB4 is required to suppress the expression of CmbHLH2 and its H3 acetylation levels. To do this, we transiently overexpressed CmMYB4 with the mutated EAR motif (three leucines were replaced with alanine in the EAR motif, from LNLELR to ANAEAR). As expected, when the EAR motif was mutated, there was no statistically significant difference in the histone H3 acetylation level between the control and the CmMYB4mEAR-OE transgenic flowers (Figure 3A), despite the transgene expression level has increased over five-fold (Supplemental Figure S4A). Neither did we identify any statistically significant change in CmbHLH2 expression levels (Supplemental Figure S4B) in the ray flower petals of the transiently transformed flowers, indicating that the EAR motif is required for CmMYB4 to function as a CmbHLH2 repressor.
We then conducted a transactivation assay to identify whether CmMYB4 is able to regulate expression of the firefly luciferase (LUC) gene driven by the CmbHLH2 promoter in N. benthamiana leaves. The CmMYB4 driven by the 35S promoter (pORE-R4-CmMYB4) was co-infiltrated with pCmbHLH2:LUC reporter into N. benthamiana leaves, while co-infiltration of empty pORE-R4 plasmid and pCmbHLH2:LUC served as a control. We found that the wild-type CmMYB4 strongly repressed activity of the LUC reporter, while the mutated CmMYB4 did not (Figure 3B). Taken together, CmMYB4 can suppress CmbHLH2 expression level by altering the histone H3 acetylation level of the CmbHLH2 promoter. However, whether the suppression of CmbHLH2 by CmMYB4 also requires the participation of CmTPL remains unknown. To test this, we first performed a Y2H assay between CmMYB4 and CmTPL and we found that CmMYB4 interacts with CmTPL, but failed to do so when the CmMYB4 EAR motif was mutated (Figure 3C), confirming that the EAR motif is required for CmMYB4 to interact with CmTPL. To further confirm the interaction between CmMYB4 and CmTPL, we performed a pull-down assay of CmMYB4 and CmTPL. GST-CmMYB4 and His-CmTPL proteins were expressed and purified from Escherichia coli BL21 (DE3). After incubation, the protein complexes were precipitated using anti-GST antibodies and then probed with anti-His antibodies. The results showed that CmMYB4 was co-precipitated with CmTPL, but failed to do so when the CmMYB4 EAR motif was mutated (Figure 3D). To test if CmTPL and CmMYB4 interact in planta, we performed a BiFC assay. In the BiFC assay, the full-length CmMYB4 and CmTPL was transiently expressed as fusion proteins of the N- and C-halves of enhanced yellow fluorescence protein, respectively, in N. benthamiana leaves by infiltration. The reconstituted fluorescence signal was observed in the nucleus and merged with the signal of the nuclear marker mRFP-NLS, while the full-length of mutated CmMYB4mEAR and CmTPL did not reconstitute fluorescence signal, despite the nucleus marker fluorescence signal was readily detectable (Figure 3E). These in vitro and in planta experiments confirmed the specific interaction between CmMYB4 and CmTPL in the nucleus, depending on the EAR motif. It has been reported that TPL recruits HDAC proteins such as HDA6, HDA9, and HDA19 to modify histone H3 acetylation level of the target genes in Arabidopsis (Oh et al., 2014; Leydon et al., 2021). To test whether such HDAC proteins are recruited by CmTPL in Chrysanthemum, we performed Y2H assays between CmTPL and selected CmHDAs in Chrysanthemum and found that CmTPL interacted with CmHDA9 and CmHDA19, but not CmHDA6 (Supplemental Figure S3C).
Having established that CmMYB4 interacts with CmTPL and binds directly to the CmbHLH2 promoter, we next investigated whether the expression of CmbHLH2 depends on CmTPL. To this end, we silenced the expression of CmTPL in the CmMYB4-OE background by co-infiltrating CmTPL-amiR and CmMYB4-OE into the wild-type Chrysanthemum ray flower petals using a transient assay. As expected, CmMYB4 expression level has increased ∼5-fold when it was transiently overexpressed and CmTPL was down regulated by over 75% (Supplemental Figure S4, A and C). Compared with CmMYB4-OE alone, which significantly suppressed the CmbHLH2 expression by 74%, silencing CmTPL in the CmMYB4-OE background resulted in a significant higher CmbHLH2 expression level than the wild-type (Supplemental Figure S4B), suggesting that CmTPL and CmMYB4 cooperatively suppress the expression level of CmbHLH2. Taken together, these results indicate that CmMYB4 interacts with CmTPL to directly inhibit CmbHLH2 expression by impairing CmbHLH2 histone H3 acetylation.
CmMYB4 plays a minor role in the accumulation of anthocyanin under the high R/FR light ratio treatment
Heretofore, we have demonstrated that CmMYB4 represses anthocyanin biosynthesis under the low R/FR light ratio by downregulating the CmbHLH2 expression level. However, whether it participates in the anthocyanin biosynthesis regulation under the high R/FR light ratio remains unclear. If CmMYB4 plays a major role in regulating Chrysanthemum petal anthocyanin accumulation, it would be expected to be significantly downregulated under the high R/FR light ratio treatment. However, CmMYB4 was only slightly lower yet statistically significantly down regulated when the anthocyanin accumulation in the ray flower petals was significantly increased under the high R/FR light ratio treatment (Figure 1B and Supplemental Figure S2A), suggesting that CmMYB4 might play a minor role in regulating anthocyanin biosynthesis under the high R/FR light ratio. To further corroborate this, we transiently overexpressed CmMYB4 in the Chrysanthemum ray flower petals and subjected the infiltrated plants to the high R/FR light ratio treatment. We found that even though CmMYB4 expression level has increased by five-fold, the expression of CmbHLH2 was slightly inhibited and the anthocyanin content was only reduced by ∼15% in the ray flower petals (Figure 4, A–D). However, this stands in contrast with the overexpression of CmMYB4 under the WL, which reduced the anthocyanin content and the CmbHLH2 expression by >60% and 70%, respectively (Figure 4, C and D), suggesting that the high R/FR light ratio had significantly compromised the function of CmMYB4.
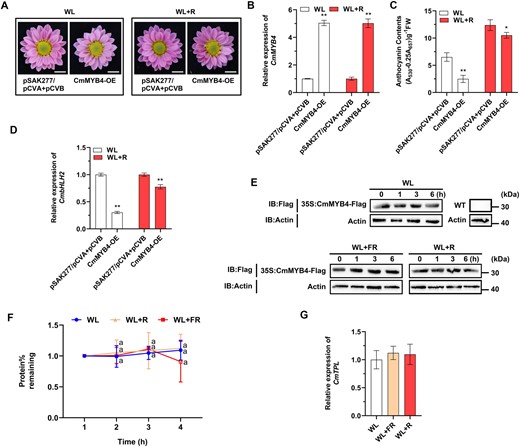
CmMYB4 plays a minor role in the accumulation of anthocyanin under the high R/FR light ratio. A, Phenotypes of flower color in the transiently transformed flowers treated under different light conditions. Scale bars, 1 cm. R, red light. B, Comparisons of CmMYB4 expression levels in the ray flower petals when plants were under different light treatments. C, Anthocyanin content in the ray flower petals of transiently transformed flowers treated under different light conditions. Error bars indicate the sd of three biological replicates, significant differences are indicated with asterisks (*P < 0.05, **P < 0.01, ANOVA, Tukey’s correction) for B and C. D, Relative expression of CmbHLH2 in the ray flower petals of transiently transformed flowers treated under different light conditions. Error bars indicate the sd of three biological replicates. Significant differences are indicated with asterisks (**P < 0.01, ANOVA, Tukey’s correction). CmEF1α and CmActin were used as the internal control. E, Protein abundance of CmMYB4 in the leaves of “Nannong Fencui” cultivar cutting seedlings transiently overexpressing CmMYB4 with pSAK277-CmMYB4 (35S:CmMYB4-Flag) at different time points treated under different light conditions, as determined using an anti-Flag antibody. Actin in total protein extracts was used as the loading control. CmMYB4-Flag proteins in the leaves of wild-type cutting seedlings from a separate immunoblot were used as a negative control. IB, immunoblot. F, Quantification of CmMYB4-Flag protein levels using Image J software. The protein stability assay in E was performed three times and the protein quantities relative to the levels at initial time 0 were counted and used to calculate the sd. Samples denoted by the same letter are not significantly different (P < 0.05, ANOVA, Tukey’s correction). G, Relative expression of CmTPL in the ray flower petals of wild-type flowers in response to different light conditions for 6 h. Error bars indicate the sd of three biological replicates. CmEF1α and CmActin were used as the internal control.
One possible explanation is that CmMYB4 protein was disproportionally degraded under the high R/FR light ratio even though the transcript expression level was high. To test this, protein stability assay was performed to examine whether different R/FR light ratios can affect the CmMYB4 protein stability differently. The 35S:CmMYB4-Flag transiently transformed “Nannong Fencui” cultivar cutting seedlings were treated under WL, low R/FR light ratio, and high R/FR light ratio for different durations, respectively. Total proteins in the leaves of the infiltrated cutting seedlings were extracted for western blotting assay with an anti-Flag antibody and we found that, compared with the WL treatment, neither low nor high R/FR light ratio had significantly affected the CmMYB4 protein stability (Figure 4, E and F), indicating that CmMYB4 protein stability was unlikely to contribute to the disparity of anthocyanin accumulation patterns under different light conditions.
Since the suppression of anthocyanin biosynthesis by CmMYB4 also requires CmTPL, we speculated that the high R/FR light ratio might have weakened the expression level of CmTPL, thereby compromising the formation of the CmMYB4–CmTPL complex, leading to higher anthocyanin accumulation in the ray flower petals. However, gene expression analysis in the ray flower petals indicates that CmTPL expression pattern was not significantly changed across different light treatments (Figure 4G), indicating that CmTPL was unlikely to be the culprit to the reduction of anthocyanin accumulation in the Chrysanthemum ray flower petals either.
CmbHLH16 interacts with CmMYB4 to destabilize the CmMYB4–CmTPL protein complex
Since neither CmMYB4 nor CmTPL transcript level was dramatically affected by the high R/FR light ratio; therefore, we hypothesized that additional interacting partners might be involved in anthocyanin regulation by destabilizing the formation of CmMYB4–CmTPL protein complex or alternatively, high R/FR light ratio might trigger another yet unknown anthocyanin biosynthesis activator independent of the CmMYB4–CmTPL module. To distinguish these possibilities, we first setup to identify this putative protein interacting partner with a Y2H screening approach using CmMYB4 as a bait. One of the several colonies we obtained contained a partial cDNA fragment of the CmbHLH16 gene. CmbHLH16 was most closely related to PtPIF8a, harboring a conserved active APB motif (Supplemental Figure S5, A and B). PIF family transcription factors are known to be light responsive and participate in anthocyanin biosynthesis regulation (Liu et al., 2015). However, whether PIF8 also plays a role in regulating anthocyanin biosynthesis remains unclear. Similar to PtPIF8a, we found that CmbHLH16 protein was light-stable in response to different R/FR light ratio treatments in the ray flower petals of the transiently transformed 35S:CmbHLH16-Flag flowers (Supplemental Figure S5C). CmbHLH16 was significantly up regulated by high R/FR light ratio and substantially down regulated by low R/FR light ratio in the ray flower petals (Figure 5A), positively corresponding with the change of anthocyanin contents under different light conditions (Figure 1B). Moreover, CmbHLH16 did not directly regulate anthocyanin-associated structural genes as revealed by Y1H assay (Supplemental Figure S6), suggesting that CmbHLH16 might interact with CmMYB4 to fine-tune anthocyanin accumulation in response to different R/FR light ratios. Y2H assays showed that CmMYB4 interacted with CmbHLH16, but CmTPL did not (Figure 5B). The interaction between CmMYB4 and CmbHLH16 was further validated by in vitro pull-down assays (Figure 5C). A BiFC assay showed that CmbHLH16 interacted with CmMYB4 in the nucleus in the N. benthamiana leaves, but not with CmTPL either (Figure 5D). CmMYB4 carries an R2R3 domain at the N-terminus and an EAR motif at the C-terminus, and we found that both domains could interact with CmbHLH16 (Supplemental Figure S7). Taken together, our results suggest that CmbHLH16 might specifically compete with CmTPL for binding with CmMYB4 in Chrysanthemum. To directly test the competitive binding of CmMYB4 by CmTPL and CmbHLH16, a yeast three-hybrid (Y3H) assay was conducted in which CmbHLH16 transcription was regulated by the Met-repressible pMET25 promoter. The CmMYB4–CmTPL hetero-dimerization was largely suppressed by CmbHLH16 under methionine-deficient conditions, as shown by yeast colony survival (Figure 5E). We then tested the competitive binding ability of the three proteins in a pull-down experiment. GST-CmTPL and His-CmMYB4 were incubated with increasing amounts of His-CmbHLH16. Precipitation with an anti-GST antibody revealed gradually decreasing amounts of CmTPL (Figure 5F), further supporting that CmbHLH16 interfered with the formation of the CmMYB4–CmTPL protein complex.
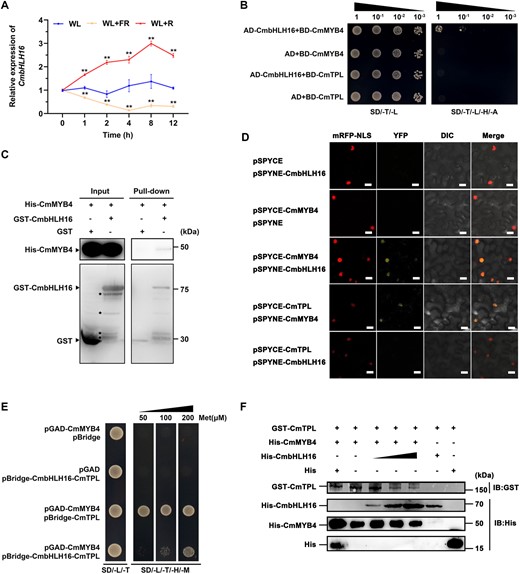
CmbHLH16 interacts with CmMYB4 to destabilize the CmMYB4–CmTPL protein complex. A, Relative expression of CmbHLH16 in the ray flower petals of wild-type plants treated under different light conditions. Error bars indicate the sd of three biological replicates. Significant differences are indicated with asterisks (**P < 0.01, ANOVA, Tukey’s correction). CmEF1α and CmActin were used as the internal control. B, Interaction between CmMYB4 or CmTPL and CmbHLH16 in yeast cells. SD/-T/-L indicates Trp and Leu synthetic dropout medium. SD/-T/-L/-H/-A indicates Trp, Leu, His, and Ade synthetic dropout medium. C, Interaction between CmMYB4 and CmbHLH16 in an in vitro pull-down assay. Asterisks indicate nonspecific binds. D, Interaction between CmMYB4 and CmbHLH16 in a BiFC assay in N. benthamiana leaves. Scale bars, 20 μm. E, CmbHLH16 competes with CmTPL for binding to CmMYB4 in yeast cells. Met, Methionine. F, Competitive binding assays of CmTPL and CmbHLH16 to CmMYB4. The gradient indicates the increasing amount of His-CmbHLH16 added in the reaction system.
CmbHLH16 fine-tunes anthocyanin biosynthesis in response to different R/FR light ratios
To investigate whether CmbHLH16 can promote the expression of CmbHLH2 by destabilizing the CmMYB4–CmTPL protein complex, a dual-LUC assay was conducted in the N. benthamiana leaves. We found that compared with the control (co-infiltration of empty pORE-R4 plasmid with pCmbHLH2:LUC), the co-expression of 35S:CmMYB4 with pCmbHLH2:LUC had significantly decreased the LUC:REN ratio. However, when 35S:CmMYB4 was co-expressed with 35S:CmbHLH16, the decreased LUC:REN ratio was partially restored (Figure 6A), indicating that the increasing amount of CmbHLH16 can attenuate the repression of CmbHLH2 promoter activity exerted by CmMYB4, which is consistent with our hypothesis. Y1H assay showed that CmbHLH16 did not regulate CmbHLH2 directly as yeast cells co-expressing pHIS2-CmbHLH2pro and pGADT7-CmbHLH16 could not grow on the selected medium (Figure 6B). Moreover, we found that in the leaves of CmMYB4-amiR transiently transformed cutting seedlings, overexpression of CmbHLH16 under WL did not further significantly promote the expression of CmbHLH2 (Supplemental Figure S8). These indicate that CmbHLH16 might promote the transcriptional activity of CmbHLH2 promoter by disrupting the formation of the CmMYB4–CmTPL protein complex, rather than directly activating the CmbHLH2 gene expression.
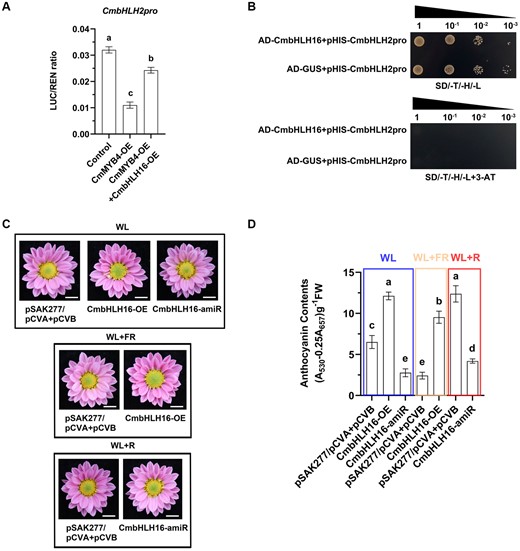
CmbHLH16 fine-tunes anthocyanin biosynthesis in response to different R/FR light ratios. A, The ratio of LUC to REN activity. Error bars indicate the sd of six biological replicates. Samples denoted by different letters are significantly different (P < 0.01, ANOVA, Tukey’s correction). B, Test of the interactions between CmbHLH16 and the CmbHLH2 promoter in yeast cells. pGADT7-GUS was used as a negative control. SD/-T/-H/-L indicates Trp, His, and Leu synthetic dropout medium. 3-AT concentrations: 80 mM. C, Phenotypes of flower color in the transiently transformed flowers treated under different light conditions. Scale bars, 1 cm. D, Anthocyanin content in the ray flower petals of transiently transformed flowers. Error bars indicate the sd of three biological replicates. Samples denoted by different letters are significantly different (P < 0.01, ANOVA, Tukey’s correction).
To further corroborate this, we transiently overexpressed CmbHLH16 in the Chrysanthemum flowers, which led to higher expression levels of CmbHLH2 and anthocyanin accumulation in the ray flower petals both under the WL and low R/FR light ratio conditions (Figure 6, C and D and Supplemental Figure S9, A and B). Furthermore, when CmbHLH16 was knocked down under the high R/FR light ratio, CmbHLH2 was also down regulated, leading to lower accumulation of anthocyanin in the ray flower petals (Figure 6, C and D and Supplemental Figure S9, A and B). These results indicate that CmbHLH16 fine-tunes anthocyanin biosynthesis in response to different R/FR light ratios.
Discussion
Light is essential throughout the life cycle of plants. From the top of the canopy to the ground, the R/FR light ratio decreases as the result of the photosynthetic apparatus absorbing the visible spectrum while filtering out the FR light. The exposure to different light ratios can elicit differential developmental responses from plants (Martínez-García et al., 2014). In addition to being an environmental signal to help plants perceive their surrounding environments, light constitutes a photosynthetic energy source (Goudriaan et al., 1985). Adequate effective light is essential for plants to maintain normal growth and reproduction. However, excessive light could lead to tissue damages (Ksas et al., 2015). Therefore, photomorphogenesis, SARs, and anthocyanin biosynthesis are intricately intertwined for plants to maintain an equilibrium between growth and reproduction. The R/FR light ratio signal is sensed by the phy photoreceptors (Holalu and Finlayson, 2017). Upon light illumination, the activated phys and PIFs induce a wide range of developmental responses in plants, including anthocyanin biosynthesis. Here, we report, CmbHLH16, a putative Chrysanthemum ortholog of the Arabidopsis PIF8, which is responsive to different R/FR light ratios and fine-tunes anthocyanin biosynthesis: under the low R/FR light ratio environment, CmbHLH16 expression is suppressed and anthocyanin repressor CmMYB4 is substantially upregulated, which facilitates the formation of the anthocyanin suppression complex CmMYB4–CmTPL. This complex directly binds to and deacetylates histone H3 in the promoter of the positive anthocyanin regulator CmbHLH2, leading to the downregulation of CmbHLH2. Hence, less anthocyanin is accumulated in the petals. Under the high R/FR light ratio environment, CmbHLH16 expression is elevated, the accumulation of CmbHLH16 leads to the direct competition with CmTPL for binding CmMYB4, therefore, destabilizing the CmMYB4–CmTPL repression complex and releasing the suppression of CmbHLH2, resulting in higher anthocyanin accumulation in the ray flower petals (Figure 7).
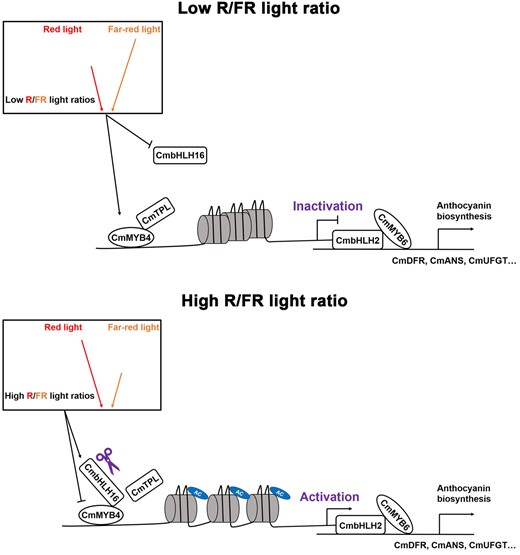
A model of CmbHLH16 fine-tuning anthocyanin biosynthesis in response to different R/FR light ratios in Chrysanthemum. Under the low R/FR light ratio environment, CmbHLH16 expression is suppressed and anthocyanin repressor CmMYB4 is substantially upregulated, which facilitates the formation of the anthocyanin suppression complex CmMYB4–CmTPL. This complex directly binds to and deacetylates histone H3 in the promoter of the positive anthocyanin regulator CmbHLH2, leading to the downregulation of CmbHLH2. Hence, less anthocyanin is accumulated in the petals. Under the high R/FR light ratio environment, CmbHLH16 expression is elevated, the accumulation of CmbHLH16 leads to the direct competition with CmTPL for binding CmMYB4, therefore, destabilizing the CmMYB4–CmTPL repression complex and releasing the suppression of CmbHLH2, resulting in higher anthocyanin accumulation in the ray flower petals.
CmbHLH16 forms a clade together with AtPIF8 and PtPIF8a, suggesting that they may share similar functions. Similar to other PIFs in this clade, CmbHLH16 lacks the core APA motif, but harbors a conserved active APB motif, which is central to the interaction between PIFs and the Pfr form of phyB (Khanna et al., 2004). As a result, AtPIF8 binds to phyB, but only weakly interacts with phyA due to the lack of the APA domain (Oh et al., 2020). In Arabidopsis, phys (phyA–phyE) are responsible for detecting R and FR light. The activated phys enter the nucleus to inhibit PIFs partially by ubiquitin E3 ligase complex COP1/SPAs (Zhu et al., 2015; Park et al., 2018). In the dark, COP1 directly interacts with AtPIF8 to mediate its degradation (Oh et al., 2020). However, whether PIF8 plays a role in regulating anthocyanin biosynthesis in Arabidopsis remains unclear. In Populus, RNAi knockdown and overexpression of PtPIF8a resulted in lower and higher anthocyanin accumulation in the stems of the trees after 2-week of long-day treatment (Ding et al., 2021), respectively, indicating that PtPIF8a is a positive regulator of anthocyanin biosynthesis. However, the exact molecular mechanism of how PtPIF8a regulates anthocyanin biosynthesis remains unknown. PIF transcription factors have been identified in nearly all land plants and had evolved long before the emergence of land plants (Possart et al., 2017). Given the similarity of the anthocyanin phenotypes and the shared protein features between Chrysanthemum and Populus, it would not be unexpected that the molecular mechanisms underpinning the PIF8-mediated anthocyanin biosynthesis are similar between the two species. However, it is also important to note that not all PIFs perform similar functions: under the R light, PIF4 and PIF5 have been demonstrated to negatively regulate anthocyanin biosynthesis by directly binding the promoters of the anthocyanin biosynthesis structural genes, such as F3′H and DFR. PIF1 is a negative regulator for FR light promotion of anthocyanin biosynthesis as well. The quadruple mutant of pif1,3,4,5 (pifq) exhibits the highest increase in anthocyanin accumulation (Liu et al., 2015; Pacín et al., 2016), suggesting the antagonizing functions among these PIFs in regulating anthocyanin biosynthesis. It has been reported that low R/FR light ratio decreases anthocyanin accumulation in many horticulture plant species, including baby leaf lettuce (Lactuca sativa L.), red pak choi (Brassica rapa var. chinensis, “Rubi F1”), and broccoli (Brassica oleracea var. Italica) (Li and Kubota, 2009; Mickens et al., 2019; Nguyen et al., 2021). However, high R/FR light ratio only promotes anthocyanin accumulation in broccoli, but has no effect on the anthocyanin accumulation in baby leaf lettuce and red pak choi (Li and Kubota, 2009; Samuolienė et al., 2012;,Mickens et al., 2019), suggesting that high R/FR light ratio promotes anthocyanin biosynthesis, depending on species specificity. Evolution of PIFs in different species might fine-tune their unique adaptive strategies, though this is less so for Chrysanthemum, which is primarily used as a horticultural crop. Extending the study to other Chrysanthemum species in nature might help further reveal the importance.
In Arabidopsis, COP1/SPA ubiquitin ligase affects PAP1/2 both transcriptionally and post-translationally (Maier et al., 2013). The same complex also degrades PIF8 in the dark (Oh et al., 2020). This synergistic regulation of the photomorphogenesis and anthocyanin genes provides a plausible mechanism for how plants quickly respond to the changing surrounding light environments. However, whether PIF8 participates in anthocyanin regulation and the molecular relationships between the PIF8 and R2R3 MYB remains unclear. We found that CmbHLH16 interacts with R2R3 MYB repressor CmMYB4, thereby titrating the formation of the CmMYB4–CmTPL complex, which in turn promotes the anthocyanin biosynthesis. These suggest that besides the suppression of anthocyanin activators, the active participation of anthocyanin repressor also plays an important role in fine-tuning anthocyanin biosynthesis for plants in response to changing light conditions. Another noticeable difference from our study is that CmMYB4, instead of interacting with CmbHLH2 to destabilize the MBW complex, thereby actively repressing anthocyanin biosynthesis at the translational level as in other systems (Aharoni et al., 2001; Jun et al., 2015; Xu et al., 2017), recruits the corepressor CmTPL and possibly other components of the histone modification apparatus such as CmHDA9 and CmHDA19 to deacetylate histone H3 and repress the expression of CmbHLH2 at the transcriptional level. In Medicago, when the EAR motif was mutated in the MYB2, complementary YFP signal can still be observed between the mutated MYB2 and TT8 in BiFC assay (Jun et al., 2015), suggesting that the EAR motif is not required to mediate the interaction between the MYB and bHLH protein, but to facilitate the recruitment of TPL-like proteins to fine-tune the anthocyanin biosynthesis. Our study expanded the function of the SG4 MYBs, which not only exert their anthocyanin biosynthesis repression functions by inhibiting the formation of the MBW complex, but can also fine-tune the anthocyanin biosynthesis regulation at the transcriptional level and reveals another layer of intricate regulation of anthocyanin biosynthesis.
Plants grown in nature never experience several times higher ratio of R/FR light than the natural light, but in protected cultivation, supplementing different quality of light with LEDs is an effective strategy to regulate yield and quality in some crops. For example, in baby leaf lettuce, supplementing R light on the WL background substantially increases the concentration of phenolics (Li and Kubota, 2009). Therefore, taking advantage of manipulating CmbHLH16 (PIF8) to achieve various levels of anthocyanin accumulation in flower petals is an attainable goal in plant breeding practice, potentially with less energy input. However, other factors must also be taken into consideration. CmbHLH16 is a putative phy interacting partner, it is conceivable that alteration of CmbHLH16 might illicit a range of other developmental phenotypes related to photomorphogenesis. Additionally, anthocyanins are secondary metabolites, besides their aesthetic appeal, they are important components for plants to cope with biotic and abiotic stresses, whether manipulating CmbHLH16 would show any of these pleiotropic effects remains to be illuminated. Applying a flower-specific promoter to manipulate the expression of CmbHLH16 or functionally interrogate the promoter of CmbHLH16 to isolate the cis-element that is responsible for flower to response to different R/FR ratio present promising technical routes moving forward. A detailed understanding of how plants react to light quality under artificial setting is essential for building a more ecological and environmentally sustainable future.
Materials and methods
Plant material and growth conditions
Chrysanthemum (C. morifolium) cultivar “Nannong Fencui” were obtained from the Chrysanthemum Germplasm Resource Preservation Center, Nanjing Agricultural University, China. “Nannong Fencui” tissue cultures were maintained on Chrysanthemum subculture medium (Murashige and Skoog medium supplemented with 2 mg·L−1 6-benzylaminopurine and 0.5 mg·L−1 1-naphthylacetic acid) at 24°C under a 16–8 h light–dark photoperiod. The cuttings of 2-month-old tissue cultures were rooted in plug trays for 15 days and then cultivated in a greenhouse for another 45 days under the same conditions. Subsequently, the cuttings from seedlings of similar size were continuously rooted in the plug tray for 15 days and then transplanted into smart glass greenhouse for 3 months at a day/night temperature of 24°C/18°C under an 8-h/16-h light/dark photoperiod and 70% relative humidity for flowering induction. At the budding stage, the plants were treated under natural light (PPFD: ∼135.29 ± 1.46–173.98 ± 1.20 µmol m−2 s−1 and R/FR light ratio: ∼1.09 ± 0.01), natural light supplemented with FR light using LEDs (AC86-260V-40W; EPILED) (PPFD: ∼135.37 ± 1.46–173.81 ± 1.07 µmol m−2 s−1 and R/FR light ratio: ∼0.22 ± 0.04), or natural light supplemented with R light using LEDs (AC86-260V-40W; EPILED) (PPFD: ∼146.84 ± 1.41–191.50 ± 0.94 µmol m−2 s−1 and R/FR light ratio: ∼7.18 ± 1.42). The flower colour phenotype was observed about 1 week after blooming. For short-term light treatment for RT-qPCR assays and protein stability assays, Chrysanthemum plants were maintained in a plant incubator under WL (PPFD: 175 µmol m−2 s−1 and R/FR light ratio: ∼1.13) and WL supplemented with R (PPFD: 182.13 µmol m−2 s−1 and R/FR light ratio: ∼7.35) or FR (PPFD: 175.07 µmol m−2 s−1 and R/FR light ratio: ∼0.19) light with 70% relative humidity. WL was supplied using white fluorescent bulbs (TSZJD2-T5-28W; TOPSTAR) and FR and R light using LEDs (AC86-260V-10W; EPILED). The R/FR light ratio for the natural light was calculated as the quantum flux density from 655 to 665 nm divided by the quantum flux density from 725 to 735 nm; while the R/FR light ratio for the fluorescent light in a plant incubator was calculated as the quantum flux density from 650 to 700 nm divided by the quantum flux density from 700 to 750 nm. The spectra of the light sources are provided in Supplemental Figure S10.
Vector construction and genetic transformation
To construct the 35S:CmMYB4-GFP vector, the full-length cDNA of CmMYB4 was cloned into the pORE-R4 vector (Coutu et al., 2007) under the control of the 35S promoter. To construct the transient overexpression vector, the full-length cDNAs of CmMYB6, CmbHLH2, CmMYB4, CmbHLH16, and CmTPL were cloned into the pSAK277 vector (Hellens et al., 2005) under the control of the 35S promoter, respectively. To construct the transient gene silencing vectors, CmMYB4, CmbHLH2, CmbHLH16, and CmTPL were cloned into the pCVA transient gene silencing vector, respectively. pCVB was used as an auxiliary vector for pCVA. For stable genetic transformation of 35S:CmMYB4-GFP transgenic plants, the resultant vector was transformed into “Nannong Fencui” tissue cultures using Agrobacterium tumefaciens strain EHA105, as described by Simmons et al. (2009). All primers used in this study are listed in the Supplemental Table S1.
Transient genetic transformation of the inflorescences was performed as described by Deng et al. (2012), with minor modifications. Briefly, at the budding stage, approximately 2-cm-long scapes (inflorescence stems) were wounded at 360° by scratching, and the wounds were covered with Agrobacterium-wetted cotton pellets for bacterial inoculation (approximately 1–2 mL for each scape) on the surface of the wounded area. Next, the infected plants were covered with black plastic bags for 3 days. Finally, the plastic bags were removed and the plants were treated under different light conditions to observe the flower color phenotype after blooming. The positively infected flowers were randomly divided into several groups for various experiments (e.g. RT-qPCR assay and anthocyanin content detection), and at least three biological replicates were set for each group to calculate standard deviation (SD).
The transient genetic transformation of the “Nannong Fencui” cultivar cutting seedlings was performed as described by Xu et al. (2020), with minor modifications. Briefly, the cuttings of 2-month-old tissue cultures were rooted in plug trays for 15 days and then immersed in the infiltration buffer with bacterial solution and vacuumed for 8 min under 0.7 MPa for thrice. After washing by deionized water, the infected cutting seedlings were placed at 8°C in the dark for 3 days and then transplanted in pots and grown under the normal conditions (24°C/18°C, 70% relative humidity, and 16-h light/8-h dark). Two weeks later, a leaf of each infected cutting seedlings was collected to extract DNA for agarose gel electrophoresis analysis for identifying positive infectors.
Gene expression analysis
Total RNA was isolated from Chrysanthemum plants using a Quick RNA isolation kit (Huayueyang, Beijing, China), following the manufacturer’s instructions. First-strand cDNA was synthesized using HiScript II Q Select RT SuperMix for qPCR (Vazyme Biotech Co. Ltd., Nanjing, China), following the manufacturer’s instructions. Next, cDNA was diluted to 100 ng μL−1 with ddH2O and RT-qPCR was conducted using the TB Green Premix Ex Taq II (Tli RNaseH Plus) (Takara, Dalian, China) in a reaction volume of 20 μL. CmEF1α and CmActin were used as the internal control.
Yeast libraries construction
Total RNA (1118 ng/μL, 559 μg, A260/A280 = 2.16) of “Nannong Fencui” ray flower petals was isolated to construct the Y1H and Y2H libraries. The construction of yeast libraries was performed according to the standard library preparation protocol (Invitrogen, Shanghai, China). The Y1H library capacity reached 8.7 × 106 cfu (colony forming unit), while the Y2H library capacity reached 1.1 × 107 cfu.
Y1H assay
For Y1H assays, full-length coding sequences (CDSs) of CmMYB4, CmbHLH16, and CmbHLH2 were inserted into the pGADT7 vector as preys. The promoter fragments of CmbHLH2, CmCHS, CmCHI, CmF3H, CmDFR, CmANS, and CmUFGT were cloned into the pHIS2 vector as baits. After cotransformation of the prey and bait into the yeast strain Saccharomyces cerevisiae Y187 using the lithium acetate method, the resultant yeast cells were plated onto a selective medium lacking Trp, Leu, and His (SD/-Trp/-Leu/-His). Subsequently, the positive colonies were inoculated on a Trp/-Leu/-His medium supplemented with an appropriate concentration of 3-AT (50 mM for CmCHIpro, CmF3Hpro, CmDFRpro, and CmANSpro; 80 mM for CmUFGTpro and CmbHLH2pro; and 100 mM for CmCHSpro) and grown for 3 days at 28°C to identify possible interactions. The CDS of β-glucuronidase (GUS) was inserted into the pGADT7 vector as the negative control.
Y2H assay
The Y2H assay was conducted as described by Zhou et al. (2019). Briefly, full-length CDSs of CmTPL, CmbHLH16, CmMYB4, CmMYB6, CmHDA6, CmHDA9, and CmHDA19 were inserted into the pGADT7 vector as preys. Full-length CDSs of CmMYB4, CmMYB4-mEAR, and CmTPL, domain-deletion constructs of CmMYB4 [N-(1-120aa), C-(121-281aa)] and CmMYB6 [N-(1-124aa)] were inserted into the pGBKT7 vector as baits. The resultant preys and baits were then cotransformed into S. cerevisiae strain Y2H using the lithium acetate method. After inoculating on a selective medium lacking Trp and Leu (-T/-L), the positive colonies were inoculated on a selective medium lacking Trp, Leu, His, and adenine (-L/-T/-H/-A) and grown for 2 days at 28°C to identify possible interactions. The empty pGADT7 vector was used as a negative control.
Y3H assay
The Y3H assay was conducted as described by Zheng et al. (2019). Briefly, the full-length CDS of CmMYB4 was inserted into the pGADT7 vector as a prey. The full-length CDS of CmbHLH16 was inserted into the pBridge vector under the control of Met-repressible pMET25 promoter. Subsequently, the full-length CDS of CmTPL was amplified and fused with the pBridge-CmbHLH16 plasmid to generate the pBridge-CmbHLH16-CmTPL vector as a bait. Next, the prey and bait were cotransformed into S. cerevisiae strain Y187 using the lithium acetate method. After inoculating on a selective medium lacking Trp and Leu (-T/-L), the positive colonies were inoculated on a selective medium lacking Trp, Leu, His, and Met (-L/-T/-H/-M) adding different concentration of Met (50, 100, and 200 μM) and grown for 3 days at 28°C to identify possible interactions.
In vitro pull-down assay
The in vitro pull-down assay was performed as described by Zhou et al. (2017a). The CDSs of CmMYB4, CmMYB4mEAR, and CmbHLH16 were amplified and cloned into the PGEX-4T-1 vector for GST-tag fusion. The CDSs of CmTPL and CmMYB4 were cloned into the pET32a vector for His-tag fusion. All proteins were individually expressed in E. coli BL21 (DE3) and purified with glutathione magnetic agarose beads (for the GST tag) or Ni-NTA magnetic beads (for the His tag) (both from Thermo Fisher Scientific, Shanghai, China). Subsequently, recombinant GST-tag fusion and His-tag fusion proteins were mixed in equal amount and, following incubation, purified with glutathione magnetic agarose beads. The pellet fraction was then detected via western blotting assays using anti-GST and anti-His (both from Thermo Fisher Scientific) antibodies to identify possible interactions.
BiFC assay
For BiFC assays, the CDSs of CmMYB4, CmMYB4mEAR, CmbHLH16, and CmbHLH2 were cloned into the pSPYNE vector, while the CDSs of CmTPL, CmMYB4, and CmMYB6 were cloned into the pSPYCE vector (Waadt et al., 2008). The resultant vectors were introduced into A. tumefaciens strain EHA105 and then transiently co-infiltrated in N. benthamiana leaves and visualized by the high-resolution confocal laser microscope (ZEISS, LSM800). The samples were visualized at the red channel for mRFP-NLS (laser wavelength: 561 nm: 0.27%, detection wavelength: 568–650 nm; detector gain: 777 V), green channel for YFP (laser wavelength: 488 nm: 3.6%, detection wavelength: 500–595 nm; detector gain: 710 V), and DIC (detection wavelength: 400–700 nm), respectively. The co-expressed 35S:D53-RFP construct was used as a nuclear marker.
In vitro competitive binding assay
In vitro competitive binding assays were conducted as described by Zheng et al. (2019), with minor modifications. Briefly, 5 μg of GST-CmTPL mixed with 1, 5, and10 μg His-CmbHLH16, respectively, was incubated with the Ni-NTA Magnetic Beads (Thermo Fisher Scientific) containing 5 μg His-CmMYB4 using 500 μL of pull-down buffer (150 mM NaCl, 20 mM Tris, 1 mM PMSF, 0.2% [v/v] Triton X-100, and 1% [v/v] protease inhibitor cocktail; pH 8.0) at 4°C for 6 h. Subsequently, the beads were washed five times with the pull-down buffer mentioned above. Proteins were eluted from beads, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and analyzed using anti-GST antibodies (Thermo Fisher Scientific). In vitro-translated His protein (5 μg) was used as the internal control. The combinations which 5 μg in vitro-translated His protein or 5 μg His-CmbHLH16 protein incubated with 5 μg of GST-CmTPL were used as negative controls.
EMSA
For EMSA, the CmMYB4 CDS was cloned into the pET32a vector for His-tag fusion. Next, the His-CmMYB4 protein was expressed in E. coli BL21 (DE3) and purified with Ni-NTA magnetic beads (Thermo Fisher Scientific, Shanghai, China). The probes were labeled with biotin at the 3′ ends. The unlabeled wild-type probes or the unlabeled mutant probes were added as cold competitors. EMSA was performed using the LightShift EMSA Optimization and Control Kit (Thermo Fisher Scientific) and Chemiluminescent Nucleic Acid Detection Module Kit (Thermo Fisher Scientific), following the manufacturer’s instructions.
ChIP-PCR assay
The leaves of 35S:CmMYB4-GFP transgenic tissue cultures were subjected to ChIP-PCR assays using Pierce ChIP-grade Protein A/G Magnetic Beads (Thermo Fisher Scientific). The 35S:GFP transgenic tissue cultures were used as the negative control. GFP recombinant rabbit monoclonal antibody (Thermo Fisher Scientific) was used. Subsequently, the enriched DNA fragments were examined by RT-qPCR.
To detect histone H3 acetylation level, the ray flower petals of plants generated by transient genetic transformation of constructs denoted in Figure 3A were used. The anti-acetyl-histone H3 antibodies (Millipore, Germany) were used.
Dual-LUC reporter assay
For dual-LUC reporter assay, the promoter fragment of CmbHLH2 was cloned into the pGreenII 0800-LUC vector (Zhou et al., 2021) to generate the reporter construct. The CDSs of the CmMYB4, CmMYB4mEAR, and CmbHLH16 were cloned into the pORE-R4 vector under the control of the 35S promoter to generate the effector constructs. Subsequently, the A. tumefaciens containing the reporter constructs and effector constructs, respectively, were transiently co-infiltrated in N. benthamiana leaves. The LUC-to-REN activity ratio was measured using the Infinite M200 luminometer (Tecan, Männedorf, Switzerland) with the Dual-Glo Luciferase Assay System (Promega, Beijing, China).
Subcellular localization
Subcellular localization assay was conducted as described by Chong et al. (2020). Briefly, the 35S:CmMYB4-GFP or 35S:CmbHLH16-GFP construct and the negative control 35S:GFP empty vector were transiently expressed in N. benthamiana epidermal cells. After holding at 22°C for 12–16 h in the dark, the N. benthamiana plants were cultured at a day/night temperature of 24°C/18°C under an 8-h/16-h light/dark photoperiod and 70% relative humidity for 2 days. Subsequently, the samples (prepared from leaves) were monitored for GFP activity with the same microscopy setup as described in the BiFC assay.
Anthocyanin content measurement
Total anthocyanin contents were quantified as described by Zheng et al. (2019).
Statistical analysis
All statistical analyses were performed using the data processing system (DPS) software (Tang and Zhang, 2013). ANOVA-Tukey correction tests were applied to determine the statistical significance among different treatments (P < 0.01, very significant; P < 0.05, significant; P ≥ 0.05, not significant).
Phylogenetic analysis
Alignments of MYB proteins or PIF proteins were made with Clustal W method. Phylogenetic tree and distance matrix table of MYB genes or PIF genes were generated in MEGA X using maximum likelihood (ML) method, based on James–Taylor–Thornton (JTT) model and 1000 bootstrap, and pair wise method, respectively.
Accession numbers
Sequence data from this article can be found in the database of the National Center for Biotechnology Information (NCBI) under the accession numbers: CmMYB4 (MW962301), CmMYB6 (KR002097.1), CmbHLH2 (KT724056.1), CmbHLH16 (MW962302), CmTPL (MW962303), CmCHS (MW368977), CmCHI (MW368978), CmF3H (MW368979), CmDFR (MW368982), CmANS (MW368983), and CmUFGT (MW368984).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Chrysanthemum “Nannong Fencui” cultivars were treated under different light conditions.
Supplemental Figure S2. Molecular characterizations of CmMYB4 in Chrysanthemum and the analysis of its phylogenetic relationships.
Supplemental Figure S3. CmMYB4 does not interact with CmbHLH2 and CmTPL interacts with CmHDA9 and CmHDA19.
Supplemental Figure S4. Relative expression of genes in the ray flower petals of the transiently transformed flowers.
Supplemental Figure S5. CmbHLH16 is a PIF family protein and is light-stable in response to different R/FR light ratios.
Supplemental Figure S6. CmMYB4 and CmbHLH2, but not CmbHLH16 directly regulate anthocyanin-associated structural genes.
Supplemental Figure S7. Interaction among CmbHLH16 and different fragments of CmMYB4.
Supplemental Figure S8. Relative expression of genes in the leaves of the transiently transformed Chrysanthemum cutting seedlings.
Supplemental Figure S9. Relative expression of genes in the ray flower petals of transiently transformed flowers in Figure 6.
Supplemental Figure S10. Spectra of light sources used in the experiments.
Supplemental Table S1. The sequence and usage of primers.
Data Availability
The authors confirm that all experimental data are available and accessible via the main text and/or the supplemental data.
F.C. and L.-J.Z. conceived and designed the research. Yu.W. and L.-J.Z. performed the experiments. Yi.W. and A.S. provided technical assistance. J.J., S.C., and Z.G. coordinated sequencing efforts. L.-J.Z. and B.D. analyzed the data and wrote the manuscript with input from all the authors.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://dbpia.nl.go.kr/plphys/pages/General-Instructions) is Fadi Chen ([email protected]).
Acknowledgments
We thank Dr. Yuehua Ma (Central Laboratory of the School of Horticulture, Nanjing Agricultural University) for assistance in using the high-resolution confocal laser microscope (ZEISS, LSM800).
Funding
This work was financially supported by grants from the National Natural Science Foundation of China (31902053, 31870279, 31730081), China Postdoctoral Science Foundation (2018M642273), Jiangsu Planned Projects for Postdoctoral Research Funds (2019K169), the Fundamental Research Funds for the Central Universities (KYQN202031), the National Key Research and Development Program of China (2019YFD1001500, 2020YFD1000400), the earmarked fund for Jiangsu Agricultural Industry Technology System, and A project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of interest statement. The authors declare that they have no conflicts of interest.
References
Samuolienė G, Brazaitytė A, Sirtautas R, Sakalauskienė S, Jankauskienė J, Duchovskis P, Novičkovas A, , , , , , (
Author notes
These authors contributed equally (L.-J.Z. and Yu.W.)



