-
PDF
- Split View
-
Views
-
Cite
Cite
Jathish Ponnu, Breaking bud: a gentian FLOWERING LOCUS T controls budbreak and dormancy, Plant Physiology, Volume 189, Issue 2, June 2022, Pages 457–458, https://doi.org/10.1093/plphys/kiac079
Close - Share Icon Share
Unlike annual plants that complete their lifecycle within one growing season, perennial plants experience repetition of growth cycles extended across multiple seasons: active vegetative and reproductive growth occur in summers followed by dormancy or resting period in winters. Dormancy in perennial species protects vegetative buds from harsh environmental conditions, such as chilling temperatures and frost. Depending on the origin of signals, dormancy in buds can be classified into paradormancy (from distal organs), endodormancy (internally from the buds), and ecodormancy (from the environment). Various endogenous and environmental factors, such as phytohormones, sugar levels, day length, and temperature, control the phases of bud dormancy (Maurya and Bhalerao, 2017; Lloret et al., 2018). FLOWERING LOCUS T (FT), the component of florigen that plays a key role in floral transition (Srikanth and Schmid, 2011), also regulates growth cessation and dormancy in perennial plants (Singh et al., 2017). In the annual plant Arabidopsis (Arabidopsis thaliana), many internal and external cues regulate the expression of FT and other floral integrators, which ultimately leads to the activation of floral meristem identity genes and flowering initiation (Srikanth and Schmid, 2011). In perennial species like poplar, grapevine, and peach, the putative orthologs of FT regulate dormancy and phase transitions by acting along with phytohormones and sugars (Singh et al., 2017).
In this issue of Plant Physiology, Takahashi and co-workers (Takahashi et al., 2022) establish the unconventional role of an FT ortholog (GtFT2) in dormancy release and budbreak in Japanese gentians (Gentiana triflora, a beautiful flowering perennial belonging to the family Gentianacae) (Figure 1). A previous study had identified two gentian FT orthologs, GtFT1 and GtFT2, implicated in floral induction (Imamura et al., 2011). The expression of GtFT1 increases during floral transition in early-flowering gentian varieties, confirming the role of GtFT1 as a floral promoter. However, the expression of GtFT2 does not differ between early- and late-flowering varieties, suggesting that GtFT2 may have unknown major functions (Imamura et al., 2011).
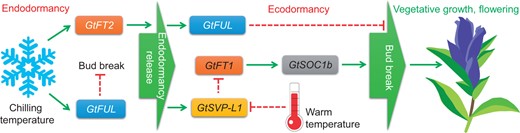
A hypothetical model of GtFT2 function in endodormancy release. The green arrows denote activation and the red dashed lines represent suppression. Modified from Takahashi et al. (2022). Gt, Gentiana triflora; FT, FLOWERING LOCUS T; FUL, FRUITFUL; SVP-L1, SHORT VEGETATIVE PHASE-LIKE 1; and SOC1b, SUPPRESSOR OF OVEREXPRESSION OF CO 1 b.
As a first step to functionally differentiate the FT orthologs, Takahashi et al. monitored expression levels of GtFT1 and GtFT2 in the leaves and apices of field-grown gentian plants throughout the year. In Japanese gentians, the vegetative overwintering buds are produced underground, enter into endodormancy during winter, and subsequently transition to ecodormancy via the process known as endodormancy release. During spring, the buds that experienced endodormancy release, undergo budbreak, and produce new vegetative shoots that continue to grow and flower for several months (Takahashi et al., 2014). The expression of GtFT2 peaked during the transition between endodormancy and ecodormancy, indicating that GtFT2 functions in endodormancy release. In addition, the expression of GtFT2 increased in overwintering buds exposed to chilling temperatures (4°C) that release endodormancy (Takahashi et al., 2014), confirming the role of GtFT2 in dormancy phase transitions. Consistent with the minor role of GtFT2 in flowering, Gtft2 mutants generated via CRISPR-Cas9 genome-editing did not show a substantial difference in flowering time compared with wild-type (WT) gentian plants. However, the overwintering buds of Gtft2 mutants lagged in budbreak for at least 3 weeks and exhibited a significantly lower budbreak frequency compared with the WT buds. While the expression of many floral integrator genes did not differ, the increased expression of gentian FRUITFUL (GtFUL) and SHORT VEGETATIVE PHASE-LIKE 1 (GtSVP-L1) observed in WT upon chilling exposure was absent in Gtft2 plants, indicating that GtFUL and GtSVP-L1 act downstream of GtFT2 in endodormancy release (Figure 1). As GtFUL expression increased during ecodormancy and Gtft2 mutation significantly affected GtFUL expression, the authors generated Gtful mutant plants via CRISPR-Cas9 to study the function of GtFUL in ecodormancy. The Gtful mutants showed a hastened budbreak but a severe delay in further growth and flowering, indicating that GtFUL functions in multiple pathways, such as flowering and dormancy. By preventing precocious budbreak, the expression of GtFUL ensures a timely release of endodormancy and subsequent transition to flowering.
Endodormancy release in Japanese gentians may involve additional unknown factors, as the transition to ecodormancy was delayed but not completely abolished in Gtft2 mutants. In contrast to GtFT2, GtFT1 may have multiple functions, as the expression of GtFT1 peaked at two stages—budbreak and floral initiation. The dissimilar functions of gentian FT orthologues may in part be due to the different genes they activate at various phase transitions. For example, GtFT2 may activate GtFUL and GtSVP-L1 expression specifically during endodormancy release. Future research on the phase-specific activation of downstream genes may provide further insights into the diverse functions of gentian FT orthologs. By demonstrating the distinct role of GtFT2 in endodormancy release, the work by Takahashi and co-workers (Takahashi et al., 2022) proposes that the perennial orthologs of FT may have evolved to perform diverse functions at various phase transitions.
Conflict of interest statement. None declared.



